Abstract
Objective
To examine the independent associations of leisure-time aerobic physical activity (PA) and resistance exercise (RE) on all-cause mortality in cancer survivors.
Patients and Methods
Patients included 2,863 male and female cancer survivors, aged 18 to 81 years, who received a preventive medical examination between April 8, 1987 and December 27, 2002 while enrolled in the Aerobics Center Longitudinal Study in Dallas, Texas. PA and RE was assessed by self-report at the baseline medical examination. Cox regression analysis was performed to determine the independent associations of PA and RE on all-cause mortality in participants who reported a history of cancer.
Results
PA in cancer survivors was not associated with a lower risk of all-cause mortality. In contrast, RE was associated with a 33% lower risk of all-cause mortality (95% CIs: 0.45–0.99) after adjusting for potential confounders, including PA.
Conclusions
Individuals who participated in RE during cancer survival had a lower risk for all-cause mortality, and the association was stronger in older individuals. The current findings provide preliminary evidence for benefits of RE during cancer survival. Future randomized controlled trials examining RE and its impact on lean body mass, muscular strength and all-cause mortality in cancer survivors are warranted.
Keywords: Physical activity, Aerobics Center Longitudinal Study, Metabolic equivalent
INTRODUCTION
Cancer is the second leading cause of death and accounts for 23% of all deaths in the United States.1 In 2013, it was estimated that approximately 1.6 million new cancer cases will be diagnosed, and 68% of survivors will live more than 5 years.1 The number of cancer survivors will continue to increase each year with improvements to early detection and treatment. Although commonly associated with the period following treatment, cancer survival is defined as the time between cancer diagnosis and mortality. Cancer survival is associated with decrements in health status, and increases the risk for all-cause mortality.2 Physical activity (PA) is a modifiable risk factor known to decrease the occurrence of disease and all-cause mortality,3 and may improve a cancer survivor’s quantity and quality of life.4–6
Individuals diagnosed with cancer have an approximately 50% higher risk of non-cancer mortality than the general population.7 There is growing evidence to suggest PA is beneficial for individuals diagnosed with cancer.8–13 Regular PA during cancer survival can lead to the maintenance and/or improvements in body composition, physical function, and overall quality of life.9 In addition, PA following diagnosis reduces the risk of cancer-specific mortality in breast cancer survivors,14–16 and decreases all-cause mortality in colorectal and prostate cancer survivors.17,18 It is rational to think resistance exercise (RE) training may also have similar benefits in cancer survivors as it does in healthy populations;19 however, there is limited research on the impact of RE on all-cause mortality in cancer survivors. Furthermore, many questions remain on which type of PA may be most beneficial for cancer survivors. Therefore, the purpose of this study was to examine the effects of leisure-time aerobic PA and RE on all-cause mortality in cancer survivors. It was hypothesized that both PA and RE would be associated with a decreased risk of all-cause mortality in cancer survivors.
METHODS
Study Population
Between April 8, 1987 and December 27, 2002, 3,388 men and women ages 18 to 81 with a previous diagnosis of cancer received a comprehensive preventive medical examination at The Cooper Clinic in Dallas, Texas and were enrolled in the Aerobics Center Longitudinal Study, a prospective epidemiological investigation. It should be noted that specific information related to the cancer diagnosis and treatment (i.e., type, stage, location) was not available at the time of baseline examination, and therefore anyone who responded positively to the question “have you had any type of cancer” was included in the current analysis. Detailed information about the study population has been published previously.20 Participants were sent to the clinic by their employers for examination, referred by their personal physician, or self-referred. Participants were volunteers and did not receive monetary assistance for participation. The study protocol was approved annually by the institutional review board of the Cooper Institute.
Participants were excluded from the final analysis if they were underweight (body mass index or BMI <18.5) (n=101); reported myocardial infarction (n=127) or stroke (n= 32); died during first year of follow-up (n=115); or had missing data on RE (n=78) or PA (n=72). These criteria resulted in 2,863 participants (859 women), who were followed until the date of death or December 31, 2003. Participants were predominantly white, well-educated, and within the middle to upper socioeconomic strata.21
Baseline Examination
Participants completed a comprehensive medical examination which included a physical evaluation by a physician, personal and family medical history questionnaire, anthropometry, blood pressure, and fasting blood chemistry. Detailed procedures regarding baseline measurements have been described previously.20 Height and weight were measured, and BMI was computed as weight per meter squared (kg/m2). Resting blood pressure was measured by trained technicians using standard ausculatory methods in a seated position and was recorded as the first and fifth Korotkoff sounds, respectively. Two readings separated by one minute were averaged. Overnight fasting serum concentrations of total cholesterol, triglycerides, and glucose were analyzed using standardized automated bioassays at the Cooper Clinic chemistry laboratory.
Baseline medical conditions were determined as having a physician diagnosis or measured phenotypes that met clinical thresholds. Hypercholesterolemia was defined as having total cholesterol levels ≥240 mg/dL (6.2 mmol/L) or physician diagnosis. Diabetes was defined as have fasting glucose levels ≥126 mg/dL (7.0 mmol/L), the use of insulin, or physician diagnosis. Hypertension was defined as having a resting systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or physician diagnosis. Parental history of cancer, smoking habits (current smoker or not) and alcohol intake (number of drinks per week) were obtained from the medical questionnaire. Heavy drinking was defined as consuming ≥ 7 drinks per week for women and ≥ 14 drinks per week for men.
Leisure-time Aerobic PA
Self-reported PA during the past 3 months was obtained from the medical questionnaire at baseline examination. Detailed procedures regarding the assessment of PA has been described previously.22 In brief, a metabolic equivalent (MET) value was assigned to each PA contained within the medical questionnaire, and then multiplied by the frequency and duration of each PA performed. PA values were summated and represent the total volume of PA, which is expressed as the total MET-minutes per week. Meeting the current PA guidelines was defined as performing ≥ 500 MET-minutes per week. Additionally, we grouped participants into physically active and inactive based on walking and jogging because they were the most common activities for the ACLS population23.
Resistance Exercise
RE was assessed by self-report on the medical history questionnaire. Participants were asked to provide yes/no answers to the following questions: 1). “Are you currently involved in a muscle-strengthening program?” 2). Can you specify the muscle-strengthening activity as “Calisthenics”, “Free Weights”, “Weight Training Machines”, or “Other”? 3). How many days per week do you do these exercises? Those that responded “Yes” to free weights or weight training and had exercised at least one day per week were classified as positive for RE.
Mortality Surveillance
Study participants were followed up for all-cause mortality from baseline examination through the end of December 2003. Official death certificates were obtained from the National Death Index and recorded throughout all analysis. The National Death Index has been shown to be an accurate method of ascertaining deaths in observational studies, having high sensitivity (96%) and specificity (100%).24
Statistical Analysis
Continuous variables were summarized using mean ± standard deviation and categorical variables were summarized using frequency (%). Continuous variables were compared using Student’s t-test and categorical variables were compared using the Chi-square test. Follow-up time was computed as the difference between the date of the baseline examination and date of death for decedents or through the end of 2003 for survivors. Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality according to categories of RE or leisure-time aerobic PA in order to quantify the strength of these associations. Adjusted models were used to control for potential confounding factors at baseline examination. Model 1 adjusted for age, gender, and examination year, and model 2 adjusted for variables in model 1 plus BMI, current smoking (yes or no), heavy drinking (yes or no), hypertension (present or not), diabetes (present or not), hypercholesterolemia (yes or no), and parental history of cancer (yes or no). Model 3 adjusted for variables in model 2 plus leisure-time aerobic PA (MET-minutes/week when resistance exercise was the exposure) or RE (yes or no when aerobic activity was the exposure). The Kaplan-Meier method was used to calculate survival curves. The log rank test was used to compare survival between RE and no RE. Finally, we conducted stratified analysis to test physical activity-related interaction on the association between RE and all-cause mortality using interaction term. All of the statistical analyses were performed by SAS software (SAS Institute, Cary, NC), and all the P values are 2 sided, with an α-level of .05.
RESULTS
Among 2,863 men and women with a cancer diagnosis there were a total of 121 deaths (4.2%) during an average 7.3 years of follow-up. The baseline characteristics of the study population are presented in Table 1. Participants were middle aged (54 ± 11 years), mostly men (70%), slightly overweight (BMI, 25.9 ± 4.1 kg/m2), predominantly active (60.9%), and non-smokers (91.2%). Participants who performed RE had a lower BMI, total cholesterol, triglycerides, fasting blood glucose, and incidence of hypercholesterolemia and hypertension (Table 1). In addition, cancer survivors who performed RE engaged in more PA than their counterparts who did not perform RE.
Table 1.
Baseline characteristics of cancer survivors in the Aerobics Center Longitudinal Study, 1987 to 2002.
| All (n=2,863) | Resistance exercise
|
P value | ||
|---|---|---|---|---|
| No (n=1612) | Yes (n=1251) | |||
| Age (year) | 54.4 (10.5) | 54.5 (10.5) | 54.2 (10.5) | .42 |
| Female (%) | 30.0 | 32.0 | 27.4 | .008 |
| Body mass index (kg/m2) | 25.9 (4.1) | 26.5 (4.4) | 25.2 (3.6) | <.001 |
| Lipid Profile (mg/dL) | ||||
| Total cholesterol | 204.2 (38.5) | 207.8 (39.0) | 199.5 (37.2) | <.001 |
| Triglycerides | 127.3 (88.4) | 135.7 (89.1) | 116.4 (86.4) | <.001 |
| Fasting blood glucose (mg/dL) | 99.4 (17.0) | 100.4 (19.3) | 98.1 (13.4) | <.001 |
| Blood Pressure (mm Hg) | ||||
| Systolic | 124 (17) | 125 (17) | 124 (17) | .13 |
| Diastolic | 81 (10) | 82 (10) | 81 (10) | .005 |
| Leisure-time aerobic physical activity (MET-minutes/week) | 1019.4 (1313.2) | 748.5 (1048.7) | 1368.5 (1521.4) | <.001 |
| Meet current guidelinesa (%) | 61.0 | 48.9 | 76.6 | <.001 |
| Current smoker (%) | 8.8 | 9.7 | 7.6 | .04 |
| Heavy drinkerb (%) | 13.6 | 12.5 | 15.0 | .06 |
| Baseline medical conditionsc (%) | ||||
| Hypercholesteremia | 36.8 | 39.3 | 33.5 | .001 |
| Diabetes | 6.6 | 7.2 | 5.8 | .12 |
| Hypertension | 38.4 | 40.3 | 36.0 | .02 |
| Skin cancer | ||||
| Parental history of cancer | 9.9 | 9.3 | 10.6 | .27 |
Values are reported as mean ± SD.
Defined as performing ≥ 500 MET-minutes/week.
Defined as consuming ≥ 7 drinks/week for women and ≥ 14 drinks/week for men.
Defined as the presence of hypercholesterolemia [history of physician diagnosis or total cholesterol level ≥240 mg/dL (6.20 mmol/L)]; diabetes [history of physician diagnosis, use of insulin, or fasting glucose level ≥126 mg/dL (7.0 mmol/l)]; or hypertension (history of physician diagnosis, resting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg).
Abbreviations: dL, decaliter. kg, kilograms. L, liters. MET, metablic equivalent. m, meters. mg, milligrams. mm Hg, millileters of mercury. mmol, millimole. min, minutes.
Table 2 shows the independent association between PA and all-cause mortality in cancer survivors. The association between PA and all-cause mortality was examined using 3 different models. For all 3 models, PA was not associated with a decreased risk of all-cause mortality in cancer survivors. The fully adjusted model showed a 1% non-significant higher risk (P=.97) of mortality in participants who performed 500 or more MET-minutes per week of PA than those who did not.
Table 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality by leisure-time aerobic physical activity (PA) levels among cancer survivors.
| No. of Death/Total | Model 1 a HR (95% CI) |
Model 2 b HR (95% CI) |
Model 3 c HR (95% CI) |
|
|---|---|---|---|---|
| Leisure-time aerobic PA | ||||
| < 500 MET-min/wk | 46/1117 | 1.00 | 1.00 | 1.00 |
| ≥ 500 MET-min/wk | 75/1746 | 0.91 (0.63 – 1.32) | 0.99 (0.67–1.46) | 1.01 (0.68 – 1.49) |
| P value | .62 | .95 | .97 | |
Adjusted for age, gender, and examination year.
Adjusted for variables in Model 1 plus body mass index, current smoking (yes or no), heavy drinking (yes or no), hypertension (present or not), diabetes (present or not), hypercholesterolemia (yes or no), and parental history of cancer (yes or no).
Adjusted for variables in Model 2 plus resistance exercise (days/wk).
Abbreviations: CI, confidence interval. HR, hazard ratio. No, number.
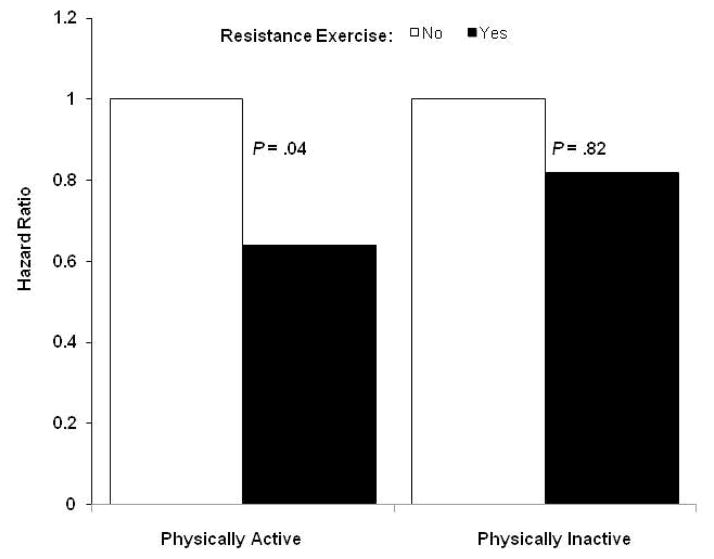
Table 3 shows the independent association between RE and all-cause mortality in cancer survivors. The risk of all-cause mortality is lower in participants who performed RE than those who did not. After adjustment for covariates (age, gender, examination year, smoking status, alcohol intake, BMI, chronic conditions, and family history of cancer), participants who performed RE (≥1 day per week) had a 33% reduction in all-cause mortality compared to those who did not (P<.05). Additional adjustment for PA did not materially change the above association. The Kaplan-Meier survival curves also indicate that cancer survivors who performed RE had greater survival probability as compared with those who did not (Figure 1). Additional analysis was performed to examine if physical activity moderated the association between resistance exercise and all-cause mortality (Figure 2). Indeed, there was an inverse relationship between RE and all-cause mortality in those who were physically active (P=.04), whereas this association was not observed in those who were physically inactive (P=.82).
Table 3.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause mortality by resistance exercise among cancer survivors.
| No. of Death/Total | Model 1 a HR (95% CI) |
Model 2 b HR (95% CI) |
Model 3 c HR (95% CI) |
|
|---|---|---|---|---|
| Resistance exercise | ||||
| No | 82/1612 | 1.00 | 1.00 | 1.00 |
| Yes | 39/1251 | 0.67 (0.46 – 0.99) | 0.67 (0.45 – 0.99) | 0.67 (0.45 – 0.99) |
| P value | .045 | .04 | .049 | |
Adjusted for age, gender, and examination year.
Adjusted for variables in Model 1 plus body mass index, current smoking (yes or no), heavy drinking (yes or no), hypertension (present or not), diabetes (present or not), hypercholesterolemia (yes or no), and parental history of cancer (yes or no).
Adjusted for variables in Model 2 plus leisure-time aerobic PA (in MET-minutes/week). Abbreviations: CI, confidence interval. HR, hazard ratio. min, minutes. No, number. PA, physical activity.
Figure 1.
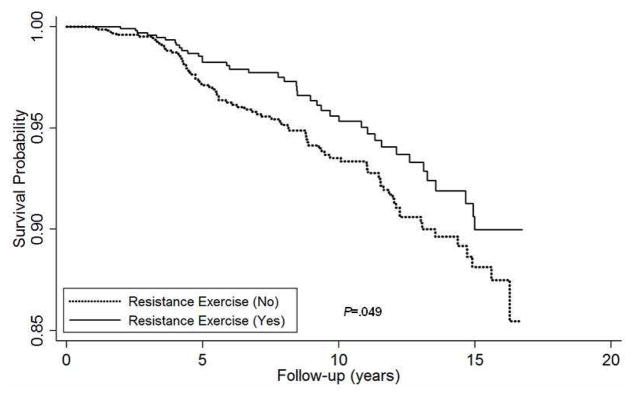
Kaplan-Meier survival curves for all-cause mortality by resistance exercise among cancer survivors, Aerobics Center Longitudinal Study, Dallas, Texas, 1987 to 2003.
Figure 2.
Multivariate*-adjusted hazard ratios of all-cause mortality according to resistance exercise performed at least 1 day per week across physical activity groups, Aerobics Center Longitudinal Study, Dallas, Texas, 1987 to 2003. *adjusted by age, gender, body mass index, current smoking (yes or no), heavy drinking (yes or no), hypertension (present or not), diabetes (present or not), hypercholesterolemia (yes or no), and parental history of cancer (yes or no).
DISCUSSION
With improvements in cancer diagnosis and treatment, the number of individuals living with cancer will continue to increase in forthcoming years. Identifying modifiable factors that increase the quality of life and reduce all-cause mortality risk during cancer survival is of great importance. Increased levels of PA have been shown to improve health outcomes following cancer diagnosis; however, the type of PA which is most beneficial for long-term cancer survival is not known. In the current study, cancer survivors who reported performing RE at least one day of the week had a 33% reduction in all-cause mortality compared with individuals who did not report participation in RE. Furthermore, there was an inverse relationship between RE and all-cause mortality in those that were physically active, but not in those that were physically inactive. Although leisure-time PA was not associated with decreased all-cause mortality, the current results support the benefits of RE and PA during cancer survival. By identifying the role of PA and RE on all-cause mortality following cancer diagnosis, clinicians may be more likely to promote these practices following cancer diagnosis.
To the best of our knowledge, this is the first prospective study to examine the associations between RE and all-cause mortality in cancer survivors. Previous reports have demonstrated positive relationships between muscular strength and decreased risk of cancer mortality in healthy men.25,26 In addition, clinically important outcomes (i.e., improved physical function and quality of life) can be achieved with RE training in cancer patients.8–13 These benefits may translate into increased survival; however, future research is needed to address this important question in cancer survivors. Our results demonstrate RE was associated with a reduction in all-cause mortality, which should be considered when providing advice to cancer survivors. With improvements in cancer treatment following diagnosis, many cancer survivors are now living longer than 65 years of age. It is well established that aging is associated with musculoskeletal perturbations (i.e., osteoporosis and sarcopenia), and therefore patients may experience age-related decrements to health at the time of or following diagnosis. In addition, reductions to bone mineral density and skeletal muscle mass can be observed during cancer treatment,27,28 which may lead to an even greater risk of all-cause mortality in older cancer survivors. Therefore, behavioral modifications that promote overall health and reduce adverse effects to treatment are of upmost importance during cancer survival.
Several mechanisms may be responsible for a reduced risk of all-cause mortality with RE during cancer survival. One primary benefit of RE training and muscle strengthening activities is increased loading to bone and skeletal muscle tissue. The musculoskeletal system has the ability to translate mechanical forces into biochemical signals in a process known as mechanotransduction.29 This response results in enhanced bone formation and muscle protein accretion following loading.30,31 When mechanical loading is continued over time, as with exercise training, it can lead to the maintenance and/or increase in bone mineral density and skeletal muscle mass. Thus, the adaptive response to RE is associated with improved muscle mass and muscular strength, which can translate into improved physical function and quality of life in cancer patients.8–13 Research has demonstrated a positive relationship between muscle strength and physical function in cancer survivors.8 Interestingly, these benefits can occur independent of changes in endocrine or immune function,8 demonstrating a unique role of mechanical loading and muscle contraction on the regulation of muscle plasticity during wasting conditions. Collectively, the importance of RE training for improvements on physical function in cancer survivors cannot be understated.
Additional biological mechanisms associated with decreased all-cause mortality with RE training may be related to modifications in glucose homeostasis, insulin and IGF-1 signaling, and inflammation. It has been reported over one-third of cancer patients demonstrate glucose intolerance and insulin resistance,32 which may contribute to increased co-morbidities and mortality in cancer survivors. In addition, insulin resistance may contribute to muscle wasting due to a decreased anabolic state of skeletal muscle. Acute physical exercise increases skeletal muscle glucose uptake independent of insulin action,33 and exercise training is associated with improved insulin sensitivity in cancer survivors.34 Additionally, elevated systemic inflammation during cancer contributes to muscle wasting and decreased survival.25,35 There is a strong inverse relationship between circulating inflammation and both cancer-specific and non-cancer survival.36 Based on the current body of literature, treatment modalities promoting the preservation of skeletal muscle mass and function are associated with improvements in cardiovascular risk factors and improved health outcomes,37,38 and may decrease an individual’s risk of all-cause mortality during cancer survival.
Interestingly, self-reported PA in the current study was not associated with decreased all-cause mortality in the current cohort of cancer survivors. This is in contrast to previous studies reporting positive benefits of PA and exercise during cancer survival.16,17,39,40 For example, Haydon et al.39 demonstrated improved disease-specific [HR = 0.73 (0.54–1.00), P=.05] and overall survival [HR = 0.77 (0.58–1.03), P=.08] in colorectal cancer patients that participated in exercise prior to diagnosis.39 In addition, Meyerhardt et al.40 found significant trends for post-diagnosis PA and improved disease-free (P for trend=.01), recurrence-free (P for trend=.03), and overall survival (P for trend=.01) in stage III colon cancer patients40 Furthermore, a reduced risk of cancer-specific death (>9 MET-hr/wk) and all-cause mortality (≥8.75 MET-hr/wk) have been reported in breast cancer survivors and colorectal survivors, respectively.16,17 In the current study, approximately 61% of the study population had reasonable PA at the time of examination. Additionally, the small number of deaths, relatively small sample size and self-report nature of PA status of the current cohort might limit the ability to detect significant changes, and thus the results reported here should be interpreted with caution. Taken collectively, there is sufficient evidence in the literature to support beneficial effects of PA on cancer recurrence and survival, and should be recommended to improve health outcomes in cancer survivors.
The current study has several limitations that should be addressed. The primary limitations were the small sample size and the assessment of PA and RE through self-report. As previously discussed, the relatively small number of deaths and sample size limited our ability to examine several factors such as the overall PA dose or the role of sex on PA associations. Further, it is well established self-reported exercise habits are subject to recall bias and is often over-reported or misclassified. Our observation of an inverse relation between RE and mortality rates only among physically active participants may reflect more precise reporting in this subgroup. Future studies should utilize objective measures, such as accelerometry or strength measurements, to provide proper classification and minimize subject bias. Additionally, the volume and intensity of RE activities was not quantified in the current study. It is known that manipulations to training intensity can result in different musculoskeletal, cardiovascular and metabolic adaptations. Thus, further research should establish optimal training parameters for the maintenance or improvement of clinically important outcomes during cancer survival. Moreover, dietary habits were not included in the current analysis, and should be considered in subsequent studies. Furthermore, the current population consisted of well-educated men and women, of middle to upper class socioeconomic status, with relatively high PA, which limits the generalization of the current findings. Also, due to the limited information, we were unable to determine the types of cancer. It has been suggested certain types of cancer may be more sensitive to changes in PA status. Finally, and probably most importantly, we were not able to demonstrate whether the association between higher reported RE and improved mortality was causal or whether selection bias (i.e., healthier and stronger cancer survivors may be more likely to perform and/or report higher levels of RE) is responsible for this powerful association, which even appears to be totally independent of leisure-time aerobic PA. Future research is needed to include this information in order to clearly establish the role of RE and PA on longevity in cancer survivors.
CONCLUSION
In summary, this study provides initial evidence that RE at least 1 day per week was associated with reduced risk of all-cause mortality in cancer survivors. The current findings along with previous evidence provides additional clinical significance and rationale for the integration of RE during cancer survival. If these findings are replicated in other studies, medical practitioners and clinicians should be aware of these benefits and discuss the importance of PA, particularly RE, during and after cancer treatment. The mechanisms associated with these benefits have yet to be clearly defined and further research on this issue is needed. In addition, it is necessary to determine if a specific type of PA may be more beneficial for certain cancers. Therefore, future prospective randomized controlled trials should be designed to address potential mechanisms between RE and health outcomes, including all-cause and disease-specific mortality, during cancer survival.
Acknowledgments
Financial support and disclosure: The research was supported by the US National Institutes of Health grants AG06945, HL62508, and DK088195.
The research was supported by the US National Institutes of Health grants (AG06945, HL62508, and DK088195). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the Cooper Clinic physicians and technicians for collecting the baseline data, and staff at the Cooper Institute for data entry and data management.
ABBREVIATIONS
- BMI
body mass index
- HRs
hazard ratios
- MET
metabolic equivalent
- PA
Physical activity
- RE
Resistance exercise
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 178(3):339–349. doi: 10.1093/aje/kws580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- 6.Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 7.Baade PD, Fritschi L, Eakin EG. Non-cancer mortality among people diagnosed with cancer (Australia) Cancer Causes Control. 2006;17(3):287–297. doi: 10.1007/s10552-005-0530-0. [DOI] [PubMed] [Google Scholar]
- 8.De Backer IC, Schep G, Backx FJ, Vreugdenhil G, Kuipers H. Resistance training in cancer survivors: a systematic review. Int J Sports Med. 2009;30(10):703–712. doi: 10.1055/s-0029-1225330. [DOI] [PubMed] [Google Scholar]
- 9.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal RJ, Reid RD, Courneya KS, et al. Randomized controlled trial of resistance or aerobic exercise in men receiving radiation therapy for prostate cancer. J Clin Oncol. 2009;27(3):344–351. doi: 10.1200/JCO.2007.15.4963. [DOI] [PubMed] [Google Scholar]
- 11.Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer. 2006;106(9):2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- 12.Herrero F, San Juan AF, Fleck SJ, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med. 2006;27(7):573–580. doi: 10.1055/s-2005-865848. [DOI] [PubMed] [Google Scholar]
- 13.Segal RJ, Reid RD, Courneya KS, et al. Resistance Exercise in Men Receiving Androgen Deprivation Therapy for Prostate Cancer. J Clin Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–765. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 15.Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers and Prev. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 16.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 17.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of Recreational Physical Activity and Leisure Time Spent Sitting With Colorectal Cancer Survival. J Clin Oncol. 2013;31(7):876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 18.Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J Clin Oncol. 2011;29(6):726–732. doi: 10.1200/JCO.2010.31.5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 21.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness. Evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 22.Lee DC, Sui X, Ortega FB, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. Br J Sports Med. 2011;45(6):504–510. doi: 10.1136/bjsm.2009.066209. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AS, Sui X, O’Connor DP, et al. Longitudinal cardiorespiratory fitness algorithms for clinical settings. American journal of preventive medicine. 2012;43(5):512–519. doi: 10.1016/j.amepre.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 25.Moses AG, Maingay J, Sangster K, Fearon KC, Ross JA. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep. 2009;21(4):1091–1095. doi: 10.3892/or_00000328. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz JR, Sui X, Lobelo F, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1468–1476. doi: 10.1158/1055-9965.EPI-08-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad S, Tan BH, Cui H, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin Nutr. 2012;31(1):74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Poterucha T, Burnette B, Jatoi A. A decline in weight and attrition of muscle in colorectal cancer patients receiving chemotherapy with bevacizumab. Med Oncol. 2012;29(2):1005–1009. doi: 10.1007/s12032-011-9894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H, Kamm RD, Lee RT. Cell mechanics and mechanotransduction: pathways, probes, and physiology. Am J Physiol Cell Physiol. 2004;287(1):C1–11. doi: 10.1152/ajpcell.00559.2003. [DOI] [PubMed] [Google Scholar]
- 30.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57(5):344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 31.Baar K, Nader G, Bodine S. Resistance exercise, muscle loading/unloading and the control of muscle mass. Essays Biochem. 2006;42:61–74. doi: 10.1042/bse0420061. [DOI] [PubMed] [Google Scholar]
- 32.Tayek JA. A review of cancer cachexia and abnormal glucose metabolism in humans with cancer. J Am Coll Nutr. 1992;11(4):445–456. doi: 10.1080/07315724.1992.10718249. [DOI] [PubMed] [Google Scholar]
- 33.Kristiansen S, Hargreaves M, Richter EA. Progressive increase in glucose transport and GLUT-4 in human sarcolemmal vesicles during moderate exercise. Am J Physiol. 1997;272(3 Pt 1):E385–389. doi: 10.1152/ajpendo.1997.272.3.E385. [DOI] [PubMed] [Google Scholar]
- 34.Lee DH, Kim JY, Lee MK, et al. Effects of a 12-week home-based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Support Care Cancer. 2013 doi: 10.1007/s00520-013-1822-7. [DOI] [PubMed] [Google Scholar]
- 35.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 36.McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41(1–2):64–69. doi: 10.1080/01635581.2001.9680613. [DOI] [PubMed] [Google Scholar]
- 37.Artero EG, Lee DC, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351–358. doi: 10.1097/HCR.0b013e3182642688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artero EG, Lee DC, Ruiz JR, et al. A prospective study of muscular strength and all-cause mortality in men with hypertension. J Am Coll Cardiol. 2011;57(18):1831–1837. doi: 10.1016/j.jacc.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55(1):62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]