Abstract
Background
This study evaluates the effect of a program combing specialized medication packaging and telephonic medication therapy management on medication adherence, health care utilization, and costs among Medicaid patients.
Research Design
A retrospective cohort design compared Medicaid participants who voluntarily enrolled in the program (n = 1007) compared with those who did not (n = 13,614). Main outcome measures were medication adherence at 12 months, hospital admissions and emergency department visits at 6 and 12 months, and total paid claim costs at 6 and 12 months. Multivariate regression models were used to adjust for the effect of age, sex, race, comorbidities, and 12-month preenrollment health care utilization.
Results
Measures of medication adherence were significantly improved in the program cohort compared with the usual care cohort. At 6 months, adjusted all-cause hospitalization was marginally less in the program cohort compared with the usual care cohort [odds ratio = 0.73, 95% confidence interval (CI), 0.54–1.0, P = 0.05]. No statistically significant differences were observed between the 2 cohorts for any of the other adjusted utilization endpoints at 6 or 12 months. Adjusted total cost at 6 and 12 months were higher in the program cohort (6-month cost ratio = 1.76, 95% CI, 1.65–1.89; 12-month cost ratio = 1.84, 95% CI, 1.72–1.97), primarily because of an increase in prescription costs. Emergency department visits and hospitalization costs did not differ between groups.
Conclusions
The program improved measures of medication adherence, but the effect on health care utilization and nonpharmacy costs at 6 and 12 months was not different from the usual care group. Reasons for these findings may reflect differences in the delivery of the specialized packaging and the medication therapy management program, health care behaviors in this Medicaid cohort, unadjusted confounding, or time required for the benefit of the intervention to manifest.
Keywords: adherence, Medicaid, outcomes, pharmacy service
As costs associated with medication use and medication-related problems continue to rise in the United States,1 there has been a greater focus on identifying effective methods for enhancing quality of care while simultaneously reducing costs. Medication therapy management (MTM), defined as “a distinct service or group of services that optimize therapeutic outcomes for individual patients,”2 has been identified as 1 promising method. Pharmacists are the most commonly utilized provider for part D MTM programs3 and these services represent an opportunity for pharmacists to provide more direct patient care and expand the scope of the profession’s role beyond medication preparation and dispensing.4 Core elements of MTM include: (a) completing a medication therapy review to identify medication-related problems; (b) developing a personal medication record and “action plan” for the patient; (c) implementing interventions and/or providing referral(s) when warranted; and (d) providing follow-up care.5 In recent years, MTM has become more integrated into Medicare, because all part D sponsors are required to offer this service to optimize medication use through promoting patient understanding of medications, enhancing medication adherence, and preventing/detecting adverse drug events.6 In addition to part D programs, MTM services have been provided to private-pay patients, through employer-sponsored insurance programs, and through state-sponsored programs such as Medicaid. Although published studies examining MTM interventions provided by pharmacists have yielded positive results,7–11 questions remain regarding the optimal design for maximizing positive health outcomes. Furthermore, few published reports have described evaluations of MTM services in state Medicaid populations.12–16 Typically, MTM programs have targeted specific disease states,13 used process measures or surrogate outcomes as endpoints,12,15 and used self-reported cost avoidance estimates16 rather than actual costs.
This report describes a program evaluation of a pharmacy service delivery model that combined a telephonic MTM service with specialized medication packaging. Combining strategies for improving medication adherence including specialized medication packaging and telephonic MTM has been supported by recent reviews.17,18 The current program is intended to: (a) promote medication adherence through specialized packaging during the drug dispensing process; and (b) engage pharmacists and patients in discussions to facilitate identification and resolution of medication-related problems and concerns. The current evaluation builds on existing literature by examining the effect of this pharmacy service model on medication adherence, health care utilization, and health care spending in a cohort of Medicaid beneficiaries.
METHODS
Program Description
A private pharmacy services company provided a proprietary multifaceted program that combined both specialized medication packaging with telephonic MTM provided by pharmacists. The program was intended to obviate the need for multiple pharmacy trips, simplify and improve accessibility to prescription meds, and improve the overall quality of the medication regimen. The specialized packaging organized prescription medications, over-the-counter medications, and vitamins into presorted packets (with all medications combined) that were clearly marked with the date and time that the dose(s) is to be taken. Packets are assembled into a dispensing box containing a 28-day supply delivered by mail to the patient or caregiver’s home. Transfer of the patients’ medications typically occurred over a period of 1–2 months during which the patient profile was built, all new and existing prescription records were transferred to the program from all other pharmacies, and the patients’ prescriptions were synchronized to enable dispensing of all medications on the same day of each month. Before each monthly dispensing box was mailed, a proactive telephone call to the patient by a pharmacy technician occurred 1–2 weeks prior to verify the medication regimen and update any changes.
In addition, an MTM program was conducted through telephone for all program patients by pharmacists who received special training in the delivery of MTM along with a manual detailing the components of MTM based on national recommendations.5 During the initial MTM phone call, the pharmacist reviewed the entire drug profile with the patient and/or their caregiver(s) to verify the safety of prescription combinations, identify any drug-related therapy problems, and develop a plan to resolve any identified problems (eg, avoid/mitigate adverse effects, add/increase medications to treat conditions, remove/decrease unnecessary medications).19 The pharmacist liaised with other health care providers or initiated further follow-up contact with the patient, as needed, to resolve problems and improve the effectiveness of the overall medication regimen. Program patients paid no additional fees to receive the program, and out-of-pocket copayment costs for medications received through the program were identical to copayments for medications received through traditional community pharmacies.
Program Enrollment
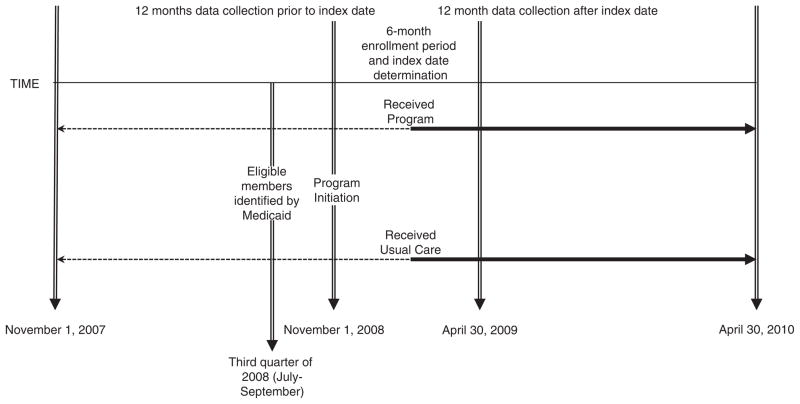
Beginning on November 1, 2008, the program was offered as a covered benefit for Indiana Medicaid members under their managed-care program, which provides comprehensive care coordination, disease management, and utilization management for members who are aged, blind, or disabled; including individuals with physical disabilities as well as intellectual and developmental disabilities and the seriously mentally ill population.20 During the third quarter of 2008, Medicaid selected members for the program. Eligible members: (a) were enrolled, as of July 1, 2008, in the Medicaid managed-care program; (b) remained continuously eligible for this service through the covered benefit start date (November 1, 2008); and (c) received ≥5 Medicaid-covered prescription medications during the third quarter of 2008. Members who received home-based or community-based waiver services were ineligible (Figs. 1 and 2).
FIGURE 1.
Study timeline.
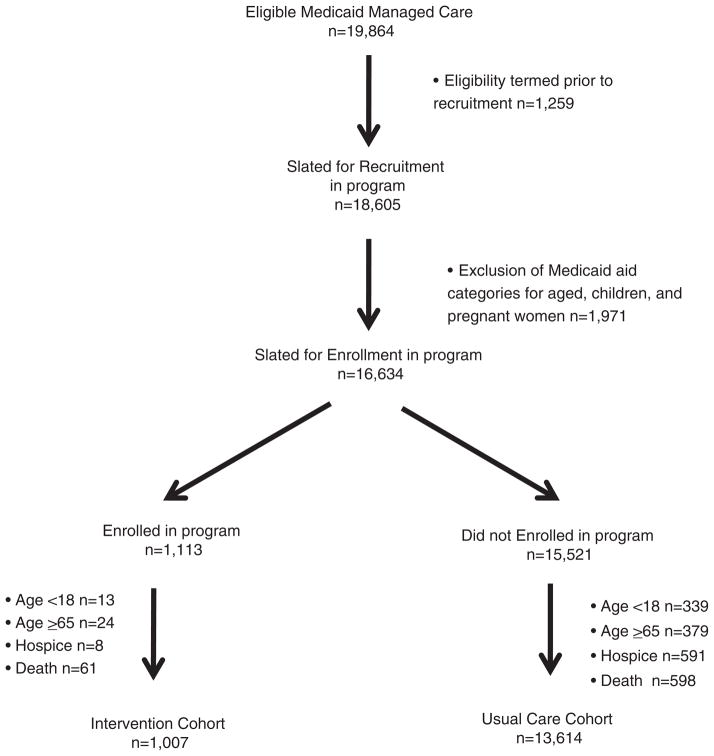
FIGURE 2.
Flow diagram of recruitment and enrollment.
During the first 6 months of the program, telephone calls were placed by Medicaid to members in randomized block sequence, according to their zip code of residence. During the call, the program was explained and members who expressed immediate interest were transferred to the enrollment center. Members who expressed some interest provided their contact information for subsequent follow-up. Three telephone calls were placed during varying days and times before a member was classified as unreachable. In addition, a letter was mailed to all eligible members describing the program and encouraging participation by contacting the enrollment center directly. Entry to the program was voluntary and required eligible members to opt in. Once enrolled, members could opt out of the program at any time for any reason.
Design
The authors were part of an evaluation team hired by Medicaid to conduct an evaluation of program effectiveness. We used a retrospective cohort design to evaluate medication adherence and utilization of health services and costs during a 12-month program period among eligible Medicaid participants who opted to enroll in the program compared with those who did not. For the retrospective cohorts, selection of patients into the program cohort included members who opted into the program and received at least 1 month of medication dispensing between November 1, 2008 and June 30, 2009. The comparison (usual care) cohort included all eligible Indiana Medicaid members who were offered participation in the program but chose not to participate. Patients were excluded from the study cohorts if they were (a) less than 18 years of age at the time of enrollment; (b) grouped into Medicaid aid categories for children, pregnant women, or aged (ie, members 65 years of age and older at time of enrollment or expected to turn 65 during the follow-up period); (c) receiving hospice care in the year prior to enrollment; or (d) died during the follow-up period.
All individuals were followed for 12 months from an index date; for the intervention cohort, this date was defined by the date of the first prescription claim from the program after enrollment. For the usual care cohort, patients were randomly allocated to an index date that coincided with the proportion of intervention participants with index dates during each month of the enrollment period. The study was approved by the Indiana University and Purdue University Indianapolis institutional review boards and the Veterans Affairs Research and Development Committee.
Data Sources and Data Collection
Data for the entire cohort were extracted from Indiana Medicaid eligibility, medical, and prescription claims files. Eligibility files included data characterizing age, sex, race, marital status, date of death, regional location of residence, and type of Care Select plan. Medical and pharmacy claims data were extracted for 12 months before and 12 months after the index date for each patient. Medical claims data included hospitalization dates, emergency department (ED) visit dates, outpatient treatment dates, paid claim costs, and primary and secondary diagnosis codes according to the International Classification of Diseases, Ninth Revision (ICD-9). ICD-9 codes were used to estimate medical comorbidities using the Elixhauser method.21 Prescription claims data included drug name, national drug code number, American Hospital Formulary Service pharmacologic-therapeutic classification code, quantity dispensed, dates of fills and refills, paid claim costs, and name of dispensing pharmacy. Additional data on the number of MTM phone contacts and the number and type of identified drug-therapy problems (DTP) for the program cohort was extracted from the telephonic MTM software (Assurance—Medication Management Systems; Eden Prairie, MN). This software was used by the pharmacists as an electronic chart to record their patient care activities.
Measured Outcomes
Outcomes included medication adherence, health care utilization and total health care costs from the Medicaid perspective, and descriptive information related to the MTM program. Medication adherence was evaluated using 2 measures, compliance and persistence, as defined by the International Society Pharmacoeconomic and Outcomes Research.22 For both measures, prescription medications and therapeutic classes that were used for chronic disease treatment (eg, hypertension, diabetes, asthma) were included, whereas medications for acute conditions, nonprescription medications, or medications intended for as-needed use were excluded (list available from the authors). Medication compliance was computed using the medication possession ratio (MPR) defined by Steiner and Prochazka23 as a continuous, multiple-interval measure of medication availability. To account for medication switches and concurrent therapy, an average of all drugs’ MPRs within a therapeutic class was computed to produce 1 averaged MPR for each class; the average MPR was then dichotomized with noncompliance defined as <0.8 and compliance defined as ≥0.8.24 If the average MPR was >1.0, it was truncated at 1.0. Medication persistence was calculated using the proportion of days covered (PDC). The PDC was sensitive to gaps of ≤15 days for all drugs and was chosen to reflect one half of the usual days’ supply of medication for most prescriptions (eg, 30-d supplies). The PDC was dichotomized using the ≥0.8 threshold for persistence.25 Health care utilization outcomes were defined as the number of all-cause hospitalizations and all-cause ED visits as well as both hospitalizations and ED visits for ambulatory care sensitive conditions.26 Total costs represented the sum of paid claims for inpatient, outpatient, ED, and medications filled under the Medicaid system. A 90-day lag period was used to account for claims lags in the data; this period was estimated by Medicaid to provided adequate time to capture >95% of all claims.
Statistical Analyses
This study compared the program cohort to the usual care cohort during the 12-month follow-up period using an intent-to-treat analysis. To compare medication adherence, a multivariate logistic regression model was constructed for each therapeutic medication class. Separate models used the dependent variable of compliance or persistence (ie, MPR or PDC ≥ 0.8) with the independent variables of age, sex, ethnicity, marital status, county of residence, type of care management, total number of Elixhauser comorbidities,21 and number of unique prescriptions medications taken during the 12-month preindex period. For health care utilization, separate multivariate logistic regression models were constructed for each of the dependent variables for the 6- and 12-month periods after the index date. Independent variables included those listed above, along with the number of preindex ED visit(s) and the number of pre-index hospitalization(s).
Similar multivariate regression models were constructed to examine total health care costs in the 6- and 12-month postindex periods. To address issues of skewness and violations of normality assumptions, a generalized linear model with a gamma distribution and a log-link function was used. Estimated parameters were obtained using the maximum likelihood technique,27–29 and adjusted results are presented as cost ratios with 95% confidence interval (CI) estimates. A-priori significant differences between groups were set for P < 0.05. Analyses were also conducted for subgroups with specific comorbid conditions: diabetes, hypertension, chronic pulmonary disease (asthma and chronic obstructive pulmonary disease), heart failure, and mental health (depression and psychoses). Finally, to assess treatment fidelity, an analysis was conducted for a subgroup of the program cohort that received “an adequate dose of the program” defined as ≥6 months of continuous specialized packaging plus ≥3 MTM telephone contacts.
RESULTS
Characteristics of the Study Cohorts
Medicaid members (n = 19,864) eligible for enrollment in the program were mailed a recruitment letter and/or received a telephone call (Fig. 2). Ultimately, 1007 members were included in the program cohort and 13,614 were included in the usual care cohort. The program cohort (Table 1) had a greater proportion of women and African Americans than did the usual care cohort (P < 0.05). The average number of comorbid conditions was similar in both groups, but the proportion of patients with diabetes, chronic pulmonary disease, obesity, and hypertension was higher in the program cohort (P < 0.05). The proportion of patients with alcoholism and psychoses was higher in the usual care cohort (P < 0.05).
TABLE 1.
Baseline Characteristics for Both Cohorts
| Characteristics | Program Cohort N (%) | Usual Care Cohort N (%) |
|---|---|---|
| N | 1007 (100) | 13,614 (100) |
| Female, N (%)* | 739 (73) | 8575 (62) |
| Age (y), mean (SD) | 49 ± 9.0 | 48 ± 10 |
| Marital status | ||
| Divorced, N (%)* | 520 (52) | 6346 (47) |
| Married, N (%) | 198 (20) | 2685 (20) |
| Single, N (%)* | 289 (29) | 4581 (34) |
| Race/ethnicity | ||
| White, N (%)* | 806 (80) | 11,409 (84) |
| Black, N (%)* | 180 (18) | 1780 (13) |
| Other, N (%) | 21 (2) | 425 (3) |
| Regional location of cohort participants* | ||
| Central, N (%) | 302 (30) | 2922 (21) |
| East Central, N (%) | 122 (12) | 1604 (12) |
| North Central, N (%) | 72 (7) | 998 (7) |
| Northeast, N (%) | 111 (11) | 1212 (9) |
| Northwest, N (%) | 108 (11) | 1861 (14) |
| Southeast, N (%) | 98 (10) | 1885 (14) |
| Southwest, N (%) | 108 (11) | 1882 (14) |
| West Central, N (%) | 86 (9) | 1250 (9) |
| Recruitment phone call disposition group*† | ||
| (A) Initially agreed to participate, N (%)* | 721 (72) | 1650 (12) |
| (B) Initially declined to participate, N (%)* | 100 (10) | 5088 (37) |
| (C) Unable to be contacted through telephone, N (%)* | 141 (14) | 5522 (41) |
| (D) No attempt to contact through telephone, N (%)* | 45 (5) | 1354 (10) |
| Managed care plan level of service*‡ | ||
| (1) Lowest, N (%) | 513 (51) | 7037 (52) |
| (2) Medium, N (%)* | 327 (32) | 3452 (25) |
| (3) High, N (%)* | 95 (9) | 880 (6) |
| (4) Highest, N (%) | 11 (1) | 140 (1) |
| Unknown, N (%)* | 61 (6) | 2105 (15) |
| Comorbid conditions§ | ||
| Heart failure, N (%) | 98 (10) | 1141 (8) |
| Hypertension, N (%)* | 514 (51) | 6045 (44) |
| Chronic pulmonary disease, N (%)* | 320 (32) | 3941 (29) |
| Diabetes, uncomplicated, N (%)* | 335 (33) | 3590 (26) |
| Diabetes, complicated, N (%)* | 79 (8) | 852 (6) |
| Renal failure, N (%) | 40 (4) | 687 (5) |
| Liver disease, N (%) | 40 (4) | 566 (4) |
| Obesity, N (%)* | 130 (13) | 1299 (10) |
| Alcohol abuse, N (%)* | 28 (3) | 623 (5) |
| Drug abuse, N (%) | 56 (6) | 951 (7) |
| Psychoses, N (%)* | 100 (10) | 1618 (19) |
| Depression, N (%) | 110 (11) | 1473 (11) |
| No. comorbidities, mean (SD) | 2.6 ± 2.1 | 2.3 ± 2.1 |
Statistically significant difference between program and usual care cohorts using t tests or χ2 as appropriate (P < 0.05).
Refers to the initial disposition of each subject regarding their receipt of a telephone call for recruitment into the program; A, all subjects in both cohorts were contacted through the telephone call and initially agreed to participate in the program; those in the program cohort enrolled in the program, whereas those in the usual care cohort did not enrolled in the program despite initially agreeing to participate; B, all subjects in both cohorts were contacted through the telephone call and initially refused to participate in the program; those in the program cohort ultimately enrolled in the program despite their initial refusal to participate (possibly because of the mailed invitation), whereas those in the usual care cohort did not enroll in the program; C, all subjects in both cohorts were unable to be contacted through telephone; those in the program cohort ultimately enrolled in the program (possibly because of the mailed invitation), whereas those in the usual care cohort did not enroll in the program; D, all subjects in both cohorts were never attempted to be contacted through telephone; those in the program cohort ultimately enrolled in the program (possibly because of the mailed invitation), whereas those in the usual care cohort did not enroll in the program.
Managed-care stratification plans are categorized from 1 to 4, with 1 representing the least care management provided to Medicaid members and 4 representing the most intense care management provided to members.
Calculated from the Elixhauser comorbidity index.21
Program Fidelity and MTM
Individuals enrolled in the program received an average of 13.6 ± 9.5 prescription fills during an average of 6.4 ± 3.5 months, and the average number of months that participants continuously received at least 1 medication (without a gap of 15 d from the previous fill) from the program was 5.4 ± 3.5. Among the 1007 participants enrolled in the program, MTM pharmacists made 3545 telephone calls to 684 participants during the 12-month follow-up period and averaged 3.5 ± 3.5 telephone calls per participant (range, 0–16). There were 323 participants who received no telephone call for reasons including patient refusal, unable to be contacted, and an information technology malfunction that prevented patients from being loaded into the queue for outbound phone calls. The average number of DTP per participant identified by the pharmacists was 31.6 ± 30.2 (range, 1–141) with a documented complete resolution for 3.8% of all problems.
Medication Adherence
Results for medication compliance and persistence (Table 2) reveal that across each of the 10 therapeutic classes, the proportion of patients who had an MPR ≥ 0.8 and/or a PDC ≥ 0.8 was significantly higher in the intervention cohort compared with usual care. The overall measure that includes ≥1 medications within each therapeutic class demonstrated that the proportion of patients who were compliant [80.8% vs. 36.4%; odds ratio (OR) = 7.8, 95% CI, 6.5–9.4, P < 0.001] and/or persistent (56.9% vs. 22.1%; OR = 5.1, 95% CI, 4.4–6.0, P < 0.001) was more than twice as high in the intervention cohort when compared with usual care.
TABLE 2.
Medication Adherence Outcomes at 12-month Follow-up
| Measures* | Program | Usual Care | P | Odds Ratio (95% CI)† | P |
|---|---|---|---|---|---|
| Medication compliance (MPR) | |||||
| Overall, N (%) | 785 (80.8) | 4367 (36.4) | < 0.0001 | 7.8 (6.5–9.4) | < 0.001 |
| Drug class groups | |||||
| Neurological, N (%) | 468 (83.0) | 3031 (49.9) | < 0.0001 | 4.9 (3.8–6.3) | < 0.0001 |
| Oral hypoglycemics, N (%) | 245 (78.0) | 1156 (39.1) | < 0.0001 | 7.2 (5.2–10.0) | < 0.0001 |
| Gastrointestinal, N (%) | 407 (80.1) | 1923 (39.6) | < 0.0001 | 6.2 (4.8–8.0) | < 0.0001 |
| Hormone, N (%) | 263 (66.6) | 1410 (37.7) | < 0.0001 | 3.6 (2.8–4.6) | < 0.0001 |
| Antihypertensives, N (%) | 594 (83.7) | 3237 (41.4) | < 0.0001 | 7.6 (6.1–9.6) | < 0.0001 |
| Lipotropics, N (%) | 465 (83.3) | 2362 (43.7) | < 0.0001 | 6.8 (5.3–8.9) | < 0.0001 |
| Psychoactives, N (%) | 550 (81.5) | 3025 (43.0) | < 0.0001 | 5.8 (4.6–7.3) | < 0.0001 |
| Respiratory, N (%) | 188 (69.9) | 659 (34.5) | < 0.0001 | 4.4 (3.1–6.1) | < 0.0001 |
| Blood modifiers, N (%) | 127 (84.1) | 587 (43.6) | < 0.0001 | 8.9 (5.3–14.9) | < 0.0001 |
| Antiarrhythmics, N (%) | 28 (87.5) | 167 (40.4) | < 0.0001 | 10.8 (3.2–36.5) | < 0.0001 |
| Medication persistence (PDC) | |||||
| Overall, N (%) | 553 (56.9) | 2649 (22.1) | < 0.0001 | 5.1 (4.4–6.0) | < 0.0001 |
| Drug class groups | |||||
| Neurological, N (%) | 364 (64.5) | 1822 (30.0) | < 0.0001 | 4.7 (3.8–5.9) | < 0.0001 |
| Oral hypoglycemics, N (%) | 200 (63.7) | 850 (28.8) | < 0.0001 | 6.5 (4.8–8.9) | < 0.0001 |
| Gastrointestinal, N (%) | 328 (64.6) | 1453 (30.0) | < 0.0001 | 5.1 (4.0–6.4) | < 0.0001 |
| Hormone, N (%) | 236 (59.8) | 1185 (31.7) | < 0.0001 | 3.5 (2.7–4.6) | < 0.0001 |
| Antihypertensives, N (%) | 467 (65.8) | 2096 (26.8) | < 0.0001 | 5.5 (4.6–6.7) | < 0.0001 |
| Lipotropics, N (%) | 365 (65.4) | 1622 (30.0) | < 0.0001 | 5.0 (4.0–6.3) | < 0.0001 |
| Psychoactives, N (%) | 408 (60.4) | 2099 (29.8) | < 0.0001 | 3.7 (3.1–4.5) | < 0.0001 |
| Respiratory, N (%) | 149 (55.4) | 547 (28.7) | < 0.0001 | 3.8 (2.7–5.3) | < 0.0001 |
| Blood modifiers, N (%) | 104 (68.9) | 425 (31.5) | < 0.0001 | 6.5 (4.1–10.1) | < 0.0001 |
| Antiarrhythmics, N (%) | 23 (71.9) | 122 (29.5) | < 0.0001 | 6.8 (2.5–18.6) | 0.0002 |
Compliance and persistence were defined as MPR and PDC ≥ 0.8, respectively, for each patient.
Estimated using multivariate logistic regression models for each therapeutic medication class. Separate models used the dependent variable of compliance or persistence with the independent variables of age, sex, ethnicity, marital status, county of residence, type of care management, total number of Elixhauser comorbidities,21 and number of unique prescriptions medications taken during the 12-month preindex period.
CI indicates confidence interval; MPR, medication possession ratio; PDC, proportion of days covered.
Health Care Utilization
Six month all-cause adjusted inpatient utilization was marginally less in the intervention cohort compared with the usual care cohort (OR = 0.73, 95% CI, 0.54–1.00, P = 0.05; Table 3). There were no other significant differences for any of the adjusted endpoints, at 6 or 12 months, between the 2 groups. Among the subgroup of participants with diabetes, the adjusted odds of all-cause ED visits at 6 months for intervention patients was 27% less than those in the usual care cohort (OR = 0.73, 95% CI, 0.54–0.97, P < 0.05); however, this effect was not sustained at the 12-month follow-up. Similar analyses for participants with hypertension, chronic pulmonary disease, heart failure, and mental health did not reveal significant differences between the intervention and usual care cohorts. In addition to the intention-to-treat analysis, intervention effects were evaluated by the “dose” of the intervention that patients received; participants who received ≥6 months of continuous specialized medication packaging plus ≥3 MTM telephone contacts (n = 372) did not differ from the usual care group with respect to health care utilization.
TABLE 3.
Health Care Utilization Endpoints—All Participants
| Measure and Timepoint | Program | Usual Care | P | Odds Ratio (95% CI)* | P |
|---|---|---|---|---|---|
| No. emergency department visits | |||||
| Preindex, N (%) | 529 (52) | 6881 (51) | 0.22 | — | — |
| 6 mo follow up, N (%) | 442 (44) | 5873 (43) | 0.64 | 0.87 (0.74–1.03) | 0.11 |
| 12 mo follow up, N (%) | 593 (59) | 7617 (56) | 0.07 | 0.92 (0.78–1.09) | 0.33 |
| No. AHRQ-defined emergency department visits† | |||||
| Preindex, N (%) | 132 (13) | 1613 (12) | 0.24 | — | — |
| 6 mo follow up, N (%) | 145 (14) | 1821 (13) | 0.36 | 1.03 (0.82–1.30) | 0.80 |
| 12 mo follow up, N (%) | 169 (17) | 2127 (16) | 0.33 | 1.05 (0.85–1.30) | 0.67 |
| No. inpatient visits | |||||
| Preindex, N (%) | 279 (28) | 3788 (28) | 0.94 | — | — |
| 6 mo follow up, N (%) | 62 (6) | 983 (7) | 0.20 | 0.73 (0.54–1.00) | 0.05 |
| 12 mo follow up, N (%) | 211 (21) | 2558 (19) | 0.10 | 1.09 (0.89–1.34) | 0.39 |
| No. of AHRQ-defined inpatients visits† | |||||
| Preindex, N (%) | 37 (4) | 485 (4) | 0.85 | — | — |
| 6 mo follow up, N (%) | 3 (0.3) | 75 (1) | 0.37 | 0.42 (0.12–1.42) | 0.16 |
| 12 mo follow up, N (%) | 28 (3) | 349 (3) | 0.68 | 0.85 (0.53–1.36) | 0.49 |
Estimated using multivariate logistic regression models adjusted for age, sex, ethnicity, marital status, county of residence, type of care management, total number of Elixhauser comorbidities,21 and number of unique prescriptions medications taken during the 12-month preindex period, preindex emergency department visit(s), and preindex hospitalization(s).
Agency for Healthcare Research and Quality definition of Prevention Quality Indicators.26
CI indicates confidence interval.
Health Care Costs
Adjusted total cost for the intervention cohort at the 12-month postindex period was 84% higher than the usual care cohort (Table 4; P < 0.0001). Adjusted results demonstrate higher expenditures for outpatient, prescription, and total costs for the intervention cohort compared with the usual care cohort. Costs for inpatient and ED care did not differ between cohorts. Analyses of the subgroup that received ≥6 months of continuous specialized packaging plus ≥3 MTM telephone contacts and comorbid subgroups such as diabetes, hypertension, chronic lung disease, heart failure, and mental health produced similar nonsignificant findings.
TABLE 4.
Cost Components of Program (N = 1,007) and Usual Care (N = 13,614) Cohorts
| Measure and Timepoint | Program Unadjusted Costs | Usual Care Unadjusted Costs | P | Cost Ratio (95% CI)* | P |
|---|---|---|---|---|---|
| Inpatient Costs (I) | |||||
| Preindex, Mean (SD) | 2968 (8178) | 3251 (10,564) | 0.4063 | — | — |
| 6 mo follow up, mean (SD) | 1213 (4993) | 1503 (7234) | 0.2103 | 0.93 (0.80–1.07) | 0.3918 |
| 12 mo follow up, mean (SD) | 3179 (9803) | 3413 (14,185) | 0.6075 | 0.95 (0.84–1.08) | 0.2305 |
| Emergency department costs (ED) | |||||
| Preindex, mean (SD) | 285 (461) | 301 (618) | 0.4131 | — | — |
| 6 mo follow up, mean (SD) | 132 (253) | 135 (324) | 0.7700 | 1.05 (0.98–1.13) | 0.2260 |
| 12 mo follow up, mean (SD) | 269 (467) | 261 (597) | 0.7108 | 1.01 (0.97–1.05) | 0.1488 |
| Outpatient costs (O) | |||||
| Preindex, mean (SD) | 1316 (2202) | 1341 (4082) | 0.8476 | — | — |
| 6 mo follow up, mean (SD) | 651 (1942) | 570 (2071) | 0.2317 | 1.11 (1.02–1.20) | 0.0202 |
| 12 mo follow up, mean (SD) | 1234 (1309) | 1078 (3513) | 0.1721 | 1.12 (1.04–1.21) | 0.0091 |
| Pharmacy costs (P) | |||||
| Preindex, mean (SD) | 6706 (8821) | 4751 (19,907) | 0.0020 | — | — |
| 6 mo follow up, mean (SD) | 4936 (6589) | 2009 (10,153) | < 0.0001 | 2.28 (2.15–2.42) | < 0.0001 |
| 12 mo follow up, mean (SD) | 10,282 (13,728) | 3782 (17,880) | < 0.0001 | 2.50 (2.36–2.66) | < 0.0001 |
| Total cost (I+ED+O+P) | |||||
| Preindex, mean (SD) | 11,275 (12,770) | 9644 (24,160) | 0.0339 | — | — |
| 6 mo follow up, mean (SD) | 6931 (9199) | 4217 (12,974) | < 0.0001 | 1.76 (1.65–1.89) | < 0.0001 |
| 12 mo follow up, mean (SD) | 14,963 (18,863) | 8534 (24,571) | < 0.0001 | 1.84 (1.72–1.97) | < 0.0001 |
Multivariate model adjusted for age, sex, ethnicity, preindex ED visit, total number of Elixhauser comorbidities,21 number of unique prescriptions medications taken during the 12-month preindex period, preindex ED visit(s), preindex hospitalization(s), preindex medical and pharmacy costs, using Gamma distribution with a log-link function.
CI indicates confidence interval.
DISCUSSION
This intervention program combined several strategies for improving medication adherence and medical outcomes, including specialized medication packaging, synchronization of dispensing along with home delivery, and telephonic MTM provided by pharmacists.17,30–32 Results of this program evaluation were mixed—although measures of medication adherence were significantly higher in the intervention cohort, there were minimal differences in associated ED visits and hospitalization outcomes. Studies that have improved medication adherence have generally been associated with some improvements in health care outcomes33; although a recent systematic review suggests that “even the most effective interventions do not translate into large improvements in adherence and treatment outcomes,” with effect sizes that are variable for interventions and not consistently related to adherence measures.17,18 The findings from the current study support these assertions. Most studies of interventions to improve medication adherence have evaluated intermediate outcomes such as blood pressure, low-density lipoprotein (LDL), or hemogloblin A1c17; while these are clinically important, the temporally distant causal link with associated health care utilization is less well established. Few studies have evaluated major outcomes such as ED visits, hospitalizations, morbidity, or mortality.17,34–36 The lack of improved health care outcomes, despite large improvements in estimates of medication adherence in the current study, may be explained by several study design and program implementation factors including the duration of follow-up, the method of medication delivery and assessment of medication adherence, the design and execution of the telephonic MTM program, a nonrandomized design, and other study limitations including the targeted population.
For an intervention designed to improve medication adherence, the downstream effect on outcomes such as blood pressure, LDL, and hemogloblin A1c often can be captured with 6–12 months of monitoring; however, the more distal outcomes, such as ED visits and hospitalizations might require longer periods of follow-up. Specifically, in this evaluation, the timeline for patients to begin receiving all medications synchronized into a single home delivery box along with the initial MTM session occurred 3 months after program enrollment. A previously published evaluation of a multicomponent intervention using specialized packaging and telephonic education and monitoring by pharmacists found that medication adherence improved at 6 months and was associated with improved systolic blood pressure and LDL37; however, the study did not evaluate long-term endpoints such as hospitalization. In contrast, in a 2-year study of periodic telephone counseling provided by pharmacists, medication adherence was improved and associated with a 41% reduction in all-cause mortality.36
This intervention program used a proactive medication refill process, synchronization of refills, and home delivery. Although the claims-based measures of medication adherence demonstrated significant improvements, these measures may overestimate true medication taking behavior—one cannot assume that simply providing more medication and making it accessible to the patient is synonymous with increased medication consumption (ie, enhanced adherence). Although the concept of specialized packaging and refill synchronization is attractive and likely reduces many of the burdens associated with medication acquisition for patients, the observed lack of correlation between our measures of adherence and health care utilization suggest a need for measurement studies to estimate the extent to which specialized packaging and synchronization translates into actual changes in medication consumption. Furthermore, studies are needed to determine the independent effects of MTM and specialized packaging. In the study reported here, the intervention elements were delivered in combination. For some patients the packaging might be more beneficial—for example, patients for whom the medical regimen is stable.38 For patients whose regimens are in flux, prepackaging (eg, a month’s supply) might not offer a logical approach to medication management. On average, participants continuously received medications an average of 5.4 months, and fewer than 4% of all participants received all 12 months of continuous specialized medication packaging, suggesting a need to examine the reasons underlying lack of fidelity to the program in this population.
The telephonic MTM component of the intervention provided a method for communication between the pharmacist, the patient, and the patients’ other health care providers to identify and resolve DTPs. The MTM program reached approximately two thirds of the intervention patients, and pharmacists spoke with these patients an average of 3.5 times during the follow-up period. During the calls, pharmacists identified a large number of DTPs (n = 22,187), yet only a small proportion (3.8%) of the DTPs were documented as having been fully resolved at the 12-month study completion. Evaluation of an MTM program in Connecticut reported cost savings and greater therapeutic goal achievement when approximately 80% of DTPs were resolved by pharmacists.39 A likely barrier to DTP resolution in the current intervention program is insufficient collaboration between participating pharmacists and patients’ physicians. Without active responses by physicians to identified DTPs, it is doubtful that benefits can be fully achieved. Pharmacists participating in the Connecticut project39 provided MTM on-site at the medical office and had access to patients’ medical and pharmacy records that assisted them in identifying and resolving DTPs. In addition, evaluation of a Minnesota Medicaid MTM program14 determined that the highest DTP resolution rates were among pharmacists practicing in an integrated care delivery system in close proximity to prescribers. In that study, 18% of resolved DTPs required collaboration with prescribers.14 Similarly, an evaluation of MTM services provided to Blue Cross/Blue Shield beneficiaries found that 22% of resolved DTPs required collaboration between the pharmacist and physician.9 Closer examination of the DTPs in the current evaluation suggests that 27.3% of the DTPs involved communication and collaboration with the prescriber to resolve the problem. Although the MTM system captured data relevant to telephone contact with patients, limited data were captured to further characterize communication with prescribers. This may have been an even greater challenge in the current population as we found a very high percentage of patients were utilizing the emergency department; this may indicate limited utilization of primary care physicians with whom the pharmacists may have attempted to contact with medication recommendations.
Accessing primary care physicians and establishing collaborative working relationships have been cited as barriers to MTM provision.40 Factors associated with developing collaborative relationships with physicians, such as establishing bidirectional communication and building trust through consistent provision of high-quality recommendations have been identified; however, these aspects of relationship development take time to mature and generally require active outreach by the pharmacist.41–44 Furthermore, in the current study model of telephonic MTM, collaboration with prescribers and outreach efforts to enhance collaboration can be especially difficult as the pharmacist and physician are practicing at a distance and the physician may be unfamiliar both with the individual pharmacist and with the overall concept of MTM. Maturation of the MTM program may be needed to accurately assess the full effect of these programs, whereas the current evaluation included early implementation of the MTM program.
Limitations of the study should be considered. First, the nonrandomized study design may produce selection bias and unobserved differences such as the healthy user effect that cannot be adjusted for in the analyses.45 Second, given the Medicaid populations’ socioeconomic difficulties and related challenges with access and routine use of primary care, this type of pharmacy program may not effectively change this populations’ health care seeking behaviors. In fact, the state of Indiana had created a separate robust full-time care management program that found similar limited results in the ability of their program to modify Medicaid members’ behavior and realize cost savings.20,46 It is possible that the program evaluated in the current study may be more applicable to other populations such as an ambulatory, geriatric program like Medicare part D but future studies are required.
CONCLUSIONS
The program improved estimates of medication adherence, but the measurable effect on health care utilization and nonpharmacy costs at 6 and 12 months was not different from the usual care group. Reasons for these findings may reflect differences in the delivery of the specialized packaging and the MTM program, health care behaviors in this Medicaid cohort, unadjusted confounding, and time required for the benefit of the intervention to manifest. Further rigorous studies are required to understand disease-specific changes in health care utilization and appropriateness of MTM programs in the Medicaid population.
Acknowledgments
Supported by Indiana Office of Medicaid Policy and Planning. The sponsor had no role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data, or preparation or approval of the manuscript. The sponsor provided a review of the manuscript. A portion of A.J.Z.’s time was supported by a Career Development award from the Department of Veterans Affairs, Health Services Research and Development (RCD 06-304-1), and M.E.S.’s effort was supported in part by KL2 RR025760 (A. Shekhar, PI.). J.H.’s collaboration was supported in part by a Senior Scholar Award from Fulbright New Zealand.
Footnotes
The corresponding author had full access to all study data and takes responsibility for the integrity of the data and accuracy of the analyses.
All authors report no conflicts of interest for the past 3 years.
References
- 1.Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 2001;41:192–199. doi: 10.1016/s1086-5802(16)31229-3. [DOI] [PubMed] [Google Scholar]
- 2.Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003) 2005;45:566–572. doi: 10.1331/1544345055001274. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed December 5, 2011];2010 CMS MTM Fact Sheet. [cited December 5, 2001]. Available at: https://www.cms.gov/PrescriptionDrugCovContra/Downloads/MTMFactSheet_2010_06-2010_final.pdf.
- 4.Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–543. [PubMed] [Google Scholar]
- 5.American Pharmacists Association and the National Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0) J Am Pharm Assoc. 2008;48:341–353. doi: 10.1331/JAPhA.2008.08514. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid Services. Department of Health and Human Services. Medicare prescription drug benefit final rule. Fed Regist. 2005;70:4194–4585. [Google Scholar]
- 7.Cranor CW, Bunting BA, Christensen DB. The Asheville Project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc (Wash) 2003;43:173–184. doi: 10.1331/108658003321480713. [DOI] [PubMed] [Google Scholar]
- 8.Fera T, Bluml BM, Ellis WM. Diabetes ten city challenge: final economic and clinical results. J Am Pharm Assoc (2003) 2009;49:383–391. doi: 10.1331/JAPhA.2009.09015. [DOI] [PubMed] [Google Scholar]
- 9.Isetts BJ, Schondelmeyer SW, Artz MB, et al. Clinical and economic outcomes of medication therapy management services: the Minnesota experience. J Am Pharm Assoc. 2008;48:203–211. doi: 10.1331/JAPhA.2008.07108. [DOI] [PubMed] [Google Scholar]
- 10.Pindolia VK, Stebelsky L, Romain TM, et al. Mitigation of medication mishaps via medication therapy management. Ann Pharmacother. 2009;43:611–620. doi: 10.1345/aph.1L591. [DOI] [PubMed] [Google Scholar]
- 11.Planas LG, Crosby KM, Mitchell KD, et al. Evaluation of a hypertension medication therapy management program in patients with diabetes. J Am Pharm Assoc. 2009;49:164–170. doi: 10.1331/JAPhA.2009.08164. [DOI] [PubMed] [Google Scholar]
- 12.Doucette WR, McDonough RP, Klepser D, et al. Comprehensive medication therapy management: identifying and resolving drug-related issues in a community pharmacy. Clin Ther. 2005;27:1104–1111. doi: 10.1016/s0149-2918(05)00146-3. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch JD, Rosenquist A, Best BM, et al. Evaluation of the first year of a pilot program in community pharmacy: HIV/AIDS medication therapy management for Medi-Cal beneficiaries. J Manag Care Pharm. 2009;15:32–41. doi: 10.18553/jmcp.2009.15.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isetts BJ. Evaluating effectiveness of the Minnesota Medication Therapy Management Care Program. [Accessed March 1, 2011];Final report to Medicaid. 2007 Available at: http://www.dhs.state.mn.us/main/groups/business_part-ners/documents/pub/dhs16_140283.pdf.
- 15.Michaels NM, Jenkins GF, Pruss DL, et al. Retrospective analysis of community pharmacists’ recommendations in the North Carolina Medicaid medication therapy management program. J Am Pharm Assoc (2003) 2010;50:347–353. doi: 10.1331/JAPhA.2010.09021. [DOI] [PubMed] [Google Scholar]
- 16.Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16:185–195. doi: 10.18553/jmcp.2010.16.3.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;2:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 18.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice: The Clinician’s Guide. 2. New York: McGraw-Hill, Medical Division; 2004. [Google Scholar]
- 20.Indiana Medicaid. Indiana Care Select - (Medicaid Care Management for Aged, Blind, Disabled) and Prior Authorization Changes. [Accessed March 29, 2012];Indiana Medicaid Provider Bulletin. 2007 Sep 13; Available from: http://provider.indianamedicaid.com/ihcp/bulletins/bt200723.pdf.
- 21.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 24.Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 25.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–457. [PubMed] [Google Scholar]
- 26.Prevention Quality Indicators overview. Agency for Healthcare Research and Quality; [Accessed March 29, 2012]. Available from: http://www.qualityindicators.ahrq.gov/Modules/pqi_overview.aspx. [Google Scholar]
- 27.Blough DK, Ramsey SD. Using generalized linear models to assess medical care costs. Health Serv Outcome Res Methodology. 2000;1:185–202. [Google Scholar]
- 28.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23:525–542. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20:461–494. doi: 10.1016/s0167-6296(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 30.Heneghan CJ, Glasziou PP, Perera R. Reminder packaging for improving adherence to self-administered long-term medications. Cochrane Database Syst Rev. 2006;1:CD005025. doi: 10.1002/14651858.CD005025.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 32.Roter DL, Hall JA, Merisca R, et al. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998;36:1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 33.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 34.Sokol MC, McGuigan KA, Verbrugge RR, et al. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 35.Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303–312. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]
- 36.Wu JY, Leung WY, Chang S, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ. 2006;333:522–527. doi: 10.1136/bmj.38905.447118.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 38.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171:814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 39.Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood) 2011;30:646–654. doi: 10.1377/hlthaff.2011.0002. [DOI] [PubMed] [Google Scholar]
- 40.Lounsbery JL, Green CG, Bennett MS, et al. Evaluation of pharmacists’ barriers to the implementation of medication therapy management services. J Am Pharm Assoc (2003) 2009;49:51–58. doi: 10.1331/JAPhA.2009.017158. [DOI] [PubMed] [Google Scholar]
- 41.Brock KA, Doucette WR. Collaborative working relationships between pharmacists and physicians: an exploratory study. J Am Pharm Assoc (2003) 2004;44:358–365. doi: 10.1331/154434504323063995. [DOI] [PubMed] [Google Scholar]
- 42.Doucette WR, Nevins J, McDonough RP. Factors affecting collaborative care between pharmacists and physicians. Res Social Adm Pharm. 2005;1:565–578. doi: 10.1016/j.sapharm.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Snyder ME, Zillich AJ, Primack BA, et al. Exploring successful community pharmacist-physician collaborative working relationships using mixed methods. Res Social Adm Pharm. 2010;6:307–323. doi: 10.1016/j.sapharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zillich AJ, McDonough RP, Carter BL, et al. Influential characteristics of physician/pharmacist collaborative relationships. Ann Pharmacother. 2004;38:764–770. doi: 10.1345/aph.1D419. [DOI] [PubMed] [Google Scholar]
- 45.Brookhart MA, Patrick AR, Dormuth C, et al. Adherence to lipid-lowering therapy and the use of preventive health services: an investigation of the healthy user effect. Am J Epidemiol. 2007;166:348–354. doi: 10.1093/aje/kwm070. [DOI] [PubMed] [Google Scholar]
- 46.Indiana Family and Social Services. [Accessed March 29, 2012];Changes to the Care Select Program Annouced. 2010 Available from: http://provider.indianamedicaid.com/news,-bulletins,-and-banners/archived-news-2010/changes-to-the-care-select-program-announced.aspx.