Abstract
Banana resistant starch (BRS) was extracted to investigate the structural properties of BRS, its effects on the gastrointestinal transit, and dejecta of normal and experimentally constipated mice. The mouse constipation model was induced by diphenoxylate administration. The BRS administered mice were divided into three groups and gavaged with 1.0, 2.0, or 4.0 g/kg body weight BRS per day. The small intestinal movement, time of the first black dejecta, dejecta granules, weight and their moisture content, body weight, and food intake of mice were studied. Results showed that the BRS particles were oval and spindly and some light cracks and pits were in the surface. The degree of crystallinity of BRS was 23.13%; the main diffraction peaks were at 2θ 15.14, 17.38, 20.08, and 22.51. The degree of polymerization of BRS was 81.16 and the number-average molecular weight was 13147.92 Da, as determined by the reducing terminal method. In animal experiments, BRS at the dose of 4.0 g/kg body weight per day was able to increase the gastrointestinal propulsive rate, and BRS at the doses of 2.0 and 4.0 g/kg body weight per day was found to shorten the start time of defecation by observing the first black dejecta exhaust. However, there were no influences of BRS on the dejecta moisture content, the dejecta granules and their weight, body weight, or daily food intake in mice. BRS was effective in accelerating the movement of the small intestine and in shortening the start time of defecation, but did not impact body weight and food intake. Therefore, BRS had the potential to be useful for improving intestinal motility during constipation.
Key Words: : banana, constipation, laxative, resistant starch, structure
Introduction
According to the definition by European Flair Concerted Action on Resistant Starch (EURESTA), resistant starch (RS) encompasses all starch and products of starch degradation not absorbed in the small intestine of healthy individuals,1 and it is divided into four types.2
RS is beneficial for bowel health (pH, epithelial thickness, and apoptosis of colorectal cancer cells), loosening constipation, decreasing postprandial glycemia, increasing insulin sensitivity, improving intestinal microflora, and regulating body weight.3,4
Chronic constipation is a very frequent disorder that affects about 10–20% of the population of Europe.5 Based on epidemiologic studies, the median prevalence of constipation was 16% (range, 0.7–79%) in adults overall and 33.5% in adults aged 60 to 101 years.6 Many constipation sufferers use laxative medicines, such as anthranoid-containing laxatives—aloe, cascara, frangula, and rheum. However, data reveal a relative risk for colorectal cancer as a result of anthranoid laxative abuse.7 Thus, it is necessary to find some natural safe ingredients, which are effective for facilitating coprostasis.
The banana is a high-yield fruit planted in the tropical and subtropical area. Many reports state that the green banana is rich in the type II resistant starch-native granular starch consisting of ungelatinized granules (RS2).2,8 Langkilde et al. studied the effects of high-resistant starch banana flour (RS2) on in vitro fermentation and the small-bowel excretion of energy, nutrients, and sterols by ileostomy research.9 Lehmann et al. reported the characterization of resistant starch type III (RS3) from banana, which was prepared by debranching of the native starch and a retrogradation method.10 Aparicio-Saguilán et al. studied the physicochemical and functional properties of cross-linked banana resistant starch (BRS).11 Cheng et al. investigated the effects of spray drying technologies on the retention rate of BRS (RS2).12 In this study, the effects of resistant starch (RS2) from the banana, in a mouse model of constipation induced by diphenoxylate, were investigated, including the bowel movement and defecation condition, to find whether the BRS has the ability to aid the evacuation of feces.
Materials and Methods
Raw material
Bananas (Musa ABB Bluggoe) with totally green peels were purchased from a local market. All chemical reagents used were of analytical grade.
Preparation of BRS
Based on the method described by Cheng et al.,12 the banana was peeled, pulped, and degraded by pectinase and amylase to remove pectin, cellulose, protein, and digested starch in the banana pulp. The degraded pulp was centrifugated and the deposit was collected and dehydrated at 50°C. The dried deposit was crushed into powder and preserved at 5°C and subjected for use in mouse experiments. BRS was analyzed and found to be 98.8% RS, using the method described by Goni et al.13
Structural observations of BRS
Light microscopy and scanning electron microscopy observations
The BRS samples were dissolved in glycerol (at a 50% concentration) and observed under a microscope (Vanox BHS-2; Olympus Corporation) using natural light.
Particles of BRS powder were scanned using an S3700N scanning electron microscope (Hitachi). Samples were fixed on an objective table coated with a platinum layer with a thickness of 10–20 nm. Both observations and photographs were produced using the scanning electron microscope.
X-ray diffraction analyses
Cu-Kα radiation was used to scan BRS samples over the 2θ=4–60° range, with a step interval of 0.04°, a scanning rate of 17.7 s per step, a voltage of 40 kV, and a current of 40 mA. The D8 ADVANCE X-ray diffractometer from Bruker Corporation was utilized for X-ray diffraction (XRD) analyses.
Starch–iodine absorption spectra
Spectra of iodine-bound starch samples were determined using the method described by Klucinec and Thompson.14 A 50 mg sample of BRS was first dispersed into 10.0 mL of DMSO containing 10% 6.0 M urea. Subsequently, 2.0 mL of the dispersed solution, 25 mL of distilled water, and 1.0 mL of an aqueous I2-KI solution (2.0 mg I2/mL and 20.0 mg KI/mL) were pipetted into a 50-mL volumetric flask and mixed. The mixed solution was brought to a volume of 50 mL with distilled water. Control solutions were created by following the same procedure, but omitting the addition of a starch sample. A UV–visible spectrophotometer (UV-1800; Shimadzu Co.) was used to scan each sample from 500 to 800 nm. The λmax for each sample was defined as the wavelength in this range that produced the highest absorbance value.
Molecular weight and degree of polymerization
Based on the method of Takeda et al.,15 the molecular weight (Mn) and degree of polymerization (DP) of BRS were determined and calculated as follows:
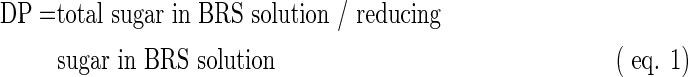 |
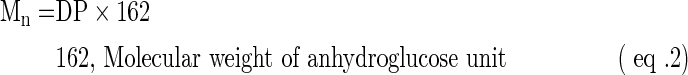 |
Animals and drugs
Animals
Male SPF-grade Kunming mice were supplied by the Medical Laboratory Animal Centre of Guangdong Province, China, on animal license no. SCXK 2003-0002, 2007A006.
Drugs
Diphenoxylate (H32022716) was produced by Changzhou Kangpu Medicine Industry Co., Ltd.
Effects of BRS on small intestinal movement of mice
The intestinal movement was evaluated by the method described by Wang et al.16 Fifty healthy male Kunming mice were divided into five groups: control, model, high-dose, middle-dose, and low-dose groups (n=10 mice each). An activated-carbon suspension solution (CSS), a diphenoxylate suspension solution, and a BRS solution were prepared. According to the species differences, mice should be treated with 10-fold concentrations of drugs relative to the concentrations used for humans. BRS was administered at a dose of 1, 2, 4 g/kg body weight per day for low-, middle-, and high-dose group mice, respectively. BRS was administered orally to the mice in the high-, middle-, and low-dose test groups and the mice in the control and model groups were gavaged with distilled water during the feeding period. All mice were fed a common mouse chow, which contains protein 18.5%, fat 4.2%, Ca 1.2%, P 0.72%, and cellulose 4%. Body weight and the quantity of food consumed were recorded daily for each mouse.
After BRS had been administered for 7 days, each group of mice was fasted for 24 h, but allowed water ad libitum to empty the intestines. Diphenoxylate was administered to the mice to induce constipation. The three test groups and the model group were then treated with diphenoxylate (5 mg/kg body weight). The control group was given distilled water.
Thirty minutes after the administration of diphenoxylate, the test groups were gavaged with CSS containing BRS (BRS concentration in the CSS was corresponding to the low-, middle-, and high-dose groups). The control and model groups were given 0.2 mL of CSS only.
After 25 min, the mice were anesthetized with ether, killed, and incisions made in their abdomens. The intestine was cut away from the pylorus to cecum with scissors and its length measured. The advance of the CSS was measured as the distance from the pylorus to the leading edge of the black sediment. The formula given below (eq. 3) was used to calculate the CSS advance ratio:
 |
For statistical analysis, the CSS advance ratio was converted using the following formula (eq. 4):
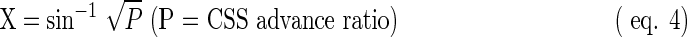 |
The constipation model induced by diphenoxylate is successful if the CSS advance ratio (P) of the model group is significantly lower compared with the control group. The test result of the small intestinal movement is positive if the CSS advance ratio (P) of the test group is significantly higher compared with the model group when the constipation model induced by diphenoxylate is successful.
Effects of BRS on dejecta granules and weight of mice
The three test groups and the model group were given diphenoxylate (10 mg/kg body weight) to induce constipation. After the mice had been administered CSS, the time until the first black feces appeared was noted and the total number and weight of dejecta granules were measured for each mouse at 3 h and at 6 h. The water content of the feces was determined for each group of mice. Other treatments were as described in the small intestinal movement of the mouse experiment above.
The constipation model induced by diphenoxylate is successful, if the time of appearance of the first black dejecta of mice in the model group is significantly longer compared with the control group.
The test result of the time of the first black dejecta is positive if the time of the appearance of the first black dejecta of mouse in the test group is significantly shorter compared with the model group when the constipation model induced by diphenoxylate is successful.
The test result of dejecta granules is positive if the dejecta granules of the test group are significantly more compared with the model group when the constipation model induced by diphenoxylate is successful.
The test result of the dejecta weight is positive if the dejecta weight of the test group is significantly heavier compared with the model group when the constipation model induced by diphenoxylate is successful.
Data analysis
The statistical analysis software (SAS version 8.1) was used to analyze the experimental data. Analysis of variance and multiple comparison programs were applied. P<.05 was considered statistically significant.
Results and Discussion
Structural properties of BRS
Light microscopy and scanning electron microscopy observations
As seen in Figure 1a, most BRS particles were oval in shape, whereas certain particles were spindly. There were some light cracks and pits in the surface of BRS, as indicated in Figure 1b. This might be due to the enzyme-catalyzed hydrolysis of BRS.
FIG. 1.
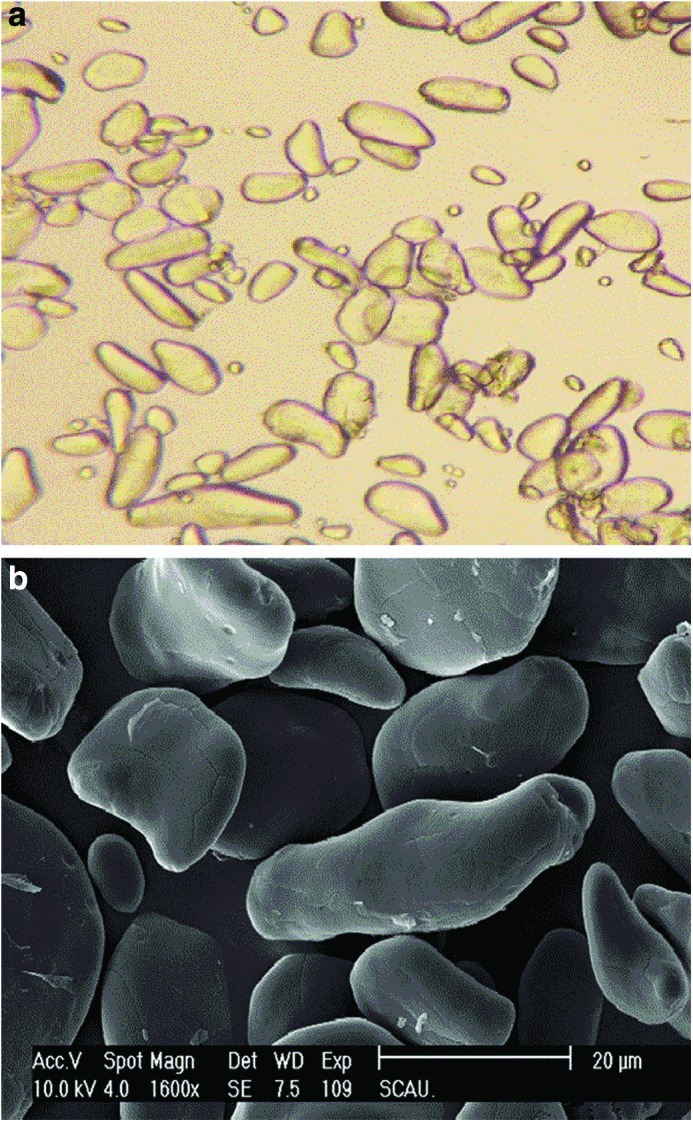
Optical microscopy and scanning electron microscopy images of banana resistant starch samples. (a) Optical microscopy images (×10). (b) Scanning electron microscopy images (×500). Color images available online at www.liebertpub.com/jmf
XRD analyses
Table 1 shows that the degree of crystallinity of BRS was 23.13%, and the main diffraction peaks were at 2θ 15.14, 17.38, 20.08, and 22.51.
Table 1.
X-ray Diffraction Patterns of Banana Resistant Starch
| Diffraction peak intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2θ | 15.14 | 16.36 | 17.38 | 20.08 | 22.51 | 23.51 | 30.38 | 32.58 | 34.51 |
| Peak intensity | 115 | 431 | 333 | 187 | 178 | 396 | 81 | 226 | 81 |
| Peak width | 2.09 | 8.70 | 2.09 | 3.12 | 3.88 | 7.61 | 2.61 | 8.84 | 2.61 |
| Area of crystalline region | 239.46 | — | 691.95 | 581.76 | 690.08 | — | 209.53 | — | 210.23 |
| Area of noncrystalline region | — | 3733.30 | — | — | — | 2997.20 | — | 1987.81 | — |
| Degree of crystallinity | 23.13% | ||||||||
Starch–iodine absorption spectra
The starch–iodine absorption spectrum for the BRS is illustrated in Figure 2. The maximum absorption wavelengths (λmax) of BRS ranged from 625 to 659 nm. According to Klucinec et al.,14 the maximum absorption wavelengths of amylose range from 643 to 655 nm. Therefore, it can be suggested that BRS contains high concentrations of amylose.
FIG. 2.
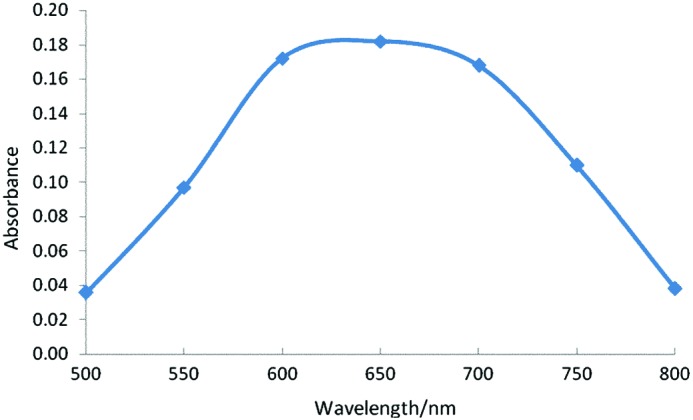
Starch–iodine absorption spectra of banana resistant starch. Color images available online at www.liebertpub.com/jmf
Molecular weight and degree of polymerization
Based on the reducing terminal method, the determined degree of polymerization of BRS was 81.16 and the average molecular weight was 13147.92 Da. However, this method was an approximative test, and a more accurate method is needed for further research.
Effects of BRS on small intestinal movement
As can be seen in Table 2, during the small intestinal movement experiment period, there were no significant differences in the original body weight, final body weight, or daily food intake among the five groups of mice. This suggests that BRS has no negative effects on body weight, growth, or food intake in mice.
Table 2.
Effects on the Body Weight and Food Intake of Mice ( ±s)
±s)
| Group | Animal No. | BRS dose (g/kg body weight per day) | Original body weight (g) | Final body weight (g) | Daily food intake (g) |
|---|---|---|---|---|---|
| Control | 10 | 0 | 25.7±1.7 | 28.6±2.0 | 6.2±1.3 |
| Model | 10 | 0 | 27.6±1.6 | 30.9±2.3 | 6.5±1.1 |
| Low | 10 | 1.0 | 26.4±1.8 | 29.6±2.2 | 6.3±1.3 |
| Middle | 10 | 2.0 | 26.6±1.4 | 30.1±1.8 | 6.3±0.9 |
| High | 10 | 4.0 | 26.4±2.5 | 29.6±2.8 | 5.9±1.1 |
Compared with control group P<.05.
BRS, banana resistant starch.
The CSS advance ratio P and the value of X calculated from P were found to be significantly lower compared with the model group, indicating that the intestinal advance of the CSS was greater in the control mice (Table 3), which means that the constipation model induced by diphenoxylate was successful. The average values for the low- and middle-dose groups showed no significant difference compared with that for the model group. However, the CSS advance ratio P of the high-dose groups was found to be significantly higher compared with the model group, implying that only the high dose of BRS increased the gastrointestinal propulsive rate and is effective in accelerating the movement of the small intestine.
Table 3.
Activated-Carbon Suspension Solution Advance Ratio ( ±)
±)
| Group | Animal no. | BRS dose (g/kg body weight per day) | CSS advance ratio P (%) | Calculated value of X |
|---|---|---|---|---|
| Control | 10 | 0 | 71.5±7.0* | 1.34±0.05* |
| Model | 10 | 0 | 44.7±6.6 | 1.63±0.12 |
| Low | 10 | 1.0 | 43.6±10.6 | 1.66±0.19 |
| Middle | 10 | 2.0 | 52.3±9.8 | 1.53±0.12 |
| High | 10 | 4.0 | 62.6±14.0* | 1.43±0.12* |
Compared with model group *P<.05.
CSS, activated-carbon suspension solution.
Effects of BRS on Stool Characteristics
Table 4 shows that no significant differences in body weight and food intake among the five groups of mice were found during the defecation test days. These results were consistent with the performance in the small intestinal movement experiment.
Table 4.
Effects on the Body Weight and Food Intake of Mice ( ±s)
±s)
| Group | Animal no. | BRS dose (g/kg body weight per day) | Original body weight (g) | Final body weight (g) | Daily food intake (g) |
|---|---|---|---|---|---|
| Control | 10 | 0 | 16.4±0.5 | 25.7±1.5 | 4.6±0.7 |
| Model | 10 | 0 | 16.6±0.6 | 27.1±1.3 | 4.9±0.8 |
| Low | 10 | 1.0 | 16.4±0.5 | 26.1±1.6 | 4.5±0.7 |
| Middle | 10 | 2.0 | 16.0±0.6 | 26.4±1.3 | 4.8±0.8 |
| High | 10 | 4.0 | 16.4±0.8 | 25.0±2.8 | 4.5±0.7 |
Compared with control group P<.05.
As shown in Table 5, the time to the first black dejecta in the control group was significantly shorter than that in the model group, which points that the constipation model induced with diphenoxylate was successful. The time to the first black dejecta in the middle- and high-dose groups was significantly shorter than that in the model group. This indicated that the start time of defecation in mice was shortened by middle- and high-dose BRS, indicating that low-dose BRS was not sufficient to reduce the time of the first black dejecta ejection, but the middle and high doses were sufficient.
Table 5.
Effects on the Time of the First Black Dejecta and the Dejecta Moisture Content ( ±s)
±s)
| Group | Animal no. | BRS dose (g/kg body weight per day) | Time of the first black dejecta (min) | Dejecta moisture content (%) |
|---|---|---|---|---|
| Control | 10 | 0 | 73.4±14.5* | 24.26±2.18 |
| Model | 10 | 0 | 117.5±9.3 | 24.88±0.73 |
| Low | 10 | 1.0 | 113.7±31.8 | 26.26±1.30 |
| Middle | 10 | 2.0 | 86.7±20.5* | 25.70±0.93 |
| High | 10 | 4.0 | 94.2±25.3* | 28.79±2.26 |
Compared with model group *P<.05.
There was no significant difference in the moisture content of the dejecta between the three BRS-treated groups, the control group, and the model group (Table 4), suggesting that the water holding capacity of BRS may not be the chief reason for the laxative effects of BRS.
The control group and the three BRS-treated groups did not differ significantly from the model group in the number of dejecta granules or the dejecta weight produced at 3 and 6 h (Table 6). Therefore, BRS had no influence on the size or weight of the dejecta granules.
Table 6.
Effects on the Dejecta Granules and Their Weight in Mice ( ±s)
±s)
| Group | Animal no. | BRS dose (g/kg body weight per day) | Dejecta granules at 3 h | Dejecta weight at 3 h (mg) | Dejecta granules at 6 h | Dejecta weight at 6 h (mg) |
|---|---|---|---|---|---|---|
| Control | 10 | 0 | 8.8±3.2 | 87±19 | 26.0±4.5 | 255±20 |
| Model | 10 | 0 | 6.4±4.0 | 88±36 | 19.9±7.2 | 238±32 |
| Low | 10 | 1.0 | 4.7±3.8 | 65±43 | 20.8±5.6 | 210±55 |
| Middle | 10 | 2.0 | 5.4±4.6 | 66±14 | 19.3±5.9 | 219±18 |
| High | 10 | 4.0 | 4.8±3.6 | 51±2 | 16.1±6.5 | 240±4 |
Compared with model group P<.05.
In conclusion, from the structural tests, it can be determined that most BRS particles were oval and spindly as seen in the light microscopy picture. Some cracks and pits caused by the hydrolysis of amylase were observed in the starch surface when viewing the scanning electron microscopy image. In XRD analyses, the degree of crystallinity of BRS was 23.13% and the main diffraction peaks were at 2θ 15.14, 17.38, 20.08, and 22.51. The degree of polymerization of BRS was 81.16 and the average molecular weight was 13147.92 Da, as calculated by the reducing terminal method.
The laxative effects of RS, isolated from unripe bananas, were studied using a murine constipation model. Based on the research outcomes, it was found that BRS was able to accelerate the movement of the small intestine in experimentally constipated mice at the dose of 4.0 g/kg body weight per day. In addition, BRS showed the capacity for shortening the time of the first black dejecta at the dose of 2.0 g/kg body weight per day. BRS had no influence on the dejecta moisture content. According to the defecation condition test, BRS did not impact the dejecta granules and weight of experimental mice. During the research periods, there were no negative effects of BRS on the body weight and food intake of mice.
Some references suggest that the physiological functions of RS may result from the fermentation and boosts to the proliferation of probiotics in intestine.2,8 Therefore, further studies about BRS will involve their effects on enteric microorganisms. Moreover, other prebiotics in banana like dietary fiber, oligosaccharides, and polysaccharides will also be investigated in detail in the future.
Acknowledgments
This research was supported by The National Natural Science Foundation of China (Grant No. 31301530); the Fundamental Research Funds for the Central Universities (Grant No. 2013ZM0063); the Science and Information Technology of Guangzhou, China (Grant No. 2013J4100056, 12C12101661); and the special funds in the introduction and cultivation of improved banana varieties of Guangdong Province in 2009.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Asp NG, ed.: Resistant starch. Proceedings from the second plenary meeting of EURESTA: European FLAIR Concerted Action No. 11 on physiological implications of the consumption of resistant starch in man. Eur J Clin Nutr 1992;46:S1. [PubMed] [Google Scholar]
- 2.Niba LL: Resistant starch: a potential functional food ingredient. Nutr Food Sci 2002;32:62–67 [Google Scholar]
- 3.Higgins JA, Brown IL: Resistant starch: a promising dietary agent for the prevention/treatment of inflammatory bowel disease and bowel cancer. Curr Opin Gastroenterol 2013;29:190–194 [DOI] [PubMed] [Google Scholar]
- 4.Robertson MD: Dietary-resistant starch and glucose metabolism. Curr Opin Clin Nutr 2012;15:362–367 [DOI] [PubMed] [Google Scholar]
- 5.Keller J, Layer P: Conservative treatment options for constipation due to primary dysfunctions of the colon—from irritable bowel syndrome to slow transit constipation. Viszeralmedizin 2012;28:243–249 [Google Scholar]
- 6.Bharucha AE, Pemberton JH, Locke GR: American Gastroenterological Association technical review on constipation. Gastroenterology 2013;144:218–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegers CP, von Hertzberg-Lottin E, Otte M, Schneider B: Anthranoid laxative abuse—a risk for colorectal cancer? Gut 1993;34:1099–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson IT, Gee JM: Resistant starch. Nutr Food Sci 1996;96:20–23 [Google Scholar]
- 9.Langkilde AM, Champ M, Andersson H: Effects of high-resistant-starch banana flour (RS2) on in vitro fermentation and the small-bowel excretion of energy, nutrients, and sterols: an ileostomy study. Am J Clin Nutr 2002;75:104–111 [DOI] [PubMed] [Google Scholar]
- 10.Lehmann U, Jacobasch G, Schmiedl D: Characterization of Resistant Starch Type III from Banana (Musa acuminata). J Agric Food Chem 2002;50:5236–5240 [DOI] [PubMed] [Google Scholar]
- 11.Aparicio-Saguilán A, Gutiérrez-Meraz F, García-Suárez FJ, Tovar J, Bello-Pérez LA: Physicochemical and functional properties of cross-linked banana resistant starch. Effect of pressure cooking. Starch 2008;60:286–291 [Google Scholar]
- 12.Cheng YF, Yang GM, Wang J, Du B, Bao JY, Wen ShN, Ding WR: Effects of spray drying technologies on the retention rate of banana resistant starch. Trans CSAE 2008;24:282–286 [Google Scholar]
- 13.Goni I, Garcia-Diz L, Manas E, Saura-Calixto F: Analysis of resistant starch: a method for food sand food products. Food Chem 1996;56:445–449 [Google Scholar]
- 14.Klucinec JD, Thompson DB: Fractionation of high-amylose maize starches by differential alcohol precipitation and chromatography of the fractions. Cereal Chem 1998;75:887–896 [Google Scholar]
- 15.Takeda Y, Maruta N, Hizukuri S: Examination of the structure of amylose by tritium labelling of the reducing terminal. Carbohyd Res 1992;227:113–120 [Google Scholar]
- 16.Wang J, Huang HH, Cheng YF, Yang GM: structure analysis and laxative effects of oligosaccharides isolated from bananas. J Med Food 2012;15:930–935 [DOI] [PubMed] [Google Scholar]


