Abstract
Macrophage foam cell formation by oxidized low-density lipoprotein (oxLDL) is a key step in the progression of atherosclerosis, which is involved in cholesterol influx and efflux in macrophages mediated by related proteins such as peroxisome proliferator-activated receptor γ (PPARγ), CD36, PPARα, liver-X receptor α (LXRα), and ATP-binding cassette transporter A1 (ABCA1). The aim of this study was to investigate the beneficial effects of kimchi methanol extract (KME) and a kimchi active compound, 3-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid (HDMPPA) on cholesterol flux in THP-1-derived macrophages treated with oxLDL. The effects of KME and HDMPPA on cell viability and lipid peroxidation were determined. Furthermore, the protein expression of PPARγ, CD36, PPARα, LXRα, and ABCA1 was examined. OxLDL strongly induced cell death and lipid peroxidation in THP-1-derived macrophages. However, KME and HDMPPA significantly improved cell viability and inhibited lipid peroxidation induced by oxLDL in THP-1-derived macrophages (P<.05). Moreover, KME and HDMPPA suppressed CD36 and PPARγ expressions, both of which participate in cholesterol influx. In contrast, KME and HDMPPA augmented LXRα, PPARα, and ABCA1 expression, which are associated with cholesterol efflux. Consequently, KME and HDMPPA suppressed lipid accumulation. These results indicate that KME and HDMPPA may inhibit lipid accumulation, in part, by regulating cholesterol influx- and efflux-related proteins. These findings will thus be useful for future prevention strategies against atherosclerosis.
Key Words: : ABCA1, CD36, HDMPPA, Kimchi, OxLDL
Introduction
Kimchi is a Korean fermented vegetable side dish made with vegetables and condiments such as red pepper, garlic, ginger, and onion.1 Three hundred different kinds of kimchi are available in Korea. The nutritional value of kimchi is considered high because kimchi is rich in dietary fiber, vitamins, and minerals. Kimchi became a worldwide food due to its functional properties. Health benefits of kimchi such as its antioxidant,2 lipid-lowering,3,4 antitumor,5 and antiatherogenic effects6,7 are well documented. Recently, antiviral effects of kimchi, particularly against severe acute respiratory syndrome, had drawn considerable attention. This antivirus effect is believed to be attributable to the lactic acid of kimchi. On the other hand, an antiviral effect of another product of lactic acid bacteria isolated from kimchi has been reported.8 3-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid (HDMPPA), with a molecular weight 226 kDa, was isolated and identified as an active compound from the dichloromethane fraction of Korean cabbage kimchi.7 HDMPPA demonstrated an antioxidant activity in vitro.9 HDMPPA also revealed preventive and therapeutic properties in hypercholesterolemic rabbits. The serum cholesterol level increase was suppressed and intimal thickness of aorta was reduced in rabbits, and these effects were comparable to those of simvastatin, a hypocholesterolemic drug.10–12 The mechanisms of action of HDMPPA for preventing atherosclerosis were involved in modulating the expressions of adhesion molecules and suppression of inflammatory molecule expressions. The protein levels of vascular adhesion molecule and intercellular adhesion molecule at aorta were decreased and the nuclear factor kappa B (NF-κB) protein level was also decreased in the aorta of apoE KO mice.12
Oxidized low-density lipoprotein (oxLDL), known as a key factor in the development of atherosclerosis, is involved in cholesterol accumulation in macrophages by means of internalizing them into the cells through binding with scavenger receptors on the membrane, which subsequently develop into foam cells. Cholesterol accumulation in macrophages is a result in the imbalance of cholesterol influx and efflux.13 Generally, scavenger receptors recognize modified LDL and bind with it. Scavenger receptors are categorized into scavenger receptor class A (SR-A), SR-B, and others (including CD68). Among them, CD36 belongs to SR-B and is particularly involved in internalizing oxLDL, leading to promote the cellular accumulation of cholesterol.14–17 ATP-binding cassette transporters A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) are responsible for efflux of cholesterol in macrophages.18
CD36, an 88 kDa transmembrane glycoprotein, is a fatty acid translocase.19 CD36 was originally identified as a glycoprotein IV on platelets, but it is also expressed by various cells such as monocytes, macrophages, smooth muscle cells, and endothelial cells.20 CD36 acts as a receptor for many ligands, but predominantly binds to oxLDL, and initiates translocation of cholesterol in the cell where it may promote transcriptional changes.21 Upregulation of CD36 by oxLDL transactivates peroxisome proliferator-activated receptor γ (PPARγ) through the mitogen-activated protein kinase pathway, subsequently forming a heterodimer with the retinoid X receptor (RXR).17,22 In addition, protein kinase C (PKC) and protein kinase B (PKB) are activated by CD36 expressions.23,24 In particular, the upregulation of CD36 induces the differentiation of macrophages into foam cells,25 thus initiating the progression of atherosclerosis. Furthermore, CD36 expression is also involved in cholesterol efflux; it regulates PPARs, liver X receptor (LXR), and ABCA1.26,27 LXR activation by lipoprotein-derived cholesterol has the potential to not only activate the cholesterol efflux pathway but also to modulate the intracellular distribution of cholesterol.26 Taken together, these observations show that the control of CD36 expression is strongly associated with the etiology of atherosclerosis. Recently, synthetic compounds such as PPAR agonists and LXR agonists have been shown to delay atherosclerosis by stimulating reverse cholesterol transport from macrophages.28 Therefore, searching for natural compounds that modulate macrophage functions represents an attractive strategy for the prevention and treatment of cardiovascular diseases.
In this study, we investigated the beneficial effects of kimchi methanol extract (KME) and a kimchi active compound, HDMPPA, in THP-1-derived macrophages. The effects of KME and HDMPPA on cell viability and lipid peroxidation were studied. In addition, the expression of protein modulators of cholesterol flux, including CD36, PPARγ, LXRα, PPARα, and ABCA1, was examined. Finally, the inhibitory effects on lipid accumulation in macrophages were examined.
Materials and Methods
Materials
Phorbol 12-myristate 13-acetate (PMA) and LDL were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), RPMI 1640, fetal bovine serum (FBS), and penicillin–streptomycin were obtained from Invitrogen Co. (Carlsbad, CA, USA). Antibodies for PPARγ, CD36, PPARα, LXRα, ABCA1, and β-actin and horseradish peroxidase-conjugated anti-rabbit and mouse Ig were purchased from SantaCruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence (ECL) advanced detection kits were purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA). All other chemicals used were of ACS grade.
Preparation of KME and HDMPPA
Fermented Korean cabbage kimchi (pH 4.2) was freeze-dried and sample powders prepared. Chemically synthesized HDMPPA was kindly provided by Professor Suh (Department of Chemistry, P University, Busan, Korea). HDMPPA was also isolated and identified from dichloromethane fraction of Korean cabbage kimchi by our team and its chemical characterization was already published.9 Figure 1 shows the chemical structure of HDMPPA.
FIG. 1.
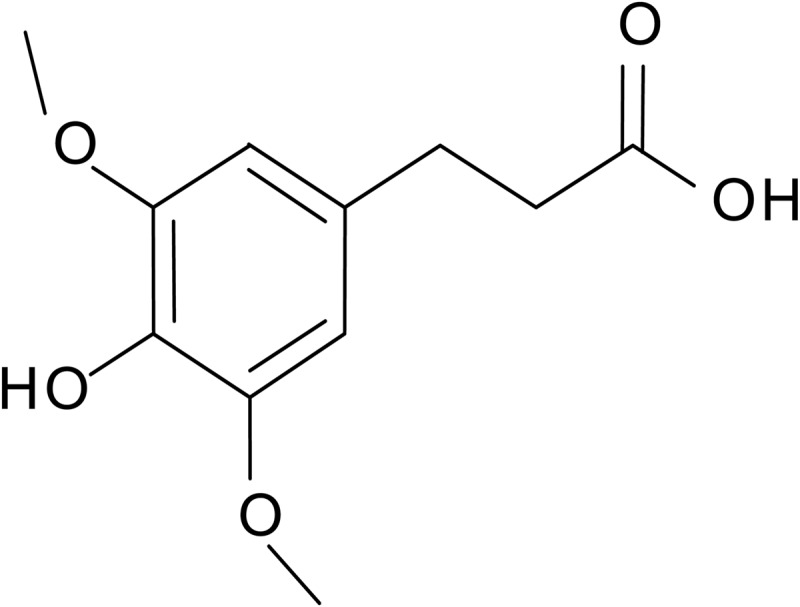
Chemical structure of 3′-(4′-hydroxyl-3′,5′-dimethoxyphenyl)propionic acid (HDMPPA).
Oxidation of LDL-C
Lyophilized LDL cholesterol (LDL-C) was dissolved in phosphate-buffered saline (PBS) and dialyzed against PBS at 4°C for 20 h. The total protein content of the LDL-C preparation was measured. Thereafter, LDL-C oxidation was performed by incubating with 10 μM CuSO4 at 37°C for 24 h. Lipid peroxidation by oxLDL was determined as thiobarbituric acid reactive substances (TBARS) and expressed as malondialdehyde (MDA) equivalents.
Cell culture
The human monocyte line THP-1 was obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea). THP-1 cells were grown in the RPMI 1640 medium supplemented with 10% heat-inactivated FBS, 20 IU/mL penicillin, and 20 μg/mL streptomycin. THP-1 cells were seeded into 96-well plates at a density of 1×105 cells/well in the RPMI 1640 medium and maintained at 37°C in a humidified atmosphere of 5% CO2. For differentiation into macrophages, 200 ng/mL PMA was added to THP-1 cells and incubated for 24 h. Furthermore, THP-1 macrophages were incubated with 100 μg/mL oxLDL for 48 h to induce foam cell formation. The FBS-free RPMI 1640 medium was used in this study.
Cell viability assay
Cell viability was measured by MTT (a yellow tetrazole) assay. Briefly, THP-1-derived macrophages were incubated with or without KME (100, 250, and 500 μg/mL) and HDMPPA (10, 25, and 50 μg/mL) for 24 h. Thereafter, 100 μg/mL oxLDL was added to each well for 48 h. At the end of culture, 100 μL MTT (5 mg/mL in PBS) was added and incubated for 4 h. The media were removed and 100 μL dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals. Absorbance at 540 nm was measured using a microplate reader.
Lipid peroxidation assay
Lipid peroxidation was measured by using a TBARS assay. THP-1-derived macrophages were incubated with or without KME (100, 250, and 500 μg/mL) and HDMPPA (10, 25, and 50 μg/mL) for 24 h followed by the addition of 100 μg/mL oxLDL in each well for 48 h. Two hundred microliters of each medium supernatant was mixed with a 400 μL TBARS solution and then boiled at 95°C for 30 min. Absorbance at 532 nm was measured and calculated from the standard curve. TBARS values were expressed as equivalent nmoles of MDA.
Western blot analysis
THP-1-derived macrophages were incubated with or without KME (500 μg/mL) and HDMPPA (50 μg/mL) for 24 h followed by the addition of 100 μg/mL oxLDL to each well for 48 h. THP-1-derived macrophages were washed with cold PBS and lysed with the lysis buffer. After allowing the mixture to stand on ice for 30 min, it was then centrifuged at 4°C, 12,000 g, for 20 min to obtain the cell lysate. The protein concentration was determined by the Bradford assay. Fifty micrograms of the cell lysate was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes. The membrane was then blocked in 1% skim milk for 2 h at room temperature (RT). The blocked membrane was incubated with specific primary antibodies (CD36, PPARγ, PPARα, LXRα, or ABCA1 at 1:200 dilution, and β-actin at 1:1000 dilution) for 2 h at RT. After washing with PBST (0.1% Tween 20 in PBS), the membrane was incubated with a species-specific horseradish peroxidase-conjugated secondary antibody (goat anti-mouse IgG at 1:5000 dilution) for 1 h at RT. Finally, the membrane was washed and the antigen signal was detected by ECL. Band intensities were quantified by ImageJ Software (NIH) and normalized to the internal control β-actin. Percent changes of target gene expression were calculated by comparing their expression levels in cells with different treatments to that in control cells.
Oil Red O staining assay
To determine lipid accumulation in THP-1-derived macrophages, cells were plated at a density of 1.0×106 cells/well in six-well plates and incubated with 100 μg/mL oxLDL for 48 h. Cells were washed three times with PBS and fixed with 10% formalin in PBS for 1 h at RT. Cells were stained with 0.1 mL/mL Oil Red O solution for 2 h, washed three times with water, and all the water was allowed to evaporate (at 32°C for 45 min). Stained cells were observed in situ under a microscope (100 × fields, Olympus IX70; Olympus Tokyo, Japan), and lipid accumulation was quantified using the isopropanol extracts from the stained cells by using a spectrophotometer at 510 nm.28
Statistical analysis
All experiments were repeated three times. Results are expressed as mean±SD. Statistical analyses among the groups were performed using a SAS program. Statistical significance was determined by one-way ANOVA followed by a Duncan's multiple-range test. In all statistical analyses, P-values below .05 were considered significant.
Results
KME and HDMPPA improved the cell viability of THP-1 cells by delaying LDL oxidation
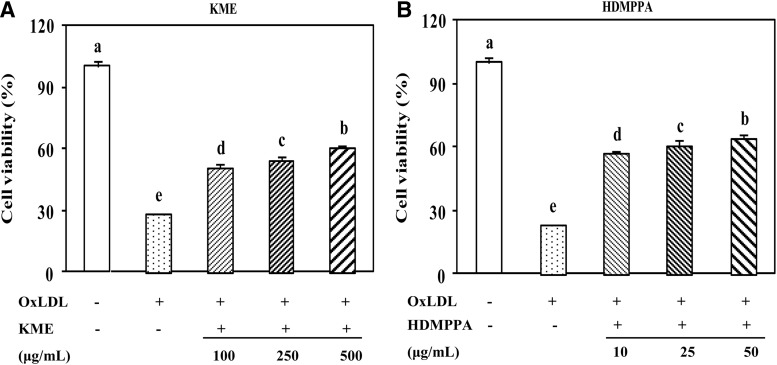
As shown in Figure 2, cell viability was significantly reduced because of LDL-C oxidation as the peroxidized lipid accumulation in the cell medium increased (Fig. 3). The cell viability that was decreased by oxLDL was increased in the presence of KME or HDMPPA in a dose-dependent manner (Fig. 2A, B). KME (500 μg/mL) and HDMPPA (50 μg/mL) significantly elevated cell viability to 60.51% and 60.16%, respectively (P<.05). HDMPPA, the active principle compound in Korean cabbage kimchi, displayed an antioxidant activity9 owing to the hydroxyl groups in its structure (Fig. 1).
FIG. 2.
Protective effect of KME (A) and HDMPPA (B) on cell viability. THP-1-derived macrophages were incubated with different concentrations of KME (100, 250, and 500 μg/mL) or HDMPPA (10, 25, and 50 μg/mL) for 24 h followed by the addition of oxLDL (100 μg/mL) for 48 h. Cell viability was then examined by MTT assay. Data are expressed as mean±SD (n=3 in triplicates, P<.05). a–eIndicates significant difference.
FIG. 3.
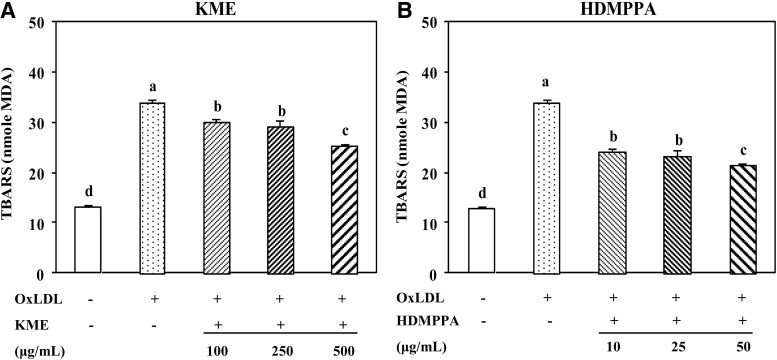
Inhibitory effect of KME (A) and HDMPPA (B) on lipid peroxidation.THP-1-derived macrophages were incubated with different concentrations of KME (100, 250, and 500 μg/mL) or HDMPPA (10, 25, and 50 μg/mL) for 24 h followed by addition of oxLDL (100 μg/mL) for 48 h and examined by TBARS assay. Data are expressed as mean±SD (n=3 in triplicates, P<.05). a–dIndicates significant difference.
KME and HDMPPA partially attenuated cholesterol influx by downregulating CD36 expression
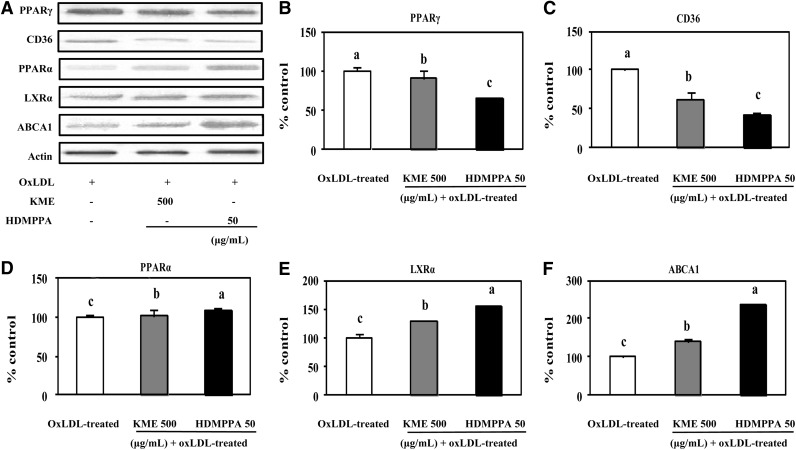
To elucidate the effects of KME and HDMPPA in THP-1-derived macrophages by oxLDL, the expression of atherosclerosis-related proteins such as CD36, PPARγ, PPARα, LXRα, and ABCA1 were examined by Western blot (Fig. 4A). As shown in Figure 4, PPARγ (B) and CD36 (C) protein expression levels were significantly decreased following the treatment of KME and HDMPPA compared to the levels in the control. On addition of KME, the percentage (%) controls of PPARγ and CD36 were 90.60% and 62.08%, respectively, whereas the values after the treatment of HDMPPA were 64.07% and 41.17%, respectively. In addition, PPARα, LXRα, and ABCA1 protein expression levels in THP-1-derived macrophages treated with oxLDL were determined. As shown in Figure 4D, PPARα expression was significantly increased by KME and HDMPPA compared with the expression levels in the control. This phenomenon was also observed for the expression of LXRα; KME increased LXRα protein expression by 28.92%, and HDMPPA elevated LXRα expression by 53.52% (P<.05). As expected, the expression of ABCA1 was also significantly increased by KME (28.06%) and HDMPPA (137.96%) compared with the control (P<.05).
FIG. 4.
Regulatory effect of KME (500 μg/mL) and HDMPPA (50 μg/mL) on protein expression. (A) Western blot analysis of PPARγ, CD36, PPARα, LXRα, and ABCA1 protein expression. THP-1-derived macrophages were incubated with or without KME or HDMPPA for 24 h followed by the addition of oxLDL (100 μg/mL) for 48 h. Densitometric analysis of PPARγ (B), CD36 (C), PPARα (D), LXRα (E), and ABCA1 protein (F). Western blot was performed by densitometric analysis and normalized to β-actin levels. Percent changes of target gene expression were calculated by comparing their expression levels in cells with different treatments to that in oxLDL-treated cells. Data are expressed as mean±SD (n=3 in triplicates, P<.05). a–cIndicates significant difference.
KME and HDMPPA attenuated lipid accumulation
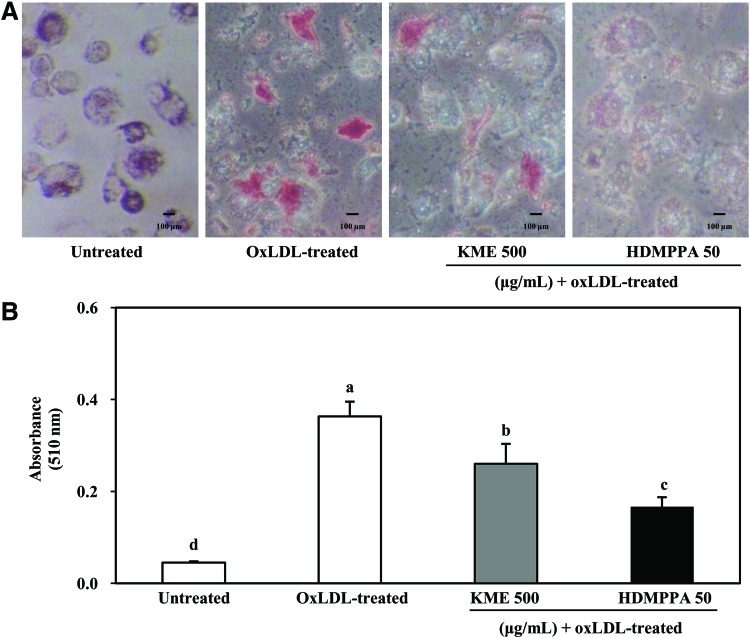
As shown in Figure 5A, lipid accumulation in THP-1-derived macrophages was evidently suppressed in the presence of KME and HDMPPA. KME and HDMPPA significantly decreased lipid accumulation in THP-1-derived macrophages (Fig. 5B). Since these results were observed in cells that survived following oxLDL damage, the data cannot be directly compared with the cell viability results shown in Figure 2. However, these results indicate that lipid accumulation in macrophages is apparently enhanced by oxLDL.
FIG. 5.
Inhibitory effect of KME (500 μg/mL) and HDMPPA (50 μg/mL) on lipid accumulation by Oil Red O staining. THP-1-derived macrophages were incubated with or without KME or HDMPPA for 24 h followed by addition of oxLDL (100 μg/mL) for 48 h, following which Oil Red O staining was performed. Stained cells were observed under a microscope (A) and quantified at 510 nm (B). Data are expressed as mean±SD (n=3, P<.05). a–dIndicates significant difference. Color images available online at www.liebertpub.com/jmf
Discussion
Macrophages play a critical role in the pathogenesis of atherosclerosis by facilitating cholesterol accumulation in foam cells. In particular, CD36 plays a pivotal role in atherosclerosis.29 Numerous studies have shown that oxLDL generally upregulates CD36 expression, leading to the formation of foam cells. Eventually, CD36 upregulation is strongly involved in the development of atherosclerosis. PPARγ is an important transcription factor with crucial regulatory roles in adipogenesis and lipid metabolism.30 The activation of PPARγ by oxLDL induces the expression of the CD36 scavenger receptor,31 whereas PPAR agonists have been shown to upregulate CD36 expression.32 Taken together, CD36 downregulation is necessary to prevent the formation of foam cells. To date, various substances have shown to control the expression of CD36. For instance, cholesterol-lowering statin drugs suppressed the expression of CD36 in macrophages. Consequently, the downregulation of CD36 would inhibit the formation of foam cells.33 In this study, CD36 expression stimulated by oxLDL was suppressed in KME- and HDMPPA-treated THP-1 cells as a result of PPARγ downregulation, suggesting that KME and HDMPPA could prevent the formation of foam cells by downregulating the expression of CD36. Uptake of oxLDL through CD36 leads to activation of PPARγ mediated, at least in part, through its content of oxidized lipids.17 However, direct assay, for example, using a 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocyanide percholorate (DiI)-labeled oxLDL34 is needed to support our results and determine whether oxLDL migration was delayed by kimchi.
PPARα, LXRα, and ABCA1 proteins are known as antiatherogenic factors that modulate cholesterol efflux and subsequently decrease cellular cholesterol accumulation. According to the study of Chinetti et al.,27 cholesterol removal from macrophage foam cells is mediated by ABCA1; PPARα activation is required for the stimulation of the ABCA1 pathway that is in line with our results. PPARα regulates lipid homeostasis by stimulating the peroxisomal β-oxidation of fatty acids. In particular, the activation of PPARα leads to the upregulation of fatty acid transport protein expression and the long-chain acyl-coenzyme A synthase (LACS) gene in the liver.35 Consequently, PPARα not only stimulates energy production but also shortens long-chain fatty acids thereby preventing lipid accumulation. LXRs, another group of regulators of lipid homeostasis, play critical roles as effectors in feed-forward mechanisms that prevent the cellular accumulation of cholesterol. There is a suggestion that LXR agonists can be used as potent agents for the prevention of atherosclerosis.36,37 ABCA1 is a member of the superfamily of ABC transporters involved in cellular cholesterol and phospholipid homeostasis.38 ABCA1 mediates cholesterol efflux most efficiently to lipid-poor apolipoprotein A1, whereas ABCG1 promotes cholesterol export to lipidated particles such as high-density lipoprotein (HDL). Larrede S et al.18 reported that efflux of cholesterol from human foam cell macrophages to HDL is independent of ABCG1 expression, but specifically requires the expression of ABCA1, suggesting that the ABCA1 pathway is important for transporting cholesterol from human macrophages. Generally, oxLDL only reduces ABCA1 expression. These findings indicate that KME and HDMPPA play a significant role in cholesterol homeostasis through the regulation of proteins, including PPARα, LXRα, and ABCA1. In the study of cholesterol homeostasis regulation, 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-23,24-bisnor-5-cholen-3-ol (NBD-cholesterol) is used to determine cholesterol efflux directly; however, ABCA1 protein expression is also considered as one of the major and important regulatory factors in the cholesterol efflux mechanism study.39–41
The possible mechanisms involved in these processes outlined above might be due to the suppressed lipid accumulation by PPARα upregulation and PPARγ downregulation. As mentioned above, KME and HDMPPA enhanced PPARα expression and inhibited PPARγ expression. In addition, KME and HDMPPA enhanced the ABCA1-mediated cholesterol efflux from lipid-laden foam cells through transcriptional mechanisms pertaining to PPARγ and LXRα signaling. Taken together, KME and HDMPPA confer a protective activity against lipid accumulation through the regulation of the expression of these related proteins. In our study, we did not investigate other scavenger receptor mechanisms such as SR-A, SR-B1, or CD68, which are also known factors involved in cholesterol influx to the macrophage. SR-A16 and SR-B114 both mediate the internalization of oxLDL to the macrophage, but foam cell formation was only observed in SR-A-mediated cholesterol influx. It seems that SR-B1 also modulates cholesterol efflux.15 CD36, but not SR-A, is transcriptionally induced by PPARγ agonists in THP-1 cells.17 SR-B1 is able to bind mature HDL and, through a process termed selective lipid uptake, is able to mediate bulk transfer of cholesterol esters from the bound HDL to the cell. SR-B1 is prominently expressed in the liver and other steroidogenic organs but can also be found in macrophages and endothelial cells.15 ABCG1 is another major receptor that mediates cholesterol efflux. As mentioned earlier, cholesterol efflux from human foam cell macrophages to HDL is independent of ABCG1 expression, but specifically requires the expression of ABCA1. PPARγ positively controls cholesterol efflux in macrophages by enhancing the expression of SR-B1 and ABCA1. PPARα and PPARγ ligands induced the expression of the cholesterol transporter ABCA1 and stimulated cholesterol efflux in macrophages by a mechanism involving induction of the LXRα.27
In conclusion, KME and HDMPPA had a protective effect on the cell viability of THP-1-derived macrophages through the inhibition of lipid peroxidation. In particular, KME and HDMPPA have significant roles in the regulation of CD36 and ABCA1 expression, both of which at least partially participated in cholesterol influx and efflux. Based on these results, the intake of kimchi might be beneficial for the treatment of degenerative diseases such as cardiovascular disease by attenuating cholesterol accumulation in macrophages through the regulation of CD36 and ABCA1 expression. Future studies should utilize direct assays for measuring cholesterol flux in macrophages and examine regulatory genes responsible for cholesterol transport to confirm the effects of kimchi.
Acknowledgment
This work was supported by a 2-Year Research Grant of Pusan National University.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Kim MJ, Ha JY, Yun YR, Noh JS, Song YB, Song YO: Extension of shelf life of kimchi by addition of encapsulated mustard oil. Food Sci Biotechnol 2006;15:884–888 [Google Scholar]
- 2.Park JM, Shin JH, Gu JG, Yoon SJ, Song JC, Jeon WM, Suh HJ, Chang UJ, Yang CY, Kim JM: Effect of antioxidant activity in kimchi during a short-term and over-ripening fermentation period. J Biosci Bioeng 2011;112:356–359 [DOI] [PubMed] [Google Scholar]
- 3.Kim EK, An SY, Lee MS, Kim TH, Lee HK, Hwang WS, Choe SJ, Kim TY, Han SJ, Kim HJ, Kim DJ, Lee KW: Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr Res 2011;31:436–443 [DOI] [PubMed] [Google Scholar]
- 4.Park JA, Tirupathi Pichiah PB, Yu JJ, Oh SH, Daily JW, III, Cha YS: Anti-obesity effect of kimchi fermented with Weissella koreensis OK1-6 as starter in high-fat diet-induced obese C57BL/6J mice. J Appl Microbiol 2012;113:1507–1156 [DOI] [PubMed] [Google Scholar]
- 5.Kong CS, Bahn YE, Kim BK, Lee KY, Park KY: Antiproliferative effect of chitosan-added kimchi in HT-29 human colon carcinoma cells. J Med Food 2010;13:6–12 [DOI] [PubMed] [Google Scholar]
- 6.Kwon MJ, Song YS, Choi MS, Song YO: Red pepper attenuates cholesteryl ester transfer protein activity and atherosclerosis in cholesterol-fed rabbits. Clin Chim Acta 2003;332:37–44 [DOI] [PubMed] [Google Scholar]
- 7.Kwon MJ, Song YS, Choi MS, Park SJ, Jeong KS, Song YO: Cholesteryl ester transfer protein activity and atherogenic parameters in rabbits supplemented with cholesterol and garlic powder. Life Sci 2003;72:2953–2964 [DOI] [PubMed] [Google Scholar]
- 8.Rhee SJ, Lee JE, Lee CH: Importance of lactic acid bacteria in Asian fermented foods. Microb Cell Fact 2011;10:S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YM, Kwon MJ, Kim JK, Suh HS, Choi JS, Song YO: Isolation and identification of active principle in Chinese cabbage Kimchi responsible for antioxidant effect. J Korean Soc Food Sci Nutr 2004;36:129–133 [Google Scholar]
- 10.Kim HJ, Lee JS, Chung HY, Song SH, Suh H, Noh JS, Song YO: 3-(4'-Hydroxyl-3',5'-dimethoxyphenyl)propionic acid, an active principle of Kimchi, inhibits development of atherosclerosis in rabbits. J Agric Food Chem 2007;55:10486–10492 [DOI] [PubMed] [Google Scholar]
- 11.Noh JS, Kim HJ, Kwon MJ, Song YO: Active principle of kimchi, 3-(4'-hydroxyl-3',5'-dimethoxyphenyl)propionic acid, retards fatty streak formation at aortic sinus of apolipoprotein E knockout mice. J Med Food 2009;12:1206–1212 [DOI] [PubMed] [Google Scholar]
- 12.Noh JS, Choi YH, Song YO: Beneficial effects of the active principle component of Korean cabbage kimchi via increasing nitric oxide production and suppressing inflammation in the aorta of apoE knockout mice. Br J Nutr 2012;13:1–8 [DOI] [PubMed] [Google Scholar]
- 13.Ross R: Atherosclerosis-an inflammatory disease. N Engl J Med 1999;340:112–126 [DOI] [PubMed] [Google Scholar]
- 14.Krieger M, Kozarsky K: Influence of the HDL receptor SR-B1 on atherosclerosis. Curr Opin Lipidol 1999;10:491–497 [DOI] [PubMed] [Google Scholar]
- 15.Silver DL, Tall AR: The cellular biology of scavenger receptor class B type 1. Curr Opin Lipidol 2001;12:497–504 [DOI] [PubMed] [Google Scholar]
- 16.Podrez EA, Febbraio M, Sheibani N, et al. : Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 2000;105:1095–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM: PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998;93:241–252 [DOI] [PubMed] [Google Scholar]
- 18.Larrede S, Quinn CM, Jessup W, et al. : Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol 2009;29:1930–1936 [DOI] [PubMed] [Google Scholar]
- 19.Abumrad NA, El-Maghrabi MR, Mari EZ, Lopez E, Grimaldi PA: Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation-homology with human CD36. J Biol Chem 1993;268:17665–17668 [PubMed] [Google Scholar]
- 20.Daviet L, McGregor JL: Vascular biology of CD36: role of this new adhesion molecule family in different disease states. Thromb Haemost 1997;78:65–69 [PubMed] [Google Scholar]
- 21.Shiffman D, Mikita T, Tai JT, et al. : Large scale gene expression analysis of cholesterol-loaded macrophages. J Biol Chem 2000;275:37324–37332 [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Liu Y, Wang X, New L, Han J, Brunk UT: Activation of the p38 MAP kinase pathway is required for foam cell formation from macrophages exposed to oxidized LDL. APMIS 2002;110:458–468 [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Han J, Pearce SF, Silverstein RL, Gotto AM, Jr, Hajjar DP, Nicholson AC: Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res 2000;41:688–696 [PubMed] [Google Scholar]
- 24.Munteanu A, Taddei M, Tamburini I, Bergamini E, Azzi A, Zingg JM: Antagonistic effects of oxidized low density lipoprotein and alpha tocopherol on CD36 scavenger receptor expression in monocytes: involvement of protein kinase B and peroxisome proliferator-activated receptor-gamma. J Biol Chem 2006;281:6489–6497 [DOI] [PubMed] [Google Scholar]
- 25.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL: A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab 2006;4:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chawla A, Boisvert WA, Lee CH, et al. : A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 2001;7:161–171 [DOI] [PubMed] [Google Scholar]
- 27.Chinetti G, Lestavel S, Bocher V, et al. : PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med 2001;7:53–58 [DOI] [PubMed] [Google Scholar]
- 28.Helen V, Lisa P, Tracey LG, et al. : The peroxisome proliferator-activated receptor promotes lipid accumulation in human macrophages. J Biol Chem 2001;276:44258–44265 [DOI] [PubMed] [Google Scholar]
- 29.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum J: Beyond cholesterol. Modification of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320:915–924 [DOI] [PubMed] [Google Scholar]
- 30.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ: Nuclear receptors and lipid physiology: opening the X-files. Science 2001b;294:1866–1870 [DOI] [PubMed] [Google Scholar]
- 31.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM: Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 1998;93:229–240 [DOI] [PubMed] [Google Scholar]
- 32.Sato O, Kuriki C, Fukui Y, Motojima K: Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferators-activated receptor α and γ ligands. J Biol Chem 2002;277:15703–15711 [DOI] [PubMed] [Google Scholar]
- 33.Han J, Zhou X, Yokoyama T, Hajjar DP, Gotto AM, Jr, Nicholson AC: Pitavastatin downregulates expression of the macrophage type B scavenger receptor, CD36. Circulation 2004;109:790–796 [DOI] [PubMed] [Google Scholar]
- 34.Morihara N, Ide N, Weiss N: Aged garlic extract inhibits homocysteine-induced scavenger receptor CD36 expression and oxidized low-density lipoprotein cholesterol uptake in human macrophages in vitro. J Ethnopharmacol 2011;134:711–716 [DOI] [PubMed] [Google Scholar]
- 35.Repa JJ, Turley SD, Lobaccaro JA, et al. : Regulation of absorption and ABCA1-mediated efflux of cholesterol by RXR heterodimers. Science 2000;289:1524–1529 [DOI] [PubMed] [Google Scholar]
- 36.Joseph SB, McKilligin E, Pei L, et al. : Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci USA 2002;99:7604–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kratzer A, Buchebner M, Pfeifer T, et al. : Synthetic LXR agonist attenuates plaque formation in apoE-/- mice without inducing liver steatosis and hypertriglyceridemia. J Lipid Res 2009;50:312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz G, Langmann T: Structure, function and regulation of the ABC1 gene product. Curr Opin Lipidol 2001;12:129–140 [DOI] [PubMed] [Google Scholar]
- 39.Tani M, Kawakami A, Mizuno Y, et al. : Small dense LDL enhances THP-1 macrophage foam cell formation. Atheroscler Thromb 2011;18:698–704 [DOI] [PubMed] [Google Scholar]
- 40.Hoang MH, Jia Y, Jun HJ, Lee JH, Lee BY, Lee SJ: Fucosterol is a selective liver x receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J Agric Food Chem 2012; 60:11567–11575 [DOI] [PubMed] [Google Scholar]
- 41.Park SH, Kim JL, Kang MK, et al. : Sage weed (Salvia plebeia) extract antagonizes foam cell formation and promotes cholesterol efflux in murine macrophages. Int J Mol Med 2012;30:1105–1112 [DOI] [PubMed] [Google Scholar]