Abstract
This study is aimed to examine the muscle fiber type, composition and satellite cells in young male soccer players and to correlate them to cardiorespiratory indices and muscle strength. The participants formed three Groups: Group A (n = 13), 11.2 ± 0.4yrs, Group B (n=10), 13.1 ± 0.5yrs and Group C (n = 9), 15.2 ± 0.6yrs. Muscle biopsies were obtained from the vastus lateralis. Peak torque values of the quadriceps and hamstrings were recorded and VO2max was measured on the treadmill. Group C had lower type I percentage distribution compared to A by 21.3% (p < 0.01), while the type IIA relative percentage was higher by 18.1% and 18.4% than in Groups A and B (p < 0.05). Groups B and C had higher cross-sectional area (CSA) values in all fiber types than in Group A (0.05 < p < 0.001). The number of satellite cells did not differ between the groups. Groups B and C had higher peak torque at all angular velocities and absolute VO2max in terms of ml·min-1 than Group A (0.05 < p < 0.001). It is concluded that the increased percentage of type IIA muscle fibers noticed in Group C in comparison to the Groups A and B should be mainly attributed to the different workload exercise and training programs. The alteration of myosin heavy chain (MHC) isoforms composition even in children is an important mechanism for skeletal muscle characteristics. Finally, CSA, isokinetic muscle strength and VO2max values seems to be expressed according to age.
Key Points.
Fifteen years old soccer players have higher IIA percentage distribution than the younger players by approximately 18%.
The age and the training status play a crucial role in muscle fibers co-expression.
Specific training in young athletes seems to alter significantly the muscular metabolic profile.
Key words: MHC isoforms, satellite cells, muscle fiber type, maximal oxygen uptake, isokinetic muscle strength, young soccer players
Introduction
The effect that physical training might have on the development of cardiorespiratory and muscle strength during childhood and adolescence has been a matter of long – lasting controversy (Degache et al., 2010; Gravina et al., 2008; Vamvakoudis et al., 2007). Several studies demonstrated an improvement in aerobic capacity (Baxter-Jones et al., 1993; Ekblom, 1969) and muscle strength (Faigenbaum, 2000; Iga et al., 2009; Rochcongar et al., 1988) in young children, while other studies, focusing on prepubescent children, have shown little or no improvement in peak oxygen consumption relative to body weight (Kobayashi et al., 1978; Williams et al., 2000) and muscle strength (Burnie and Brodie, 1986).
As far as the muscular characteristics of prepubescent children are concerned, there are no substantial disagreements or disputes simply because there is an insufficient research on the effect on muscular adaptations of the fiber types due to training, compared to research conducted in adults. In adult elite soccer players it has been reported (Bangsbo, 1994) that there are no significant differences between type I and type II fiber and that type I makes up approximately 60%, type IIA ca 30% and type IIX ca 15% indicating limits which have previously been mentioned for young athletes of various sports using myofibre histochemical ATP-ase staining methodology (Bell et al., 1980; Mero et al., 1991). To the best of our knowledge no study has examined the interaction of age, during prepuberty – puberty, and long-term soccer training with the structural and functional properties of human skeletal muscle, using sensitive immunohistochemical analytic methods.
Satellite cells have been reported that can be divided or multiplied by strength training in adults (Kadi, 2000). The question which remains, however, is at what age this becomes apparent. It has been suggested that in adults resistance training can increase the proportion of satellite cells and the number of myonuclei (Kadi and Thornell, 2000; Kadi et al., 2004). However, no study has dealt with the effect of long-term soccer training on the muscle metabolism of prepubertal and pubertal soccer players.
We hypothesized that young athletes of different age and training experience would have different characteristics in muscle fiber tissues. Based on the above mentioned reports, the purpose of this study was to examine and corellate cardiorespiratory capacity and isokinetic muscle strength, as well as the specific muscle fiber characteristics in three different age groups of young soccer players. Additionally, regarding the muscle profile, it was of particular interest to study the pattern of myosin heavy chain (MHC) protein isoform expression, fiber type cross-sectional area (CSA) and the distribution of satellite cells in slow and fast fibers from the vastus lateralis muscle.
Methods
Participants
The participants were divided into three Groups: Group A (n = 13) consisted of elite soccer players aged 11.2 ± 0.4yrs old with 3.7 ± 1.5yrs of training, Group B (n = 10) was made up of players aged 13.1 ± 0.5yrs old with 6.6±1.6yrs of training and Group C (n = 9) included soccer players aged 15.2 ± 0.6yrs old with 8.0 ± 1.1yrs of training. All participants were informed of the procedures, risks, and benefits and provided written consent signed by their parents before participating in the study. It was a randomized cross-sectional study and it was approved by the local ethical committee of the Department of Physical Education and Sports Science in accordance with the ethical standards in sport and exercise research and the Declaration of Helsinki.
Since the biological maturity status significantly influences the functional capacity of muscle fibers, the level of maturation was assessed by a physician using Tanner’s photographs for the 5 stages of maturity (Tanner, 1975). Considering the nature of this comparative study all participants of each group had the same maturational status and Tanner’s stages were 2, 3 and 4 for groups A, B and C, respectively.
Training program
The young soccer players were recruited based on the modern model of development that occurs at young ages and applies the results of the laboratory testing in training practice in order to improve the sport performance of young soccer players. The duration of the training season for all athletes was 41 weeks per year. In particular, the subjects of Group A systematically performed 2 training sessions per week of 75min each, while the athletes of Groups B and C participated in 3 and 4 training sessions of 90min each per week, respectively. Furthermore, all athletes had 1 additional specific personal training session every 15 days for individual improvement in certain skills. In total, the annual amount of training sessions for groups A, B and C were 100, 140 and 180, respectively. Moreover, all subjects competed in one game per week throughout the season. The training protocol was based on the normal technical-tactical and physiological elements of soccer indicated for improvement according to developmental age. The experiment was performed in mid-season, that is, three months after the beginning of the competitive season followed by the physiological tests 2 months later.
Anthropometric measurements
The height and body weight were measured using an electronic digital scale (Seca 220e, Hamburg, Germany). All participants underwent anthropometric examinations, including body fat assessment with skinfold measurements (four-site method): biceps (S1), triceps (S2), suprailiac (S3) and subscapular (S4) by specific calliper (Lafayette, Ins. Co., Indiana, USA). Estimation of the body density was calculated according to standard equations for males under 16 years old (Durnin and Rahaman, 1967; Siri, 1956). Additionally, the lean body mass (LBM) was calculated from the body weight and body fat measurements.
Muscle biopsies
Muscle biopsies (approximately 120mg) were obtained from the middle portion of the vastus lateralis muscle of the dominant leg (18.3 ± 5.1, 19.3 ± 3.4 and 20.4 ± 2.2cm from proximal to the patella, respectively) by a physician using Weil-Blakesley’s choncotom technique. Afterwards local anaesthesia was applied in the skin penetrating the underlying fascia. The muscle tissue was embedded in an embedding medium (Jung Tissue Freezing) and immediately frozen in isopentane, cooled in liquid nitrogen and stored at -80°C until analyzed. On average, 505 ± 102 muscle fibers were classified in each sample of each participant. A mean total of these fibers was measured for satellite cells per fiber type I and II.
Immunohistochemistry
Serial transverse sections 5-7 μm thick were cut in microtome at -22°C and mounted on glass slides. Muscle biopsies were air dried, rinsed for 20 min in phosphate buffered saline (PBS), and incubated for 20 min with diluted normal horse serum. Sections were incubated overnight at +4°C with the primary monoclonal antibodies (mAbs) diluted in bovine serum albumin (BSA). The following day, the slides were washed in PBS for 20 min and incubated for 1 hour with the diluted biotinylated horse anti-mouse secondary antibody (Vector BA-9200, Burlingame, California). The slides were then washed for 20 min in PBS and incubated for 1 hour with a Vectastain ABC reagent. For the visualization of the primary antibody binding, the diaminobenzidine (DAB) substrate kit for peroxidase (Vector, SK-4100, Burlingame, California) was used. MHC expression was assessed using well-characterized monoclonal antibodies (mAbs) tested against human MHC I (mAb A4.840) and MHC I & IIA (mAb N2.261) (Hughes et al., 1993; Kadi and Thornell, 2000). The mAb A4.840 strongly stained type I fibers, whereas type IIA, IIAX and IIX remained unstained (Figure 1a). The mAb N2.261 strongly stained type IIA fibers, whereas type I and IIAX fibers were equally weakly stained and type IIX fibers were unstained (Figure 1b). Type IIC fibers were strongly stained with mAb N2.261 and moderately stained with mAbs A4.840. CSA of muscle fibers was measured using TEMA image analysis system (Scanbeam, a/s, Handsund, Denmark).
Figure 1.
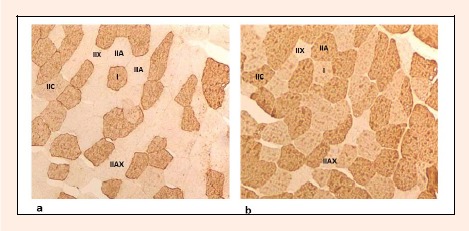
Identification of muscle fiber type in cross-section of vastus lateralis biopsy from a young soccer player using immunohistochemical analysis. In (a) serial sections stained using mAb a4.840 and in (b) using mAb N2.261.
Satellite cells were analyzed using a monoclonal antibody directed against the neural cell adhesion molecule (NCAM/CD56) (Becton Dickinson, San Jose, California) (Charifi et al., 2003; Kadi, 2000). For the visualization of satellite cells, the sections were counterstained with Mayer’s hematoxylin. Satellite cells were stained brown and images were acquired with a digital camera (SPOT Insight; Diagnostics Ins, Sterling Heights, Michingan) connected to a light microscope (Nikon Eclipse E400, Badhoevedorp, The Netherlands) (Figure 2a, 2b and 2c). The visualization of satellite cells was done at high magnification (objective, X40 or X60). Immunochistochemistry analysis was performed by a technician with expertise in this area.
Figure 2.
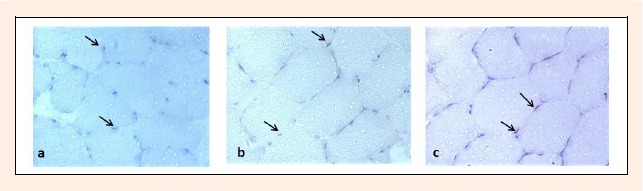
Representative images of stallite cells from vastus lateralis muscle in (a) 11 yrs, (b) 13 yrs and (c) 15 yrs old soccer players using immunohistochemical analysis.
Cardiorespiratory endurance
Maximal oxygen uptake (VO2max) was performed on a motorized treadmill (h/p/cosmos pulsar, Nussdorf-Traunstein, Germany) using a continuous exercise testing protocol consisting of ten 1-minute stages. The initial grade and speed were set at 0% at 8 and 10km/h, respectively, and followed by an increase in speed of 1km/h per stage with a 2% stable grade until exhaustion. VO2max values and cardiorespiratory indices were measured via breath by breath automated pulmonary/metabolic gas exchange system (Oxycon Pro-Jaeger, Wurzburg Germany). The heart rate (HR) was recorded by means of a 12-lead electrocardiogram (Viasys) connected to the ergospirometric system. During the test, the following additional cardiorespiratory parameters were determined: exercise duration; the maximal pulmonary ventilation (VEmax); the heart rate (HRAT) and the VO2 of anaerobic threshold; the speed of anaerobic threshold (UAT); the maximal heart rate (HRmax) and the respiratory exchange ratio (RER). VO2max was assumed when at least three of the four following criteria were met: (a) the HR during the last minute exceeded 95% of the expected maximal HR predicted 220-age (b) a respiratory gas exchange ratio (VCO2/VO2) of either 1.1 or higher was reached; (c) the subjects were no longer able to maintain running despite verbal encouragements and (d) blood lactate higher than 6 mmol(l-1. Blood samples were drawn from the fingertip and the concentration of blood lactate was determined after the end of VO2max test, in the 5th min of the recovery phase using a lactate photometer analyzer (Accusport, Boehringer Mannheim, Germany).
Strength measurements
Before testing protocol the participants performed a standardized warm-up on a Monark cycle ergometer and two trial efforts were executed. The strength of knee flexors and extensors in the dominant leg was measured using an isokinetic dynamometer Humac Norm CSMI (Model 770, Stoughton, MA, USA). The participant was seated on the dynamometer in an adjustable chair; the upper body was stabilized with straps secured diagonally across the chest and the hips. Maximal isokinetic strength was recorded as the torque of the quadricep and hamstring muscles throughout the whole range of motion (ROM) at angular velocities of 60, 180 and 300º·sec-1. Gravity correction was performed for the tested leg. The knee to be tested was positioned at 90ο flexion (0ο corresponding to fully extended knee) to align the axis of the dynamometer lever arm with the distal point of the lateral femoral condyle. The length of the level arm was individually determined and the resistance pad placed proximal to the medial malleolus. The non-tested leg was hanging freely. Knee extension started when the knee positioned at 90ο of flexion, while the knee flexion started when the knee was at full extension (0º). Subjects were instructed to cross their arms over their chest and to kick the leg as hard and as fast as they could through a complete ROM. Three repetitions were carried out at each angular velocity and the best torque value was used. A 30-second rest period was taken between each trial and a 60-second rest period was taken between each velocity measurement.
Statistical analysis
All values are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Scheffe’s post-hoc test was used to determine differences between groups. Correlation analysis was performed according to Pearson since there was a normal distribution in the samples. The statistical analysis was performed via SPSS (version 18.0, Chicago, Illinois, USA). Significance for all tests was set a priori at p < 0.05.
Results
The physical characteristics and the results of the maximal cardiopulmonary exercise testing of the players are shown in Table 1. Both Groups B and C presented significantly higher height, weight, BSA and lean body mass (LBM) compared to Group A. Although there was a tendency for higher body fat in Groups B and C compared to A, this finding was not proven to be of significance (p > 0.05).
Table 1.
Physical characteristics of young soccer players. Data are means (±SD).
| Group A (n = 13) |
Group B (n = 10) |
Group C (n = 9) |
|
|---|---|---|---|
| Height (m) | 1.51 (.06) | 1.73 (.06) ††† | 1.74 (.05) *** |
| Weight (kg) | 43.1 (7.1) | 63.2 (11.0) ††† | 66.5 (5.9) *** |
| BSA (m2) | 1.35 (.13) | 1.75 (.17) ††† | 1.80 (.09) *** |
| Body fat (%) | 10.5 (2.8) | 12.3 (4.1) | 13.1 (3.2) |
| LBM (kg) | 38.5 (6.2) | 55.2 (8.3) ††† | 57.8 (5.9) *** |
| HRrest (b·min-1) | 68.5 (11.1) | 71.1 (7.1) | 67.5 (9.4) |
| sBP (mmHg) | 117.8 (6.4) | 119.0 (7.8) | 124.1 (6.4) |
| dBP (mmHg) | 64.5 (7.9) | 61.7 (8.0) | 66.8 (8.9) |
| Time to exhaustion (min) | 7.89 (1.87) | 9.14 (2.87) ††† | 9.04 (1.46) ‡‡‡ |
| HRmax (b·min-1) | 196.2 (8.0) | 200.5 (4.0) | 197.0 (5.1) |
| RER | 1.10 (.05) | 1.11 (.04) | 1.10±0.04 |
| VO2max (ml·min-1) | 2395 (238) | 3669 (678) ††† | 3890 (593) ***,‡‡ |
| VO2max (ml·kg-1·min-1) | 56.37 (6.62) | 58.20 (4.75) | 58.51 (7.01) |
| BLamax (mmol·l-1) | 6.87 (1.53) | 8.31 (.72) † | 8.41 (.97) * |
*** p < 0.001
** p < 0.01
* p < 0.05 AvsC
††† p < 0.001
† p < 0.05 ΑvsΒ
‡‡‡ p < 0.001
‡‡ p < 0.01Βvs C.
Groups B and C exhibited a longer time to reach the stage of exhaustion compared to Group A by 15.8% and 14.6%, respectively (p < 0.001). Furthermore, Group C had a higher VO2max (ml·min-1) and VO2 uptake at the anaerobic threshold by 33.7% and 6.0% compared to A and B, respectively, but HRAT was significantly different only between Groups A and B by 4.3% (Figure 3a). VO2max expressed in ml·min-1 was significantly higher in Groups B and C in comparison to Group A (p < 0.001) (Figure 3b). However, VO2max in terms of ml·kg-1·min-1 did not differ significantly between the three Groups with the relative mean values lying between 56 and 59 ml·kg-1·min-1 in all Groups (Figure 3c). Group C had significantly higher maximal blood lactate values compared to Group A by 22.4%, while B had higher lactate values than A by 21.0% (p < 0.05).
Figure 3.
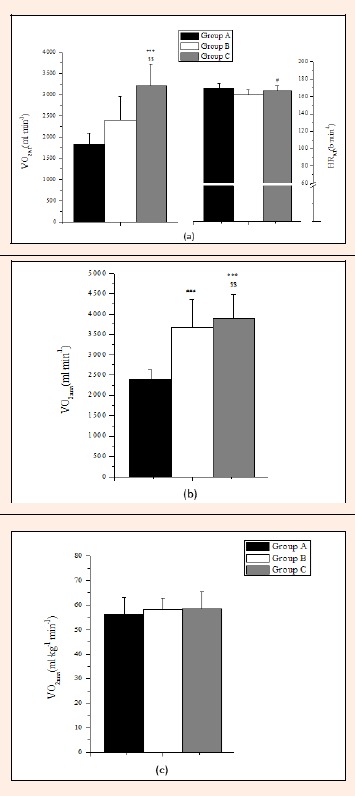
(a) Oxygen uptake values and HR at the anaerobic threshold; VO2max values expressed as; (b) ml·min-1; (c) ml·min-1·kg-1. (mean±SD).*** p < 0.001 A vs C; §§ p < 0.01 B vs C; # p < 0.05 A vs B.
The data of fiber type distribution in the dominant vastus lateralis muscle of the players are presented in Figure 4a. The 15 year old soccer players had significantly lower type I percentage distribution compared to the younger ones (Group A) by 21.3% (p < 0.01), while their type IIA relative percentage was found to be higher by 18.1% in comparison to the respective distribution in Group A (p < 0.05).The results of the mean fiber CSA in the dominant vastus lateralis muscle of the subjects are shown in Figure 4b. Groups B and C showed significantly higher CSA values in all muscle fiber types (I, IIA, IIX, IIAX and IIC) compared to Group A (p ranged from < 0.05 to < 0.001). Furthermore, Group C exhibited higher CSA in type I muscle fiber by 28.6% compared to Group B (p < 0.01).
Figure 4.
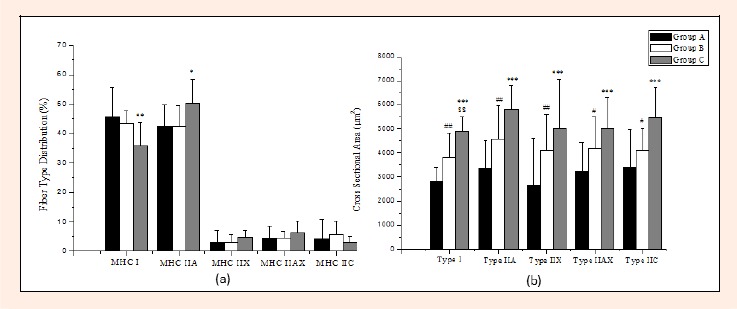
(a) Fiber type distribution determined by immunohistochemical staining and (b) CSA of vastus lateralis muscle in young soccer players (mean±SD). *** p < 0.001, ** p < 0.01, * p < 0.05 A vs C; §§ p < 0.01 B vs C; ## p < 0.01, # p < 0.05 A vs B.
The number of muscle fibers (Group A: 482 ± 182; Group B: 508 ± 203; Group C: 527 ± 312) and satellite cells (Group A: 80 ± 40; Group B: 97 ± 40; Group C: 87 ± 40) in the studied muscular sections as well as their ratio (Group A: 0.17 ± 0.05; Group B: 0.19 ± 0.03; Group C: 0.17 ± 0.05) did not differ significantly between the three Groups. Furthermore, no differences were found between the Groups in satellite cells per fiber type I and type II. Correlation analyses showed a significant correlation coefficient between CSA of type I and the maximal strength of quadriceps at 60ο·sec-1 in Group C (r = 0.669, p = 0.049) while the percentage distribution of the type I fibers was correlated with the VO2max in Group B (r = 0.661, p = 0.038). Significant coefficients were established in Group A between the distribution of type I fibers and the maximal strength of quadriceps at 60ο (r = 0.845, p < 0.001) as well as the distribution of IIA fibers and the maximal strength of hamstrings at 300ο>sec-1 (r = 0.611, p = 0.026).
The peak torque values of hamstrings and quadriceps are demonstrated in Figures 5a and 5b. Groups B and C presented higher peak torques at all angular velocities compared to Group A (p ranged from < 0.05 to < 0.001). Additionally, the soccer players of Group C had significantly higher peak torque values of quadriceps at the angular velocity of 300ο·sec-1 compared to the players of Group B by 31.3% (p < 0.05).
Figure 5.
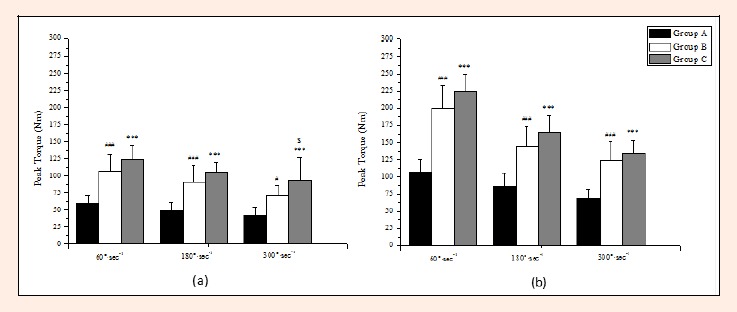
Comparison of hamstrings (a) and quadriceps (b) peak torque values at 60, 180 and 300°·sec-1 angular velocities between three groups (mean±SD). *** p < 0.001 A vs C; § p < 0.05 B vs C; ### p < 0.001, # p < 0.05 A vs B.
Discussion
The main findings of the present study were that older soccer players with more training experience demonstrated a different pattern of fiber type composition, MHC protein isoforms co-expression, CSA, cardiorespiratory values and muscle strength. The fiber type composition determined by MHC isoforms analysis showed a higher percentage of MHC IIA in Group C (15 yrs old) compared to the other two Groups A and B (11, 13 yrs old). However, the percentage of slow twitch oxidative muscle fiber, type I, was significantly lower in Group C compared to Group A. As expected, the cardiorespiratory capacity and muscle strength of the hamstrings and quadriceps were significantly higher in the oldest Group C compared to the two younger Groups, A and B. The higher proportion of MHC IIA in the distribution of the muscle fibers, which was noted in Group C, may indicate a simultaneous adaptive response in both extensive and simultaneous anaerobic – strength and aerobic endurance soccer training.
The present study indicates that variables such as age and training status play a role in muscle fibers co-expression. This study showed that changes in fast twitch muscle fibers (type II), due to the high percentage of fast twitch oxidative (type IIA) and the low percentage of fast glycolytic (type IIX), and co-expession commonly occur in prepubescent and pubescent trained athletes. This co-expression also appeared in pubescent adolescents and as the chronological and training aged increase muscle fibers may be transformed to the type II fiber spectrum. These significant differences that were found in the muscle composition of type I and IIA fibers between Groups should be a reflection of specific athletic requirements, even though genetic influences cannot be disregarded. It is worth mentioning that an athlete in Group C was found to have a percentage of fast twitch muscle fiber over 70%.
In adults, a large proportion of individual muscle fibers has been found to contain more than one MHC isoforms in different ratios (i.e. I/IIA, IIA/IIX, I/IIA/IIX) (Klitgaard et al., 1990; Mandroukas et al., 2010; Staron and Hikida, 1992). In youth, it is yet unknown whether muscle fiber has had different attributes, either during isolated incidents, over various periods of time, or by uniting with other fiber types, namely hybrid muscle fibers. The results of the present study show the existence of “hybrid” muscle fibers, despite the fact that there were no significant differences in the percentage of muscle fibers according to age.
Thus, it becomes apparent that both age and training experience play a role in the co-expression of different muscle fibers. While in adults the phenomenon of fluctuation between type II and co-expression is common, in children the phenomenon is less obvious. However, our study demonstrates just that condition, which may be due to the influence of training, thus the co-expression of muscle fibers is also evident in Group A. This leads us to believe that the muscle fiber has been predisposed to be in a position of transition between type II by factors such as age and in terms of a coaching regimen.
The current study demonstrates that age in tandem with systematic long-term training may possibly affect muscle fibers, as shown by the results from Group C. The results lead to two ways of interpretation in correlation with the high percentage of MHC IIA combined with the low percentage of ΜHC Ι, respectively in Group C. Firstly, it seems that the influence of training on pubertal boys is evident as it corroborates with the results of training in adult athletes. The documented influence of intensive training on MHC composition increases MHC IIA which is mainly due to the conversion of fibers containing MHC IIX to MHC IIA (Adams et al., 1993; Kesidis et al., 2008; Staron et al., 1994). Secondly, it seems that the influence of training may affect the reduction of oxidative muscle fibers type I.
The significantly higher CSA of Group C was an expected result in comparison to Group A. However, what was unexpected was the significant difference in CSA observed between Groups A and B, considering the minor age difference between the two Groups. The difference noted may be attributed to the different stages of physical maturation between the two Groups. Interestingly, the results analysis showed elevated CSA in type IIC muscle fiber, the role of which is still questioned, as there is an unresolved question over whether IIC muscle fiber is an intermediate type between fast oxidative IIA and oxidative type I, or a fiber type in development perhaps close to cellular death and degradation (Mandroukas et al., 2010). It is worth mentioning that previous studies have observed this type of muscle fiber to present large amounts of CSA and oxidative potential (Ingjer, 1978). In comparison to Groups A and B, Group C demonstrated faster exercise times on the treadmill, along with a the higher concentration of blood lactate, VO2max, the distribution of muscle fiber type IIA, higher muscle strength and CSA, all of which show that the group displays appropriate energy (aerobic and anaerobic), enzymatic (mitochondria and anaerobic enzymes) hormonal and metabolic fundamentals to train at a high level and to activate fast oxidative muscle fibers type IIA able to cause adaptations.
Although the muscle fibers characteristics in male adult soccer players have been studied extensively there is no information regarding muscle fiber alterations after long-term training in young soccer players. The question of at what age can exercise begin to cause adaptations is not new. Even in longitudinal studies which monitor the same subjects over the course of a whole year report the changes that occur reflect each subject’s continued development, as well as the changes that occur as a result of training. The difficulty of interpreting results in this type of athletic research in young athletes is in differentiation between the results caused by biological development from the changes that can be attributed to soccer training.
The present study is the first to examine the MHC isoforms in young soccer players. The question that arises is whether long-term training is effective at causing adaptations that become apparent in the muscle profile of young soccer players, so that every person/athlete can be selected for the appropriate sport. However, a fundamental difficulty in conducting research in young subjects is the fact that is not as clear whether the changes observed are the result of training, growth, or a combination of the two factors.
The higher aerobic capacity of Group C may be attributed not only to training, but also to hereditary endowment. The selection process for the soccer players of the current study was based on anthropometric criteria and technical skills and took place long before research for the study began. Some cross-sectional studies show variation in related maximal oxygen uptake values at developmental ages are probably due to hereditary factors and differences in the levels of physical activity. Our results are comparable to those reported by Hansen and Clausen (2004) for elite young Danish soccer players aged 10.5-13 yrs. The results of the present study support that test velocity in absolute peak torque values increase significantly with age (Forbes et al., 2009). The thirteen and fifteen year old soccer players of the present study had significantly higher peak torque values of hamstrings and quadriceps at all angular velocities than the eleven year old players. Controversially, between thirteen and fifteen year old soccer players a significant difference was found only in hamstrings, at just the velocity of 300°·sec-1. This finding is also supported by Forbes et al. (2009) who studied 156 young soccer players aged 11-17 years old and found a particular percentage increase at the age of 14 years old (approximately 35%). This increase in peak torque may be related to the natural growth- and strength-spurt under the known androgenic hormonal effect during puberty.
Malina (1968) and later Beumen and Malina (1988) reported that the biological events that occur are complex and include changes to the nervous and endocrine systems, in coordination with anthropometric and physiological changes. During these pubertal ages other physiological changes take place, resistance and strength are also improved and training can be a stimulus for higher growth hormone and testosterone levels (Hansen et al., 1999; Zakas et al., 1994). Although the present study did not investigate hormonal production, it seems that the intensity and duration of training are important factors to consider on the evolution of growth. Zakas et al. (1994) who investigated boys aged, 10, 13 and 16yrs old found that after 3 months of training secreted growth hormone and testosterone levels in prepubertal participants (10yrs old) were not changed but in 13 and 16yr olds the indices pattern undergoes a remarkable alteration. The marked increase in physical performance in these ages occurs due to the muscular, neuronal, hormonal and biomechanical factors. It has been argued that due to lack of circulating androgens in prepubertal children, the strength improvements are largely caused by neurological, rather than muscular factors (Malina, 2006). The significant higher body weight in 15yrs old soccer players could be attributed to the growth spurt and increase of height and weight that is observed at this age. The processes of maturation do not occur at the same chronological age in all the subjects and the 90% percentile range of peak growth age is approximately 4.5 years (Borsboom et al., 1996).
In adults, resistance training increased the number of satellite cells in young men and that there was a significant decrease after detraining (Kadi et al., 2006). Complete and systematic studies on the behavior of satellite cells in relation to training at a young age do not exist. It has been shown that satellite cells are equally distributed in type I and II fibers which suggests that the loading pattern of vastus lateralis muscle does not require a specific distribution of satellite cells among type I and II muscle fibers. It so appears that the strength training performed at young ages, mainly using personal body weight, during soccer training is not sufficient to cause an increase in satellite cells. Our results are in agreement with the study of Kadi et al. (2006), who found no significant difference between the percentage of satellite cells in contact with type I and II fibers in healthy subjects.
The findings of the present study should be interpreted within a realm of the inherent limitations; mainly the absence of an untrained control group and the relatively moderate subject sample. Furthermore, our findings may have been influenced by genetic factors, the selection of the young subjects for the specific sport and adaptations caused by training. However, the current study could initiate further research regarding the effects of age, training and their combination on muscle fibers in prepubertal and pubertal boys. It is still unclear how large and how relevant to muscle function the adaptation of fiber type distribution to training can be (Ingalls 2004). It would be interesting for future studies to investigate and follow the young soccer players at regular intervals over time, examining the adaptations of muscle fiber to training (despite the inherent difficulties) in order to distinguish development/maturation from the influence of training. The knowledge gained from further research will allow for improved training regimens capable of maximizing performance, as well as provide clearer explanations as to what degree the possible alterations are due to growth and biological maturity and how substantial the contribution of soccer training is. When working with young athletes, such information is needed to enable quantitative biochemical characterization of muscle fiber types and to understand better the metabolism and degree of adaptability. This knowledge will help coaches design safer and more effective soccer training programs.
Conclusion
In conclusion, the increased percentage of muscle fibers ΙΙΑ noticed in 15year old compared to the other groups should be mainly attributed to the different training programs as well as to the age factor, since these fibers are strongly activated during increased workload exercise. CSA, muscle strength and VO2max values seem to be expressed according to age. Nevertheless, the participants and heredity do play an important role in the formation of the results. It is suggested that the application of specific training during the developmental ages could lead to a differentiation of the muscular metabolic properties to maximize muscular performance. It seems that some adaptations are linked to specific characteristics of training intensity, age and previous training experience. However, the alteration of MHC isoforms composition, even in children is an important mechanism for skeletal muscle characteristics.
Acknowledgements
There was not any kind of funding for this work. This work did not receive any type of external funding.
Biographies
Thomas I. METAXAS
Employment
Department of Physical Education and Sports Science, Aristotle University of Thessaloniki
Degree
Ass. Professor
Research interests
Exercise testing, Field tests, Exercise Physiology of Sports, Soccer training
E-mail: tommet@phed.auth.gr
Athanasios MANDROUKAS
Degree
BSc, MSc of
Research interests
Exercise Physiology, Biomechanics of Sports
E-mail: thanmandrou@hotmail.com
Efstratios VAMVAKOUDIS
Employment
Department of Physical Education and Sports Science, Aristotle University of Thessaloniki
Degree
Ass. Professor
Research interests
Exercise Physiology of Team Sports
E-mail: vamvak@phed.auth.gr?
Kostas KOTOGLOU
Degree
Physiotherapist
Research interests
Rehabilitation of Sports Injuries
Björn EKBLOM
Employment
Åstrand Laboratory of Work Physiology
Degree
Emeritus Professor
Research interests
Physiology, Physical Activity, Training Adaptations, Doping
E-mail: bjorn.ekblom@gih.se
Konstantinos MANDROUKAS
Employment
Department of Physical Education and Sports Science, Aristotle University of Thessaloniki
Degree
Professor
Research interests
Exercise Physiology of Sports
E-mail: kmandrou@phed.auth.gr
References
- Adams G.R., Hather B.M., Baldwin K.M., Dudley G.A. (1993) Skeletal muscle myosin heavy chain composition and resistance training. Journal of Applied Physiology 74, 911-915 [DOI] [PubMed] [Google Scholar]
- Bangsbo J. (1994) The physiology of soccer – with special reference to intense intermittent exercise. Doctoral thesis, University of Co-penhagen, Copenhagen [PubMed] [Google Scholar]
- Baxter-Jones A., Goldstein H., Helms P. (1993) The development of aerobic power in young athletes. Journal of Applied Physiology 75, 1160-1167 [DOI] [PubMed] [Google Scholar]
- Bell R.D., MacDougall J.D., Billeter R., Howald H. (1980) Muscle fiber types and morphometric analysis of skeletal muscle in six-year old children. Medicine and Science in Sports and Exercise 12, 28-31 [PubMed] [Google Scholar]
- Beunen G., Malina R.M. (1988) Growth and physical performance relative to the timing of the adolescent spurt. Exercise and Sport Sciences Reviews 16, 503-540 [PubMed] [Google Scholar]
- Borsboom G.J., Van Pelt W., Quanjer P.H. (1996) Interindividual variation in pubertal growth patterns of ventilatory function, standing height, and weight. American Journal of Respiratory and Critical Care Medicine 153, 1182-1186 [DOI] [PubMed] [Google Scholar]
- Burnie J., Brodie D.A. (1986) Isokinetic measurement in preadolescent males. International Journal of Sports Medicine 7, 205-209 [DOI] [PubMed] [Google Scholar]
- Charifi N., Kadi F., Feasson L., Denis C. (2003) Effects of endurance training on satellite cell frequency in skeletal muscle of old men. Muscle Nerve 28, 87-92 [DOI] [PubMed] [Google Scholar]
- Degache F., Richard R., Edouard P., Oullion R., Calmels P. (2010) The relationship between muscle strength and physiological age: Across-sectional study in boys aged from 11 to 15. Annals of Physical and Rehabilitation Medicine 53, 180-188 [DOI] [PubMed] [Google Scholar]
- Durmin S.V.G.A., Rahaman M.M. (1967) The assessment of the amount of fat in the human body from measurements of skinfold thickness. British Journal of Sports Medicine 21, 681-689 [DOI] [PubMed] [Google Scholar]
- Ekblom B. (1969) Effect of physical training in adolescent boys. Journal of Applied Physiology 27, 350-355 [DOI] [PubMed] [Google Scholar]
- Faigenbaum A.D. (2000) Strength training for adolescent and children. Clinical Sports Medicine 19, 593-619 [DOI] [PubMed] [Google Scholar]
- Forbes H., Bullers A., Lovell A., McNaughton L.R., Polman R.C., Siegler J.C. (2009) Relative torque profiles of elite male youth footballers: effects of age and pubertal development. International Journal of Sports Medicine 30, 592-597 [DOI] [PubMed] [Google Scholar]
- Gravina L., Gil S.M., Ruiz F., Zubero J., Gil J., Irazusta J. (2008) Anthropometric and physiological differences between first team and reserve soccer players aged 10-14 years at the beginning and end of the season. Journal of Strength and Conditioning Research 22, 1308-1314 [DOI] [PubMed] [Google Scholar]
- Hansen L., Bangsbo J., Twisk J., Klausen K. (1999) Development of muscle strength in relation to training level and testosterone in young male soccer players. Journal of Applied Physiology 87, 1141-1147 [DOI] [PubMed] [Google Scholar]
- Hansen L., Klausen K. (2004) Development of aerobic power in pubescent male soccer players related to hematocrit, hemoglobin and maturation. A longitudinal study. Journal of Sports Medicine and Physical Fitness 44, 219-223 [PubMed] [Google Scholar]
- Hughes S.M., Cho M., Karsch-Mizrachi I., Travis M., Silberstein L., Leinwand L.A., Blau H.M. (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Developmental Biology 158, 183-199 [DOI] [PubMed] [Google Scholar]
- Iga J., George K., Lees A., Reilly T. (2009) Cross-sectional investigation of indices of isokinetic leg strength in youth soccer players and untrained individuals. Scandinavian Journal of Medicine & Science in Sports 19, 714-719 [DOI] [PubMed] [Google Scholar]
- Ingalls C.P. (2004) Nature vs. nurture: can exercise really alter fiber type composition in human skeletal muscle. Journal of Applied Physiology 97, 1591-1592 [DOI] [PubMed] [Google Scholar]
- Ingjer F. (1978) Maximal aerobic power related to the capillary supply of the quadriceps femoris muscle in man. Acta Physiologica Scandinavica 104, 238-240 [DOI] [PubMed] [Google Scholar]
- Kadi F. (2000) Adaptation of human skeletal muscle to training and anabolic steroids. Doctoral thesis, University of Umeä, Sweden [PubMed] [Google Scholar]
- Kadi F., Charifi N., Henriksson J. (2006) The number of satellite cells in slow and fast fibres from human vastus lateralis muscle. Histochemistry and Cell Biology 126, 83-87 [DOI] [PubMed] [Google Scholar]
- Kadi F., Schjerling P., Andersen L.L., Charifi N., Madsen J.L., Christensen L.R., Andersen J.L. (2004) The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. The Journal of Physiology 558, 1005-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F., Thornell L-E. (2000) Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochemistry and Cell Biology 113, 99-103 [DOI] [PubMed] [Google Scholar]
- Kesidis N., Metaxas T.I., Vrabas I.S., Stefanidis P., Vamvakoudis E., Christoulas K., Mandroukas A., Balasas D., Mandroukas K. (2008) Myosin heavy chain isoform distribution in single fibres of bodybuilders. European Journal of Applied Physiology 103, 579-583 [DOI] [PubMed] [Google Scholar]
- Klitgaard H., Bergman O., Betto R., Salviati G., Schiaffino S., Clausen T., Saltin B. (1990) Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflugers Archive 416, 470-472 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Kitamura K., Miura M., Sodeyama H., Murase Y., Miyashita M., Matsui H. (1978) Aerobic power as related to body growth and training in Japanese boys: a longitudinal study. Journal of Applied Physiology 44, 666-672 [DOI] [PubMed] [Google Scholar]
- Malina R.M. (1968) Growth and maturation of young athletes: is training for a sport factor. : Sports and Children. Chang, Micheli KML. Baltimore: Williams & Wilkins; 133-161 [Google Scholar]
- Malina R.M. (2006) Weight training in youth-growth, maturation, and safety: an evidence-based review. Clinical Journal of Sport Medicine 16, 478-487 [DOI] [PubMed] [Google Scholar]
- Mandroukas A., Metaxas T., Kesidis N., Christoulas K., Vamvakoudis E., Stefanidis P., Heller J., Ekblom B., Mandroukas K. (2010) Deltoid muscle fiber characteristics in adolescent and adult wrestlers. Journal of Sports Medicine and Physical Fitness 50, 113-120 [PubMed] [Google Scholar]
- Mero A., Jaakkola L., Komi P.V. (1991) Relationships between muscle fibre characteristics and physical performance capacity in trained athletic boys. Journal of Sports Sciences 9, 161-171 [DOI] [PubMed] [Google Scholar]
- Rochcongar P., Morvan R., Jan J., Dassonville D., Belliot J. (1988) Isokinetic investigation of knee extensors and knee flexors in young French soccer players. International Journal of Sports Medicine 9, 448-450 [DOI] [PubMed] [Google Scholar]
- Siri W.E. (1956) The gross composition of the body. Advances in Biological and Medical Physics 4, 239-280 [DOI] [PubMed] [Google Scholar]
- Staron R.S., Hikida R.S. (1992) Histochemical, biochemical, and ultrastructural analyses of single human muscle fibers, with special reference to the C-fiber population. Journal of Histochemistry & Cytochemistry 40, 563-568 [DOI] [PubMed] [Google Scholar]
- Staron R.S., Karapondo D.L., Kraemer W.J., Fry A.C., Gordon S.E., Falkel J.E., Hagerman F.C., Hikida R.S. (1994) Skeletal muscle adaptations during early phase of heavy-resistance training in men and women. Journal of Applied Physiology 76, 1247-1255 [DOI] [PubMed] [Google Scholar]
- Tanner J.M. (1975) Growth and endocrinology of the adolescent. : Endocrine and Genetic Diseases of Childhood. Gardner L.J.Philadelphia: PA Brauner [Google Scholar]
- Vamvakoudis E., Vrabas I.S., Galazoulas C., Stefanidis P., Metaxas T.I., Mandroukas K. (2007) Effects of basketball training on maximal oxygen uptake, muscle strength, and joint mobility in young basketball players. Journal of Strength and Conditioning Research 21, 930-936 [DOI] [PubMed] [Google Scholar]
- Williams C.A., Armstrong N., Powell J. (2000) Aerobic responses of prepubertal boys to two modes of training. British Journal of Sports Medicine 34, 168-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakas A., Mandroukas K., Karamouzis M., Panagiotopoulou G. (1994) Physical training, growth hormone and testosterone levels and blood pressure in prepubertal, pubertal and adolescent boys. Scandinavian Journal of Medicine & Science in Sports 4, 113-118 [Google Scholar]


