Abstract
During rugby game, or intensive rugby training there are many high intensity explosive exercises and eccentric muscle contractions, therefore adequate recovery is very important to rugby players. In the present study we have tested the effects of cold water immersion (CWI) after game-simulated (80 min.) rugby training on muscle power recovery and blood markers of muscle damage. Twenty well-trained collegiate male rugby players (age: 20.3 ± 0.6 years old, body height: 1.74 ± 0.05 m, body weight: 85.4 ± 2.0 kg, body fat: 18.2 ± 1.4 %) volunteered for this study. This study was conducted as a cross-over design; i.e., the subjects were randomly assigned either to CWI (n = 10) or passive rest condition (n = 10) for the 1st trial and 1 week later the subjects were switched conditions for the 2nd trial. After the simulated rugby training, including tackles and body contacts, muscle functional ability and blood markers of muscle damage were tested immediately, after CWI or passive rest, and again 24 hours later. Statistical analysis of all muscle functional tests (10 m dash, counter movement jump, reaction time, side steps) except for 10 seconds maximal pedaling power and blood makers of muscle damage (aspartate aminotransferase, lactate dehydrogenase, creatine kinase, and creatinine) revealed significant main effects for time (p < 0.05). However, no statistically significant interactions were found in any of the muscle functional tests and blood markers between groups and time courses. Our results suggest that a rugby game induces muscle damage and reduces muscle function. However, CWI has no significant restorative effect after an 80-minute rugby game in terms of muscle damage.
Key Points.
Cold water immersion study for the recovery of rugby players
Muscle strength and muscle power were mainly evaluated as well as muscle enzymes of muscle break down
Subjects were highly trained rugby players with control group
Key words: Cold water immersion, muscle power, muscle damage, rugby
Introduction
Despite the popularity of cold water immersion (CWI) as a recovery modality, little research has been conducted in this area. CWI therapy for acute injuries has been used to explain the purported physiologic effects on post-exercise recovery. However, positive effect of CWI on the recovery of muscle strength and power or on muscle damage after severe muscle activity has not been demonstrated clearly. Bailey et al. (2007) reported that CWI immediately after 90 minutes of intermittent shuttle run that induced marked muscle damage attenuated the severity of muscle soreness, temporary muscle dysfunction, and the elevation of some serum markers of muscle damage at 1, 24 and 48 hours post-exercise. Pournot et al. (2011) revealed significant improvements in maximum voluntary contraction and power output for 30 seconds in the CWI and contrast water therapy (CWT) groups 1 hour after a 20-minute exhaustive intermittent exercise when compared to pre-exercise. However, Kevin et al., (1996) suggested that 20 minutes of CWI at 13°C significantly decreased vertical jump and shuttle run performances compared to a non-CWI control group. Sellwood et al., (2007) suggested that 3×1 minutes of CWI at 5°C was ineffective in attenuating markers of delayed-onset muscle soreness (DOMS) in untrained individuals. Despite many studies to date, the effects of CWI on muscle strength and power in athletes have been a controversial issue (Bleakly et al., 2012). The differences in CWI method (duration and temperature), exercise mode (endurance or power and sprint type) and subjects (athlete and non-athlete), along with the statistical methods used may offer an explanation for the contradictory results.
Several of the preliminary studies were conducted in laboratory settings. On the other hand, to clarify whether CWI is effective for rugby players after daily rugby training or a rugby match, the experiment must be conducted in a realistic environment in which there are many body contacts and stop-and-go movements accompanied by high-intensity eccentric muscle contraction. The number of body contact in rugby play positively correlates with the increase in muscle damage markers, such as creatine kinase (CK) and myoglobin (Takarada, 2003). In fact, Gill et al., (2006) revealed that CWT was more effective than passive rest to reduce the increased CK in rugby players after a rugby match. Unfortunately, the changes in muscle power and performance have not been measured in this study. Elias et al., (2012) evaluated the effects of CWI on muscle power and soreness during post-training recovery using Australian footballers. In this study, the authors have indicated positive effects of CWI on muscle power. However, data expression included only the mean and standard deviation, and no further statistical analyses were conducted. Therefore, it is valuable to study the effects of CWI on muscle damage and muscle power after a rugby game with severe body contact and high intensity muscle contractions. We hypothesized that the rugby training including body contacts and tackles results in muscle damage, leading to the decrease in muscle power output, while CWI protects against muscle damage and the decline of muscle performance. This study examined the effect of CWI used after a rugby game simulation training, including severe body contacts, on biochemical parameters, representing exercise-induced muscle damage as well as on muscular functional tests in rugby players.
Methods
Subjects
Twenty well-trained collegiate male rugby players (age: 20.3±0.6 years old, body height: 1.74 ± 0.05 m, body weight: 85.4 ± 2.0 kg, body fat: 18.2 ± 1.4 %) volunteered for this study. Participants were at the beginning of competitive season and were free of injury at the time of testing. Ethics approval was obtained from the Human Research Ethics Committee of the Doshisha University (application number: 1036). Participants were informed of the risks and benefits associated with the study and provided written informed consent prior to participation.
Study design
The time frame of this study was shown in Figure 1. We conducted the same experimental trials twice. Twenty subjects were randomly assigned to the CWI condition (n = 10) or control condition (n = 10) with matched height and body weight for 1st trial and 1 week later the subjects were switched for CWI and control conditions (cross-over design) for 2nd trial. Therefore, the number of subjects were 20 for both CWI and control conditions.
Figure 1.
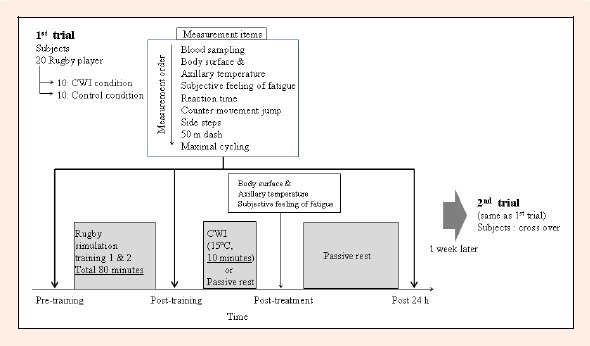
Representation of the study protocols.
Testing procedure: Rugby simulation training
All subjects underwent 80 minutes of rugby game simulation training, including tackles and body contacts as the 1st trial. Training consisted of 2 types of exercises: 1) In training (Figure 2), 2 players were assigned either as attacker or defender, 10 m from side to side. The attacker was required to attack, while the defender was going to tackle the attacker. Then, the attacker was going to push the defense to the starting line. After that, they were set 15 m from side to side, followed by 22 m from side to side, repeating the same exercise. All of the subjects performed 3 trials (10, 15, and 22 m) of attacking and defending. After that, they switched positions and repeated the exercise sequence. This was counted as 1 set and a total of 10 sets were completed. 2) In the game (Figure 3), ten players played the role of offense (attacking group) and another 10 players played as defense (defending group), using a rugby ball. From the 10 m distance between groups, the offense executed the attack, while the defense tried to stop the attacking group. If the attacking group was successfully stopped, the scrum half of the attacking group tried to execute a new attack from side to side. This exercise was conducted for 50 minutes. Offensive and defensive groups were switched periodically along with the positions of the players both in offense and defense to ensure the same amount of exercise volume and tackle. During training, heart rate (HR) and the running distance were recorded from randomly selected 6 subjects, using VXsport with built-in HR and GPS sensors, which was attached to the back of the subjects.
Figure 2.
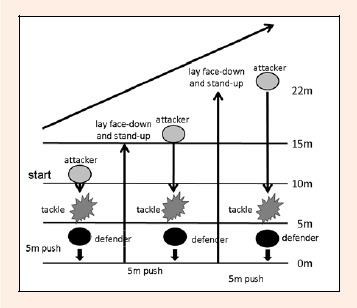
Illustration of rugby game simulation training 1.
Figure 3.
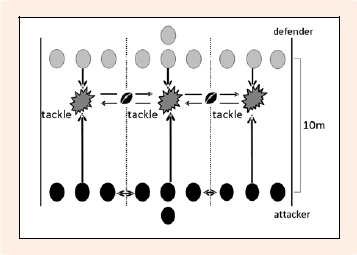
Illustration of rugby game simulation training 2.
Measurement items
Immediately (2 minutes) after the simulated training, blood sample was taken and then muscle power was measured as follows. For the indicators of muscle damage, 5 cc of blood sample was taken from the antecubital vein of the subjects and analyzed for serum creatine kinase (CPK), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and creatinine (Cre). Serum CPK, AST, and LDH were analyzed according to the transferable method of the Japan Society of Clinical Chemistry. Serum Cre was analyzed with an enzymatic method (Moss et al., 1975), using a Hitachi creatinine auto-analyzer, model 7170 (Hitachi, Tokyo, Japan) and enzyme solution (Preauto-SCrE-N; Daiichi Pure Chemicals Co, Tokyo, Japan. Creatine, produced by hydrolysis, was acted upon by creatine kinase, and then by pyruvate kinase and lactate dehydrogenase, to result in a change in absorbance at 340 nm. The amount of creatinine present is related to the rate of change in A340 and is determined from a standard curve. Blood lactate (La) was analyzed from capillary blood via a finger prick as an index of fatigue and intensity of exercise, using a Lactate Pro (Arkray, Kyoto, Japan) handheld device.
As the indices of muscle power and neuromuscular performance, 50 m dash running performance, vertical jump (counter movement jump), reaction time, side steps for 20 seconds, and maximal anaerobic cycling power for 10 seconds were measured. In the 50 m dash time trial, a photocell was used, i.e., the player was positioned in the standing position at the starting line. The first light sensor was placed at the start line, 50 cm above the ground and was activated as the subject moved from the starting position. The second light sensor was placed at the finish line. The muscle power was defined as the ability to perform maximal- or high-intensity exercise. To test the muscle power, the cycling test was conducted on a power max VIII (Combi Wellness) cycle ergometer, in which magnetostrictive brake was used. Subjects were asked to try 3 times of 10 seconds maximal pedaling with 2 minutes rest between trials. The pedaling load was set at 4 kp for less than 80 kg of body weight and 5 kp for more than 80 kg of body weight for 1st trial. At the 2nd trial, the load was set depending on the results of 1st trail, i.e., at 5 kp for less than 150 rpm in 1st trail, at 6 kp for more than 150 rpm to less than 180 rpm, and at 7 kp for more than 180 rp, in 1st trial. At the 3rd test, the load was also set depending on the results of 2nd test, ie., at 7 kp less than 150 rpm at 5 kp, at 7 kp for less than 140 rpm at 6 kp, 8 kp for more than 140 rpm to less than 150 rpm at 6 kp, at 9 kp for less than 150 rpm at 6 kp, at 9 kp for more than 150 rpm at 6 kp, at 9 kp for less than 150 rpm at 7 kp, at 10 kp for more than 150 rpm at 7 kp, at 10 kp for less than 150 rpm at 10 kp, and at 11 kp for more than 150 rpm at 8 kp, in 2nd trial. The power was calculated based on the following formula; power (p) = kpx6x14/52xN, where kp was the load of pedaling, 6 was the distance of wheel for 1 rotation, 14/52 was gear ratio of wheel and pedal, and N was the number of revolution of wheel per minutes. The data were expressed as absolute maximal value of power (watts) and relative power which was divided by body weight during three trials.
Subjective feeling of fatigue (arbitrary 10-unit scale) was also measured. This was a visual scale ranging from 1 to 10, where 1 means no fatigue at all and 10 means the most fatigue the subject has ever experienced. The subjects were familiarized with the scale before the study.
Body temperature was evaluated by 2 methods such as axillary temperature and body surface temperature. Body surface temperature of the front and back of the whole body were measured using thermography (Thermo Tracer, TH6200, NEC) inside a room where temperature was kept constant (22°C) and sunlight was blocked. From the data of body surface temperature, 7 points of the body surface such as forehead, forearm, umbilical region, back of the hand, top of the foot, cervix, and lower part of the thigh were selected and averaged. Ideally core body temperature should be measured as a body temperature. Core body temperature was not measured in this study, because the measurement of core body temperature in the water was technically difficult (even in small amount of infusion of water to the body affects to the temperature) and this measurement also imposes a burden to the subjects. Axillary temperature was measured under the left forearm by thermometer (ET-C231P, Termo). Although the body temperature of an individual can vary, a healthy human body can maintain a fairly consistent body temperature between 36.8°C and 37.0°C (Mackowiak et al., Sund-Levander et al, 2002). There are different values for normal temperatures regarding oral, rectal, tympanic, and axillary body temperatures (Sund-Levander et al, 2002). The range for oral temperature is 33.2°C–38.2°C, for rectal 34.4°C–37.8°C, for tympanic 35.4°C–37.8°C, and for axillary 35.5°C–37.0°C in men. Body surface temperature does not reflect core body temperature (Rust et al., 2012). We assumed that axillary temperature reflected core body temperature well and body surface temperature can vary from core body temperature but it reflected the effects of CWI.
These items were measured before, immediately after and 24 hours after the training. Body temperature and feeling of fatigue were also evaluated after the CWI within 30 seconds. One week later, a 2nd trial was conducted as interchangeably classified groups, with cross-over design. All measurements, training and CWI methods were the identical to the 1st trial.
Cold water immersion
Immediately (1 minute) after the blood sampling and muscle power measurements, CWI group was immersed with the entire body excluding neck and head in cold water (15°C for 10 minutes) immediately (5 minutes) after the training. Most CWI studies have used 10-15°C temperature for 10-15 minutes for CWI. In our experience with our subjects, 10 °C was too cold for the subjects to hold still for 10 minutes, while 15 minutes of CWI at 15 °C has been considered too long (severe) for the subjects. So, we used 10 minutes duration at 15°C of CWI. CWI was achieved with water and ice cubes in a convenience bath with 3 m depth, 2 m width, and 80 cm height. Water temperature was monitored with a standard thermometer and kept constant throughout the protocol. Control group was at rest in a seated position on a chair for the same duration (15 minutes) as the CWI group.
Statistical analyses
Normality of the data was checked by the Kolmogorov-Smirnov test. Statistical method was determined at first by test of equal variances of the Levene. To identify the difference between the two conditions (CWI and control), 2-way ANOVA design with repeated measures was used (Excel statistics 2012 for Windows) for the time points of pre-training, immediately after and 24 hours after training. After the evaluation for interaction of time and group, simple main effect was assessed. If significant differences were found in simple main effect, multiple comparisons (Bonferoni) were used to clarify the difference of time points. Significance level was set at p = 0.05. Data were expressed as mean value ± standard deviation.
Results
Taken together the training and the simulated game, total running distance was 4.7 ± 0.8 km, average maximal velocity of running was 22.2 ± 1.6 km·h-1, total number of tackles was 37.6 ± 3.0 times, total number of contacts was 10.4 ± 2.5 times, average HR during training was 134.2 ± 8.7, average maximal heart rate (HRmax) was 186.8 ± 5.3 b·min-1, and total duration of training was 83.4 ± 5.4 minutes.
Significant interaction was found between time and group in body temperature. In the assessment of simple main effect, body axillary temperature (Table 1) at pre-training and post-training in both CWI and control conditions were almost the same, however, body surface temperature significantly decreased immediately after both CWI and passive rest. Moreover, significant difference was found in body surface temperature of the groups after treatment (20.5 ± 1.0°C for CWI vs. 28.2 ± 1.4°C for control condition). At post-24 hours, no significant differences were found in the body temperature between the groups.
Table 1.
Changes in body temperature, feeling of fatigue, muscle power, and blood markers before, after, and 24 hours after the simulated rugby training and after the CWI treatment.
| Pre-training | Post-training | Post-treatment | Post 24 h | ||
|---|---|---|---|---|---|
| Axillary temp., °C | CWI | 36.5 ±.5 | 36.5 ±.6 | 35.6 ± .5 b,d# | 36.4 ± .3 f |
| Control | 36.6 ± .5 | 36.5 ± .4 | 36.3 ± .3 b,d | 36.6 ± .4 f | |
| Body surface temp., °C | CWI | 30.4 ± .9 | 30.4 ± 1.0 | 20.5 ± 1.0 b,d# | 30.4 ± 2.7 f |
| Control | 30.2 ± .8 | 30.6 ± .9 | 28.2 ± 1.4 b,d | 29.8 ± 2.5 f | |
| Feeling of fatigue | CWI | 3.9 ± 1.8 | 6.7 ± 1.5 a | 4.9 ± 1.6 b,d# | 4.8 ± 1.1e |
| Control | 3.7 ± 1.6 | 7.3 ± 1.0 a | 6.7 ± 1.0 b | 5.1 ± 1.4 c,e,f | |
| AST (IU/L) | CWI | 25.0 ± 7.0 (28.4) | 30.0 ± 8.0 (27.0) a | 29.0 ± 7.1 (24.6) c | |
| Control | 25.0 ± 6.4 (25.7) | 29.0 ± 6.5 (22.3) a | 30.0 ± 8.2 (26.9) c | ||
| LDH (IU/L) | CWI | 215 ± 38 (17.6) | 276 ± 44 (15.8) a | 242 ± 36 (15.0) c,e | |
| Control | 229 ± 55 (24.1) | 288 ± 64 (22.3) a | 249 ± 47 (18.8) c,e | ||
| CPK (IU/L) | CWI | 350 ± 149 (43.7) | 459 ± 179 (38.4) a | 621 ± 261 (40.4) c,e | |
| Control | 431 ± 276 (48.8) | 598 ± 325 (48.1) a | 850 ± 491 (57.8) c,e | ||
| Cre (mg/dl) | CWI | .96 ± .10 (10.4) | 1.23 ± .17 (13.8) a | .99 ± .13 (13.4) e | |
| Control | .94 ± .10 (11.0) | 1.23 ± .18 (14.6) a | .97 ± .14 (14.3) e | ||
| La (mmol/l) | CWI | 1.2 ± .2 | 6.3 ± 2.7 a | 1.3 ± .3e | |
| Control | 1.1 ± .2 | 5.5 ± 1.5a | 1.5 ± .3e | ||
a, b, c; significantly different from pre-training.
d, e; significantly different from post-training.
f; significantly different from post-treatment.
#; significantly different from control.
AST: asparate aminotransferase, LDH: lactate dehydrogenase, CPK: creatine kinase, Cre: creatinine, La: lactate. The data expressed in the parentheses of AST, LDH, CPK, and Cre indicate coefficient variations (CV%).
Significant group by time interaction was found in subjective feeling of fatigue. In the assessment of simple main effect, subjective feeling of fatigue increased after training in both groups (Table 1). However, subjective feeling of fatigue in CWI condition significantly decreased immediately after CWI and it was significantly lower than that of the control condition (4.9 ± 1.6 for CWI vs. 6.7 ± 1.0 for control condition). At post-24 hours, subjective feeling of fatigue of both conditions were significantly lower than that of post-training, but no significant difference was found between conditions.
No significant interactions were found between time and group in any of blood markers. Blood markers of muscle damage, such as AST, LDH, CPK, and Cre significantly increased after the training in both conditions (Table 1). AST remained unchanged at post-24 hours, and LDH significantly decreased in both conditions and remained significantly elevated at post-24 hours, compared to pre-training. CPK significantly increased at post-24 hours from post-training in both conditions, and CPK of CWI condition tended to be lower than that of the control condition (621 ± 261 in CWI vs. 850 ± 491 in control), but this was not statistically significant. Cre significantly decreased at post-24 hours in both conditions. Blood La significantly increased at post-training in both groups and returned to the same level as pre-training at post-24 hours. When we consider the results of statistical analysis, main effects of time courses of all blood markers were statistically significant. However, no statistically significant interactions were found in any of blood makers between groups and time courses.
The results of muscle functional test are summarized in Table 2. No significant interactions were found between time and group in any of muscle functional tests. 50 m dash time increased significantly in the CWI group at post-training. At post 24 hours, 50 m dash time remained significantly longer compared to pre-training, but did not differ from post-training. In control conditions, the 50 m dash time significantly increased from post-training to post-24 hours. Counter movement jump, reaction time, side steps, maximal cycling power for 10 seconds (both in watts and watts/kg) did not change at post-training. However, reaction time and side steps significantly declined from pre-training or post-training to post-24 hours only in the control condition. When we look at the results of statistical analysis of all muscle functional tests except for 10 seconds maximal pedaling power, main effects of time courses were statistically significant. However, no statistically significant interactions were found in any of muscle functional tests between groups and time courses.
Table 2.
Changes in muscle power before, after, and 24 hours after the training and after the CWI treatment.
| Pre-training | Post-training | Post 24 h | ||
|---|---|---|---|---|
| 50 m dash (sec) | CWI | 7.11 ± .35 | 7.22 ± .36 a | 7.27 ± .35 c |
| Control | 7.19 ± .40 | 7.16 ± .43 | 7.31 ± .36 c,e | |
| Counter movement jump (cm) | CWI | 47.1 ± 6.9 | 44.3 ± 7.6 | 43.6 ± 5.6 |
| Control | 45.1 ± 9.3 | 43.9 ± 8.4 | 41.4 ± 9.6 c | |
| Reaction time (sec.) | CWI | .302 ± .047 | .292 ± .055 | .310 ± .038 |
| Control | .301 ± .036 | .287 ± .035 | .320 ± .043 e | |
| Side steps (times) | CWI | 55.7 ± 3.3 | 53.9 ± 5.9 | 53.5 ± 5.1 |
| Control | 56.2 ± 3.9 | 55.2 ± 4.8 | 52.6 ± 3.7 c,e | |
| Maximal cycling power (watts) | CWI | 1102 ± 148.6 | 1078 ± 152.7 | 1105 ± 146.3 |
| Control | 1098 ± 172.4 | 1081 ± 146.4 | 1071 ± 125.7 | |
| Maximal cycling power (watts·kg-1) | CWI | 13.1 ± 1.8 | 12.5 ± 1.5 | 12.8 ± 2.0 |
| Control | 13.2 ± 1.7 | 12.5 ± 1.3 | 12.7 ± 1.4 | |
a, b, c; significantly different from pre-training.
d, e; significantly different from post-training
Discussion
In CWI studies of team sports with ball games (Elias et al., 2012), as reported previously, there have been no body contacts and tackles, while exercise duration was shorter than the actual game. Moreover, the temperature and the duration of CWI varied in those investigations (Bleakly et al., 2012; Elias et al., 2012; Kevin et al., 1996; Pournot et al., 2011; Sellwood et al., 2007) and blood markers of muscle damage were not analyzed. The statistical analysis was also inappropriate in most studies. These limitations of the available data make it difficult to understand the effects of CWI therapy on muscle damage and muscle function. In this study, we used a sufficient sample size of elite rugby players with almost the same exercise duration and content, including body contacts and tackles (simulation training) of a real rugby game with a well-controlled research design.
Total running distance (4.7 ± 0.8 km) during training might be a little lower than that of real game. However, HRmax (186.8 ±5.3 b·min-1), average HR (134.2 ± 8.7 b·min-1), duration of training (83.4 ± 5.4 min), maximal running speed (22.2 ± 1.6 km·h-1), total number of tackles (37.6 ± 3.0 times), and total number of contacts (10.4 ± 2.5 times) indicate adequate exercise stimulus to the subjects. As a result, we confirmed that feeling of fatigue and blood La increased significantly at post-training (6.3 ± 2.7 mmol·l-1 in CWI condition and 5.5 ± 1.5 mmol·l-1 in control condition). Moreover, all blood markers of muscle damage (AST, LDH, CPK, and Cre) significantly increased at post-training, supporting the fact that the exercise demand was similar to an actual game. Takarada, (2003) reported a significant increase in CPK after a rugby game with further increase after 24 hours. In our study, CPK significantly increased at post-24 hours from the level measured immediately after the training. Similarly, Banfi et al., (2007) reported significant increase in CPK after a rugby training session. Our result confirmed those previous findings, thus, it is clear that significant muscle damage has occurred after the severe 80 minutes rugby simulation training, including tackles and body contacts.
In our study, we hypothesized that muscle damage after the rugby training might result in the decrease in power output and CWI might protect against the decline of muscle performance. We have set the temperature of CWI to 15°C and the duration of CWI to 10 minutes. The explanation for using this condition lies in preliminary studies and our prior experience with this protocol. Most CWI studies have used 10-15 °C temperature. Vaile et al. (2008) reported that intermittent CWI at 10 °C, 15 °C, 20 °C, and continuous CWI at 20 °C were more effective for recovery of 15 minutes bicycle power output than active recovery in hot environment. In our experience, 15 minutes of CWI at 15 °C has been considered severe for the subjects. So, we used 10 minutes duration of CWI at this temperature. The body surface temperature as measured by thermography decreased significantly at post CWI with no significant reduction in control condition (20.5 ± 1.0°C for CWI vs 28.2 ± 1.4°C for control condition). It would be desirable to measure body core temperature in CWI studies. However as explained in the methods section, in practice the measurement of core temperature was impossible in the water. Therefore, unfortunately we could not report body core temperature in our study, which is a limitation of the present study. Whether the decline of body surface temperature observed in our study was sufficient to decrease core body temperature remains undetermined. However, the temperature and the duration of CWI in our study were similar to those used regularly for athletes.
Feeling of fatigue significantly decreased at post-treatment in CWI condition with no significant reduction in control condition. This difference in the subjective feeling of fatigue at post-treatment was statistically significant for interaction between groups. Bleakley et al., (2012) reviewed CWI studies and concluded that CWI has been effective for preventing and treating muscle soreness after exercise. Therefore, it is possible to say that CWI has a positive effect on the reduction of feeling of fatigue after severe exercise with muscle damage. CWI could not reduce muscle damage as determined by blood markers such as AST, LDH, CPK, and Cre, because there was no significant interaction effect between CWI and control conditions after exercise in our study. However, there was a trend of decreased CPK in the CWI compared to the control group at 24 hours post-training, which taken together with the reduced feeling of fatigue may represent an advantage of CWI over passive rest after severe exercise. On the other hand, Banfi et al., (2007) measured CPK level before and after a rugby training session and immediately after a CWI session or passive recovery. However, no clear difference in CPK after treatment was found between groups in that study, although no statistical analysis was conducted between groups.
It has been observed that the declines of functional test performance such as 50 m dash time trial, countermovement jump, reaction time, side step and maximal cycling power for 10 seconds were modest. In post-training analysis, 50 m dash time trial significantly decreased in the CWI group. Other measurement items tended to decrease in both condition at post-training. At post-24 hours, the decline of functional test performance such as 50 m dash, countermovement jump, reaction time and side steps were more pronounced in both conditions. Therefore, the rugby simulation training for 80 minutes, including body tackles and contacts in our study had negative effects on muscle performance in our subjects. Highton et al., (2009) reported that exercise-induced muscle damage after 100 plyometric jumps resulted in significantly increased muscle soreness and reduced neuromuscular performance, such as isokinetic peak torque at 60 and 270 deg/sec., 5 m and 10 m sprint time, agility time, and ground contact time at the agility turn point at 24 hours. In accordance with these findings, loss of muscle performance after exercise-induced muscle damage was apparent in our study.
A reduction of neuromuscular function after exercise-induced muscle damage has also been suggested (Byrne et al., 2004). A reduction in neuromuscular efficiency, as indicated by a decrease in the force output: integrated electromyographic (iEMG) activity ratio of the knee extensors, has been observed after eccentric exercise (Deschenes et al., 2000; Komi and Viitasalo, 1977). This infers that a greater central activation (nervous stimulation) is required to achieve a given submaximal or maximal force. Deschenes et al. (2000) reported that the impairment in neuromuscular efficiency outlasted other symptoms of damage, such as strength loss, muscle soreness, and increased circulating levels of myofibrillar proteins. Exercise-induced muscle damage may lead to changes in recruitment patterns or changes in the temporal sequencing of muscle activation patterns. This can result in changes in muscle co-ordination and segment motion (Cheung et al., 2003). Miles et al. (1997) observed several indicators of impaired neuromuscular control using EMG analysis during elbow flexion for up to five days after performing 50 high-force, eccentric contractions of the elbow flexors. They reported longer movement times, time to peak EMG of the biceps, time to peak velocity and slowing of peak velocity. They attributed the slowing of peak velocity to selective damage of fast twitch fibers.
If we consider the effects of CWI on deteriorated muscle performance, there was a tendency to attenuate the reduction of muscle performance after rugby training with CWI. Because, the performance decline in 50 m dash time trial, reaction time, and side steps from post-training to post-24 hours was observed only in the control, but not in the CWI group. Although, unfortunately interaction effects between groups at post-24 hours were not significant in any of these three tests, we believe that muscle function was clearly preserved from post-training to post-24 hours following CWI treatment. Therefore, although rugby simulation training resulted in the deterioration of muscle performance in rugby players, CWI could not suppress the markers of muscle damage post 24 hours, but mitigated the decline in muscle power when administered instead of passive rest. It should be noted, however, that the results may have been even more pronounced if the subjects had been treated with CWI frequently after training, and if the evaluations of blood markers and functional tests had been conducted at 48, 72 hours after treatment. Unfortunately, controlling all aspects of this study was difficult in practice because our subjects were elite players at the Japanese university level.
It is worth mentioning that prior eccentric and concentric training attenuates the decline of muscle performance after severe eccentric exercise (Margison & Eston, 2002). Our subjects were highly trained rugby players, all of them trained for more than 6 years in rugby. Therefore, the attenuation of muscle damage and decline of power after severe rugby training might be limited in these elite players. This might help in the understanding of the effects of CWI on muscle damage and muscle power output in this study.
Conclusion
Our results suggest that a rugby game induces muscle damage and reduces muscle function. However, CWI has no significant restorative effect after 80-minute rugby game in terms of muscle damage markers. On the other hand, based on the measure of the subjective feeling of fatigue and some muscle function tests, CWI may be a useful recovery intervention after severe exercise. Future studies should also investigate multiple application of CWI with longer recovery times, such as 48 and 72 hours to determine the most effective recovery strategy for competitive athletes in high-intensity contact sports.
Acknowledgements
This study was supported by research grant from experimental practice research fund. The authors acknowledge the contribution of rugby players of Doshisha University as the volunteers of this study. All authors declare that they have no conflict of interest.
Biographies

Masaki TAKEDA
Employment
Professor at Faculty of Health and Sports Science, Doshisha University, Kyoto Japan
Degree
PhD
Research interests
The physiological adaptation of endurance, high altitude and muscle strength training. Physiology of cross-country skiing
E-mail: mtakeda@mail.doshisha.ac.jp
Takashi SATO
Employment
Kobe Steel Rugby Club
Degree
MSc
Research interests
Training and coaching for rugby, The effects of cold water immersion for recovery from the training and competition
E-mail: sugar610_9@hotmail.com
Tatsushi HASEGAWA
Employment
Master student of Faculty of Sports Science, Waseda University, Tokorozawa, Japan
Degree
BS
Research interests
Sports Marketing
E-mail: dd.lac-fo.k1@ezweb.ne.jp
Hiroto SHINTAKU
Employment
Haseko Corporation
Degree
BS
Research interests
Training for rugby and American football
E-mail: begins-to-shine@ezweb.ne.jp
Hisashi KATO
Employment
Doctoral student of Health and Sports Science, Doshisha University, Kyoto, Japan
Degree
MSc
Research interests
High altitude training for endurance athletes and body fat metabolism
E-mail: ehn0002@mail4.doshisha.ac.jp
Yoshihiko YAMAGUCHI
Employment
Yamaguchi Orthopedics Hospital
Degree
MD
Research interests
Orthopedic problems in sports training and competition
E-mail: yama-oc@nike.eonet.ne.jp
Zsolt RADAK
Employment
Semmelweis University, Budapest, Hungary
Degree
PhD
E-mail: radak@tf.hu
References
- Bailey D.M., Erith S.J., Griffin P.J., Dowson A., Brewer D.S., Gant N., Williams C. (2007) Influence of cold-water immersion on indices of muscle damage following prolonged intermittent shuttle running. Journal of Sports Sciences 25 (11), 1163-1170 [DOI] [PubMed] [Google Scholar]
- Banfi G., Melegati G., Valentini P. (2007) Effects of cold-water immersion of legs after training session on serum creatine kinase concentrations in rugby players. British Journal of Sports Medicine 41 (5), 339-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakley C., McDonough S., Gardner E., Baxter G.D., Hopkins J.T., Davison G.W. (2012) Cold-water immersion (cryotherapy) for preventing and treating muscle soreness after exercise (Review). The Cochrane Database of Systematic Review 15 (2), CD008262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C., Twist C., Eston R. (2004) Neuromuscular function after exercise-induced muscle damage. Theoretical and applied implications. Sports Medicine 34 (1), 49-69 [DOI] [PubMed] [Google Scholar]
- Cheung K., Hume P.A., Maxwell L. (2003) Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Medicine 33 (2), 145-164 [DOI] [PubMed] [Google Scholar]
- Deschenes M.R., Brewer R.E., Bush J.A., McCoy R.W., Volek J.S., Kraemer W.J. (2000) Neuromuscular disturbance outlasts other symptoms of exercise-induced muscle damage. Journal of the Neurological Sciences 174 (2), 92-99 [DOI] [PubMed] [Google Scholar]
- Elias G.P., Varley M.C., Wyckeisma V.L., McKenna M.J., Minahan C.L., Aughey R.J. (2012) Effects of Water Immersion on Posttraining Recovery in Australian Footballers. International Journal of Sports Physiology 7 (4), 357-366 [DOI] [PubMed] [Google Scholar]
- Gill N.D., Beaven C.N., Cook C. (2006) Effectiveness of post-match recovery strategies in rugby Players. British Journal Sports Medicine 40 (3), 260-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton J.M., Twist C., Eston R.G. (2009) The effects of exercise-induced muscle damage on agility and sprint running performance. Journal of Exercise Science and Fitness 7 (1), 24-30 [Google Scholar]
- Kevin M.C., Rick W.W., David H.P. (1996) Functional performance following an ice immersion to the lower extremity. Journal of Athletic Training 31 (2), 113-116 [PMC free article] [PubMed] [Google Scholar]
- Komi P.V., Viitasalo J.T. (1977) Changes in motor unit activity and metabolism in human skeletal muscle during and after repeated eccentric and concentric contractions. Acta Physiologica Scandinavica 100 (2), 246-254 [DOI] [PubMed] [Google Scholar]
- Mackowiak P.A., Wasserman S.S., Levine M.M. (1992) A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. Journal of American Medical Association 268 (12), 1578-1580 [PubMed] [Google Scholar]
- Marginson V., Eston R.G. (2002) Symptoms of exercise-induced muscle damage in boys and men following two bouts of eighty plyometric jumps. Journal Physiology 539, 75P [Google Scholar]
- Miles M.P., Ives J.C., Vincent K.R. (1997) Neuromuscular control following maximal eccentric exercise. European Journal of Applied Physiology 76 (4), 368-374 [DOI] [PubMed] [Google Scholar]
- Moss G.A., Bondar R.J., Buzzelli D.M. (1975) Kinetic enzymatic method for determining serum creatinine. Clinical Chemistry 21 (10), 1422-1426 [PubMed] [Google Scholar]
- Pournot H., Bieuzen F., Duffield R., Lepretre P.M., Cozzolino C., Hausswirth C. (2011) Short term effects of various water immersions on recovery from exhaustive intermittent exercise. European Journal of Applied Physiology 111 (7), 1287-1295 [DOI] [PubMed] [Google Scholar]
- Rüst C.A., Knechtle B., Rosemann T. (2012) Changes in body core and body surface temperatures during prolonged swimming in water of 10°C-a case report. Extreme Physiology and Medicine 1 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellwood K., Brukner P., Williams D., Nicol A., Hinman R. (2007) Ice-water immersion and delayed-onset muscle soreness: a randomised controlled trial. British Journal of Sports Medicine 41 (6), 392-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sund-Levander M., Forsberg C., Wahren L.K. (2002) Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scandinavian Journal of Caring Science 16 (2), 122-128 [DOI] [PubMed] [Google Scholar]
- Takarada Y. (2003). Evaluation of muscle damage after a rugby match with special reference to tackle plays. British Journal of Sports Medicine 37(5), 416-419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaile J., Halson S., Gill N., Dawson B. (2008) Effect of cold water immersion on repeat cycling performance and thermoregulation in the heat. Journal Sports Science 26 (5), 431-440 [DOI] [PubMed] [Google Scholar]


