Abstract
An estimated 22 350 patients had multiple myeloma diagnosed in 2013, representing 1.3% of all new cancers; 10 710 deaths are projected, representing 1.8% of cancer deaths. Approximately 0.7% of US men and women will have a myeloma diagnosis in their lifetime, and with advances in therapy, 77 600 US patients are living with myeloma. The 5-year survival rate was 25.6% in 1989 and was 44.9% in 2005. The median age at diagnosis is 69 years, with 62.4% of patients aged 65 or older at diagnosis. Median age at death is 75 years. The rate of new myeloma cases has been rising 0.7% per year during the past decade. The most common indication for autologous stem cell transplantation in the United States is multiple myeloma, and this article is designed to provide the specifics of organizing a transplant program for multiple myeloma. We review the data justifying use of stem cell transplantation as initial management in myeloma patients. We provide selection criteria that minimize the risks of transplantation. Specific guidelines on mobilization and supportive care through the transplant course, as done at Mayo Clinic, are given. A review of the data on tandem vs sequential autologous transplants is provided.
Introduction
A 50-year-old woman had asymptomatic multiple myeloma identified in December 1992. A bone marrow biopsy showed 49% plasma cells. She initially had expectant management only. In April 1996, anemia developed (hemoglobin, 8.6 g/dL), a bone marrow biopsy showed 73% plasma cells, and her immunoglobulin (Ig) G level was 5740 mg/dL. She had compression fractures of L2 and L5. She began treatment with vincristine, doxorubicin, and dexamethasone. After 4 cycles, stem cells were collected and cryopreserved. She deferred stem cell transplantation and was treated with vincristine, carmustine, melphalan, cyclophosphamide, and prednisone between November 1996 and October 1997. She had progression in September 1998, with an IgG that rose from 1960 to 2940 mg/dL. She went directly to stem cell transplantation and was conditioned with melphalan (200 mg/m2). At day 100, she had achieved a very good partial response (VGPR); she remained in remission for 43.6 months, when IgG levels doubled to 1420 mg/dL. She underwent a second stem cell transplantation (conditioned with melphalan [200 mg/m2]) in September 2004 using cells cryopreserved in October 1996. She achieved a complete response (CR) and has been in continuous remission for 9 years and 3 months since then; she now survives 17.7 years after development of overt myeloma. She represents the 10% of patients whose response duration after a second transplantation exceeds that of the first.
Is there any role for stem cell transplantation in the novel-agent era?
In the 2 largest studies,1,2 autologous stem cell transplantation (ASCT) showed a 12-month improvement in overall survival compared with nontransplant cohorts. Unfortunately, these studies predated novel agents, and with current survival rates, it is fair to ask whether ASCT still has a role. A randomized trial comparing chemotherapy plus lenalidomide with ASCT followed by maintenance with lenalidomide-prednisone or lenalidomide alone in patients with newly diagnosed myeloma demonstrated significantly prolonged progression-free survival (PFS) in the transplant arm (60% vs 38% at 3 years).3 Randomized trials to address the value of ASCT are ongoing. The Nordic trial (NCT01208766) (Table 1) incorporates induction with bortezomib, melphalan, and prednisone (VMP) and randomizes to 1 or 2 courses of high-dose melphalan followed by either 4 consolidation courses of VMP or no consolidation. Maintenance lenalidomide is given until progression. The second trial (NCT01208662) uses lenalidomide, bortezomib, and dexamethasone induction and then randomizes ASCT. Lenalidomide maintenance is given to all. This trial should definitively answer the question of early vs delayed first transplant, which in 1 prospective analysis13 and 2 retrospective analyses14,15 failed to show improved survival.
Table 1.
Induction consolidation and maintenance studies in myeloma
| Group | Induction | Transplant type: response | Consolidation | Maintenance | Reference |
|---|---|---|---|---|---|
| Medical Research Council | CTd | Single: CR in 50% | No | Thalidomide vs 0 | 4 |
| Dana-Farber Cancer Institute | VRd | Single in 28: PFS in 92% at 1 y | No | Not in transplanted patients | 5 |
| European Myeloma Network | Carfilzomib, thalidomide, dexamethasone | Single: ≥VGPR in 60% | Carfilzomib, thalidomide, dexamethasone | No | 6 |
| Intergroupe Francophone du Myelome | Vd | Single: ≥VGPR in 54% | Lenalidomide ×2 | Lenalidomide vs 0 | 7 |
| Program a Espanol de Tratamientos en Hematologica | VTd | Single: ≥CR in 46% | No | Interferon vs thalidomide vs bortezomib thalidomide | 8 |
| Italian Group for Adult Hematologic Diseases | VTd | Tandem: ≥VGPR in 89% | VTd ×2 | Dexamethasone | 9 |
| Dutch-Belgian Hemato-Oncology Cooperative Group and the German Multicenter Myeloma Group | PAD | Single (n = 210) and tandem (n = 142): CR or near-CR in 49% | No | PAD ×2 y | 10 |
| European Myeloma Network | VMP ×4 | Single vs tandem* | VRd ×2 vs 0 | Lenalidomide | 11 |
| Dana-Farber Cancer Institute | VRd | Single vs 0* | No | Lenalidomide | 12 |
| International | CRd | Single vs CRd: PFS in 60% vs 38% at 3 y | CRd in nontransplant arm | Lenalidomide vs lenalidomide prednisone | 3 |
CRd, cyclophosphamide, lenalidomide, and dexamethasone; CTd, cyclophosphamide, thalidomide, and dexamethasone; PAD, bortezomib, doxorubicin, and dexamethasone; Vd, bortezomib, dexamethasone.
Clinical trial in progress.
The Center for the International Blood and Marrow Transplant Registry showed that the risk of death after ASCT in the years 2000-2004 and 2005-2010 has decreased.16 In the Eastern Cooperative Oncology Group E4A03 trial,17 a retrospective analysis compared patients who underwent early stem cell transplantation with those who did not undergo high-dose therapy. For patients <65 years old, overall survival at 3 years was 94% with early stem cell transplantation vs 78% in patients continuing protocol therapy. In the >65 age group, 1-year mortality rates were similar between those who did or did not undergo early stem cell transplantation. Moreover, in the >70 age group, stem cell transplantation seemed to mitigate the toxicity and survival disadvantage associated with high-dose dexamethasone.
Transplant trials that use novel agents clearly showed that responses are deepened so that the fraction of patients with CRs and VGPRs increase between the induction phase and the post-ASCT consolidation phase. A recent transplant study in patients aged 65 to 75 years reported an increase in patients with VGPR or better from 55% after induction to 76% posttransplantation.19 This sequential increase in deep response rates has been a consistent finding after ASCT.20 In the novel-agent era, CR has been a surrogate marker for improved PFS and overall survival.21 Induction with bortezomib, thalidomide, and dexamethasone (VTd) before stem cell transplantation resulted in previously unreported high levels of CRs.9 These findings led the International Myeloma Working Group to conclude that autotransplantation after novel-agent induction improved the depth of response, a gain that translated into extended PFS.22 An analysis of 153 studies covering 22 700 patients concluded that time-dependent end points such as time to progression, PFS, and event-free survival predict overall survival in patients with myeloma.23
Stem cell transplantation is capable of achieving residual disease negativity, a predictor of PFS.24 The use of ASCT for multiple myeloma is increasing, and survival is increasing proportionately.25 Therefore, until trials definitively demonstrate that the platform of ASCT does not improve survival, it remains the standard of care for eligible patients.
Summary
Until proven otherwise, stem cell transplantation remains the standard of care for eligible patients.
What are the roles of induction and maintenance in transplant-eligible patients?
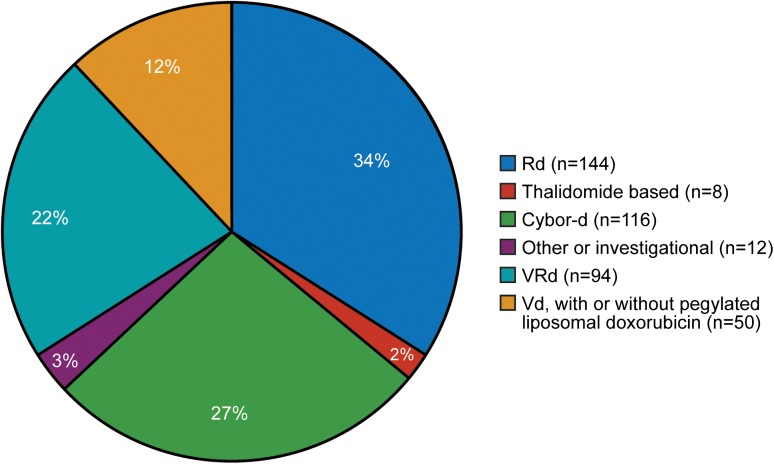
In our referral population of patients (initially treated locally and then referred for ASCT), induction is determined by the referring physician. Figure 1 shows pretransplant induction therapy for 424 consecutive patients, demonstrating the lack of consensus in our referral community. Table 1 summarizes trials published and in progress, indicating 8 totally different induction schemes (only the investigational arm is shown). It is noteworthy that 6 of the 8 are triplet combinations, whereas 2 use doublet induction. The Eastern Cooperative Oncology Group trial is comparing bortezomib, lenalidomide, and dexamethasone (VRd) with carfilzomib, lenalidomide, and dexamethasone.26
Figure 1.
Induction regimen administered before stem cell transplantation. Cybor-d, cyclophosphamide, bortezomib, and dexamethasone; Rd, lenalidomide and dexamethasone.
Two trials of posttransplant lenalidomide maintenance have been reported, with 1 showing a survival advantage27 and the other study reporting no survival benefit.28 Maintenance therapy was offered to all patients in 6 trials and randomized to maintenance or no maintenance in 2 (Table 1). The Eastern Cooperative Oncology Group offers lenalidomide to all patients either for 24 cycles or until progression.
In the Dutch-Belgian Hemato-Oncology Cooperative Group and the German Multicenter Myeloma Group (Hovon-65/GMMG) trial, bortezomib maintenance (1.3 mg/m2, administered intravenously every 2 weeks for 2 years) resulted in a 78% survival rate at 3 years compared with 71% survival in the nonbortezomib arm (P = .48).10 This bortezomib-containing induction and maintenance also improved outcome in patients with renal impairment29 and adverse genetic profiles.30 The design of the study makes it impossible to distinguish whether the survival benefit was attributable to bortezomib induction, maintenance, or both.
In Total Therapy 3, 3 years of VTd maintenance was administered31 with a 2-year event-free survival rate of 84%. Premature discontinuation of bortezomib was an independent highly adverse feature for survival (hazard ratio, 6.44; P < .001).32 At Mayo, our Stratification for Myeloma And Risk-adapted Therapy guidelines33 risk-stratify maintenance. For patients with standard risk, we give 2 cycles of lenalidomide-dexamethasone consolidation, based on the French trial.28 For patients with intermediate or high risk, we administer bortezomib-based therapy for 1 year.30,34
Summary
There is no definite “correct” induction therapy before ASCT, but it should include a novel agent. Although survival benefit is not consistently seen with posttransplant maintenance, most trials are incorporating the concept.
What are selection criteria for transplant-eligible candidates?
Selection for ASCT in the European Union has been limited to patients <65 years. In the United States, age is considered in conjunction with performance status, frailty, and comorbidities (including cardiac, pulmonary, hepatic, and renal function) when assessing suitability for ASCT.35 A scoring system based on age, comorbidities, and cognitive and physical conditions was used to categorize patients into 3 groups with different risk of severe toxicities to myeloma induction36; this scoring system could be applied to assess probability of safe transplantation in the elderly.37 We have performed transplantations in patients up to 76 years of age; currently, 460 patients >65 years old and 158 patients >70 years of age have undergone transplantation. These 158 patients were selected for their ability to tolerate transplant; 87 received melphalan (200 mg/m2) and 71 received a lower dose of melphalan. Day 100 all-cause mortality rates were 1.3% for both the >65 and >70 age groups (6/460 and 2/158, respectively). Of the 6 patients who died before day 100, 2 underwent transplantation in relapse and 1 had induction-refractory disease. Patient age did not influence outcome.38 For patients undergoing transplantation in their first plateau, a response of VGPR or better was observed in 66.9% of patients aged ≥65 years and 65.1% for patients <65 years (P = .60). Relapse-free survival for patients who were not in relapse at transplantation was 30.8 and 25.3 months for patients aged ≥65 years or <65 years, respectively (P = .14). The feasibility of ASCT in patients >70 years is well established.39-41 A phase 2 trial for patients aged 65 to 75 included 4 cycles of bortezomib, pegylated liposomal doxorubicin, and dexamethasone, tandem transplants with melphalan (100 mg/m2), followed by 4 cycles of lenalidomide-prednisone consolidation, and lenalidomide maintenance. The CR rate was 33% after transplant and 53% after maintenance. Median PFS was 48 months. During induction and transplant, 8% of patients died. The rate of death attributable to adverse events was significantly higher in patients aged >70 years.19 In a matched-pair analysis of myeloma patients aged >70 compared with patients ≤65 years (with 2 controls for each patient), more of the older patients received dose-reduced melphalan but had an overall response rate that was similar (97% vs 98%), and the median time to progression was 28.5 vs 17.8 months (P = .70).42 Median overall survival after transplant also was not significantly different by age. Therefore, in patients aged >70 years selected for transplant, the toxicity and outcome are comparable to younger patients. Others have reported similar outcomes.43 A frailty index could consider measures of activities of daily living plus cardiac, renal, and hepatic comorbidities to identify a population with low risk of transplant-related complications; this would be a more realistic approach to patient eligibility than age alone.44,45
Because 19% of patients with multiple myeloma present with renal insufficiency (creatinine ≥2.0 mg/dL) at diagnosis,46 how to use stem cell transplantation to manage cast nephropathy is an important issue. Fortunately, since the advent of novel agents, only 6% of our patients (52/863) since January 1, 2007, had a serum creatinine ≥2 mg/dL at the time of transplant. We and others have previously demonstrated38,47,48 that creatinine level does not affect CR rates or time to progression, but higher creatinine levels are associated with a higher day-100 mortality rate (2/52; 3.8%), a shorter overall survival (62% at 30 months), and delayed platelet engraftment (median, 17 days to 50/nL). Patients with elevated creatinine require a reduction in their conditioning dose of melphalan to 140 mg/m2. We recently reviewed the long-term outcomes of patients with advanced renal failure after ASCT between 2000 and 2010; we included 30 patients (2.8% of all patients who underwent ASCT) with serum creatinine >3 mg/dL and those who were dialysis-dependent. Fourteen of 15 patients who were dialysis-dependent before transplantation still required long-term dialysis, despite a hematologic response. Of 15 patients with serum creatinine >3 mg/dL not requiring dialysis, the median glomerular filtration rate increased from 15 to 19.4 mL/minute. ASCT did not lead to independence from dialysis; this suggests that if renal function does not improve after novel-agent induction, enough renal scarring has occurred to limit the amount of recovery that can be expected after ASCT.49 Bortezomib appears to be particularly important in managing myeloma-cast nephropathy before transplantation because it improves overall and CR rates as well as PFS compared with VAD induction.29
One issue to consider is the level of response needed for a successful outcome after ASCT. Before the novel-agent era, high-dose therapy could overcome drug resistance with corticosteroid- and doxorubicin-based regimens.50 Current trials designed to evaluate transplantation remove patients from the trial if, at the completion of induction, their disease is progressive. These trials do not independently report outcomes for patients failing novel agent-based induction. One trial examined registry data and assessed myeloma patients who did not have a partial response before transplantation.51 These patients were stratified into 2 groups: those who went directly to ASCT and those who received second-line salvage therapy before transplantation. In this setting, no additional benefit could be demonstrated for salvage regimens intended to upgrade response before ASCT, suggesting that patients who do not respond to induction therapy should move directly to transplantation, rather than delay with attempts to lower the tumor mass before collection.
Patients who do not have a partial response after novel agent–based induction therapy will have an inferior overall survival because their disease has shown chemotherapy resistance; however, this should not delay transplantation.52 The frequency of progressive disease with novel agents is low. In 1 phase 3 trial, 1 of 105 registered patients went off trial for progression.53 Among 604 Mayo patients who underwent transplantation after January 2010, only 7 (1.2%) did not achieve at least a minor response after induction. The median PFS for these 7 was only 16.4 months. Failure to respond to induction predicts a poor outcome because it suggests chemotherapy-resistant disease. At Mayo, the survival rate at 36 months from transplant in plateau vs at refractory relapse is 77% vs 37%, respectively.
Pretransplant studies can be performed that will predict the outcome after ASCT. A response failure before stem cell transplantation predicts significantly shorter overall and PFS. Pretransplant genetic tests such as a 1p31-32 deletion are prognostic for survival after transplant.54 Myeloma patients with translocation t(4;14) or deletion of 17p have poor outcomes after treatment with high-dose therapy, and these patients require novel agent–based induction, consolidation, and maintenance.30,55
Summary
Ability to transplant safely is the paramount decision. Frailty is a major determinant. Age, performance status, and renal function are not exclusions to safe transplantation.
How do we mobilize?
The 3 most common forms of mobilization are chemotherapy mobilization, growth factor–only mobilization, and growth factor plus plerixafor mobilization. Multiple groups have demonstrated that chemotherapy mobilization, usually cyclophosphamide (3-4 g/m2) alone or combined with etoposide, yields significantly more stem cells than growth factor–only mobilization.56-58 However, this puts the patient at risk of neutropenic fever, hospitalization, and transfusion support with both red cells and platelets. Moreover, in our experience, cyclophosphamide mobilization does not improve either the overall CR rates or time to progression.59 When we compared cyclophosphamide mobilization to growth factor–only mobilization, we found no difference in duration of hospitalization after transplantation.58 However, patients treated with cyclophosphamide had a higher incidence of nonstaphylococcal bacteremia. Our overall stem cell mobilization failure rate was approximately 10%, and those patients had increased use of growth factors and antibiotics, subsequent requirements for additional chemotherapy, more apheresis procedures, and increased transfusion support.60 We also found that after cyclophosphamide mobilization, in patients with a peripheral blood CD34 cell count below 10/µL on day 13 or 1 day after the leukocyte count was >1000/µL, plerixafor was required for a successful mobilization.
We have been unable to identify clinical characteristics of patients that can successfully predict, with high specificity, which patients will have poor mobilization.56 However, lenalidomide is known to impair mobilization of stem cells. Age, platelet count at the time of mobilization, and extent of exposure to lenalidomide independently correlate with peripheral blood CD34.56 Intravenous plerixafor has been demonstrated to be an effective strategy for mobilization in patients after lenalidomide-based initial therapy,61 and it can overcome the negative effects of most predictors of poor mobilization.62 Nevertheless, adding plerixafor to the mobilization strategy increases the cost by more than 50% for the mobilization procedure. Therefore, although it has superior efficacy for mobilizing stem cells compared with growth factor alone,63 mobilization costs are substantially higher, and the efficacy of plerixafor-based mobilization is quite comparable to cyclophosphamide-pulsed mobilization.64 The International Myeloma Working Group supports the use of cyclophosphamide and granulocyte colony-stimulating factor for patients previously exposed to lenalidomide.65 Based on these data, Table 2 lists the mobilization algorithm currently used at Mayo in an effort to maximize yield while minimizing the use of resource-intensive agents such as plerixafor. Currently, we reserve chemotherapy-based mobilization for patients with circulating clonal plasma cells at the time of apheresis and patients who are undergoing transplantation in frank relapse. As a result, the fraction of patients undergoing transplantation using chemotherapy-based mobilization has decreased from 30% (176/586; 2003-2008) to 18% (124/706; 2009-2013).
Table 2.
Recommendations for stem cell mobilization
| Chemotherapy and growth factor recommendations |
|---|
|
Chemotherapy mobilization • Cyclophosphamide (1.5 g/m2) on days 1 and 2. |
| • Granulocyte colony-stimulating factor (5 μg/kg) daily on day +3 after initiation of cyclophosphamide and daily thereafter until mobilization procedure is complete, with dose rounded to the nearest vial size (eg, 300, 480, 600, 780, 900 μg). Monitor leukocyte count daily (platelet support if <10/µL). |
| When white blood cell count is ≥10/µL, check blood CD34 levels. |
| -If CD34 is ≥10/µL, begin apheresis. |
| -If CD34 is <10/µL, continue granulocyte colony-stimulating factor and measure CD34 daily. When CD34 is >10/μL, begin apheresis. |
| -If CD34 <10/μL and white blood cells are >1/μL for 3 days, start plerixafor. When CD34 is >10/μL, begin apheresis. |
| Growth factor–only mobilization |
| • Granulocyte colony-stimulating factor (10 μg/kg) every day (day +1) and daily thereafter until mobilization procedure is complete, with dose rounded to nearest vial size (eg, 600; 780; 900; 960; 1080; 1260; 1440). On day 4, measure CD34 levels. |
| -If the goal is to collect for 1 transplant and day 4 CD34 is <10/µL, begin plerixafor and collect cells the next morning. |
| -If the goal is to collect for >1 transplant and day 4 CD34 is <20/µL, begin plerixafor and collect cells the next morning. |
| Plerixafor dosing |
| Renal clearance ≥50 mL/min: 0.24 mg/kg |
| Renal clearance <50 mL/min: 0.16 mg/kg |
| Dose never exceeds 24 mg |
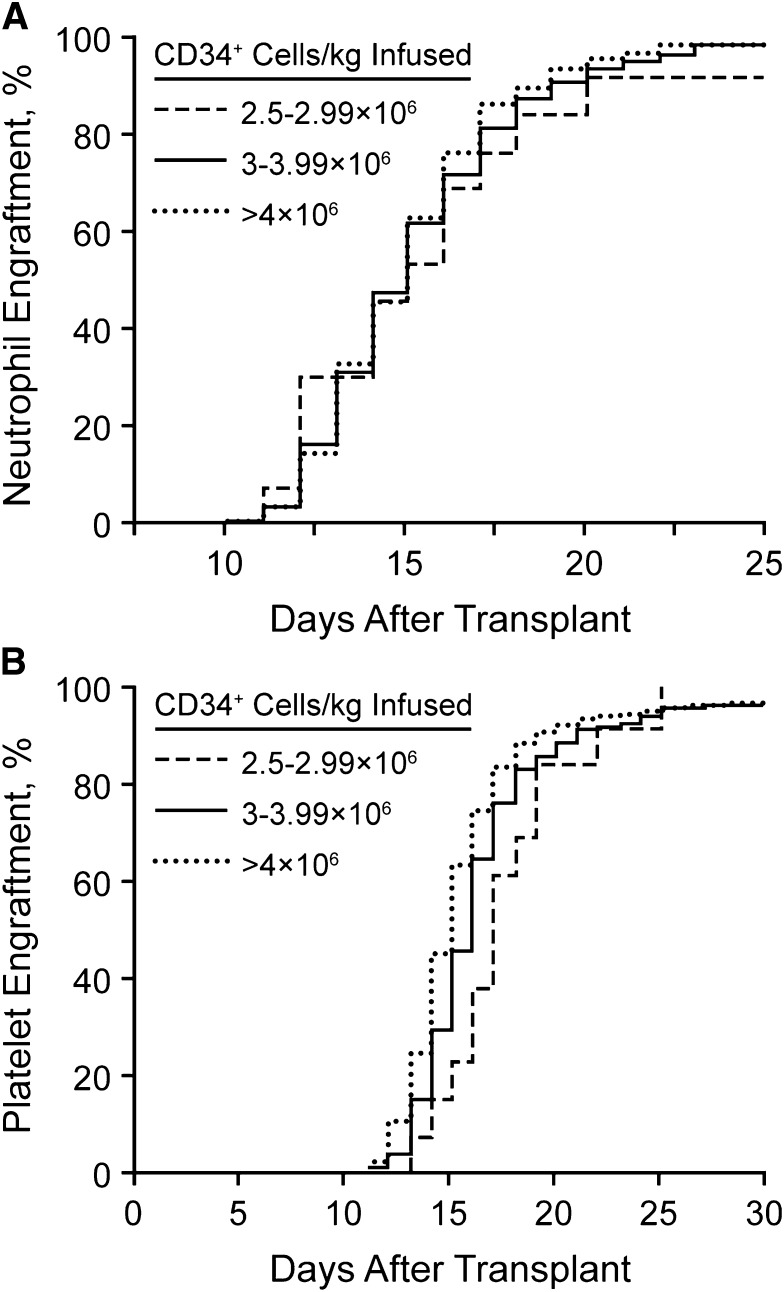
The number of stem cells that need to be collected remains a critical question. We have analyzed our rates of hematopoietic reconstitution as a function of the number of CD34+ cells infused. We do not use growth factor for enhancement of neutrophil recovery after stem cell transplantation.66 Since the cessation of growth factor use, we have not seen a change in the rates of bacteremia or days of hospitalization.66 Figure 2 shows the neutrophil and platelet recovery rates, stratified by the number of stem cells infused (2.5-2.9 × 106, 3.0-3.9 × 106, or >4.0 × 106 CD34+ cells/kg). As noted, the time to neutrophil recovery did not differ for any patient infused with at least 2.5 × 106 CD34+ cells/kg. However, 30 days after transplantation, the fraction of patients with a platelet count <50/nL was significantly greater in those who received <3 × 106 CD34+ cells/kg. Therefore, we have elected to set our threshold for a single transplant at 3 × 106 CD34+ cells/kg. We recognize that it is possible to have patients undergo transplantation with as few as 2 × 106 CD34+ cells/kg.
Figure 2.
Neutrophil and platelet recovery rates. (A) Neutrophil engraftment after stem cell transplantation without growth factor support. (B) Platelet engraftment after stem cell transplantation without growth factor support.
Because a significant pharmaceutical cost in transplant is growth factor mobilization, we are currently analyzing outcomes of patients mobilized with the recently introduced biosimilar tbo-filgrastim, which has been shown to provide similar yields of CD34+ cells.67 This agent has a similar safety profile to filgrastim but is a less expensive substitute.68-70 A single dose of pegfilgrastim also may be used for mobilization; although extensively used elsewhere, it has not been adopted by our group.71
The second most common cost relates to hospitalization. Patients at Mayo Clinic undergo mobilization, conditioning, and follow-up in the outpatient setting. Hospitalization generally is precipitated by a decline in performance status, hemodynamic instability, mucositis that renders maintaining hydration impossible as an outpatient, antibiotic requirements that cannot be satisfied with outpatient administration, and intractable nausea and vomiting. Since January 1, 2008, 53% of our patients (416/790) have not needed hospitalization. For the remaining 47% (n = 374), the median hospital stay was 6 days. The major predictor of hospitalization need is patient age; those ≥65 years had a hospitalization rate of 52% (139/266), whereas those <65 years had a hospitalization rate of 45% (235/524). We routinely use penicillin (doxycycline), levofloxacin, acyclovir, and fluconazole for antibiotic prophylaxis beginning on day 1 through engraftment at 500 neutrophils/μL. In patients that develop neutropenic fever, levofloxacin and penicillin are discontinued; they are started on vancomycin every 12 hours (dosage adjusted to blood levels and renal function) and cefepime every 12 hours. This is appropriate for the specific microbiome of the Mayo hospitals, with high rates of sensitivity for commonly encountered flora. Vancomycin is used empirically because of a high incidence of staphylococcal bacteremia (89/790 [11%]) since January 1, 2008; if blood cultures are negative, vancomycin is discontinued after 72 hours to reduce the risk of selecting vancomycin-resistant enterococcus. Antiemetics include dexamethasone (10 mg) administered on days 1 and 2, granisetron (2 mg, oral) as needed, prochlorperazine (10 mg) as needed, and lorazepam (0.5 mg) as needed.
Summary
In the era of novel agents, chemotherapy mobilization is not clearly superior to growth factor only. Long-term storage of stem cells is feasible for salvage transplantation.
How many transplants and what is their timing?
Tandem transplant was first demonstrated to improve overall survival in 2003.72 Survival benefit was seen after extended follow-up in patients who did not achieve a VGPR after the first transplant. In that same year, the Spanish Myeloma Study Group demonstrated an increase in the CR rate after a second stem cell transplant, with improved overall and event-free survival.73 However, in an intention-to-treat analysis, a third of patients with newly diagnosed symptomatic myeloma never received their second transplant. In a second feasibility trial, only 42% received a second stem cell transplant because of inadequate numbers of stem cells, toxicity with the first transplant, and early progression.74 The Bologna-96 trial compared single and tandem transplant in myeloma and showed that tandem transplantation did not prolong overall survival.75 The failure to prolong overall survival suggests that improved outcome after tandem transplant is not consistently observed. In a review of 5 trials that compared single and tandem transplant, improved PFS was seen in 3 trials; overall survival was significantly prolonged in only 1 trial and was limited to patients who did not achieve a VGPR. In a meta-analysis, tandem transplant did not result in improved overall or event-free survival, and the treatment-related mortality risk with the second transplant had a hazard ratio of 1.71.76 The recently reported Hovon-65/GMMG-HD4 trial showed that tandem transplantation (German policy) vs single transplant (Dutch policy) resulted in a significantly improved overall survival after tandem transplantation (P = .004).77
Tandem transplantation is the foundation of the Total Therapy program. In the era before novel agents, tandem high-dose therapy resulted in event-free survival >5 years in one-fourth of patients, with no relapse after 7 years. The report raised the prospect of cure for patients in CR for >7 years.78 Failure to achieve CR and loss of CR were independently associated with inferior survival.79 Mathematical modeling of CR duration in low-risk myeloma predicts a 55% cure rate.80 In Total Therapy 3, 5-year PFS is now 67%.81 In the Hovon-65/GMMG-HD4 trial, tandem transplantation was an independent predictor of overall survival, with a hazard ratio of 0.75.10 A meta-analysis of 1,894 patients from 4 studies also found tandem ASCT was predictive of improved overall survival (hazard ratio, 0.4).82
A second transplant need not always be done in tandem. The second transplant can be delayed until progression and becomes a useful salvage strategy. We generally collect stem cells for 2 or more transplants, and some patients have waited as long as 158 months between the 2 procedures. A second mobilization procedure is not required.83 Second stem cell transplantation in relapsed myeloma can be associated with superior overall and PFS compared with conventional chemotherapy if the response duration from the first transplant exceeds 9 months.84 The predictors of a shorter PFS after a second stem cell transplantation include time to progression after the first transplant, not achieving a CR, and the number of regimens preceding the second transplant.85 Currently, if we collect sufficient stem cells for 2 transplants, we perform 1 transplant after induction and have cells available for a salvage procedure. We have completed 179 second salvage transplants since 1996, with a median of 51 months between transplants and a median overall survival of 38 months after the second stem cell transplant. A matched-pair analysis comparing a second salvage autologous stem cell transplant to systemic chemotherapy alone after relapse suggested superior survival for transplanted patients (55.5 vs 25.4 months; P = .04). The transplant benefit was greater if the duration of response after the first transplant exceeded 18 months.86 The reported data on the use of a second stem cell transplant as salvage for relapsed myeloma have recently been reviewed. If the response duration after the initial transplant was less than 1 year, stem cell transplantation could not be recommended as an effective intervention.87 Currently at Mayo, we routinely collect sufficient stem cells for 2 transplants but do not perform tandem transplantations; we keep an aliquot cryopreserved for a second transplant at relapse.
Summary
Single and tandem transplants are both appropriate options for the management of myeloma. Collecting stem cells for a future salvage transplant is justifiable.
What is appropriate conditioning?
Melphalan (200 mg/m2) remains the standard conditioning regimen for multiple myeloma. However, it is still complicated by severe mucositis, possible cardiotoxicity,88 and, rarely, encephalopathy.89 A prospective phase III trial effectively eliminated the use of standard dose, total body irradiation regimens for conditioning.90 Phase II trials have studied the use of idarubicin,91 etoposide,92 busulfan,93 carmustine,94 and bortezomib for conditioning.95 Busulfan melphalan conditioning has not been demonstrated to increase CR rates or PFS.96 Evidence suggests that dexamethasone with carmustine, etoposide, cytarabine, and melphalan is an effective conditioning regimen, particularly in patients with extramedullary disease.97 Bortezomib has been added to carmustine, etoposide, cytarabine, and melphalan in the setting of salvage transplantation and resulted in a 20% therapy-related mortality rate.98 However, 2 other trials combined bortezomib with melphalan safely, with CR rates of 35% and 21%, respectively.7,95 Still, none have shown superior outcomes compared with melphalan (200 mg/m2). Current active trials on conditioning include high-dose lenalidomide, bortezomib or carfilzomib, and low-dose total body irradiation in combination with melphalan.
Previous radiotherapy has been used for conditioning and has included regimens that contain holmium-[166Ho]-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylenephoonic acid99 and 153samarium-ethylenediaminetetramethylenephosphonic acid,100 and early studies are exploring myeloablative effects of anti-CD38 antibodies that are conjugated to radiolabeled targeting agents.101
Melphalan remains the standard for transplantation, but the addition of novel agents to conditioning is being actively explored.
Conclusion
Stem cell transplantation remains an important option for eligible patients to improve depth of response, PFS, and likely overall survival. Transplantation can be safely performed as an outpatient and is cost effective, particularly when novel agents are not readily available. Transplant can be done early or late in the disease course (or both). We have provided specific guidelines with regard to the technique and the mobilization procedure (Table 3).
Table 3.
Summary of how we transplant
| Technique and the mobilization procedure |
|---|
|
Selection Up to age 76 years. No restriction for renal function. No requirement for response to induction. Mobilization (see Table 1) Patients aged <70 routinely undergo collection for 2 transplants. Plerixafor not standard. Chemotherapy mobilization if circulating cells by flow or if no response to induction. |
|
Conditioning Standard nonprotocol remains melphalan (200 mg/m2) for fit patients. Melphalan (140 mg/m2) if patient is frail or serum creatinine ≥2.0 mg/dL. |
|
Procedural Conditioning, infusion, and postinfusion monitoring usually are performed on an outpatient basis. |
| • Oral antibiotic prophylaxis: penicillin, levofloxacin, acyclovir, and fluconazole |
| • Manage breakthrough fever of >38.5°C with vancomycin (3 days if culture negative) and cefepime (outpatient). |
| • Anticipate that half of patients will complete treatment as outpatients. |
Authorship
Contribution: M.A.G. contributed to study design, analysis, data collection, and manuscript writing, and D.D. contributed to data analysis and interpretation and manuscript writing.
Conflict-of-interest disclosure: M.A.G. receives honoraria from Celgene, Onyx, Research to Practice, and ISIS. The remaining author declares no competing financial interests.
Correspondence: Morie A. Gertz, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: gertz.morie@mayo.edu.
References
- 1.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, et al. Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Gay F, Spencer A, et al. A phase III study of ASCT vs cyclophosphamide-lenalidomide-dexamethasone and lenalidomide-prednisone maintenance vs lenalidomide alone in newly diagnosed myeloma patients [abstract]. Blood. 2013;122(21):763. [Google Scholar]
- 4.Morgan GJ, Davies FE, Gregory WM, et al. National Cancer Research Institute Haematological Oncology Clinical Studies Group. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97(3):442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonneveld P, Asselberg-Hacker E, Zweegman S, et al. Dose escalation phase 2 trial of carfilzomib combined with thalidomide and low-dose dexamethasone in newly diagnosed, transplant eligible patients with multiple myeloma: a trial of the European Myeloma Network [abstract]. Blood. 2013;122(21):688. [Google Scholar]
- 7.Roussel M, Moreau P, Huynh A, et al. Intergroupe Francophone du Myélome (IFM) Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood. 2010;115(1):32–37. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 8.Rosiñol L, Oriol A, Teruel AI, et al. Programa para el Estudio y la Terapéutica de las Hemopatías Malignas/Grupo Español de Mieloma (PETHEMA/GEM) group. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120(8):1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 9.Cavo M, Tacchetti P, Patriarca F, et al. GIMEMA Italian Myeloma Network. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 10.Sonneveld P, Schmidt-Wolf IG, van der Holt B, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012;30(24):2946–2955. doi: 10.1200/JCO.2011.39.6820. [DOI] [PubMed] [Google Scholar]
- 11.Stichting Hemato-Oncologie voor Volwassenen Nederland. Study to compare VMP with HDM followed by VRD consolidation and lenalidomide maintenance in patients with newly diagnosed multiple myeloma (HO95). Available at: http://clinicaltrials.gov/show/NCT01208766. Accessed July 1, 2014.
- 12.Richardson PG Dana-Farber Cancer Institute. Randomized trial of lenalidomide, bortezomib, dexamethasone vs high-dose treatment with SCT in MM patients up to age 65 (DFCI 10-106). Available at: http://clinicaltrials.gov/ct2/show/NCT01208662?term=NCT01208662&rank=1. Accessed July 1, 2014.
- 13.Fermand JP, Ravaud P, Chevret S, et al. High-dose therapy and autologous peripheral blood stem cell transplantation in multiple myeloma: up-front or rescue treatment? Results of a multicenter sequential randomized clinical trial. Blood. 1998;92(9):3131–3136. [PubMed] [Google Scholar]
- 14.Dunavin NC, Wei L, Elder P, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013;54(8):1658–1664. doi: 10.3109/10428194.2012.751528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar SK, Lacy MQ, Dispenzieri A, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118(6):1585–1592. doi: 10.1002/cncr.26422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19(11):1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkumar SV, Jacobus S, Callander NS, et al. Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel DS, Jacobus S, Rajkumar SV, et al. Eastern Cooperative Oncology Group. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in the ECOG E4A03 randomized clinical trial. Abstract presented at 53rd American Society of Hematology (ASH) Annual Meeting and Exposition. December 10-13, 201. San Diego, CA. [Google Scholar]
- 19.Gay F, Magarotto V, Crippa C, et al. Bortezomib induction, reduced-intensity transplantation, and lenalidomide consolidation-maintenance for myeloma: updated results. Blood. 2013;122(8):1376–1383. doi: 10.1182/blood-2013-02-483073. [DOI] [PubMed] [Google Scholar]
- 20.Siddiqui M, Gertz M. The role of high-dose chemotherapy followed by peripheral blood stem cell transplantation for the treatment of multiple myeloma. Leuk Lymphoma. 2008;49(8):1436–1451. doi: 10.1080/10428190802084972. [DOI] [PubMed] [Google Scholar]
- 21.Niesvizky R, Richardson PG, Rajkumar SV, et al. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. Br J Haematol. 2008;143(1):46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- 22.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117(23):6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Félix J, Aragão F, Almeida JM, et al. Time-dependent endpoints as predictors of overall survival in multiple myeloma. BMC Cancer. 2013;13:122. doi: 10.1186/1471-2407-13-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putkonen M, Kairisto V, Juvonen V, et al. Depth of response assessed by quantitative ASO-PCR predicts the outcome after stem cell transplantation in multiple myeloma. Eur J Haematol. 2010;85(5):416–423. doi: 10.1111/j.1600-0609.2010.01510.x. [DOI] [PubMed] [Google Scholar]
- 25.McCarthy PL, Jr, Hahn T, Hassebroek A, et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995-2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant. 2013;19(7):1116–1123. doi: 10.1016/j.bbmt.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eastern Cooperative Oncology Group, National Cancer Institute. Bortezomib or carfilzomib with lenalidomide and dexamethasone in treating patients with newly diagnosed multiple myeloma. Available at: http://clinicaltrials.gov/show/NCT01863550. Accessed July 1, 2014.
- 27.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma: follow-up analysis of the IFM 2005-02 Trial [abstract]. Blood. 2013;122(21):406. [Google Scholar]
- 29.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148–154. doi: 10.3324/haematol.2013.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 31.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138(2):176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 32.Wolf N. [Creative arts activity in manually handicapped patients]. Rehabilitation (Stuttg) 1986;25(1):30–35. [German.] [PubMed] [Google Scholar]
- 33. mSMART: Stratification for Myeloma And Risk-adapted Therapy. Consensus guidelines to management of plasma cell disorders. Available at: http://msmart.org/msmart_mar09_002.htm. Accessed July 1, 2014.
- 34.Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. 2013;121(6):884–892. doi: 10.1182/blood-2012-05-432203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahindra A, Saad AA, Zhang M-J, et al. Hematopoietic cell transplant co-morbidity index (HCTCI) and multiple myeloma (MM) survival after autologous hematopoietic cell transplantation (AHCT). Abstract presented at 54th Annual American Society of Hematology (ASH) Meeting and Exposition. December 8-11, 2012. Atlanta, GA. [Google Scholar]
- 36.Larocca A, Bringhen S, Evalgelista A, et al. A simple score, based on geriatric assessment, improves prediction of survival, and risk of serious adverse events in elderly newly diagnosed multiple myeloma patients [abstract]. Blood. 2013;122(21):687. [Google Scholar]
- 37.Saad A, Mahindra A, Zhang MJ, et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2014;20(3):402-408.e1. [DOI] [PMC free article] [PubMed]
- 38.Gertz MA, Lacy MQ, Dispenzieri A, et al. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2007;39(10):605–611. doi: 10.1038/sj.bmt.1705627. [DOI] [PubMed] [Google Scholar]
- 39.Fratino L, Rupolo M, Mazzuccato M, et al. Autologus stem cell transplatation as a care option in elderly patients. A review. Anticancer Agents Med Chem. 2013;13(9):1419–1429. doi: 10.2174/18715206113136660357. [DOI] [PubMed] [Google Scholar]
- 40.Siegel DS, Desikan KR, Mehta J, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999;93(1):51–54. [PubMed] [Google Scholar]
- 41.Badros A, Barlogie B, Siegel E, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114(3):600–607. doi: 10.1046/j.1365-2141.2001.02976.x. [DOI] [PubMed] [Google Scholar]
- 42.Kumar SK, Dingli D, Lacy MQ, et al. Autologous stem cell transplantation in patients of 70 years and older with multiple myeloma: results from a matched pair analysis. Am J Hematol. 2008;83(8):614–617. doi: 10.1002/ajh.21191. [DOI] [PubMed] [Google Scholar]
- 43.Sharma M, Zhang M-J, Zhong X, et al. Multiple myeloma (MM) in older (>70 year) patients: similar benefit from autologous hematopoietic cell transplantation (AHCT) compared with younger patients [abstract]. Blood. 2013;122(21):416. [Google Scholar]
- 44.Palumbo A, Rajkumar SV, San Miguel JF, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014;32(6):587–600. doi: 10.1200/JCO.2013.48.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 46.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 47.Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114(4):822–829. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 48.Lee CK, Zangari M, Barlogie B, et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 2004;33(8):823–828. doi: 10.1038/sj.bmt.1704440. [DOI] [PubMed] [Google Scholar]
- 49.Glavey SV, Gertz MA, Dispenzieri A, et al. Long-term outcome of patients with mutiple myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013;48(12):1543–1547. doi: 10.1038/bmt.2013.109. [DOI] [PubMed] [Google Scholar]
- 50.Rajkumar SV, Fonseca R, Lacy MQ, et al. Autologous stem cell transplantation for relapsed and primary refractory myeloma. Bone Marrow Transplant. 1999;23(12):1267–1272. doi: 10.1038/sj.bmt.1701805. [DOI] [PubMed] [Google Scholar]
- 51.Vij R, Zhong X, Zhang M-J, Lonial S, Dispenzieri A, Hari P. Pre-transplant salvage therapy prior to autologous transplant (AHCT) in patients not responding to initial induction for multiple myeloma (MM). Abstract presented at 54th Annual American Society of Hematology (ASH) Meeting and Exposition. December 8-11, 2012. Atlanta, GA. [Google Scholar]
- 52.Gertz MA, Kumar S, Lacy MQ, et al. Stem cell transplantation in multiple myeloma: impact of response failure with thalidomide or lenalidomide induction. Blood. 2010;115(12):2348–2353, quiz 2560. doi: 10.1182/blood-2009-07-235531. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig H, Viterbo L, Greil R, et al. Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J Clin Oncol. 2013;31(2):247–255. doi: 10.1200/JCO.2011.39.5137. [DOI] [PubMed] [Google Scholar]
- 54.Chng WJ, Gertz MA, Chung TH, et al. Correlation between array-comparative genomic hybridization-defined genomic gains and losses and survival: identification of 1p31-32 deletion as a prognostic factor in myeloma. Leukemia. 2010;24(4):833–842. doi: 10.1038/leu.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106(8):2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa LJ, Nista EJ, Buadi FK, et al. Prediction of poor mobilization of autologous CD34+ cells with growth factor in multiple myeloma patients: implications for risk-stratification. Biol Blood Marrow Transplant. 2014;20(2):222–228. doi: 10.1016/j.bbmt.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Moog R. Management strategies for poor peripheral blood stem cell mobilization. Transfus Apheresis Sci. 2008;38(3):229–236. doi: 10.1016/j.transci.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Gertz MA, Kumar SK, Lacy MQ, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2009;43(8):619–625. doi: 10.1038/bmt.2008.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dingli D, Nowakowski GS, Dispenzieri A, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6(5):384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 60.Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010;45(9):1396–1403. doi: 10.1038/bmt.2009.370. [DOI] [PubMed] [Google Scholar]
- 61.Kumar SK, Mikhael J, Laplant B, et al. Phase 2 trial of intravenously administered plerixafor for stem cell mobilization in patients with multiple myeloma following lenalidomide-based initial therapy. Bone Marrow Transplant. 2014;49(2):201–205. doi: 10.1038/bmt.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lanza F, Lemoli RM, Olivieri A, et al. Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion. 2014;54(2):331–339. doi: 10.1111/trf.12265. [DOI] [PubMed] [Google Scholar]
- 63.Costa LJ, Abbas J, Hogan KR, et al. Growth factor plus preemptive (‘just-in-time’) plerixafor successfully mobilizes hematopoietic stem cells in multiple myeloma patients despite prior lenalidomide exposure. Bone Marrow Transplant. 2012;47(11):1403–1408. doi: 10.1038/bmt.2012.60. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhary L, Awan F, Cumpston A, et al. Peripheral blood stem cell mobilization in multiple myeloma patients treat in the novel therapy-era with plerixafor and G-CSF has superior efficacy but significantly higher costs compared to mobilization with low-dose cyclophosphamide and G-CSF. J Clin Apher. 2013;28(5):359–367. doi: 10.1002/jca.21280. [DOI] [PubMed] [Google Scholar]
- 65.Pozotrigo M, Adel N, Landau H, et al. Factors impacting stem cell mobilization failure rate and efficiency in multiple myeloma in the era of novel therapies: experience at Memorial Sloan Kettering Cancer Center. Bone Marrow Transplant. 2013;48(8):1033–1039. doi: 10.1038/bmt.2012.281. [DOI] [PubMed] [Google Scholar]
- 66.Gertz MA, Ansell SM, Dingli D, et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83(10):1131–1138. doi: 10.4065/83.10.1131. [DOI] [PubMed] [Google Scholar]
- 67.Sivgin S, Karakus E, Kaynar L, et al. The comparison of Filgrastim (Neupogen®), biosimilar filgrastim (Leucostim®) and Lenograstim (Granocyte®) as a first line peripheral blood stem cell mobilization strategy in autologous hematopoieitic stem cell transplantation: a single center experience from Turkey. Transfus Apheresis Sci. 2013;48(3):315–320. doi: 10.1016/j.transci.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Hirsch BR, Lyman GH. Will biosimilars gain momentum? J Natl Compr Canc Netw. 2013;11(10):1291–1297. doi: 10.6004/jnccn.2013.0149. [DOI] [PubMed] [Google Scholar]
- 69.Crawford J, Armitage J, Balducci L, et al. National comprehensive cancer network. Myeloid growth factors. J Natl Compr Canc Netw. 2013;11(10):1266–1290. doi: 10.6004/jnccn.2013.0148. [DOI] [PubMed] [Google Scholar]
- 70.Abraham I, Tharmarajah S, MacDonald K. Clinical safety of biosimilar recombinant human granulocyte colony-stimulating factors. Expert Opin Drug Saf. 2013;12(2):235–246. doi: 10.1517/14740338.2013.770472. [DOI] [PubMed] [Google Scholar]
- 71.Herbert KE, Gambell P, Link EK, et al. Pegfilgrastim compared with filgrastim for cytokine-alone mobilization of autologous haematopoietic stem and progenitor cells. Bone Marrow Transplant. 2013;48(3):351–356. doi: 10.1038/bmt.2012.145. [DOI] [PubMed] [Google Scholar]
- 72.Attal M, Harousseau JL, Facon T, et al. InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 73.Lahuerta JJ, Grande C, Martínez-Lopez J, et al. Grupo Español de Síndromes Linfoproliferativos/Trasplante Autólogo de Médula Osea. Tandem transplants with different high-dose regimens improve the complete remission rates in multiple myeloma. Results of a Grupo Español de Síndromes Linfoproliferativos/Trasplante Autólogo de Médula Osea phase II trial. Br J Haematol. 2003;120(2):296–303. doi: 10.1046/j.1365-2141.2003.04067.x. [DOI] [PubMed] [Google Scholar]
- 74.Corso A, Mangiacavalli S, Barbarano L, et al. HOST Group. Limited feasibility of double transplant in multiple myeloma: results of a multicenter study on 153 patients aged <65 years. Cancer. 2007;109(11):2273–2278. doi: 10.1002/cncr.22660. [DOI] [PubMed] [Google Scholar]
- 75.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25(17):2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 76.Kumar A, Kharfan-Dabaja MA, Glasmacher A, Djulbegovic B. Tandem versus single autologous hematopoietic cell transplantation for the treatment of multiple myeloma: a systematic review and meta-analysis. J Natl Cancer Inst. 2009;101(2):100–106. doi: 10.1093/jnci/djn439. [DOI] [PubMed] [Google Scholar]
- 77.Sonneveld P, Scheid C, van der Holt B, et al. Bortezomib induction and maintenance treatment improves survival in patients with newly diagnosed multiple myeloma: extended follow-up of the HOVON-65/GMMG-HD4 trial [abstract]. Blood. 2013;122(21):404. [Google Scholar]
- 78.Tricot G, Spencer T, Sawyer J, et al. Predicting long-term (> or = 5 years) event-free survival in multiple myeloma patients following planned tandem autotransplants. Br J Haematol. 2002;116(1):211–217. doi: 10.1046/j.1365-2141.2002.03231.x. [DOI] [PubMed] [Google Scholar]
- 79.Hoering A, Crowley J, Shaughnessy JD, Jr, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114(7):1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010;115(21):4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Usmani SZ, Crowley J, Hoering A, et al. Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured? Leukemia. 2013;27(1):226–232. doi: 10.1038/leu.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavo M, Sonneveld P, Moreau P, et al. Impact of bortezomib incorporated into autotransplantation on outcomes of myeloma patients with high-risk cytogenetics: an integrated analysis of 1894 patients enrolled in four European phase 3 studies [abstract]. Blood. 2012;120(21):749. [Google Scholar]
- 83.Gertz MA. Stem cell transplant: an effective salvage therapy for multiple myeloma. Leuk Lymphoma. 2011;52(8):1413–1414. doi: 10.3109/10428194.2011.583702. [DOI] [PubMed] [Google Scholar]
- 84.Cook G, Liakopoulou E, Pearce R, et al. British Society of Blood & Marrow Transplantation Clinical Trials Committee. Factors influencing the outcome of a second autologous stem cell transplant (ASCT) in relapsed multiple myeloma: a study from the British Society of Blood and Marrow Transplantation Registry. Biol Blood Marrow Transplant. 2011;17(11):1638–1645. doi: 10.1016/j.bbmt.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Gonsalves WI, Gertz MA, Lacy MQ, et al. Second auto-SCT for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2013;48(4):568–573. doi: 10.1038/bmt.2012.183. [DOI] [PubMed] [Google Scholar]
- 86.Yhim HY, Kim K, Kim JS, et al. Matched-pair analysis to compare the outcomes of a second salvage auto-SCT to systemic chemotherapy alone in patients with multiple myeloma who relapsed after front-line auto-SCT. Bone Marrow Transplant. 2013;48(3):425–432. doi: 10.1038/bmt.2012.164. [DOI] [PubMed] [Google Scholar]
- 87.Atanackovic D, Schilling G. Second autologous transplant as salvage therapy in multiple myeloma. Br J Haematol. 2013;163(5):565–572. doi: 10.1111/bjh.12579. [DOI] [PubMed] [Google Scholar]
- 88.Bleeker JS, Gertz MA, Pellikka PA, et al. Evaluation of pretransplant factors predicting cardiac dysfunction following high-dose melphalan conditioning and autologous peripheral blood stem cell transplantation. Eur J Haematol. 2012;89(3):228–235. doi: 10.1111/j.1600-0609.2012.01815.x. [DOI] [PubMed] [Google Scholar]
- 89.Alayon-Laguer D, Alsina M, Ochoa-Bayona JL, Ayala E. Melphalan culprit or confounder in acute encephalopathy during autologous hematopoietic stem cell transplantation? Case Rep Transplant. 2012;2012:942795. [DOI] [PMC free article] [PubMed]
- 90.Moreau P, Facon T, Attal M, et al. Intergroupe Francophone du Myélome. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99(3):731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 91.Capria S, Petrucci MT, Pulsoni A, et al. High-dose idarubicin, busulphan and melphalan for autologous stem cell transplantation in multiple myeloma responsive to DAV chemotherapy: comparison with a historical control. Acta Haematol. 2006;115(1-2):9–14. doi: 10.1159/000089459. [DOI] [PubMed] [Google Scholar]
- 92.Abu Zaid B, Abdul-Hai A, Grotto I, et al. Autologous transplant in multiple myeloma with an augmented conditioning protocol. Leuk Lymphoma. 2013;54(11):2480–2484. doi: 10.3109/10428194.2013.782608. [DOI] [PubMed] [Google Scholar]
- 93.Reece D, Song K, LeBlanc R, et al. Efficacy and safety of busulfan-based conditioning regimens for multiple myeloma. Oncologist. 2013;18(5):611–618. doi: 10.1634/theoncologist.2012-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen AI, Negrin RS, McMillan A, et al. Tandem chemo-mobilization followed by high-dose melphalan and carmustine with single autologous hematopoietic cell transplantation for multiple myeloma. Bone Marrow Transplant. 2012;47(4):516–521. doi: 10.1038/bmt.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lonial S, Kaufman J, Tighiouart M, et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res. 2010;16(20):5079–5086. doi: 10.1158/1078-0432.CCR-10-1662. [DOI] [PubMed] [Google Scholar]
- 96.Blanes M, Lahuerta JJ, González JD, et al. Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant. 2013;19(1):69–74. doi: 10.1016/j.bbmt.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Rasche L, Strifler S, Duell J, et al. The lymphoma-like polychemotherapy regimen “Dexa-BEAM” in advanced and extramedullary multiple myeloma. Ann Hematol. 2014;93(7):1207–1214. doi: 10.1007/s00277-014-2023-2. [DOI] [PubMed] [Google Scholar]
- 98.Wang T-F, Fiala MA, Wu N, et al. A phase II study of V-BEAM (bortezomib, carmustine, etoposide, cytarabine, and melphalan) as conditioning regimen prior to second autologous stem cell transplantation for multiple myeloma [abstract]. Blood. 2013;122(21):5492. [Google Scholar]
- 99.Christoforidou AV, Saliba RM, Williams P, et al. Results of a retrospective single institution analysis of targeted skeletal radiotherapy with (166)Holmium-DOTMP as conditioning regimen for autologous stem cell transplant for patients with multiple myeloma. Impact on transplant outcomes. Biol Blood Marrow Transplant. 2007;13(5):543–549. doi: 10.1016/j.bbmt.2006.12.448. [DOI] [PubMed] [Google Scholar]
- 100.Dispenzieri A, Wiseman GA, Lacy MQ, et al. A Phase II study of (153)Sm-EDTMP and high-dose melphalan as a peripheral blood stem cell conditioning regimen in patients with multiple myeloma. Am J Hematol. 2010;85(6):409–413. doi: 10.1002/ajh.21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Green DJ, Jones JC, Hylarides MD, et al. Anti-CD38 pretargeted radioimmunotherapy eradicates multiple myeloma xenografts in a murine model. Abstract presented at 55th American Society of Hematology (ASH) Annual Meeting and Exposition. December 7-10, 2013. New Orleans, LA. [Google Scholar]