Abstract
Cognitive impairment represents an important and often defining component of the clinical syndromes of Lewy body disorders: Parkinson’s disease and dementia with Lewy bodies. The spectrum of cognitive deficits in these Lewy body diseases encompasses a broad range of clinical features, severity of impairment, and timing of presentation. Cognitive dysfunction is now recognized to occur not only in more advanced Parkinson’s disease, but also in early, untreated patients, and even in those patients with pre-motor syndromes such as REM behavior disorder and hyposmia. In recent years, the concept of “mild cognitive impairment” as a transitional or pre-dementia state in Parkinson’s disease has emerged. While this has led to much research regarding the diagnosis, prognosis, and underlying neurobiology of mild cognitive impairment in Parkinson’s disease, it has also raised questions regarding the usefulness of this concept and its application in clinical and research settings. In addition, the conundrum of whether Parkinson’s disease dementia and dementia with Lewy bodies represent the same or different entities remains unresolved. While these disorders overlap in many aspects of their presentations and pathophysiology, they differ in other aspects such as timing of cognitive, behavioral, and motor symptoms, medication responses, and neuropathological contributions. This article examines the spectrum and evolution of cognitive impairment in Lewy body disorders and debates these controversial issues in the field using point-counterpoint approaches.
Keywords: Cognition, Dementia, Executive function, Mild cognitive impairment, Parkinson’s disease
Introduction
Cognitive impairment represents an important component of the clinical syndromes of Lewy body disorders: Parkinson’s disease (PD) and dementia with Lewy bodies (DLB). The spectrum of impairment ranges broadly in phenotype as well as timing in the disease course. Cognitive deficits can occur in one or more domains, vary in severity, and present differently at various stages of the disease. Studies of incident PD cohorts indicate that cognitive impairment is no longer just a late-stage problem.1–3 Dementia, however, remains a troubling complication for a majority of advanced PD patients4, 5 and affects quality of life,6 caregiving,7 and socioeconomics.8 Longitudinal studies reveal patient differences in the progression of cognitive deficits and in risk factors for developing PD dementia (PDD).9–11 As treatments to prevent dementia or arrest cognitive decline represent critical unmet needs in PD, recent research has focused on the state of mild cognitive impairment (MCI), which has been considered a transitional stage between normal cognition and dementia and one in which cognitive deficits have little to no impact on functional abilities. While much preceding work has been done on MCI as related to Alzheimer’s disease (AD),12, 13 the MCI construct as specifically designated in PD has only recently emerged, with diagnostic criteria recently proposed.14 At present, there are many unanswered questions regarding PD-MCI and what PD-MCI represents and whether it is a useful construct for the field will be debated in this article. In addition, this article will examine the PDD and DLB controversy, debating their similarities and differences and boundaries of motor and cognitive dysfunction in these Lewy body disorders. A better understanding of the clinical characterization, neurobiological basis, and progression of cognitive deficits in Lewy body disorders is essential for the development of therapeutic strategies, whether they be geared towards early, mild cognitive deficits or at the dementia stage.
The evolution of cognitive impairment in PD: from pre-motor to late stage
Pre-motor PD
Recent studies characterising prodromal PD have implicated cognitive changes as part of the pre-motor syndrome (summarized in Table 1). Although the studies differ regarding the nature of the cohorts examined (e.g., G2019S LRRK2 mutation carriers, hyposmic individuals, first degree relatives of PD patients, healthy individuals) and methodological design (e.g., cross-sectional vs. longitudinal, different testing paradigms and analyses), they are similar in their findings which suggest that the principal domain affected in the early stages of nigrostriatal dopamine depletion is executive function, with deficits in working memory, attentional and verbal fluency tasks reported before motor features of PD become apparent.15,16,17
Table 1.
Cognitive function in pre-motor PD
| Study | Type | Cohort | Methodology | N | Age | Outcome |
|---|---|---|---|---|---|---|
| Thaler et al, 201215 | Cross sectional | Ashkenazi healthy 1st degree relatives of PD patients | ‘Mindstreams’ computerized cognitive test battery and Stroop test performance compared across groups stratified according to LRRK2 G2019S mutation carrier status | 60 (30 carriers versus 30 non carriers) | Mean 50.9 ± 6.2 | Lower scores in G2019S carriers on executive index of computerized battery, and on Stroop interference task |
| Ross et al, 2012 (Honolulu-Asia Aging Study)16 | Prospective longitudinal | Japanese - American men born 1900–1919 | Cognitive abilities screening instrument (CASI) performed in subjects free of PD and dementia; PD incidence determined prospectively over 8 year follow-up period | 3456 | Range 71–93 | Age- and education-adjusted PD incidence of PD decreased from lowest to highest quartiles of executive function subscale (26.1, 27.0, 15, and 10.9 per 10,000 person years respectively) |
| Hawkins et al 2010 (PARS study)17 | Cross sectional | Healthy relatives of PD patients and general population | Olfactory testing (UPSIT), DAT scanning (beta-CIT SPECT) and neuropsychological test battery performed | 148 (98 hyposmic, 50 normosmic) | N/A | Correlation between global neuropsychological score and DAT uptake values; among hyposmics, those with DAT deficiency (<66% expected) had lower phonemic and semantic fluency, Trail Making Test scores and WAIS-III processing speed |
Abbreviations:
beta-CIT-SPECT = 2β-carboxymethoxy-3β(4-iodophenyl tropane) single-photon emission computed tomography, DAT = dopamine transporter, PARS = Parkinson Associated Risk Study, PD = Parkinson’s disease, UPSIT = University of Pennsylvania Smell Identification Test
Further insight into cognitive deficits in the prodromal phase of Lewy body disorders comes from rapid eye movement sleep behavior disorder (RBD) studies. RBD frequently occurs in association with alpha-synucleinopathies including PD and DLB and can predate these disorders by 5 or more years.18 About half of “idiopathic” RBD patients will develop an alpha-synucleinopathy after 12 years.19 Cognitive deficits have been documented in RBD; however, in contrast to other pre-motor PD reports, they are not restricted to executive function, but also affect memory and visuospatial abilities (reviewed in20). This may reflect the fact that RBD is a harbinger of cortical Lewy Body pathology rather than PD per se. Furthermore, it highlights the fact that cognitive dysfunction is heterogeneous even at the earliest pathological stages of Lewy body disease.
MCI prevalence in established PD
Several studies of incident PD cohorts (n=88–196) estimate the prevalence of cognitive deficits or MCI at the time of PD diagnosis to be 19–36%.1, 3, 21, 22 PD-MCI frequencies in prevalent cohorts overlap with these estimates, but due to longer disease durations and methodological differences, may be ~50% in some clinic-based cohorts.23 A multi-center analysis (n=1341, mean disease duration 6.1 years), which defined MCI on the basis of performance 1.5 standard deviations (SD) below normative means in at least 1 of 3 domains (attention/executive function, memory, and visuospatial), reported a frequency of 25.8% (95% CI 23.5–28.2).24 Similarly, a recent systematic literature review by a MDS Task Force on PD-MCI reported a prevalence of 19–38% (mean 27%).23 Longitudinal studies suggest that although cognitive function declines over time,25 MCI prevalence may remain relatively stable at least in the early years of PD. While new MCI cases emerge, a proportion of existing ones convert to PDD, and a proportion also revert to normal cognition.10, 9 Variation of PD-MCI prevalence estimates across studies reflects, at least in part, differing definitions of impairment. In a study of 119 PD patients, the prevalence of MCI varied from 14% when 2 neuropsychological tests were impaired in 1 domain at 2 SD below normative means, to 89% of patients when 1 abnormal test was required in 1 domain at 1 SD below normal.26 In another study (n=76) using impairment on at least 2 tests from a comprehensive battery to define PD-MCI, frequency varied from 38% using 2.5 SD cut-offs to 91% using 1 SD cut-offs.27
In an attempt to standardize definitions of MCI in PD, a MDS task force proposed diagnostic criteria,14 thereby defining a clinical syndrome analogous to MCI in the AD field. The criteria are closely based on the MCI criteria proposed by Petersen29 but encompass aspects specific to PD and provide additional recommendations in terms of neuropsychological testing and subtyping (Table 2). Debates regarding the merits of a distinct PD-MCI syndrome are discussed below. The PD-MCI criteria still allow for considerable diagnostic variation, depending on use of abbreviated (Level I) or comprehensive (Level II) neuropsychological assessments and setting of cut-offs for impairment between 1–2 SD below normative means. Studies to date using the MDS PD-MCI criteria illustrate that prevalence estimates remain highly variable (20–62%) (Table 3); despite adoption of these standardized criteria, differences in PD cohorts and methodologies likely contribute. The criteria require validation, but one potential difficulty is the lack of a “gold standard” given that PD-MCI is a new construct. The first study to attempt this compared MDS PD-MCI Level II criteria to a “consensus diagnosis” of PD-MCI made by 3 experts. In their clinic-based cohort (n=76), they reported an optimal combination of sensitivity (85.4%) and specificity (78.6%) for a 2 SD cut-off, compared to cut-offs ranging from 1–2.5 SD.27
Table 2.
MDS PD-MCI criteria
| Inclusion Criteria | Exclusion criteria |
|---|---|
| PD diagnosis based on UKPDS Brain Bank criteria | Diagnosis of PD dementia based on MDS dementia criteria |
| Gradual decline in cognitive ability reported by patient or informant, or observed by clinician | Another explanation for cognitive impairment (e.g. delirium, depression, medication side effects) |
| Cognitive deficits demonstrable on neuropsychological testing or a global cognitive scale | Other PD-related factors that significantly impact on cognitive testing (motor impairment, anxiety, sleepiness, psychosis) |
| Cognitive impairment does not interfere significantly with functional ability | |
| Level I criteria (abbreviated assessment) | |
| Impairment on global cognitive scale validated in PD | |
| OR | |
| Impairment on at least 2 tests from a limited neuropsychological battery (<2 tests per domain, or < 5 domains tested) | |
| Level 2 criteria (comprehensive assessment) | |
| Neuropsychological testing includes 2 tests within each of 5 cognitive domains (attention and working memory, executive functions, language, memory, visuospatial skills) | |
| Impairment on at least 2 tests: either 2 impaired tests within 1 domain, or 1 test in 2 different domains | |
| Impairment demonstrated by: score 1-2SD below appropriate norms, or significant decline on serial cognitive testing, or significant decline from estimated pre-morbid levels | |
| Subtype classification for PD-MCI (comprehensive assessment required) | |
| Single domain: abnormalities on two tests within a single cognitive domain | |
| Multiple domain: abnormalities on at least 1 test in 2 or more domains | |
Adapted from Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27(3):349–356.
Table 3.
Estimated prevalence of mild cognitive impairment in PD in studies adopting the MDS PD-MCI criteria.
| Study | Cohort type |
Criteria | SD cut- off |
N (non-demented) |
Disease duration |
% PD-MCI |
|---|---|---|---|---|---|---|
| Biundo et al, 2013129 | Prevalent, clinic-based | Level II | 1.5 | 89* | Mean (SD): 7.8 (4.4) | 38 |
| Broeders et al, 201310 | Incident, clinic-based | Level II | 1.5 | 123 88 56 |
Baseline Year 3 Year 5 |
35 53 50 |
| Goldman et al, 201327 | Prevalent, clinic-based | Level II | 2 | 76 | Mean (SD): 8.7 (2.9) - non MCI group; 9.7 (4.4) - MCI group | 62 |
| Marras et al, 201328 | Prevalent, multi-center clinic-based cohort | Level II | 1.5 | 139 | Mean (SD): 5.2 (4.6) | 33 |
| Pedersen et al, 20139 | Incident, Population-based | Level I | 1.5 | 182 178 |
Baseline Year 1 |
20 20 |
cohort size of 104 of whom 15 met criteria for PDD
Estimates of dementia frequency in PD are similarly subject to considerable variation due to methodological differences among studies and a transition over the years from Diagnostic and Statistical Manual (DSM) criteria and/or Mini-mental State Examination (MMSE) score cut-offs to the MDS proposed PDD criteria.30–33 A large systematic review reported a point prevalence for PDD of 24–31%.34 As dementia is more prevalent in later stages of PD, a more relevant question may be what proportion of PD patients followed prospectively will ultimately develop dementia. The cumulative incidence of PDD from longitudinal studies of incident PD cohorts is remarkably consistent, suggesting that around half will develop dementia within 10 years from diagnosis (Table 4).35–37
Table 4.
Risk of dementia in incident PD cohorts
| Study | Cohort type | Criteria for dementia |
N | Follow-up duration (years) |
% dementia |
|---|---|---|---|---|---|
| Williams-Gray et al, 2013 (CamPaIGN)35 | Incident, population-based | DSM-IV and MMSE≤24 | 142 | 10 | 46a |
| Auyeung et al, 201236 | Incident, clinic-based | DSM-IV | 171 | 10 | 49a |
| Perez et al, 201237 | Incident, population-based, >65yrs | DSM-IIIR and MDS criteria | 44 | 10 | 50a |
| Hely et al, 2008 (Sydney study)4 | Incident, clinical trial cohort | Impairment in memory + 2 other domains, or CDR ≥ 1 | 136 | 15 20 |
48b 83b |
Abbreviations: CDR = Clinical Dementia Rating, DSM = Diagnostic and Statistical Manual, MDS = Movement Disorder Society, MMSE = Mini-Mental State Examination
cumulative proportion
% of survivors
Neuropsychological features of PD cognitive dysfunction
Executive function represents the most common cognitive domain affected in PD, early on as well as later in disease.3, 21, 27 Deficits can be detected on tests sensitive to frontal dysfunction (e.g., tests of planning, spatial working memory and attentional set shifting)38, 39 and reflect dopaminergic dysfunction in frontostriatal networks.40 However, impairments in attention, explicit memory and visuospatial function are also demonstrable in early PD.3, 21, 41 Some of these cognitive deficits may be, at least in part, secondary to the dysexecutive syndrome (e.g., observed improvement in recall with cueing in PD suggests that memory impairment relates to faulty retrieval rather than information storage;.42, 43 impaired visuospatial performance may relate in part to problems with sequential organization).44 Executive dysfunction may occur individually, as single domain impairment, or in combination with other cognitive deficits, as multiple-domain impairments. Some, but not all, studies investigating clinical features and frequencies of cognitive impairment in PD patients without dementia reveal that non-memory (nonamnestic) single domain deficits are the most frequent cognitive subtype. In some of these studies, these non-demented but cognitively impaired PD patients were categorized as “MCI;” however, it should be noted that several studies were conducted prior to the introduction of the “MCI” term and many different neuropsychological tests and definitions for “MCI” in PD have been used.1, 3, 21, 23, 45, 46 As the field evolved, subsequent studies have further characterized these non-demented but cognitively impaired PD patients as “cognitively normal” or “MCI.”
Several studies demonstrate that cognitive deficits in PD are heterogeneous and that cohorts can be stratified by cognitive profile.1, 21, 46–48 Deficits in certain cognitive domains (e.g., attention/working memory, memory, language, or visuospatial function) may have alternate, non-dopaminergic etiologies (e.g., cholinergic dysfunction, cortical Lewy body or AD pathology). The MDS PD-MCI criteria provide a framework for domain-specific subtyping, by number (single vs. multiple) and by specific cognitive domain affected, rather than as “non-memory (nonamnestic) vs. memory (amnestic) MCI subtypes;” this is in contrast to prior MCI criteria (e.g., Petersen) and MCI/AD studies that focused on nonamnestic vs. amnestic subtypes. Subtyping of PD-MCI by MDS criteria requires a comprehensive neuropsychological assessment with at least 2 neuropsychological tests in each of 5 cognitive domains.14 Studies employing the MDS PD-MCI criteria to date demonstrate multiple-domain impairment in the majority (>90% in prevalent cohorts,27, 30 65% in an incident cohort at baseline10). This finding raises questions about whether the current criteria will enable investigations of the progression of individual cognitive domain PD-MCI subtypes to dementia.
The cognitive profile of PDD remains variable, though often affects similar cognitive domains as in PD-MCI but with more severe deficits and disrupting multiple areas. By definition, PDD includes impairment in at least 2 cognitive domains, but as per MDS criteria, does not require memory deficits.32 The predominant cognitive deficits in late PDD are similar to those in DLB, with marked visuospatial dysfunction and fluctuating attention. Impairments in executive function, working memory, and episodic memory are also common in PDD, though language, particularly as measured by object naming, tends to be relatively preserved.32
Longitudinal relationship between early cognitive impairment and dementia
Longitudinal studies provide key information on rates of cognitive decline in PD and associated risk factors. Unanswered questions include whether PD-MCI inevitably deteriorates to a state of dementia and whether individual cognitive subtypes have differing prognoses. Historically, many different neuropsychological deficits have been reported as predictors of PDD, including executive function deficits,49–51 impaired verbal fluency,51, 52 visuospatial deficits,51 memory, and language dysfunction.50, 53 However, these studies differ in their inclusion of patients at varying disease stages, use of hospital-based cohorts, or non-uniform approaches to neuropsychological testing, and thereby pose challenges for comparing studies and generalizing to broader PD populations.
Several longitudinal studies in population-based incident PD cohorts with detailed neuropsychological evaluation have been established and will facilitate better descriptions of the pattern and temporal evolution of cognitive dysfunction in PD. Most are still in their early stages,9, 54–56 but the first of these, the CamPaIGN study21 recently reported 10 year follow-up data.57 Analysis at multiple time-points in this cohort (n=142) indicated that, aside from age, the most significant baseline predictors of later dementia were impaired semantic fluency and pentagon copying (hazard ratios of 3.1 and 2.6 respectively for dementia at 10 years from diagnosis).2, 11, 57 There was no association between “frontostriatal-based” executive dysfunction and later dementia, and in fact, no decline in executive function performance over this time. Cognitive functions and certain candidate genes also dissociated in this cohort. A common variant in the MAPT (tau) region was strongly associated with earlier dementia, whereas a functional polymorphism in the dopamine-regulating enzyme COMT was associated with executive dysfunction but not dementia. Thus, cognitive impairment in early PD may be segregated into 2 types: a) executive deficits, which are primarily due to dysfunction in dopaminergic frontostriatal networks, likely to fluctuate with disease course and medications, but not clearly associated with dementia, and b) posterior cortically-based deficits, as measured by tests of language (semantic fluency) and visuospatial orientation (pentagon copying), which are due to dysfunction in non-dopaminergic systems, cortical Lewy body deposition, or AD-type pathology, and represent the early stages of a dementing process.57 Other studies support this dissociation. In a study of the PD-Cognitive Rating Scale, the transition from MCI to dementia was marked by the addition of cortically-based deficits to an underlying frontal-subcortically-based syndrome.58 A large meta-analysis of 901 non-demented PD patients followed for a mean of 29 months reported significant decline in global cognitive ability, visuospatial function and memory, but not executive function.59 MRI studies also suggest that hippocampal and temporoparietal atrophy in early PD predict global cognitive decline,60 and hypometabolism in posterior cortical regions on positron emission tomography (PET) imaging distinguishes PDD from PD-MCI.61 While these regions may also be implicated in AD, the profile of amyloid binding as measured by Pittsburgh compound B PET imaging differed in PD patients with cognitive impairment compared to AD patients.62 These studies also introduce the descriptive terminology of “executive dysfunction” and “posterior-cortical dysfunction” for specific cognitive syndromes in PD. In this article, these terms will be primarily used in the context of the United Kingdom and Spanish PD cohorts described above; however, at present, other PD studies may describe similar cognitive deficits by using the terminology “nonamnestic” or “nonamnestic/amnestic” or specific individual type of deficit such as in “executive function, language, or visuospatial” domains.
The relationship between early cognitive deficits and PDD awaits confirmation in studies adopting more standardized definitions of cognitive impairment.14 Although 2 longitudinal studies have been published to date using MDS PD-MCI criteria in incident cohorts, neither have explored associations between particular PD-MCI subtypes and dementia, due either to insufficient neuropsychological tests for Level II criteria9 or small subgroup sizes.10 Both studies demonstrated that conversion to dementia was more common among PD-MCI than non-MCI PD patients over 39 and 5 years10 from diagnosis. However, 22% reverted from PD-MCI to normal cognition over 3 years,9 and in the other study, 33% of those developing dementia at 3 years were cognitively normal at baseline.10 Future studies with larger samples of PD-MCI subjects and subtype representation will be needed to dissect out the diagnostic and prognostic values of different types of early cognitive impairment in PD.
Is MCI in PD a useful concept?
The concept of MCI as a clinical syndrome in PD has been increasingly recognized over recent years, drawing on the background and lessons of the AD/MCI field. The term MCI in general was first introduced as a stage in a global cognitive measure, but has become synonymous with a cognitive syndrome denoting a state of impaired cognitive function not normal for age and suggesting a continuum from normal cognition to dementia, with MCI representing a transitional state.13, 29, 63, 64 It is recognized, however, that not all MCI patients in general progress to a dementia (i.e., some remain stable or revert to normal)65, 66, and that not all MCI patients progress to AD (i.e., nonamnestic subtypes more frequently develop non-AD dementias).13 In addition, the cognitive deficits in MCI, in contrast to dementia syndromes, do not significantly affect a patient’s functional abilities or instrumental activities of daily living. It is also recognized, however, that MCI patients may indeed have some degree of functional impairment.67, 68 Historically, criteria for MCI developed from longitudinal, epidemiological studies of aging demonstrating cognitive decline or conversion to dementia in subsets of elderly participants.13 In recent years, application MCI criteria, or variations therein, emerged in PD populations and thereby, generated a more formalized concept of MCI in PD with proposed diagnostic criteria for PD-MCI. The concept of MCI, however, is not without debate or controversy in the AD/MCI field as well as in the PD arena. The following section examines whether or not PD-MCI is a useful concept using a point-counterpoint approach.
Point
The introduction of MCI as a concept in PD has led to a beneficial increase in awareness of the cognitive deficits that accompany PD in both the scientific and lay community. While the presence of cognitive deficits in non-demented PD had been recognized for many years, the focus of much PD research and therapeutics over the years has been on motor features. PD has now been dubbed the “quintessential neuropsychiatric disease.”69 This represents a long evolution from James Parkinson’s observation that the “senses and intellect were uninjured.” The concept of PD-MCI has advanced research in the field, with recent years witnessing an explosion of publications on “cognitive impairment in PD” and “MCI in PD” (959 and 341 articles cited in PubMed over the past 5 years, respectively); increased research programs for PD cognition; and a number of clinical trials of symptomatic therapies for PD-MCI. The emergence of PD-MCI has provided an opportunity to develop a framework for understanding the frequency and characteristics of cognitive deficits specifically within the clinical diagnosis of PD, and separate from AD, as designated “PD-MCI.”14 The increased recognition of PD-MCI may have positive implications for patients, caregivers, and physicians, such as validating previously observed cognitive changes, even early in the disease, as part of PD. This also may lead to appropriate counselling of patients and caregivers, earlier use of compensatory or cognitive strategies, discussions regarding safety and driving, and avoidance of medications with adverse central nervous system effects. Increased awareness of PD-MCI provides an opportunity for more frequent and formalized evaluation of cognition in PD patients, similar to motor assessments that are regularly tracked by clinicians. Cognitive assessments ranging from “bedside” tests to comprehensive neuropsychological evaluations can provide an objective measure of cognitive function at baseline and in follow-up.
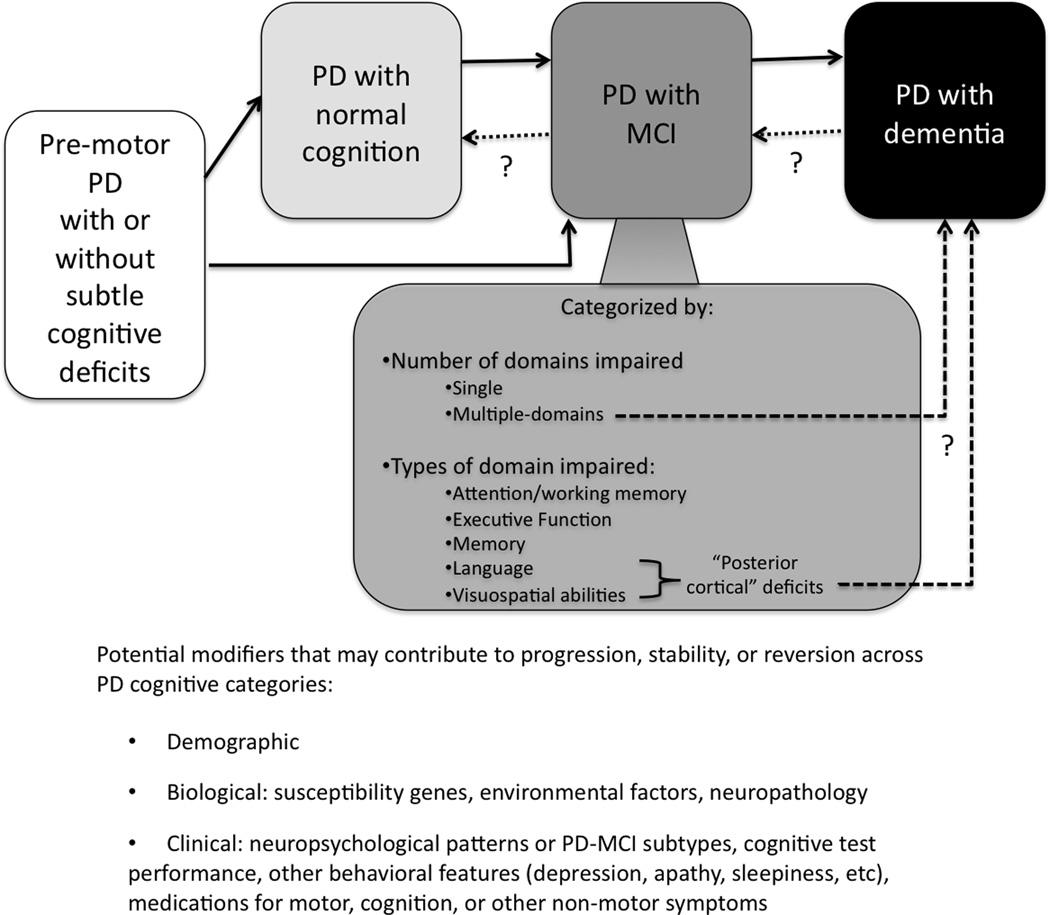
One of the main reasons for the development of PD-MCI as a concept and for standardized diagnostic criteria is the early identification of patients who are at risk of converting to dementia (Figure 1). These patients may be best suited for interventions that halt or slow down cognitive decline. An initial step of this identification process involves improving the general characterization of this patient group. Application of the PD-MCI construct provided initial reports of frequencies, clinical phenotypes, and associated biomarkers, first in cross-sectional studies and now with emerging longitudinal follow-up. The use of MCI criteria in PD, whether as modified Petersen’s criteria, MDS PD-MCI Task Force criteria, or other definitions, has enabled these patients to be characterized to a degree not previously captured. Studies of incident PD cohorts highlight that PD-MCI patients can be identified early in the course of their cognitive deficits,1, 3, 9, 21 and thus, future trials may focus on disease-modifying interventions at earlier stages. PD-MCI frequency estimates from incident and prevalent cohorts, along with information from longitudinal studies regarding change in neuropsychological test scores, clinical status, and other variables, will allow researchers to plan for clinical trials and identify the best predictors for conversion from PD-MCI to PDD. Furthermore, detailed characterization of PD-MCI patients over recent years has led to the recognition that PD-MCI is more heterogeneous than previously anticipated, with differences in cognitive profiles, underlying neurobiological mechanisms, and rates of progression.2, 23, 24, 45–47, 59 While the MDS PD-MCI criteria do not include biomarkers in the definition of MCI, there is great interest in the identification of biomarkers for PD-MCI and as predictors of conversion to dementia, whether they be CSF, imaging, genetics, or other. Future studies are needed to establish these biomarkers and whether they will be ultimately incorporated into PD-MCI criteria, as recently done in revised MCI/AD criteria.64 While these factors may make the diagnosis of PD-MCI more complex, they also provide new, important insights into PD-MCI and emphasize that it represents a clinical syndrome requiring further research.
FIG 1.
The spectrum of Parkinson’s disease cognitive impairment. MCI indicates mild cognitive impairment. Abbreviations: MCI, mild cognitive impairment; PD, Parkinson’s disease.
From these PD-MCI studies, the need for a uniform definition of PD-MCI for clinical and research purposes became apparent. While these studies added much value in descriptions of PD-MCI, interpretation was complicated by differences in the definitions and tests used, populations studied, and others.14, 23, 70 The heterogeneity of PD-MCI across different cohorts could be attributed at least partly to definitions used. Thus, the MDS Task Force PD-MCI criteria were developed with the goals of providing a standardized definition to be used in identifying (1) the earliest stage of PD cognitive impairment, (2), best predictors of conversion from PD-MCI to PDD, (3) effects of PD-MCI on quality of life and daily functioning, (4) patient populations and potential outcome measures for clinical trials, and (5) ways to improve communication among clinicians, patients, caregivers, and researchers.14 Although validation efforts are underway and several unanswered questions remain, the PD-MCI criteria provide a first step towards a uniform definition that can be used across multiple centers and in clinical research trials.
Counterpoint
In the world of AD, the concept of MCI has caused much debate and confusion. Initially MCI was seen as a useful way to define prodromal AD and thus facilitate studying patients at the earliest stage of disease when rescue of neuronal networks might be possible and could make a real difference.71 With the birth of this concept, a number of papers covering the definition, epidemiology and treatment of MCI were published. With time, however, many have started to question the concept as some MCI patients failed to progress to AD. As such, the original concept of clinically-defined MCI has evolved with greater emphasis on early diagnosis using more objective neuropsychological assessments, neuroimaging and other biomarkers.72, 73
While many see the MDS PD-MCI diagnostic criteria as a useful step forward in defining more rigorously exactly what MCI means in the context of PD, others see it as creating confusion where there was once clarity.74 For many, the utility of the term PD-MCI is in defining patients who have the earliest cognitive deficits predictive of a dementia rather than displaying cognitive deficits per se. This creates a conundrum to be debated within the field of whether PD-MCI is a static entity or a transitional period.
The heterogeneous nature of cognitive deficits within defined PD-MCI provides another point of confusion. This clinical heterogeneity may reflect a range of different pathologies, and thus lumping all PD-MCI together into a single entity creates confusion, both clinically and pathophysiologically, and in the future, likely therapeutically. Some studies demonstrate that there are two distinct types of MCI in PD, which have divergent etiologies and prognostic implications.11, 35, 75 As discussed in the previous section, studies from the CamPaIGN cohort demonstrate two phenotypes of PD-MCI, a executive dysfunction/frontostriatal type, associated with COMT polymorphisms but not dementia and a posterior-cortical type with visuospatial and semantic naming deficits, associated with MAPT tau haplotypes and heterozygous GBA mutations, and development of early PDD. Lumping these distinct forms of MCI together could interfere with disease-modifying therapy trials for PD-MCI, as not all patients will evolve into a demented state. PD-MCI is not amenable to a reductionist mechanistic approach given that these two forms of PD-MCI have different pathophysiologies. Moreover, it does not help the clinician advising the patient and caregiver as to the prognostic implications of MCI. In addition, while the COMT polymorphism and executive dysfunction/frontostriatal type suggest that there are genetic and clinical reasons why some PD-MCI do not progress to dementia, it remains to be seen what factors influence those PD-MCI who revert to “normal” on subsequent testing. Many demographic, biological or clinical variables could be hypothesized to play a role in this.
Not only are there different types of PD-MCI in well-studied cohorts of patients, but there are also other reasons why patients may have PD-MCI independent of these two disease processes already discussed. They may be impaired due to a degree of depression or apathy, use of tests that have not been fully validated in the PD population, medications taken, presence of another disease pathology, or simply being the way they are - as is seen in some case series of MCI in the elderly.76, 77
With these caveats, if the PD-MCI criteria, with their ability to classify subtypes similar to the Petersen criteria (i.e., single or multiple-domain impairment, but with specification of the affected domain[s],) can identify a group of high-risk individuals likely to develop a dementia, then they would be useful, particularly as and when disease-modifying therapies become available. Furthermore, such a position would also facilitate more detailed evaluation of PD-MCI’s underlying pathophysiologies, and in particular, what drives the early dementia of PD. If such a position is not adopted, there is concern that patients will be sent off to have extensive neuropsychological testing and many will return with the diagnosis of PD-MCI. The clinician may now feel they are now looking at a different type of PD patient compared to the cognitively normal PD patient, and the patient may feel now destined to dement. The experimental neurobiologist will want to target disease-modifying therapy to such a cohort and with time, we may end up in the confused world that has haunted the field of AD for decades.
Are PDD and DLB the same entity?
DLB is the second most common form of degenerative dementia after AD, with prevalence rates of up to 5% in the elderly and up to 30% of all dementia cases.78, 79 A related Lewy body disorder is PD, in which dementia may ensue and thereby, shares many clinical and cognitive features with DLB.32,80, 81 At early stages, DLB and PDD are easy to differentiate by the predominance of dementia in DLB and of parkinsonian motor features in PD.82 Nevertheless, in some patients, dementia and motor signs occur in close succession, provoking debate about their nosology. For research purposes, the “one-year” rule regarding timing of dementia and parkinsonian features is used.32, 79 In clinical practice, however, a diagnosis is made based on the relative prominence of the clinical features. The separation between DLB and PDD is considered by some to be artificial, since it implies that the two clinical syndromes have different pathophysiologies.83 The following point-counterpoint approach explores the conundrum of whether they are the same or different diseases looking at both similarities vs. differences and agreement vs. controversies yet to be decided.
Clinical Characteristics
Point
There is no single sign or symptom that definitively distinguishes PDD from DLB. In a longitudinal memory/aging study of 100 participants (10 non-demented controls, 40 PD patients, 15 with DLB, and 35 with AD), those DLB and PDD who came to autopsy were nearly identical in all clinical features including male sex, parkinsonian features, visual hallucinations, sleep disturbances, and neuroleptic sensitivity.81 One of the unique features of both PDD and DLB, but not AD, is cognitive fluctuations, with episodes of confusion, hypersomnolence, incoherent speech, and staring spells. These are seen in 15–80% of DLB cases84 and are also as common in PDD.85
Counterpoint
While there are similarities in the core features of PDD and DLB, the extent of clinical symptoms differs. Resting tremor is not as common in DLB.78 Parkinsonism usually presents bilaterally in DLB, with possibly more axial rigidity, whereas in PD, it more typically presents unilaterally and asymmetrically.78 Sexual disinhibition, alexia, and anomia were more common in DLB than PDD.80, 81 In contrast to DLB, motor fluctuations, particularly in later PD stages when dementia also occurs, add to the substantial burden of advanced PD.
Neuropsychiatric Features
Point
Neuropsychiatric features are hallmarks in both PDD and DLB. Depression occurs in 20–70% of PD patients,86 with risk factors including early onset of PD, hallucinations or delusions, and akinetic-rigid presentations.87, 88 Anxiety co-occurs with depression in up to 40% of PD patients.89 A history of depression has been reported in 58% of persons with PDD, 50% of patients with DLB, compared to 14% of AD cases coming to autopsy.90 Apathy is also common with a 15% frequency in community samples of PD. Similar rates of depression, anxiety, and apathy and phenomenology of mood disorders have been reported in DLB.91 Visual hallucinations are phenomenologically similar in PDD and DLB. For both conditions, they tend to be formed, detailed, and involving anonymous people, although they may also involve family members, animals, body parts, and machines.32, 79
Counterpoint
Although hallucinations may accompany both PDD and DLB, their timing may differ. Visual hallucinations in PD typically occur after chronic dopaminergic therapy, even if their pathogenesis is only partly related to dopaminergic receptor stimulation. In DLB, however, hallucinations may occur spontaneously and earlier, even prior to dopaminergic treatment.79, 92 Delusions, particularly paranoid or spousal infidelity, are less common than hallucinations in PDD, occurring in 29% of patients in one study, though more frequent in demented than non-demented PD patients.93 Delusions, however, occur in over 50% of DLB patients at first presentation and about two-thirds at some point in their illness. Delusions tend to be more common in DLB than in PDD or AD. Paranoid-type, Capgras syndrome (i.e., a delusional misidentification syndrome in which the patient thinks a close family member or friend has been replaced by an identical-looking imposter), and “phantom boarder” delusions are among the most common types in DLB.94 These may be less common in PDD, although there are cases or small series reported.95, 96 Differences in psychosis in PDD and DLB may be more quantitative rather than qualitative.
Cognitive Features
Point
Both PDD and DLB have prominent executive and visuospatial dysfunction, in contrast to AD. Compared to AD patients, DLB patients generally show milder deficits on measures of confrontation naming.97 DLB patients are equally impaired in semantic (category) and phonemic (letter) fluency tests whereas AD patients perform significantly better on the latter.98 Language impairments in PDD also tend to be mild, with rare aphasia. Verbal fluency impairments are reliably observed in PDD and occur to a greater degree in PDD than in AD.99 Semantic fluency deficits in PD, which suggest posterior cortical dysfunction, may be a risk factor for dementia.2, 100
Counterpoint
The frequency of memory deficits, however, may differ between DLB and PDD patients. Memory deficits are the presenting problem in 67% of PDD patients, compared to 94% of DLB and 100% of AD patients.101 Better recall on verbal memory tests occurs in DLB than in AD; however, this has not been consistently reported, possibly because of the difficulty in isolating pure forms of DLB at autopsy from those cases with concurrent AD pathology.102 Remote memory may also be affected by PDD.103, 104 Relative contributions of Lewy body, AD, or other pathologies may account for some of these differences in cognitive deficits between DLB and PDD.
Other non-motor features - autonomic and sleep disturbances
Point
Autonomic features such as constipation, bladder dysfunction, and orthostatic hypotension, are prominent in many cases of DLB and PDD. Orthostatic hypotension has been observed in many DLB patients and almost 50% of PDD patients with longstanding disease. However, true estimates of prevalence and severity are compounded, particularly in PDD, by other factors such as anti-parkinsonian medications that can exacerbate orthostasis, and in DLB, by limited studies.4 In both DLB and PDD, cardiac scintigraphy with [I–123] metaiodobenzyl guanidine (MIBG) is reduced; while it does not differentiate between alpha-synucleinopathies, MIBG can differentiate Lewy-body related dementias from AD with high sensitivity and specificity.105 The sleep disturbance, RBD frequently occurs in both DLB and PDD, and in both, can precede the onset of cognitive and motor symptoms by many years.18
Counterpoint
While autonomic dysfunction is common to both DLB and PDD, the criteria for DLB and PDD differ in their inclusion of this in diagnosis. Among the “supportive” features in DLB criteria are: syncope and severe autonomic dysfunction. Although these features are well recognized in PDD, it is interesting that they are not part of the diagnostic criteria for PDD.32 In the DLB clinical diagnostic criteria,79 RBD constitutes a “suggestive” diagnostic feature, and its presence may improve the accuracy of diagnostic classification for DLB.106
Medication responses
Point
For cognitive symptoms, it has been suggested that treatment with cholinesterase inhibitors (ChEIs) may be more effective in DLB and PDD, compared to AD, due to their early, prominent central nervous system cholinergic dysfunction.107 Randomized, double-blind, placebo-controlled trials support the use of rivastigmine or donepezil in DLB.108, 109 Rivastigmine is currently approved in the United States and European Union for the treatment of PDD.110 For motor deficits, levodopa and other dopaminergic medications are effective for tremor and parkinsonian motor symptoms of PD, including PDD. There are reports of small series of DLB patients whose motor impairments were successfully treated with levodopa, although doses used in DLB are generally lower than in PD.111 For psychosis treatment, atypical antipsychotics (e.g. quetiapine, clozapine) have been used in DLB and PDD, despite limited data of their use in DLB and current evidence-based medicine favoring clozapine in PD.112
Counterpoint
Only a few clinical trials of ChEIs in treating cognitive and behavioral aspects of DLB have been conducted; most guidelines are based on case reports and extension of AD therapeutic trials. There are no controlled clinical trials for treatments of parkinsonian motor features in DLB. Increased adverse events with combined use of levodopa and ChEIs in PD have been reported, though any worsening of tremor or parkinsonism has been mild.113, 108 Despite the trials, a Cochrane review, however, does not provide convincing evidence of significant benefits of ChEIs for either DLB or PDD114. For motor symptoms, levodopa and other dopaminergic medications are more effective in PD than in DLB, and higher doses can often be used. Psychosis may significantly worsen in DLB following dopaminergic therapies, even at low doses, and this poses a particular challenge.115 For psychosis treatment, severe neuroleptic sensitivity remains a risk in DLB, although this is less frequently encountered with atypical antipsychotics with greater serotonergic profiles.116
Pathology and biomarkers
Point
Studies demonstrate that Lewy bodies composed of alpha-synuclein are the predominant cause of dementia in PD as well as in DLB.117, 118 Whether the ascending progression of Lewy body pathology described by Braak and colleagues in non-demented PD also applies to DLB is unclear, with some studies suggesting the possibility of a descending pattern of progression in some cases.119–121 In nearly all studies of DLB and PDD, variable amounts of AD pathology, mostly in the form of amyloid contribute to the pathologic burden. This shared neuropathology may explain similar appearances of DLB and PDD in structural and metabolic imaging studies, though in some studies, there has been greater amyloid burden detected on imaging in DLB.122–124 There may also be variable amounts of cerebrovascular disease in both DLB and PDD – the extent to which this pathology contributes to clinical symptoms is unclear. Cerebrospinal fluid analyses for alpha-synuclein, amyloid, and tau do not clearly distinguish DLB and PDD, though results have been variable.125–127 These biomarker studies, however, may help potentially distinguish DLB and/or PDD from AD.
Counterpoint
It should be noted however, that approximately 80% of DLB cases have sufficient AD pathology to be considered a mixed dementia.99 This is not so in PDD, where three neuropathological profiles may be present: 1/3rd with only neocortical Lewy bodies, 1/3rd with AD pathology, and 1/3rd with only subcortical pathology.81 In a study examining Lewy body and AD pathology as a function of dementia severity in PDD, severity of cortical Lewy body pathology was positively associated with dementia, while 29% of all PD cases had sufficient pathology for comorbid AD.128 Combined Lewy body and AD pathology correlated with later PD onset age, higher Lewy body burden, and a greater degree of cerebral amyloid angiopathy.128 Furthermore, in a subgroup of severe PDD patients, cortical tau burden was markedly increased, suggesting that progressive dementia is a product of extensive cortical neurofibrillary tangles.118
The debate remains
If one were to draw a conclusion from these arguments, then PDD and DLB should be considered disorders that share many common features and pathologies. Yet not all features are identical. Early amyloid deposits in DLB relative to PDD may account for differences in the timing of dementia and motor findings. Later appearance of tau pathology may hasten severity of symptoms as the dementia progresses. For these reasons, studying prodromal states of DLB and PDD, including pre-dementia (i.e., MCI) or pre-motor stages, may yield the best answers as to whether they are the same or different disorders.
Conclusion
The spectrum of cognitive impairment in Lewy body disease is indeed broad, with symptoms potentially present even at pre-motor stages through advanced disease. The cognitive phenotypes of PD, PDD, and DLB are heterogeneous, thereby prompting debate regarding optimal categorizations for diagnosis and prognosis. Early cognitive deficits may further blur diagnostic boundaries between PDD and DLB, particularly when considering MCI as a transitional state, preceding dementia. Findings from recent studies and ongoing debates on PD-MCI and PDD/DLB illustrate the growth of the field of cognition in Lewy body disorders, a move away from traditional or dopaminergic models of disease, and the opportunity for well-needed, safe and effective therapies.
Footnotes
FINANCIAL DISCLOSURES
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
| Consultancies: Merz, Pfizer | Expert Testimony: none |
| Advisory Boards: none | Employment: Rush University Medical Center |
| Partnerships: none | Contracts: none |
| Honoraria: Movement Disorders Society, American Academy of Neurology, Teva, Medscape, Johns Hopkins Dystonia and Spasticity Practicum | Royalties: none |
| Grants: NIH K23NS060949, Michael J. Fox Foundation (BioFIND, site-PI), Parkinson’s Disease Foundation, Rush University, Teva (Moderato study, site-PI) | Other: none |
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: University of Cambridge |
| Partnerships: None | Contracts: None |
| Honoraria: Lundbeck | Royalties: None |
| Grants: NIHR, Academy of Medical Sciences | Other: None |
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: UCB | Expert Testimony: None |
| Advisory Boards: NTcell | Employment: University of Cambridge |
| Partnerships: None | Contracts: None |
| Honoraria: CIRM; ERC | Royalties: Wiley |
| Grants: Parkinson's UK; EU; Rosetrees Trust; Cure-PD; CHDI; Evelyn Trust; NIHR; BBSRC | Other: Editorial work on Journal of Neurology for Springer |
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: University of Pennsylvania Perelman School of Medicine and the Parkinson’s Disease Research, Education and Clinical Center, Philadelphia Veterans Affairs Medical Center |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other: None |
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: Pfizer, Eisai, Forest, Novartis, Accera | Expert Testimony: None |
| Advisory Boards: None | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria:None | Royalties: None |
| Grants: NIH, Michael J Fox Foundation, Alzheimer Association, Alzheimer Drug Discovery Foundation, Morris and Alma Schapiro Fund | Other: None |
References
- 1.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 2.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson's disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 3.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 4.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 8.Findley L, Aujla M, Bain PG, et al. Direct economic impact of Parkinson's disease: a research survey in the United Kingdom. Mov Disord. 2003;18(10):1139–1145. doi: 10.1002/mds.10507. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of Mild Cognitive Impairment in Early Parkinson Disease: The Norwegian ParkWest Study. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 10.Broeders M, de Bie RM, Velseboer DC, Speelman JD, Muslimovic D, Schmand B. Evolution of mild cognitive impairment in Parkinson disease. Neurology. 2013;81(4):346–352. doi: 10.1212/WNL.0b013e31829c5c86. [DOI] [PubMed] [Google Scholar]
- 11.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(Pt 11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thaler A, Mirelman A, Gurevich T, et al. Lower cognitive performance in healthy G2019S LRRK2 mutation carriers. Neurology. 2012;79(10):1027–1032. doi: 10.1212/WNL.0b013e3182684646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson's disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18(Suppl 1):S199–S202. doi: 10.1016/S1353-8020(11)70062-1. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins K, Jennings D, Marek K, Siderowf A, Stern M. Cognitive deficits associated with dopamine transporter loss in the pre-motor subjects in PARS cohort. Movement Disorders. 2010;25(Suppl 3):S690–S691. [Google Scholar]
- 18.Boeve BF, Silber MH, Ferman TJ, Lucas JA, Parisi JE. Association of REM sleep behavior disorder and neurodegenerative disease may reflect an underlying synucleinopathy. Mov Disord. 2001;16(4):622–630. doi: 10.1002/mds.1120. [DOI] [PubMed] [Google Scholar]
- 19.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology. 2009;72(15):1296–1300. doi: 10.1212/01.wnl.0000340980.19702.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagnon JF, Bertrand JA, Genier Marchand D. Cognition in rapid eye movement sleep behavior disorder. Frontiers in neurology. 2012;3:82. doi: 10.3389/fneur.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 22.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. Eur J Neurol. 2009;16(12):1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 23.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson's disease: Critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broeders M, Velseboer DC, de Bie R, et al. Cognitive change in newly-diagnosed patients with Parkinson's disease: a 5-year follow-up study. J Int Neuropsychol Soc. 2013;19(6):695–708. doi: 10.1017/S1355617713000295. [DOI] [PubMed] [Google Scholar]
- 26.Dalrymple-Alford JC, Livingston L, Macaskill MR, et al. Characterizing mild cognitive impairment in Parkinson's disease. Mov Disord. 2011 doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- 27.Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marras C, Armstrong MJ, Meaney CA, et al. Measuring mild cognitive impairment in patients with Parkinson's disease. Mov Disord. 2013 doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, text revision. 4th ed ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 33.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 34.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord. 2005;20(10):1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- 35.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013;84(11):1258–1264. doi: 10.1136/jnnp-2013-305277. [DOI] [PubMed] [Google Scholar]
- 36.Auyeung M, Tsoi TH, Mok V, et al. Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson's disease patients. J Neurol Neurosurg Psychiatry. 2012;83(6):607–611. doi: 10.1136/jnnp-2011-301590. [DOI] [PubMed] [Google Scholar]
- 37.Perez F, Helmer C, Foubert-Samier A, Auriacombe S, Dartigues JF, Tison F. Risk of dementia in an elderly population of Parkinson's disease patients: A 15-year population-based study. Alzheimers Dement. 2012;8(6):463–469. doi: 10.1016/j.jalz.2011.09.230. [DOI] [PubMed] [Google Scholar]
- 38.Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain. 1992;115(6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 39.Robbins TW, James M, Owen AM, et al. Cognitive deficits in progressive supranuclear palsy, Parkinson's disease, and multiple system atrophy in tests sensitive to frontal lobe dysfunction. J Neurol Neurosurg Psychiatry. 1994;57(1):79–88. doi: 10.1136/jnnp.57.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–537. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- 41.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson's disease without dementia. Dement Geriatr Cogn Disord. 2003;15(3):126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- 42.Helkala EL, Laulumaa V, Soininen H, et al. Recall and recognition memory in patients with Alzheimer's and Parkinson's disease. Ann Neurol. 1988;24:214–217. doi: 10.1002/ana.410240207. [DOI] [PubMed] [Google Scholar]
- 43.Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch Neurol. 1993;50(4):374–379. doi: 10.1001/archneur.1993.00540040036010. [DOI] [PubMed] [Google Scholar]
- 44.Stern Y, Mayeux R, Rosen J, Ilson J. Perceptual motor dysfunction in Parkinson's disease: a deficit in sequential and predictive voluntary movement. J Neurol Neurosurg Psychiatry. 1983;46(2):145–151. doi: 10.1136/jnnp.46.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson's disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 46.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson's disease. Mov Disord. 2012;27(9):1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 48.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson's disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson's disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18(3):149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 50.Levy G, Jacobs DM, Tang MX, et al. Memory and executive function impairment predict dementia in Parkinson's disease. Mov Disord. 2002;17(6):1221–1226. doi: 10.1002/mds.10280. [DOI] [PubMed] [Google Scholar]
- 51.Mahieux F, Fenelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64(2):178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R. Neuropsychological characteristics of preclinical dementia in Parkinson's disease. Neurology. 1995;45(9):1691–1696. doi: 10.1212/wnl.45.9.1691. [DOI] [PubMed] [Google Scholar]
- 53.Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson's disease in the United Kingdom. Mov Disord. 2004;19(9):1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- 54.Domellof ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson's disease. Mov Disord. 2011 doi: 10.1002/mds.23814. [DOI] [PubMed] [Google Scholar]
- 55.Yarnall AJ, Breen DP, Duncan GW, Barker RA, Burn DJ. Characterising Mild Cognitive Impairment In Incident Parkinson's Disease: The ICICLE-PD Study. Mov Disord. 2013;28(Suppl 1):505. [abstract]. [Google Scholar]
- 56.Caslake R, Taylor K, Scott N, et al. Age-, gender-, and socioeconomic status-specific incidence of Parkinson's disease and parkinsonism in North East Scotland: The PINE study. Parkinsonism Relat Disord. 2013;19(5):515–521. doi: 10.1016/j.parkreldis.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 57.Williams-Gray CH, Mason SL, Evans JR, et al. The CamPaIGN study of Parkinson’s disease: 10 year outlook in an incident population-based cohort. J Neurol Neurosurg Psychiatry. 2013 doi: 10.1136/jnnp-2013-305277. (epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 58.Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson's disease-cognitive rating scale: a new cognitive scale specific for Parkinson's disease. Mov Disord. 2008;23(7):998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 59.Muslimovic D, Schmand B, Speelman JD, de Haan RJ. Course of cognitive decline in Parkinson's disease: a meta-analysis. J Int Neuropsychol Soc. 2007;13(6):920–932. doi: 10.1017/S1355617707071160. [DOI] [PubMed] [Google Scholar]
- 60.Weintraub D, Dietz N, Duda JE, et al. Alzheimer's disease pattern of brain atrophy predicts cognitive decline in Parkinson's disease. Brain. 2012;135(Pt 1):170–180. doi: 10.1093/brain/awr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Garcia D, Clavero P, Gasca Salas C, et al. Posterior parietooccipital hypometabolism may differentiate mild cognitive impairment from dementia in Parkinson's disease. European journal of nuclear medicine and molecular imaging. 2012 doi: 10.1007/s00259-012-2198-5. [DOI] [PubMed] [Google Scholar]
- 62.Campbell MC, Markham J, Flores H, et al. Principal component analysis of PiB distribution in Parkinson and Alzheimer diseases. Neurology. 2013;81(6):520–527. doi: 10.1212/WNL.0b013e31829e6f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 64.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near-normal cognition: risk factors and prognosis. Neurology. 2012;79(15):1591–1598. doi: 10.1212/WNL.0b013e31826e26b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bangen KJ, Jak AJ, Schiehser DM, et al. Complex activities of daily living vary by mild cognitive impairment subtype. J Int Neuropsychol Soc. 2010;16(4):630–639. doi: 10.1017/S1355617710000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perneczky R, Pohl C, Sorg C, et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing. 2006;35(3):240–245. doi: 10.1093/ageing/afj054. [DOI] [PubMed] [Google Scholar]
- 69.Weintraub D, Burn DJ. Parkinson's disease: the quintessential neuropsychiatric disorder. Mov Disord. 2011;26(6):1022–1031. doi: 10.1002/mds.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldman JG, Litvan I. Mild cognitive impairment in Parkinson's disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 71.Petersen RC. Early diagnosis of Alzheimer's disease: is MCI too late? Curr Alzheimer Res. 2009;6(4):324–330. doi: 10.2174/156720509788929237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubois B, Albert ML. Amnestic MCI or prodromal Alzheimer's disease? Lancet Neurol. 2004;3(4):246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 73.Ewers M, Walsh C, Trojanowski JQ, et al. Prediction of conversion from mild cognitive impairment to Alzheimer's disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol Aging. 2012;33(7):1203–1214. doi: 10.1016/j.neurobiolaging.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burn DJ, Barker RA. Mild cognitive impairment in Parkinson's disease: millstone or milestone? Pract Neurol. 2013;13(2):68–69. doi: 10.1136/practneurol-2013-000539. [DOI] [PubMed] [Google Scholar]
- 75.Wu K, O'Keeffe D, Politis M, et al. The catechol-O-methyltransferase Val(158)Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson's disease: a PET study. Brain. 2012;135(Pt 8):2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]
- 76.Stephan BC, Savva GM, Brayne C, Bond J, McKeith IG, Matthews FE. Optimizing mild cognitive impairment for discriminating dementia risk in the general older population. Am J Geriatr Psychiatry. 2010;18(8):662–673. doi: 10.1097/jgp.0b013e3181e0450d. [DOI] [PubMed] [Google Scholar]
- 77.Stephan BC, Matthews FE, Hunter S, et al. Neuropathological profile of mild cognitive impairment from a population perspective. Alzheimer Dis Assoc Disord. 2012;26(3):205–212. doi: 10.1097/WAD.0b013e31822fc24d. [DOI] [PubMed] [Google Scholar]
- 78.Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–566. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]
- 79.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 80.Galvin JE. Cognitive change in Parkinson disease. Alzheimer Dis Assoc Disord. 2006;20(4):302–310. doi: 10.1097/01.wad.0000213858.27731.f8. [DOI] [PubMed] [Google Scholar]
- 81.Galvin JE, Pollack J, Morris JC. Clinical phenotype of Parkinson disease dementia. Neurology. 2006;67(9):1605–1611. doi: 10.1212/01.wnl.0000242630.52203.8f. [DOI] [PubMed] [Google Scholar]
- 82.Johnson DK, Galvin JE. Longitudinal changes in cognition in Parkinson's disease with and without dementia. Dement Geriatr Cogn Disord. 2011;31(2):98–108. doi: 10.1159/000323570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aarsland D, Ballard CG, Halliday G. Are Parkinson's disease with dementia and dementia with Lewy bodies the same entity? J Geriatr Psychiatry Neurol. 2004;17(3):137–145. doi: 10.1177/0891988704267470. [DOI] [PubMed] [Google Scholar]
- 84.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 85.Ballard CG, Aarsland D, McKeith I, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology. 2002;59(11):1714–1720. doi: 10.1212/01.wnl.0000036908.39696.fd. [DOI] [PubMed] [Google Scholar]
- 86.Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord. 2005;20(Suppl 11):S23–S29. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- 87.Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1992;55(5):377–382. doi: 10.1136/jnnp.55.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Starkstein SE, Petracca G, Chemerinski E, et al. Depression in classic versus akinetic-rigid Parkinson's disease. Mov Disord. 1998;13(1):29–33. doi: 10.1002/mds.870130109. [DOI] [PubMed] [Google Scholar]
- 89.Aarsland D, Larsen JP, Lim NG, et al. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67(4):492–496. doi: 10.1136/jnnp.67.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klatka LA, Louis ED, Schiffer RB. Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer's disease and Parkinson's disease comparison groups. Neurology. 1996;47(5):1148–1152. doi: 10.1212/wnl.47.5.1148. [DOI] [PubMed] [Google Scholar]
- 91.Karantzoulis S, Galvin JE. Update of Dementia with Lewy Bodies. Current Translational Geriatrics and Experimental Gerontology Reports. 2013;2:196–204. doi: 10.1007/s13670-013-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 93.Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson's disease with and without dementia. Int J Geriatr Psychiatry. 2001;16(5):528–536. doi: 10.1002/gps.389. [DOI] [PubMed] [Google Scholar]
- 94.Thaipisuttikul P, Lobach I, Zweig Y, Gurnani A, Galvin JE. Capgras syndrome in Dementia with Lewy Bodies. Int Psychogeriatr. 2013;25(5):843–849. doi: 10.1017/S1041610212002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pagonabarraga J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A, Kulisevsky J. A prospective study of delusional misidentification syndromes in Parkinson's disease with dementia. Mov Disord. 2008;23(3):443–448. doi: 10.1002/mds.21864. [DOI] [PubMed] [Google Scholar]
- 96.Roane DM, Rogers JD, Robinson JH, Feinberg TE. Delusional misidentification in association with parkinsonism. J Neuropsychiatry Clin Neurosci. 1998;10(2):194–198. doi: 10.1176/jnp.10.2.194. [DOI] [PubMed] [Google Scholar]
- 97.Williams VG, Bruce JM, Westervelt HJ, et al. Boston naming performance distinguishes between Lewy body and Alzheimer's dementias. Arch Clin Neuropsychol. 2007;22(8):925–931. doi: 10.1016/j.acn.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 98.Lambon Ralph MA, Powell J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70(2):149–156. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tarawneh R, Galvin JE. Distinguishing Lewy body dementias from Alzheimer's disease. Expert Rev Neurother. 2007;7(11):1499–1516. doi: 10.1586/14737175.7.11.1499. [DOI] [PubMed] [Google Scholar]
- 100.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 101.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. 2004;19(1):60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 102.Karantzoulis S, Galvin JE. Discriminating Alzheimer disease from other major forms of dementia. Expert Rev Neurother. 2011;11:1579–1591. doi: 10.1586/ern.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huber SJ, Shuttleworth EC, Paulson GW. Dementia in Parkinson's disease. Arch Neurol. 1986;43(10):987–990. doi: 10.1001/archneur.1986.00520100009006. [DOI] [PubMed] [Google Scholar]
- 104.Leplow B, Dierks C, Herrmann P, Pieper N, Annecke R, Ulm G. Remote memory in Parkinson's disease and senile dementia. Neuropsychologia. 1997;35(4):547–557. doi: 10.1016/s0028-3932(96)00116-9. [DOI] [PubMed] [Google Scholar]
- 105.King AE, Mintz J, Royall DR. Meta-analysis of 123I-MIBG cardiac scintigraphy for the diagnosis of Lewy body-related disorders. Mov Disord. 2011;26(7):1218–1224. doi: 10.1002/mds.23659. [DOI] [PubMed] [Google Scholar]
- 106.Ferman TJ, Boeve BF, Smith GE, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77(9):875–882. doi: 10.1212/WNL.0b013e31822c9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Curr Med Res Opin. 2006;22(1):49–59. doi: 10.1185/030079906x80279. [DOI] [PubMed] [Google Scholar]
- 108.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]
- 109.Mori E, Ikeda M, Kosaka K. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72(1):41–52. doi: 10.1002/ana.23557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.van Laar T, De Deyn PP, Aarsland D, Barone P, Galvin JE. Effects of cholinesterase inhibitors in Parkinson's disease dementia: a review of clinical data. CNS Neurosci Ther. 2011;17(5):428–441. doi: 10.1111/j.1755-5949.2010.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Molloy S, McKeith IG, O'Brien JT, Burn DJ. The role of levodopa in the management of dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2005;76(9):1200–1203. doi: 10.1136/jnnp.2004.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: Treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. 2011;26(Suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Okereke CS, Kirby L, Kumar D, Cullen EI, Pratt RD, Hahne WA. Concurrent administration of donepezil HCl and levodopa/carbidopa in patients with Parkinson's disease: assessment of pharmacokinetic changes and safety following multiple oral doses. Br J Clin Pharmacol. 2004;58(Suppl 1):41–49. doi: 10.1111/j.1365-2125.2004.01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rolinski M, Fox C, Maidment I, McShane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease. Cochrane Database Syst Rev. 2012;3:CD006504. doi: 10.1002/14651858.CD006504.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goldman JG, Goetz CG, Brandabur M, Sanfilippo M, Stebbins GT. Effects of dopaminergic medications on psychosis and motor function in dementia with Lewy bodies. Mov Disord. 2008;23(15):2248–2250. doi: 10.1002/mds.22322. [DOI] [PubMed] [Google Scholar]
- 116.Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson's disease and parkinsonian dementias. J Clin Psychiatry. 2005;66(5):633–637. [PubMed] [Google Scholar]
- 117.Hurtig HI, Trojanowski JQ, Galvin J, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54(10):1916–1921. doi: 10.1212/wnl.54.10.1916. [DOI] [PubMed] [Google Scholar]
- 118.Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kovari E. Neuropathology of dementia in a large cohort of patients with Parkinson's disease. Parkinsonism Relat Disord. 2013;19(10):864–868. doi: 10.1016/j.parkreldis.2013.05.010. discussion 864. [DOI] [PubMed] [Google Scholar]
- 119.Brunnstrom H, Lindberg E, Englund E. Staging of Lewy-related pathology in dementia. Clin Neuropathol. 2012;31(4):216–223. doi: 10.5414/NP300471. [DOI] [PubMed] [Google Scholar]
- 120.Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG. Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology. 2008;70(13):1042–1048. doi: 10.1212/01.wnl.0000306697.48738.b6. [DOI] [PubMed] [Google Scholar]
- 121.Frigerio R, Fujishiro H, Ahn TB, et al. Incidental Lewy body disease: do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging. 2011;32(5):857–863. doi: 10.1016/j.neurobiolaging.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27(8):965–973. doi: 10.1002/mds.25048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warr L, Walker Z. Identification of biomarkers in Lewy-body disorders. Q J Nucl Med Mol Imaging. 2012;56(1):39–54. [PubMed] [Google Scholar]
- 124.Sinha N, Firbank M, O'Brien JT. Biomarkers in dementia with Lewy bodies: a review. Int J Geriatr Psychiatry. 2012;27(5):443–453. doi: 10.1002/gps.2749. [DOI] [PubMed] [Google Scholar]
- 125.Stefani A, Brusa L, Olivola E, Pierantozzi M, Martorana A. CSF and clinical hallmarks of subcortical dementias: focus on DLB and PDD. J Neural Transm. 2012;119(7):861–875. doi: 10.1007/s00702-012-0820-0. [DOI] [PubMed] [Google Scholar]
- 126.Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 127.Bibl M, Mollenhauer B, Esselmann H, et al. CSF amyloid-beta-peptides in Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease dementia. Brain. 2006;129(Pt 5):1177–1187. doi: 10.1093/brain/awl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol. 2012;72(4):587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Biundo R, Weis L, Pilleri M, et al. Diagnostic and screening power of neuropsychological testing in detecting mild cognitive impairment in Parkinson's disease. J Neural Transm. 2013;120(4):627–633. doi: 10.1007/s00702-013-1004-2. [DOI] [PubMed] [Google Scholar]