Abstract
Nonalcoholic fatty liver disease (NAFLD) is defined as excessive accumulation of fatty acid in the liver, a common disease in the world. The research of single nucleotide polymorphisms (SNPs) provides a new approach for managing NAFLD. SNPs may increase or decrease the functions of the target genes and their encoding proteins. Peroxisome proliferator-activated receptor (PPAR) plays a key role in modulating metabolism of hepatic triglycerides and consequently magnitude of NAFLD. In this study, we investigated the effect of three SNPs in the PPAR-γ gene i.e. rs10865710 (C-681G), rs7649970 (C-689T) and rs1801282 (C34G, also termed Pro12Ala) on susceptibility to NAFLD. The participants were selected from our epidemiological survey. Totally 169 participants were enrolled in NAFLD group, and 699 healthy subjects were included as controls. PCR-RFLP was applied to detect the SNPs. The G allele frequency of rs10865710 in NAFLD group (41.1%) was significantly higher than that (34.8%) in controls (p = 0.03). Differences in other two loci (rs7649970 and rs1801282) were not statistically significant between the two groups (p > 0.05). This result was confirmed by haplotype analysis. The GCC haplotype (a set of 3 adjacent SNPs in linkage disequilibrium, corresponding to the three alleles of above polymorphisms in order) was a risk factor for the susceptibility to NAFLD (p = 0.03). This study has revealed that the G allele of rs10865710 in the PPAR-γ gene is associated with the increased susceptibility to NAFLD. Our findings may provide novel diagnostic biomarkers and therapeutic targets for NAFLD.
Keywords: gene, insulin resistance, non-alcoholic fatty liver disease, peroxisome proliferator-activated receptor-γ, polymorphism
Non-alcoholic fatty liver disease (NAFLD) refers to a spectrum of histological findings ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and NASH-related cirrhosis, which can progress to hepatocellular carcinoma (HCC) (Mendez-Sanchez et al. 2007). NAFLD has become a major cause of chronic liver disease globally. It is estimated that the prevalence of NAFLD ranges from 3% to 24%, with most countries’ prevalence between 6% and 14% (Williams 2006; Amarapurkar et al. 2007; Argo and Caldwell 2009). In recent years, due to alterations of lifestyle and dietary habits, the incidence of NAFLD has increased dramatically in China (Fan et al. 2005). Our previous survey revealed a prevalence of 15% of the population in southern China (Zhou et al. 2007). NAFLD is a hepatic component of metabolic syndrome (MS), which is characterized by obesity, type 2 diabetes mellitus (T2DM), dyslipidemia and hypertension with insulin resistance (IR) being the main mechanisms (Mendez-Sanchez et al. 2007). The pathogenesis of NAFLD is not completely understood. Both environmental and genetic factors are required for the development and progression of NAFLD (Day 2006). A number of studies have demonstrated that many genetic variations related to reactive oxygen species, cytokines, endotoxin receptors, fibrogenic mediators and IR were involved in the pathogenesis underlying NAFLD (Osterreicher and Brenner 2007; Wilfred de Alwis and Day 2007; Zhou et al. 2010). We have previously demonstrated that some single nucleotide polymorphisms (SNPs) were associated with the susceptibility to NAFLD (Zhou et al. 2010).
So far there have been significant advances in our understanding of the human genome and its clinical sequelae over a range of diseases. Over 3.1 million SNPs have been identified. The International HapMap Project (http://hapmap.ncbi.nlm.nih.gov) characterized patterns of SNPs across individuals from diverse ethnic backgrounds (Frazer et al. 2007; Daly et al. 2011). SNPs may increase or decrease the functions of the target genes and their encoding proteins. There are two mainstream SNP notations. The nomenclature (rs#) in SNP database (dbSNP: http://www.ncbi.nlm.nih.gov/SNP/) is unique, clear and stable, but difficult to understand. The nomenclature in HGVS (Human Genome Variation Society) guidelines (http://www.hgvs.org/mutnomen/recs-DNA.html) includes gene symbol (sequence type) and variation position. Its abbreviation is not standard, but is easy to understand and more commonly applied in literatures. In this manuscript, we used rs# notations plus HGVS nomenclature in brackets behind e.g. rs1801282 (C34G or Pro12Ala). The C34G polymorphism results in the substitution of Ala (GCA) for Pro (CCA).
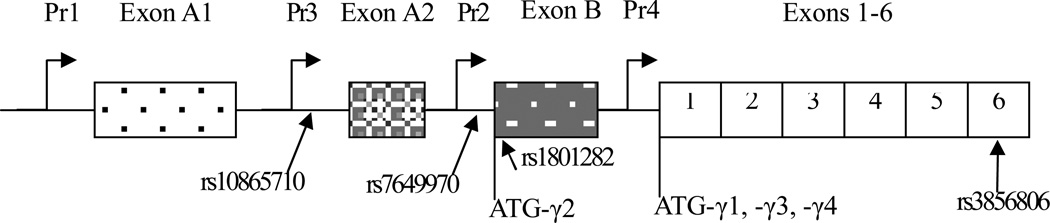
The PPARs play a key role in modulating the synthesis, storage, and export of hepatic triglycerides and consequently the magnitude of NAFLD. The PPAR family consists of PPAR-α, PPAR-γ and PPAR-β/δ nuclear receptors. These receptors exhibit different tissue distributions and functions. Among them, PPAR-γ increases insulin sensitivity and modulates glucose and lipid homeostasis, which makes it a most attractive target for the treatment of MS and NAFLD (Semple et al. 2006; Kallwitz et al. 2008). The human PPAR-γ gene, located in chromosome 3, has nine exons (exon A1, exon A2, exon B, exon 1–6 from 5’ to 3’ direction) and extends over more than 100 kilobases of genomic DNA (Fig 1). Differential promoter usage coupled with alternate splicing of the PPAR-γ gene gives rise to a variety of mRNA isoforms (PPAR-γ 1–4). Besides exons 1 to 6 commonly existing in all isoforms, PPAR-γ1 contains the untranslated exons A1 and A2; while PPAR-γ3 contains exon A2 and PPAR-γ2 contains the translated exon B. PPAR-γ1, PPAR-γ3 and PPAR-γ4 produce the same protein containing 477 amino acid residues encoded by exons 1 to 6, while PPAR-γ2 produces a different protein containing 505 amino acid residues, which may be encoded by exon B and consecutive exons 1 to 6 (Fajas et al. 1997; Gurnell 2005). The PPAR-γ gene has a number of genetic variants at several nucleotide loci, which result in conformational changes in the protein structures and functions of the gene. The SNPs of the PPAR-γ gene have been reported to be associated with the susceptibility to MS and its component diseases including NAFLD (Semple et al. 2006; Osterreicher and Brenner 2007; Wilfred de Alwis and Day 2007; Kallwitz et al. 2008). We have previously demonstrated that the SNP rs3856806 (also termed C161T or C1431T) in the PPAR-γ gene was associated with a higher susceptibility to NAFLD through the adiponectin pathway (Yang et al. 2008; Zhou et al. 2010). Human and animal studies have shown a significant improvement in biochemical and histological features in MS and NAFLD patients after PPAR-γ agonist treatment (Caldwell et al. 2006; Kallwitz et al. 2008; Blackburn 2010). However, the data mentioned above were not conclusive and even controversial (Dongiovanni et al. 2010; Rey et al. 2010).
Figure 1. Locations of four loci in the PPAR-γ gene.
The locations of four loci are indicated by arrows. Pr1 stands for promoter 1 of PPAR-γ gene, deducing the rest from this. ATG-γ1 stands for the ATG-translation initiation codon of PPAR-γ1, deducing the rest from this. Exons 1 to 6 are common to all isoforms. PPAR-γ1 contains the untranslated exons A1 and A2, PPAR-γ3 contains the untranslated exon A2. PPAR-γ2 contains the translated exon B (28 amino acids). The PPAR-γ1, PPAR-γ3, and PPAR-γ4 proteins are identical and translate by exons 1 to 6. PPAR-γ2 protein translates by exon B and exons 1 to 6.
Currently, haplotype analysis has become an important tool to study the combining effect of several SNPs on phenotype. A haplotype is a set of SNPs at a single chromosome of a chromosome pair that are statistically associated. Determination of a few alleles associations in a haplotype block can unambiguously identify all other polymorphic loci in this region. Such information is valuable for investigating the genetics behind many diseases, and has been applied to the human species by the International HapMap Projects (International HapMap Consortium. 2003; International HapMap Consortium. 2005). In the present study, HAPLOVIEW software was used to screen the tag SNPs. After the software programming, rs10865710 at PPAR-γ3 promoter, rs7649970 at PPAR-γ2 promoter, rs1801282 at PPAR-γ2 exon B and rs3856806 at PPAR-γ exon 6 were selected for further investigation (Fig 1). The literature has also documented the influence of these four SNPs on PPAR-γ activation. The G allele of rs10865710 and the T allele of rs7649970 were associated with lower PPAR-γ promoter activity (Meirhaeghe et al. 2003; Meirhaeghe et al. 2005). The G allele of rs1801282 at PPAR-γ2 exon B linked to the decrease of PPAR-γ activation by altering the 12th amino acid residue at the beginning of PPAR-γ2 protein (Deeb et al. 1998). Our previous studies showed rs3856806 in the PPAR-γ gene was associated with a higher susceptibility to NAFLD (Yang et al. 2008; Zhou et al. 2010).
Methods
Participants
The participants were selected from a cross-sectional epidemiological survey on the population of Guangdong province in southern China (Zhou et al. 2007). In the survey, 531 out of the total 3,543 participants (15.0%) were diagnosed as having NAFLD. Sampling of this study was estimated by Quanto software (version 1.2.4) with the data of NAFLD prevalence in southern China (Zhou et al. 2007). Totally 169 participants with typically clinical and ultrasonographic manifestations were recruited in the NAFLD group, and 699 healthy subjects matched with age and gender were included as controls.
Trained postgraduate students of Guangzhou Medical College, who were under the supervision of experienced investigators, conducted a face to face interview for the participants. Standard questionnaires, designed by the collaboration of epidemiologists and hepatologists, included the following items: demographics, current medication use, medical history and health relevant behaviors, i.e. alcohol consumption, smoking habits and dietary habits. Physical examination included anthropometric measurements, such as body height, body weight, waist circumference, hip circumference and other routine physical check-up measurements. Laboratory assessments included fasting plasma glucose (FPG); fasting insulin (FINS); plasma lipid profiles, such as total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDLc), low-density lipoprotein cholesterol (LDLc); serum liver functions, such as alanine transaminase (ALT), aspartate transaminase (AST), markers of hepatitis A virus (HAV), B virus (HBV) and C virus (HCV) and indices of insulin resistance estimated by the homeostasis model assessment of insulin resistance (HOMA-IR). Ultrasonography (US) was carried out for each subject on the same day as laboratory work at a mobile examination center (Zhou et al. 2007).
This study complied with the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Guangzhou Medical College. Written consent was obtained from each participant.
Anthropometric measurement
Height (m) and weight (kg) were measured to calculate BMI as weight (kg) / height (m2). Waist circumference i.e. the minimum circumference between the costal margin and the iliac crest and hip circumference (cm) were measured to calculate waist / hip ratio (WHR).
Biochemical assay
Serum and plasma samples were collected from fasting participants by routine methods and stored at −80 °C until analysis. FPG were determined by the glucose oxidase method. The plasma lipid profiles, i.e. TC, TG, HDLc, LDLc and serum liver functions, i.e. ALT, AST, BIL and albumin levels were measured on a Hitachi 7060 automatic analyzer (Hitachi, Tokyo, Japan) by enzymatic methods. Tests for the markers of HAV, HBV and HCV were also carried out. Indices of IR were estimated by using HOMA-IR as described previously (Matthews et al. 1985).
Diagnosis
NAFLD was diagnosed according to the guidelines for diagnosis and treatment issued by the Chinese Liver Disease Association (Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. 2006), which was adapted from the American Gastroenterological Association’s guidelines (American Gastroenterological Association. 2002). Briefly, the diagnosis was based on the combination of medical history, clinical symptoms and laboratory and ultrasonographic findings. Subjects with an average of weekly ethanol consumption ≥ 140 grams for men (≥ 70 grams for women) were excluded. Patients were excluded if there was evidence of other liver diseases such as viral hepatitis B and C in their clinical history or upon examinations. Liver biopsy was taken when the diagnosis was suspected. In this epidemiological study, only three subjects received biopsy. In most cases, NAFLD was diagnosed mainly by typical ultrasonographic findings after alcoholic liver disease and other chronic liver diseases were ruled out.
Based on the MS criteria proposed by the International Diabetes Federation (IDF), patients were diagnosed as having MS when their waist circumference was ≥ 90th plus at least two of the following items were present: (1) increased concentration of TG: ≥ 150 mg/dL (1.7 mmol/L) or receiving specific treatment for high TG; (2) reduced concentration of HDLc: < 40 mg/dL (1.03 mmol/L) or receiving specific treatment for this lipid abnormality; (3) elevated blood pressure: SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or receiving treatment for previously diagnosed hypertension; and (4) increased FPG concentration 100 mg/dL (5.6 mmol/L) or known T2DM (Zimmet et al. 2007).
The real-time ultrasonographic examination of upper abdominal organs was performed by two experienced physicians using a scanner equipped with a 3.5-MHz transducer (Siemens Adama, Erlangen, Germany). Physicians carrying out ultrasonography were unaware of the program of the study. Ultrasonographic patterns of fatty liver disease appear as a ‘bright’ liver (brightness and posterior attenuation) with stronger echoes in the hepatic parenchyma than in the renal parenchyma, vessel blurring and narrowing of the lumen of the hepatic veins in the absence of findings suggestive of other chronic liver diseases. (Yajima et al. 1983; Mishra and Younossi 2007; Kim et al. 2009; Shannon et al. 2011).
Genetic analysis
The SNPs (rs10865710, rs7649970, rs1801282 and rs3856806) in PPAR-γ gene were amplified by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) methods with the sets of primers (obtained from Invitrogen Co. Shanghai, China) that are listed in Table 1.
Table 1.
The primers of four SNPs at PPAR-γ gene
| PPAR-γ SNPs* | Primers | ||
|---|---|---|---|
| rs10865710 | Forward | 5’-TGTCGGGTCTCGATGTTG-3’ | |
| (C-681G) | Reverse | 5’-TGGTTATTAAGCCTAAGGTG-3’ | |
| rs7649970 | Forward | 5’-TAGAGAACTCCATTTTTTCATTATGACATAGCACTGAT-3’† | |
| (C-689T) | Reverse | 5’-ACTGACTGCTATCTAAATTCTG-3’ | |
| rs1801282 | Forward | 5’-ACTCTGGGAGATTCTCCTATTGGC-3’† | |
| (Pro12Ala) | Reverse | 5’-CTGGAAGACAAACTACAAGAG-3’ | |
| rs3856806 | Forward | 5’-GCCTGGATGACAGAGCAA-3’ | |
| (C161T) | Reverse | 5’-CAACTGGAAGAAGGGAAATG-3’ | |
Forward, forward primer; Reverse, reverse primer
The underlined mismatched base introducing a restriction endonuclease recognition site (T→G for rs7649970 and C→G for rs1801282)
Statistical analysis
The data were analyzed using SPSS13.0 for windows (Chicago, IL, USA). Sample size and power calculations were performed by Quanto software (version 1.2.4) (http://hydra.usc.edu/gxe). HAPLOVIEW software (Cambridge, MA, USA) was used to screen tag SNPs. SHEsis software (Shanghai, Bio-X Life Science Research Center, China) was applied for haplotype analysis. A haplotypes’ allele frequency ≥ 3% were accepted for assessment (Shi and He 2005). Both SNPs and their haplotypes were compared across NAFLD and control groups. Continuous data with normal distribution were expressed as mean ± standard deviation and examined using the Student’s t-test. Continuous data with skewed distribution were expressed as quartiles and examined using rank sum test. Categorical variables were expressed as a percentage and examined using the Chi-square and Fisher’s tests. Hardy–Weinberg test was performed to calculate allelic frequencies using the Chi-square test. Multivariate logistic regression analysis was performed to estimate the odds ratios (ORs) and 95% confidence intervals (CI) for the potential risk factors of NAFLD. Statistical significance was set at p < 0.05 (two-tailed).
Results
The prevalence of MS in NAFLD group (103/169, 60.9%) was significantly higher than that in controls (52/699, 7.4%) (p < 0.001). The clinical features in NAFLD and control groups were shown in Table 2. There was no significant difference in age and gender (p > 0.05). The anthropometric and biomedical variables related to MS were significantly different between the two groups (p < 0.01).
Table 2.
Demographic and biochemical features of patients with NAFLD and normal controls
| NAFLD | Controls | P-value | |
|---|---|---|---|
| n | 169 | 699 | NA |
| Gender(M/F) | 42 / 127 | 179 / 520 | 0.840 |
| Age(yrs) † | 56.00(49.00 – 65.50) | 56.00(46.00 – 64.00) | 0.55 |
| Body height(cm) | 157.27 ± 8.08 | 156.52 ± 8.08 | 0.288 |
| Body weight(kg) | 65.97 ± 10.74 | 53.69 ± 9.22 | 0.000 |
| BMI(kg/m2) | 26.59 ± 3.08 | 21.84 ± 2.86 | 0.000 |
| SBP(mmHg) | 135.90 ± 21.61 | 125. 49 ± 20.39 | 0.000 |
| DBP(mmHg) † | 84.00(78.00 – 92.00) | 80.00(70.00 – 85.00) | 0.000 |
| Waist(cm) † | 90.00(84.00 – 95.00) | 75.00(69.00 – 81.00) | 0.000 |
| Hip(cm) † | 100.00(95.00 – 103.00) | 91.00(86.75 – 95.00) | 0.000 |
| WHR† | 0.90(0.87 – 0.94) | 0.83(0.78 – 0.87) | 0.000 |
| FPG(mmol/L) | 6.37 ± 2.09 | 5.71 ± 1.68 | 0.000 |
| TG(mmol/L) | 2. 45 ± 1.90 | 1.28 ± 0.99 | 0.000 |
| HDLc(mmol/L) † | 1.20(1.02 – 1.49) | 1.60(1.36 – 1.90) | 0.000 |
| LDLc(mmol/L) | 2.74 ± 0.93 | 2.64 ± 0.90 | 0.198 |
| TC(mmol/L) | 5.69 ± 1.16 | 5.44 ± 1.09 | 0.009 |
| ALT(mmol/L) | 36.94 ± 31.83 | 17.88 ± 9.56 | 0.000 |
| AST(mmol/L) | 31.72 ± 14.87 | 27.34 ± 10.61 | 0.001 |
| GGT(U/L) | 42.54 ± 27.95 | 22.03 ± 13.27 | 0.000 |
| ALP(U/L) | 83.43 ± 28.03 | 75.99 ± 24.98 | 0.002 |
| FINS(mlU/L) † | 10.66(7.80 – 14.52) | 5.05(3.39 – 7.34) | 0.000 |
| HOMA-IR † | 2.84(2.03 – 4.03) | 1.20(0.80 – 1.83) | 0.000 |
| HSCRP(mg/L) † | 1.00(1.00 – 2.32) | 1.00(1.00 – 2.30) | 0.472 |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FINS, fasting insulin; GGT, gamma-glutamyl transpeptidase; HDLc, high-density lipoprotein cholesterol; HOMA-IR, homoeostatic metabolic assessment insulin resistance index; HSCRP, high sensitive C-reactive protein; LDLc, low-density lipoprotein cholesterol; NA, not analyzed; NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WHR, waist-to-hip ratio.
Continuous data with skewed distribution
The distributions of the three SNPs (rs10865710, rs7649970 and rs1801282) obeyed Hardy–Weinberg equilibrium in all subjects (Table 3), but rs3856806 did not (p = 0.042). Therefore, rs3856806 was excluded of further investigation. The genotypic distributions of the three loci in the PPAR-γ gene were shown in Table 4. The G allele frequency of rs10865710 (C-681G) in NAFLD group (41.1%) was significantly higher than that (34.8%) in controls (p = 0.03). The GG genotype distributions of rs10865710 in NAFLD group (17.2%) differed significantly from that (11.2%) in the control group (p = 0.03). Nevertheless, the differences at the other two SNPs (rs7649970 and rs1801282) were not significant (p > 0.05).
Table 3.
Hardy–Weinberg equilibrium for the four studied loci
| rs10865710* | rs7649970* | rs1801282* | rs3856806* | |||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P-value | χ2 | P-value | χ2 | P-value | χ2 | P-value | |
| NAFLD | 0.018 | 0.894 | 0.157 | 0.692 | 0.157 | 0.692 | 0.225 | 0.635 |
| Control | 1.293 | 0.256 | 0.546 | 0.460 | 0.704 | 0.402 | 4.108 | 0.043 |
| Total | 0.746 | 0.388 | 0.702 | 0.402 | 0.861 | 0.534 | 4.140 | 0.042 |
rs10865710=C-681G; rs7649970=C-689T; rs1801282=Pro12Ala; rs3856806 =C161T or C1431T
Table 4.
The genotypic distributions of the three loci in the PPAR-γ gene
| rs10865710 | Genotypes (%) | Allele Frequency (%) | |||
| (C-681G) | n | GG | CC + CG | C | G |
| NAFLD | 169 | 29(17.2%) | 59(34.9%) + 81(47.9%) | 199(58.9%) | 139(41.1%) |
| Controls | 699 | 78(11.2%) | 290(41.5%) + 331(47.3%) | 911(65.2%) | 487(34.8%) |
| P | 0.03 | 0.03 | |||
| rs7649970 | Genotypes (%) | Allele Frequency (%) | |||
| (C-689T) | n | CC | CT+TT | C | T |
| NAFLD | 169 | 159(94.1%) | 10(5.9%)+0 | 328(97.0%) | 10(3.0%) |
| Controls | 699 | 661(94.6%) | 38(5.4%)+0 | 1360(97.3%) | 38(2.7%) |
| P | 0.81 | 0.81 | |||
| rs1801282 | Genotypes (%) | Allele Frequency (%) | |||
| (Pro12Ala) | n | CC | CG+GG | C | G |
| NAFLD | 169 | 159(94.1%) | 10(5.9%)+0 | 328(97.0%) | 10(3.0%) |
| Controls | 699 | 656(93.8%) | 43(6.2%)+0 | 1355(96.9%) | 43(3.1%) |
| P | 0.91 | 0.91 | |||
When all variables were put into multivariate logistic regression analysis, GG genotype of rs10865710 and BMI, FBG, ALT, TG, HOMA-IR, FINS, MS were the risk factors for development of NAFLD (Table 5). The clinical features in subjects with or without rs10865710 variation were shown in Table 6. There was no significant difference in most anthropometric and biomedical variables except for FBG levels between GG (5.57 ± 1.29) and GC + CC groups (5.38 ± 1.84) (p = 0.026). Remarkably, the GG genotype of rs10865710 was a risk factor for NAFLD, but it did not reach the significance as an independent risk factor for MS as a whole (p = 0.112).
Table 5.
Multivariate regression analysis for risk factors of NAFLD (n = 868)
| B | S.E. | Wald | df | Sig. | Exp(B) | 95.0% CI.for EXP(B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Gender | 0.822 | 0.447 | 3.381 | 1 | 0.066 | 2.274 | 0.947 | 5.459 |
| Age | 0.011 | 0.016 | 0.450 | 1 | 0.502 | 1.011 | 0.980 | 1.042 |
| rs10865710* | −0.601 | 0.243 | 6.097 | 1 | 0.014 | 1.824 | 1.132 | 2.940 |
| rs7649970* | 15.782 | 1.72E4 | 0.000 | 1 | 0.999 | 7.14E6 | 0.000 | - |
| rs1801282* | −15.905 | 1.72E4 | 0.000 | 1 | 0.999 | 0.000 | 0.000 | - |
| BMI | 0.567 | 0.074 | 57.856 | 1 | 0.000 | 1.762 | 1.523 | 2.039 |
| SBP | 0.000 | 0.013 | 0.000 | 1 | 0.985 | 1.000 | 0.975 | 1.025 |
| DBP | 0.014 | 0.023 | 0.397 | 1 | 0.529 | 1.014 | 0.970 | 1.061 |
| FBG | 0.236 | 0.103 | 5.278 | 1 | 0.022 | 1.266 | 1.035 | 1.547 |
| ALT | 0.076 | 0.015 | 26.696 | 1 | 0.000 | 1.079 | 1.049 | 1.111 |
| TG | 0.238 | 0.118 | 4.079 | 1 | 0.043 | 1.269 | 1.007 | 1.599 |
| HDLc | −0.048 | 0.127 | 0.143 | 1 | 0.705 | 0.953 | 0.743 | 1.223 |
| MS | 1.270 | 0.373 | 11.597 | 1 | 0.001 | 3.560 | 1.714 | 7.395 |
| HOMA-IR | −0.273 | 0.116 | 5.532 | 1 | 0.019 | 0.761 | 0.606 | 0.956 |
| GGT | 0.011 | 0.010 | 1.170 | 1 | 0.279 | 1.011 | 0.991 | 1.031 |
| ALP | 0.003 | 0.006 | 0.197 | 1 | 0.657 | 1.003 | 0.990 | 1.016 |
| TC | 0.220 | 0.164 | 1.797 | 1 | 0.180 | 1.246 | 0.903 | 1.718 |
| FINS | 0.135 | 0.055 | 6.126 | 1 | 0.013 | 1.144 | 1.028 | 1.274 |
B, the values for the logistic regression equation for predicting the dependent variables from the independent variables; S.E., the standard errors associated with the coefficients; Wald and Sig., the Wald chi-square value and 2-tailed p-value used in test; df, the degrees of freedom for each of the tests of the coefficients; Exp(B), the odds ratios for the predictors
ALP, alkaline phosphatase ; ALT, alanine transaminase; BMI, body mass index; DBP, diastolic blood pressure; FINS, fasting insulin; FPG, fasting plasma glucose; GGT, gamma-glutamyl transpeptidase; HDLc, high-density lipoprotein cholesterol; HOMA-IR, homoeostatic metabolic assessment insulin resistance index; MS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride
rs10865710=C-681G; rs7649970=C-689T; rs1801282=Pro12Ala
Table 6.
Demographic and biochemical features of subjects with and without rs10865710* variation
| GG | GC + CC | P-value | |
|---|---|---|---|
| n | 107 | 761 | NA |
| Gender(M/F) | 27 / 80 | 194 / 567 | 0.954 |
| Body height(cm) | 158.14 ± 8.33 | 156.46 ± 8.03 | 0.033 |
| Body weight(kg) | 57.58 ± 11.73 | 55.84 ± 10. 52 | 0.134 |
| BMI(kg/m2) | 22.92 ± 3.54 | 22.73 ± 3.43 | 0.633 |
| SBP(mmHg) | 126.85 ± 21.05 | 127.60 ± 21.03 | 0.678 |
| DBP(mmHg) † | 80.00(75.00 – 86.00) | 80.00(70.00 – 88.00) | 0.398 |
| Waist(cm) | 79.26 ± 11.34 | 77.57 ± 10.29 | 0.178 |
| Hip(cm) | 93.41 ± 7.64 | 92.05 ± 7.42 | 0.095 |
| WHR | 0.85 ± 0.09 | 0.84 ± 0.08 | 0.735 |
| FPG(mmol/L) | 5.57 ± 1.29 | 5.38 ± 1. 84 | 0.026 |
| TG(mmol/L) | 1.57 ± 1.56 | 1.49 ± 1. 27 | 0.643 |
| HDLc(mmol/L) | 1.54 ± 0.46 | 1.60 ± 0. 77 | 0.396 |
| LDLc(mmol/L) | 2.57 ± 0.91 | 2.67 ± 0.90 | 0.201 |
| TC(mmol/L) | 5.41 ± 1.09 | 5.50 ± 1.11 | 0.471 |
| ALT(mmol/L) | 24.16 ± 25.15 | 21.30 ± 16.97 | 0.388 |
| AST(mmol/L) | 29.00 ± 15.85 | 28.03 ± 10.91 | 0.751 |
| GGT(U/L) | 29.95 ± 22.16 | 26.14 ± 19.17 | 0.079 |
| ALP(U/L) | 76.17 ± 24.69 | 77.88 ± 26.05 | 0.602 |
| FINS(mlU/L) † | 6.04(3.89 – 9.54) | 5.68(3.71 – 8.79) | 0.306 |
| HOMA-IR † | 1.44(0.93 – 2.45) | 1.42(0.86 – 2.28) | 0.592 |
| HSCRP(mg/L) † | 1.00(1.00 – 2.40) | 1.00(1.00 – 2.30) | 0.475 |
| MS(+/−) | 25 / 82 (23.4%) | 130 / 631 (17.1%) | 0.112 |
ALP, alkaline phosphatase ; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; DBP, diastolic blood pressure; FPG, fasting plasma glucose; FINS, fasting insulin; GGT, gamma-glutamyl transpeptidase; HDLc, high-density lipoprotein cholesterol; HOMA-IR, homoeostatic metabolic assessment insulin resistance index; HSCRP, high sensitive C-reactive protein; LDLc, low-density lipoprotein cholesterol; MS, metabolic syndrome; NA, not analyzed; NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WHR, waist-to-hip ratio.
Continuous data with skewed distribution
rs10865710=C-681G
When all the genotypes of the three loci entered haplotype analysis with SHEsis, six haplotypes were found after programming. Only two haplotypes’ allele i.e. CCC and GCC frequencies (a set of 3 SNPs at a single chromosome, corresponding to the alleles of the rs10865710, rs7649970 and rs1801282 polymorphisms in this order) were accepted for assessment (≥ 3%) between NAFLD and control groups. Haplotype GCC at PPAR-γ gene conferred the risk (OR = 1.32, 95%CI: 1.03–1.70, p = 0.03), and haplotype CCC reduced the risk (i.e. a protective factor) (OR = 0.76, 95%CI: 0.59–0.97, p = 0.03) of susceptibility to NAFLD (Table 7).
Table 7.
Haplotype frequencies of rs10865710, rs7649970 and rs1801282* run by SHEsis analysis
| Frequencies | X2 | Fisher's p | Pearson's p | OR | 95%CI | ||
|---|---|---|---|---|---|---|---|
| NAFLD | Control | ||||||
| CCC | 0.5858 | 0.6505 | 4.8395 | 0.0279 | 0.0278 | 0.7561 | 0.5891,0.9704 |
| GCC | 0.3787 | 0.3180 | 4.8395 | 0.0279 | 0.0278 | 1.3226 | 1.0305,1.6975 |
| Global | 4.8395 | 0.0279 | 0.0278 | ||||
X2, the chi-square value; Fisher's p, p value of Fisher's test; Pearson's p, p value of Pearson's test; OR, the odds ratio; 95%CI, 95% confidence interval for OR
rs10865710=C-681G; rs7649970=C-689T; rs1801282=Pro12Ala
Discussion
There is a substantial overlap in the pathogenesis of MS and NAFLD, as NAFLD represents the hepatic manifestation of MS, and the accumulation of fat in the liver impairs insulin sensitivity (Ryysy et al. 2000; Seppala-Lindroos et al. 2002; Mendez-Sanchez et al. 2007). Theoretically, genetic variations of the candidate genes found in MS patients may be related to NAFLD. In comparison to NAFLD, the relationships between the genotypes and phenotypes of MS have been examined more extensively. A large number of SNPs in the genes involved in IR and energy metabolism reported in MS might be associated with the susceptibility to NAFLD (Meirhaeghe et al. 2005; Ranjith et al. 2008). The genetic variations (SNPs) may positively or negatively affect gene activities. We have reported some SNPs increased the risk, while others decreased the risk of NAFLD development (Zhou et al. 2010).
PPAR-γ has attracted attention in basic, animal and clinical researches. PPAR-γ agonists such as thiazolidinedione have been recommended as therapeutic options for patients with T2DM as well as NAFLD (Caldwell et al. 2006; Kallwitz et al. 2008; Blackburn 2010). However, the effect of genetic polymorphisms in PPAR-γ on the pathogenesis of MS and NAFLD has not been clearly documented. The results from the literature are controversial. The SNPs at the PPAR-γ gene involved in MS may occur at many loci, such as rs10865710 (C-681G), rs7649970 (C-689T), rs1801282 (Pro12Ala), rs4135304 (G67222A), rs4135247 (A69208G), rs4135317 (G81556T), rs4135263 (T95872C), rs2959272 (T115432G), rs709151 (C127599T), and rs3856806 (C161T or C1431T), but only a few of them have been investigated extensively (Meirhaeghe and Amouyel 2004; Wei et al. 2006). The PPAR-γ gene consists of three haplotype areas with nine exons and four promoters (Fajas et al. 1997). In this study, the three SNP loci were chosen from the international hapmap website for humans (http://hapmap.ncbi.nlm.nih.gov/index.html.en) with HAPLOVIEW software (International HapMap Consortium. 2003; Barrett et al. 2005; Haiman and Stram 2008).
The role of the SNP at rs1801282 (Pro12Ala) in MS and its component diseases i.e. obesity, T2DM, dyslipidemia and hypertension has been well described. Most studies suggested an inverse association of its polymorphism with the risk of T2DM and hyperglycemia because the substitution of Ala to Pro resulted in reduced activity of PPAR-γ (Altshuler et al. 2000; Jaziri et al. 2006; Badii et al. 2008; Heude et al. 2011). However, in a few studies, the influence of the polymorphism was not significant (Tonjes and Stumvoll 2007; Yang et al. 2009). In contrast to the inverse association, Gupta et al. (2010) observed that the heterozygous genotype of Pro12Ala in NAFLD group (34.7%) was significantly more frequent than that in controls (23.9%) in a study enrolling 98 patients with biopsy confirmed NAFLD and 280 matched healthy controls. This finding leads to a conclusion that Pro12Ala variation of the PPAR-γ2 gene was associated with NAFLD. Kotronen et al. (2009) also demonstrated that the SNP at the Pro12Ala was significantly associated with hepatic fat content measured with proton magnetic resonance spectroscopy in a total of 302 patients. Yang et al. (2012) observed that Pro/Pro genotype of Pro12Ala was an independent risk factor for the development of NAFLD. In a study with 363 NAFLD patients confirmed with liver biopsies and 259 healthy controls, Rey et al. (2010) revealed that the incidence of the Ala12 mutant in NAFLD patients (3.4%) did not significantly differ from that (1.5%) in the controls, and the mutation was not associated with the progression of fatty liver disease. Dongiovanni et al. (2010) showed that the Ala to Pro substitution in PPAR-γ gene was not associated with the severity of steatosis, necroinflammation, or fibrosis of the liver. So far, findings from literature have been controversial, which might be explained by the difference of the ethnicity and populations studied and also because of the low frequency of the Ala allele in Asian. In the present study, rs1801282 variant was not associated with the susceptibility to NAFLD. As the sample size of the present study was referred to the study with Ye and Lv (2007), the frequency of the Ala allele in this study (6.10%) was eventually lower than the earlier study (14.46%). Therefore, the power of the Pro12Ala SNP’s analysis was relatively low (25.17%). A study with a bigger sample size is needed to confirm our conclusion.
The SNP rs3856806 (also termed C161T or C1431T) is another locus in PPAR-γ gene frequently reported in the literature. Our previous study demonstrated that the T allele of rs3856806 was associated with a higher susceptibility to NAFLD (Yang et al. 2008). The results were consistent with most studies on MS-related diseases published before and after ours (Liu et al. 2008; Wan et al. 2010). Unexpectedly, in this study, the distributions of rs3856806 did not obey Hardy–Weinberg equilibrium, and was excluded for further analysis.
Limited data describe the involvement of the polymorphisms at rs10865710 (C-681G) and rs7649970 (C-689T) in disorders. The SNP rs10865710 was located in a binding consensus site of signal transducer and activator of transcription 5B (STAT5B). The G allele of rs10865710 completely abolished the binding of STAT5B to the cognate promoter element as well as the transactivation of the PPAR-γ3 promoter by the growth hormone/STAT5B pathway, which could lower the activity of the PPAR-γ3 promoter and influence lipid homeostasis in humans (Meirhaeghe et al. 2003). The present study revealed that the GG genotype at rs10865710 was associated with a higher susceptibility to NAFLD, but no influence of rs7649970 was found. The results were supported by our haplotype analysis. When the combined effect of the three SNPs was investigated, haplotype GCC was found to be a risk factor, whereas haplotype CCC to be a protective factor for susceptibility to NAFLD.
To our knowledge, this is the first study to systematically investigate the association of several SNPs in PPAR-γ gene with the susceptibility to NAFLD. In addition to our previous findings on rs3856806 (also termed C161T, or C1431T) (Yang et al. 2008; Zhou et al. 2010), we demonstrated that G allele of rs10865710 (C-681G) in PPAR-γ gene increased the susceptibility to NAFLD, but no influence of rs7649970 (C-689T) and rs1801282 (Pro12Ala) was observed. The results were supported by the haplotype analysis. A limitation of our study is that the diagnosis of NAFLD was based on ultrasonographic findings, not the gold standard of histology. For ethical reasons, it is impossible to perform liver biopsy in an epidemiological survey. The lack of biopsy made it difficult to interpret the implications that the results might have for differentiating simple steatosis from NASH. Susceptibility to NAFLD may not indicate a susceptibility to NASH. In order to limit this disadvantage, we only included subjects with typical ultrasonographic patterns (medium and advanced stages). Imaging modalities such as ultrasonography have a reasonably high agreement, especially in the late stages of disease, in determining the diagnosis but not the extent of NAFLD (Yajima et al. 1983; Mishra and Younossi 2007; Kim et al. 2009; Shannon et al. 2011). Our study suggests that certain genetic variations in the PPAR-γ gene play a role in the development of NAFLD. Further studies are required to elucidate the underlying molecular mechanisms that link PPAR-γ polymorphisms to the pathogenesis of NAFLD.
Acknowledgments
This study was supported by the Foundations from Guangzhou Health Bureau, China (2008-Zdi-1 and 2009-ZDi-03).
Footnotes
Conflict of Interest
All authors have no conflict of interest.
References
- Altshuler D, Hirschhorn JN, Klannemark M, Lindgren CM, Vohl MC, Nemesh J, Lane CR, Schaffner SF, Bolk S, Brewer C, Tuomi T, Gaudet D, Hudson TJ, Daly M, Groop L, Lander ES. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 2000;26:76–80. doi: 10.1038/79216. [DOI] [PubMed] [Google Scholar]
- Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J. Gastroenterol. Hepatol. 2007;22:788–793. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- American Gastroenterological Association. American Gastroenterological Association medical position statement: nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1702–1704. doi: 10.1053/gast.2002.36569. [DOI] [PubMed] [Google Scholar]
- Argo CK, Caldwell SH. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin. Liver Dis. 2009;13:511–531. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Badii R, Bener A, Zirie M, Al-Rikabi A, Simsek M, Al-Hamaq AO, Ghoussaini M, Froguel P, Wareham NJ. Lack of association between the Pro12Ala polymorphism of the PPAR-gamma 2 gene and type 2 diabetes mellitus in the Qatari consanguineous population. Acta. Diabetol. 2008;45:15–21. doi: 10.1007/s00592-007-0013-8. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Blackburn GL. From bench to bedside: novel mechanisms and therapeutic advances through the development of selective peroxisome proliferator-activated receptor gamma modulators. Am. J. Clin. Nutr. 2010;91:251S–253S. doi: 10.3945/ajcn.2009.28449A. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, Argo CK, Al-Osaimi AM. Therapy of NAFLD: insulin sensitizing agents. J. Clin. Gastroenterol. 2006;40(Suppl 1):S61–S66. doi: 10.1097/01.mcg.0000168647.71411.48. [DOI] [PubMed] [Google Scholar]
- Daly AK, Ballestri S, Carulli L, Loria P, Day CP. Genetic determinants of susceptibility and severity in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2011;5:253–263. doi: 10.1586/egh.11.18. [DOI] [PubMed] [Google Scholar]
- Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–1028. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat. Genet. 1998;20:284–287. doi: 10.1038/3099. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Rametta R, Fracanzani AL, Benedan L, Borroni V, Maggioni P, Maggioni M, Fargion S, Valenti L. Lack of association between peroxisome proliferator-activated receptors alpha and gamma2 polymorphisms and progressive liver damage in patients with non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol. 2010;10:102. doi: 10.1186/1471-230X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, Najib J, Laville M, Fruchart JC, Deeb S, Vidal-Puig A, Flier J, Briggs MR, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, Dai F, Li F, Chen SY. Fatty liver and the metabolic syndrome among Shanghai adults. J. Gastroenterol. Hepatol. 2005;20:1825–1832. doi: 10.1111/j.1440-1746.2005.04058.x. [DOI] [PubMed] [Google Scholar]
- Fatty Liver and Alcoholic Liver Disease Study Group of the Chinese Liver Disease Association. Guidelines for diagnosis and treatment of nonalcoholic fatty liver diseases. Zhonghua Gan Zang Bing Za Zhi. 2006;14:161–163. [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, Pasternak S, Wheeler DA, Willis TD, Yu F, Yang H, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AC, Chaudhory AK, Sukriti, Pande C, Sakhuja P, Singh Y, Basir SF, Sarin SK. Peroxisome proliferators-activated receptor gamma2 Pro12Ala variant is associated with body mass index in non-alcoholic fatty liver disease patients. Hepatol. Int. 2010;5:575–580. doi: 10.1007/s12072-010-9225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnell M. Peroxisome proliferator-activated receptor gamma and the regulation of adipocyte function: lessons from human genetic studies. Best Pract. Res. Clin. Endocrinol. Metab. 2005;19:501–523. doi: 10.1016/j.beem.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Haiman CA, Stram DO. Utilizing HapMap and tagging SNPs. Methods Mol. Med. 2008;141:37–54. doi: 10.1007/978-1-60327-148-6_3. [DOI] [PubMed] [Google Scholar]
- Heude B, Pelloux V, Forhan A, Bedel JF, Lacorte JM, Clement K, Charles MA. Association of the Pro12Ala and C1431T variants of PPARgamma and their haplotypes with susceptibility to gestational diabetes. J. Clin. Endocrinol. Metab. 2011;96:E1656–E1660. doi: 10.1210/jc.2011-0381. [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium. The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- International HapMap Consortium. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaziri R, Lobbens S, Aubert R, Pean F, Lahmidi S, Vaxillaire M, Porchay I, Bellili N, Tichet J, Balkau B, Froguel P, Marre M, Fumeron F. The PPARG Pro12Ala polymorphism is associated with a decreased risk of developing hyperglycemia over 6 years and combines with the effect of the APM1 G-11391A single nucleotide polymorphism: the Data From an Epidemiological Study on the Insulin Resistance Syndrome (DESIR) study. Diabetes. 2006;55:1157–1162. doi: 10.2337/diabetes.55.04.06.db05-0676. [DOI] [PubMed] [Google Scholar]
- Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J. Gastroenterol. 2008;14:22–28. doi: 10.3748/wjg.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Park JY, Lee KU, Lee GE, Jeon SH, Kim JH, Kim CH. Effect of body weight and lifestyle changes on long-term course of nonalcoholic fatty liver disease in Koreans. Am. J. Med. Sci. 2009;337:98–102. doi: 10.1097/MAJ.0b013e3181812879. [DOI] [PubMed] [Google Scholar]
- Kotronen A, Yki-Jarvinen H, Aminoff A, Bergholm R, Pietilainen KH, Westerbacka J, Talmud PJ, Humphries SE, Hamsten A, Isomaa B, Groop L, Orho-Melander M, Ehrenborg E, Fisher RM. Genetic variation in the ADIPOR2 gene is associated with liver fat content and its surrogate markers in three independent cohorts. Eur. J. Endocrinol. 2009;160:593–602. doi: 10.1530/EJE-08-0900. [DOI] [PubMed] [Google Scholar]
- Liu DX, Hua Q, Liu LS, Guo JC. Association of peroxisome proliferator-activated receptorgamma gene Pro12Ala and C161T polymorphisms with metabolic syndrome. Circ. J. 2008;72:551–557. doi: 10.1253/circj.72.551. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Amouyel P. Impact of genetic variation of PPARgamma in humans. Mol. Genet. Metab. 2004;83:93–102. doi: 10.1016/j.ymgme.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Cottel D, Amouyel P, Dallongeville J. Lack of association between certain candidate gene polymorphisms and the metabolic syndrome. Mol. Genet. Metab. 2005;86:293–299. doi: 10.1016/j.ymgme.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Fajas L, Gouilleux F, Cottel D, Helbecque N, Auwerx J, Amouyel P. A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler. Thromb. Vasc. Biol. 2003;23:289–294. doi: 10.1161/01.atv.0000051382.28752.fe. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Tanck MW, Fajas L, Janot C, Helbecque N, Cottel D, Auwerx J, Amouyel P, Dallongeville J. Study of a new PPARgamma2 promoter polymorphism and haplotype analysis in a French population. Mol. Genet. Metab. 2005;85:140–148. doi: 10.1016/j.ymgme.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mendez-Sanchez N, Arrese M, Zamora-Valdes D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423–433. doi: 10.1111/j.1478-3231.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am. J. Gastroenterol. 2007;102:2716–2717. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- Osterreicher CH, Brenner DA. The genetics of nonalcoholic fatty liver disease. Ann. Hepatol. 2007;6:83–88. [PubMed] [Google Scholar]
- Ranjith N, Pegoraro RJ, Naidoo DP, Shanmugam R, Rom L. Genetic variants associated with insulin resistance and metabolic syndrome in young Asian Indians with myocardial infarction. Metab. Syndr. Relat. Disord. 2008;6:209–214. doi: 10.1089/met.2008.0023. [DOI] [PubMed] [Google Scholar]
- Rey JW, Noetel A, Hardt A, Canbay A, Alakus H, Zur Hausen A, Dienes HP, Drebber U, Odenthal M. Pro12Ala polymorphism of the peroxisome proliferator-activated receptor gamma2 in patients with fatty liver diseases. World J. Gastroenterol. 2010;16:5830–5837. doi: 10.3748/wjg.v16.i46.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryysy L, Hakkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Jarvinen H. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes. 2000;49:749–758. doi: 10.2337/diabetes.49.5.749. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, Feldstein AE, Nobili V. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J. Pediatr. Gastroenterol. Nutr. 2011;53:190–195. doi: 10.1097/MPG.0b013e31821b4b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- Tonjes A, Stumvoll M. The role of the Pro12Ala polymorphism in peroxisome proliferator-activated receptor gamma in diabetes risk. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:410–414. doi: 10.1097/MCO.0b013e3281e389d9. [DOI] [PubMed] [Google Scholar]
- Wan J, Xiong S, Chao S, Xiao J, Ma Y, Wang J, Roy S. PPARgamma gene C161T substitution alters lipid profile in Chinese patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc. Diabetol. 2010;9:13. doi: 10.1186/1475-2840-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Jacobs DR, Jr, Schreiner PJ, Siscovick DS, Steffes MW, Fornage M. Patterns of association between PPARgamma genetic variation and indices of adiposity and insulin action in African-Americans and whites: the CARDIA Study. J. Mol. Med (Berl) 2006;84:955–965. doi: 10.1007/s00109-006-0088-7. [DOI] [PubMed] [Google Scholar]
- Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin. Liver Dis. 2007;27:44–54. doi: 10.1055/s-2006-960170. [DOI] [PubMed] [Google Scholar]
- Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Ohta K, Narui T, Abe R, Suzuki H, Ohtsuki M. Ultrasonographical diagnosis of fatty liver: significance of the liver-kidney contrast. Tohoku J. Exp. Med. 1983;139:43–50. doi: 10.1620/tjem.139.43. [DOI] [PubMed] [Google Scholar]
- Yang H, Li YY, Nie YQ, Sha WH, Du YL, Lai XB, Zhou YJ. Effect of peroxisome proliferator-activated receptors-gamma and co-activator-1alpha genetic polymorphisms on plasma adiponectin levels and susceptibility of non-alcoholic fatty liver disease in Chinese people. Liver Int. 2008;28:385–392. doi: 10.1111/j.1478-3231.2007.01623.x. [DOI] [PubMed] [Google Scholar]
- Yang LL, Hua Q, Liu RK, Yang Z. Association between two common polymorphisms of PPARgamma gene and metabolic syndrome families in a Chinese population. Arch. Med. Res. 2009;40:89–96. doi: 10.1016/j.arcmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wen J, Li Q, Tao X, Ye Z, He M, Zhang W, Huang Y, Chen L, Ling C, Qu S, Hu R. PPARG gene Pro12Ala variant contributes to the development of non-alcoholic fatty liver in middle-aged and older Chinese population. Mol. Cell Endocrinol. 2012;348:255–259. doi: 10.1016/j.mce.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Ye Q, Lv ZS. A study of the association between PPARr2 gene Pro12Ala polymorphism and NAFLD. Zhonghua Gan Zang Bing Za Zhi. 2007;15:228–229. [PubMed] [Google Scholar]
- Zhou YJ, Li YY, Nie YQ, Ma JX, Lu LG, Shi SL, Chen MH, Hu PJ. Prevalence of fatty liver disease and its risk factors in the population of South China. World J. Gastroenterol. 2007;13:6419–6424. doi: 10.3748/wjg.v13.i47.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YJ, Li YY, Nie YQ, Yang H, Zhan Q, Huang J, Shi SL, Lai XB, Huang HL. Influence of polygenetic polymorphisms on the susceptibility to non-alcoholic fatty liver disease of Chinese people. J. Gastroenterol. Hepatol. 2010;25:772–777. doi: 10.1111/j.1440-1746.2009.06144.x. [DOI] [PubMed] [Google Scholar]
- Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr. Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]