Abstract
The ectodomain of matrix protein 2 (M2e) of influenza virus is suggested to be a rational target for a universal influenza A vaccine. However, there are some concerns that M2e vaccines might not be highly effective in the general population with diverse genetic backgrounds. Here we examined the immunogenicity and protective efficacy of the baculovirus-derived virus-like particles containing multiple M2e (M2eVLP) with AS04 adjuvant in a C57BL/6 mouse strain (H-2b). M2eVLP vaccine induced significant levels of M2e-specific IgG in C57BL/6 mice after vaccination. Furthermore, M2eVLP adjuvanted with AS04 was more effective than M2eVLP alone in conferring protection as well as in inducing recall humoral and T cell responses specific for M2e after lethal influenza virus challenge. A mechanistic study provides evidence that activation of dendritic cells by the toll-like receptor 4 agonist MPL in the AS04 adjuvant was associated with interferon-γ producing CD4 T cell responses. Our results suggest that AS04 adjuvanted M2eVLP vaccines have the potential to improve cross-protection.
Keywords: Influenza virus, M2e VLPs, adjuvants, C57BL/6
Introduction
Current influenza virus vaccine strategies based on the variable hemagglutinin (HA) protein can help provide strain-specific protection, but the emergence of drifted or pandemic strains would cause a substantial burden of disease. The extracellular domain of ion-channel protein M2 (M2e) is well conserved across human influenza A subtypes although there are few residue changes among avian and swine species origin influenza A viruses [1–2]. Therefore, M2e-based vaccines have been investigated as a potential candidate for a universal influenza vaccine [3–5]. Various studies have shown that mice immunized with M2e-carrier constructs and subsequently challenged with influenza virus were protected from death [6–14]. Nonetheless, most M2 vaccine studies were reported using BALB/c (H-2d) mice that raised high responses to M2 vaccination [8–10, 13, 15–21]. Previously, it was shown that mouse strains with H-2d, H-2k and H-2b (C57BL/6) haplotypes were high, intermediate, and very low responders respectively to HA-based vaccine [22] and influenza M2 protein expressed by recombinant adenovirus vectors [23]. It was reported that some humans exhibited a poor response after trivalent inactivated influenza [24–25] or M2 [26] vaccination. These inadequate responses have been associated with the diverse genetic polymorphism in the genes associated with antigen processing and presentation [27] and emphasize the importance of examining the vaccine efficacy in the population with various genetic backgrounds and in a large size. Previously, we developed a vaccine construct with tandem repeat of M2e, which was expressed in a membrane-anchored form using the recombinant baculovirus expression system and presented on the enveloped virus-like particle (M2eVLP) that was effective in inducing M2e-specific antibodies in BALB/c (H-2d) mice [14].
Adjuvant System 04 (AS04) consisting of MPL (3-O-desacyl-4′-monophosphoryl lipid A) toll-like receptor 4 (TLR4) ligand and aluminum hydroxide (Alum) is licensed for use in humans [28]. In this study, we determined immunogenicity and protective efficacy of adjuvanted M2eVLP with AS04, MPL, or Alum in C57BL/6 mice (H-2b).
Materials and methods
Preparation of influenza virus and M2eVLPs
Influenza virus A/PR/8/1934 (H1N1, abbreviated as A/PR8) was grown in 10-day-old embryonated hen’s eggs at 37°C for 2 days. M2eVLP that contain tandem repeat of heterologous M2e presented on VLP was prepared using the insect cell expression system as described previously [14]. The gene construct for encoding multiple M2e was genetically designed to contain a melittin signal peptide, a polypeptide sequence derived from flagellin, five copies of influenza virus M2e sequences from human type-SLLTEVETPIRNEWGSRSNDSSD (2x), swine type-SLLTEVETPTRSEWESRSSDSSD (1x, A/California/4/2009, H1N1), avian type I-SLLTEVETPTRNEWESRSSDSSD (1x, A/Vietnam/1203/04, H5N1) and avian type II-SLLTEVETLTRNGWGCRCSDSSD (1x,A/Hong Kong/156/97, H5N1), a tetramerizing leucine zipper derived from GCN4 [21], and transmembrane and cytoplasmic domains of HA derived from influenza A/PR/8/34 virus [29]. Spodoptera frugiperda Sf9 insect cells were co-infected with recombinant baculoviruses (rBVs) expressing influenza M2e5x and M1 proteins, respectively. At 2 days after infection, the infected cell culture supernatants were clarified by centrifugation (6,000 rpm, 30 minutes) and then were concentrated by the QuixStand hollow fiber-based ultrafiltration system (GE Healthcare, Piscataway, NJ). M2e5x VLPs in the culture supernatants were purified by using discontinuous sucrose gradient ultracentrifugation with layers of 20 and 60% (wt/vol) as previously described [30].
Immunization and challenge
For animal experiments, 6- to 8-week-old female C57BL/6 mice (n = 10; Harlan Laboratories) were intramuscularly immunized with 10 μg of M2eVLP (total protein) or mixed with 5 μg of MPL (Sigma-Aldrich), 50 μg of aluminum hydroxide (Alum), or AS04 (5 μg MPL plus 50 μg Alum) in the thigh muscle using an insulin syringe with 6mm 29G needle at week 0 and 4. Blood samples were collected at 3 weeks after each immunization. Immunized mice were then challenged with a lethal dose (4xLD50) of A/PR8 influenza virus at 8 weeks after boost immunization. After challenge with influenza virus, survival rate and weight loss were observed daily for 14 days post-infection (p.i.). All animal experiments presented in this study were approved by the Georgia State University IACUC review boards (IACUC A11026).
Determination of antibody responses
Influenza virus-specific or M2e-specific antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) as previously described [30].
Preparation of BALF and lung extracts
For bronchoalveolar lavage fluids (BALF), the lungs were lavaged with 1 ml PBS via a 25 gauge-catheter inserted in the trachea. Each mouse lung was homogenized and centrifuged at 1400 × g at 4°C for 10 min. Lung viral titers were determined from the 50% egg infective dose (EID50) by using embryonated hen’s eggs. Cytokine levels in BALF were determined using ELISA kits for IFN-γ and IL-6 according to the manufacturers’ instructions in duplicate against a standard curve (eBioscience, SanDiego, CA).
Determination of antibody secreting cell and T cell responses
On day 5 p.i., spleen cells were isolated from challenged mice and single cells were cultured in 96-well plates coated with M2eVLP [29]. The supernatants were harvested at 1 and 6 days of culture and two-fold diluted with PBST to determine the levels of M2e-specific IgG responses by ELISA. Absorbance was read at 450 nm. Interferon (IFN)-γ secreting cell spots were determined on Multi-screen 96 well plates (Millipore, Billerica, MA) coated with cytokine specific capture antibodies as previously described [31].
Flow cytometric analysis
To evaluate intracellular cytokine production, lung cells were stimulated with 5 μg/ml of M2 peptide (SLLTEVETPIRNEWGSRSN) at 37°C for 5 h and then stimulated lung cells were surface stained for anti-CD45-peridinin chlorophyll protein complex, anti-CD4-allophycocyanin (APC) and anti-CD8α-r-phycoerythrin (PE) antibodies and then were permeable using the Cytofix/Cytoperm kit (BD Biosciences). Intracellular cytokines were revealed by staining the cells with or anti-granzyme B-fluorescein isothiocyanate or anti-IFN-γ-APC-Cy7 antibodies. Stained cells were analyzed using LSRFortessa (BD Biosciences) and FlowJo software (Tree Star Inc.).
Preparation and in vitro stimulation of bone marrow derived dendritic cells (BMDCs)
BMDCs were prepared from bone marrow cells of C57BL/6 mice treated with 10 ng/ml of mouse granulocytes-macrophages colony stimulating factor for 6 days. BMDCs were stimulated with 5 μg/ml of M2eVLP alone or in combination with 1 μg/ml of MPL or 10 μg/ml of Alum at 2 × 105 cells/ml in 96-well plate for 2 days. IL-6, TNF-α and IL-12 cytokines were determined in the BMDC culture supernatants using ELISA as described above. For mixed lymphocyte reactions, BMDCs were first treated with M2eVLPs alone or in combination with MPL or Alum. After wash, BMDC were cocultured with CFSE-labeled allogeneic BALB/c splenocytes with the ratio of 1:10 for BMDC to splenocytes. After 5 days, the cells were washed and the proliferation and activation of the T cells were assessed by flow cytometry. Endotoxin analysis using the Limulus Amebocyte Lysate test (Lonza Inc, Walkersville, MD) indicated that all preparations were endotoxin-free.
Statistical analysis
All results are expressed as the mean ± standard error of the mean (SEM). Significant differences among treatments were evaluated by 1-way ANOVA or two-tailed student’s t-test where appropriate. P-values of less than or equal to 0.05 were considered statistically significant.
Results
Immune responses to vaccination with M2eVLP in C57BL/6 mice
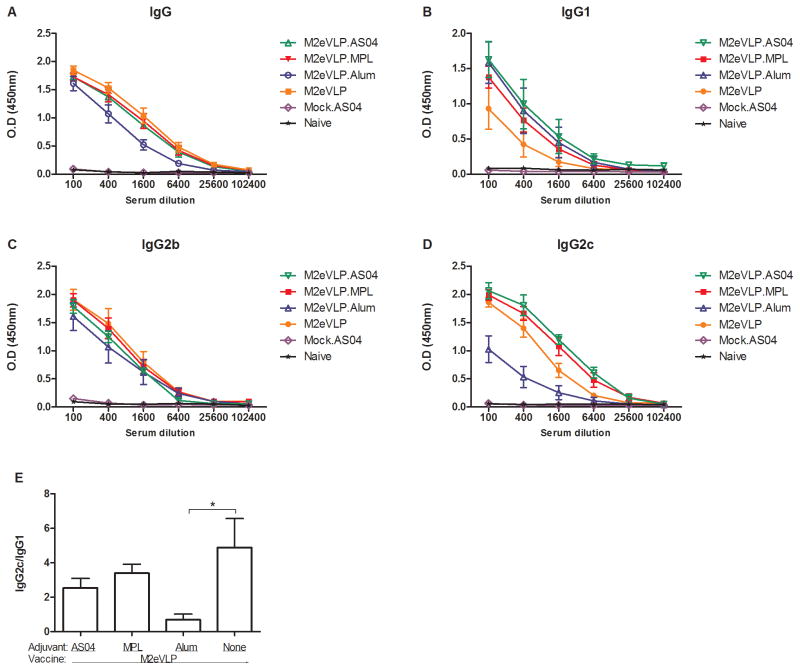
To evaluate the immunogenicity of M2eVLP vaccines adjuvanted with AS04 (M2eVLP.AS04), MPL (M2eVLP.MPL), or Alum (M2eVLP.Alum), groups of mice were intramuscularly immunized and antibody responses in sera were determined (Fig. 1). M2eVLP, M2eVLP.AS04, and M2eVLP.MPL vaccination induced high levels of M2e-specific IgG in C57BL/6 mice (Fig. 1A), indicating that M2eVLP is immunogenic in C57BL/6 mice. The M2eVLP.Alum group showed approximately 3-fold lower IgG levels than those in other groups (Fig. 1A), but there was no significant difference between groups.
Fig. 1. M2e-specific total IgG and IgG isotype antibody responses.
C57BL/6 mice (n = 10) were immunized with M2eVLP alone, or mixed with adjuvant AS04 (M2eVLP.AS04), MPL (M2eVLP.MPL), or Alum (M2eVLP.Alum). Control groups of mice included AS04 only without M2eVLP vaccine (Mock.AS04) and unimmunized naïve mice (Naïve). Blood samples were collected at 3 weeks after boost immunization. IgG (A), IgG1 (B), IgG2b (C), and IgG2c (D) was detected by M2e peptide as an ELISA-coating antigen. (E) Ratios of IgG2a/IgG1 isotype M2e-specific antibodies. Sera were serially diluted and ELISA was performed for serum antibodies specific for M2e peptide. Error bars indicates mean ± SEM.
IgG isotypes induced by M2eVLP vaccination adjuvanted with AS04, MPL, or Alum were determined (Fig. 1B–D). The anti-M2e IgG1 responses in the M2eVLP.Alum and M2eVLP.AS04 groups were higher than those in mice vaccinated with M2eVLP alone (100 x serum dilution, p < 0.05, Fig. 1B). There were no differences in levels of IgG2b antibodies among groups (Fig. 1C). Mice with the B6 background lack the Igh-1a allele that codes for IgG2a but instead express IgG2c from the Igh-1b allele, [32–33] and thus we measured IgG2c rather than IgG2a. As shown in Fig. 1D, anti-M2e IgG2c responses in the M2eVLP.MPL and M2eVLP.AS04 groups were significantly higher than those in the M2eVLP.Alum group at 100, 400, and 1,600 times serum dilution (p < 0.001) and 6,400 times diluted serum (p < 0.05), respectively. As a result of the reverse pattern of IgG isotypes between M2eVLP.Alum and other groups, the M2eVLP, M2eVLP.MPL, and M2eVLP.AS04 groups showed higher ratios of IgG2c/IgG1isotypes than the M2eVLP.Alum group (Fig. 1E).
Since a previous study [23] reported that recombinant M2 expressing DNA and adenovirus vector vaccines were highly immunogenic in BALB/c mice but not in C57BL/6 mice, we compared M2 antibody responses of M2eVLP vaccine alone and M2e peptide in BALB/c and C57BL/6 mice. Mice were intramuscularly immunized with 10 μg of M2eVLP or M2e peptide in the absence of adjuvants. Anti-M2e IgG concentrations in M2eVLP-immunized BALB/c mice (5688.0 ± 792.2 ng/ml) were observed approximately 1.2-fold higher than those in M2eVLP-immunized C57BL/6 mice (4520.3 ± 823.8 ng/ml). Anti-M2e IgG1 responses in M2eVLP-immunized BALB/c mice (2322.4 ± 1155.8 ng/ml) also showed approximately 3.5-fold higher than those in M2eVLP-immunized C57BL/6 mice (659.5 ± 582.1 ng/ml). No anti-M2e antibody response was observed in any of M2e peptide-immunized BALB/c and C57BL/6 mice (data not shown). These results suggest that M2e on VLP carrier is immunogenic in C57BL/6 mice as well as in BALB/c mice except IgG1 isotype antibody.
M2eVLP with AS04 improved protection against influenza virus
To compare the efficacy of protection, groups of mice that were intramuscularly immunized with M2eVLP adjuvanted with AS04, MPL, or Alum were challenged with a lethal dose (4xLD50) of A/PR8 virus at 8 weeks after boosting (Fig. 2). All naive and mock.AS04 control mice (AS04 without vaccine) lost over 25% in body weight and died or had to be euthanized. The survival rates of the mice vaccinated with M2eVLP.Alum, M2eVLP alone, M2eVLP.MPL, and M2eVLP.AS04 were 20%, 40%, 60%, and 100%, respectively (Fig. 2A). The groups of mice with M2eVLP alone and M2eVLP.Alum showed a substantial loss of approximately 22% in body weight post challenge. The M2eVLP.MPL group lost less weight (approximately 15%). By contrast, the M2eVLP.AS04 group showed a slight loss (approximately 11%) in body weight. Moreover, the M2eVLP.AS04 group recovered body weight significantly faster than the M2eVLP group after 10 day p.i. (p < 0.05).
Fig. 2. Vaccination with AS04-adjuvanted M2eVLP induces improved protective immunity.
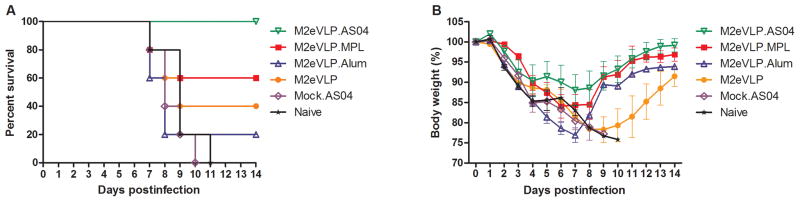
Groups of C57BL/6 mice (n = 5) were challenged with a lethal dose of A/PR/8/34 (H1N1) virus at 8 weeks after boost vaccination. Survival rates (A) and body weight (B) were monitored for 14 days. Groups are the same as described in the Fig. 1.
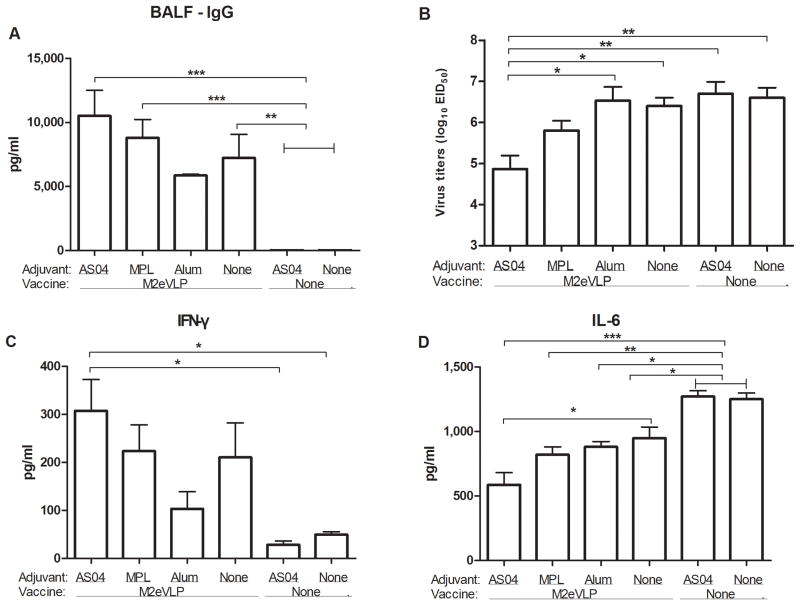
To better understand the improved protection by AS04 adjuvant, we determined immune responses and lung viral titers at an early time post challenge. M2 specific IgG concentrations in BALF from the groups of mice with M2eVLP.AS04 (p < 0.001), M2eVLP.MPL (p < 0.001), and M2eVLP alone (p < 0.01) were significantly higher than those in naïve and mock.AS04 control mice at day 5 p.i., respectively (Fig. 3A). The M2eVLP.AS04 group showed significantly lower lung viral titers compared with those in the M2eVLP or M2eVLP.Alum group (p < 0.05), and in naïve and mock.AS04 control groups (p < 0.01, Fig. 3B) at day 5 p.i.. Moreover, the lung viral titers of the M2eVLP.AS04 group (7.2 ± 0.1 Log10EID50/ml) were 4-fold lower than those in the naïve group (7.8 ± 0.1 Log10EID50/ml) even at an earlier time point day 3 p.i., which is statistically significant (p < 0.05). The difference between the M2eVLP.AS04 group and other groups was found to be bigger at the later time point day 5 p.i.. The lowest lung viral titer in the M2eVLP.AS04 group indicates a good correlation with its high protective efficacy of lowest body weight loss and 100% survival rate.
Fig. 3. Antibody responses, virus, and inflammatory cytokine levels in BALF and lungs after challenge.
Levels of IgG antibodies were determined in samples collected from the immunized mice at day 5 p.i. with A/PR/8/34 (H1N1) virus (n = 5). (A) IgG antibody levels in BALF. The antibody level was determined by ELISA using M2e peptide as a coating antigen. Lung viral titers (B), IFN-γ (C), and IL-6 (D) cytokine in BALF were determined from immunized mice at day 5 p.i.. Lung viral titers were determined by an egg infection assay. IFN-γ and IL-6 was determined by a cytokine ELISA. Data represent mean ± SEM. Groups of mice are the same as described in the Fig. 1 in terms of the presence or absence of M2eVLP vaccine and the addition of adjuvant or not (None).
The levels of IFN-γ in BALF were found to be significantly higher from the group of mice vaccinated with M2eVLP.AS04 compared to those in naïve and mock.AS04 control mice at day 5 p.i. (p < 0.05, Fig. 3C). In contrast, a reverse pattern was observed with IL-6 inflammatory cytokine. The M2eVLP.AS04 group showed the lowest level of BALF IL-6 among groups after virus challenge (Fig. 3D, p < 0.05 compared to M2eVLP). Whereas naïve and mock.AS04 control mice showed highest levels of BALF IL-6 cytokine (Fig. 3D, p < 0.05 compared to M2eVLP, M2eVLP.Alum; p < 0.01 compared to M2eVLP.MPL; p < 0.001 compared to M2eVLP.AS04).
Enhancement of antibody secreting cell responses by M2eVLP vaccination with AS04
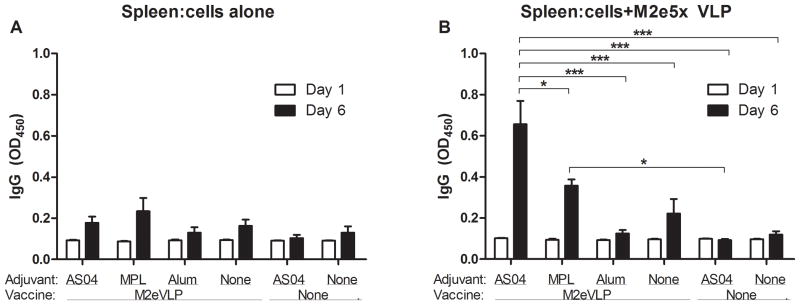
To determine antibody secreting cell responses (ASC), spleen cells were harvested at day 5 p.i. and cultured in vitro for 1 and 6 days. Antigenic challenge would expand ASC responses indicating anamnestic responses since the number of B cells in a memory state is relatively low with VLP vaccination [34]. Significantly higher levels of anti-M2e IgG were secreted into supernatants after 6 day-culture of splenocytes from the M2eVLP.AS04 group than those from other groups (Fig. 4). Moreover, the levels of IgG specific to M2e secreted into the culture supernatants were significantly increased in the M2eVLP.MPL group compared to those from the mock.AS04 group (p < 0.05).
Fig. 4. Vaccination with AS04-adjuvanted M2eVLP induces enhanced humoral immune responses.
Spleen cells were isolated from mice at day 5 p.i. (n = 5) and were incubated in the absence (A) or in the presence (B) of M2eVLP coated on the culture plates for in vitro stimulation. Culture supernatants were harvested after 1 or 6 days of culture. M2e-specific IgG levels were determined by ELISA. Data represent mean ± SEM. Groups of mice are the same as described in the Fig. 1 in terms of the presence or absence of M2eVLP vaccine and the addition of adjuvant or not (None).
Enhanced effector function of M2e-specific CD4+ T cells in lungs by M2eVLP vaccination with AS04
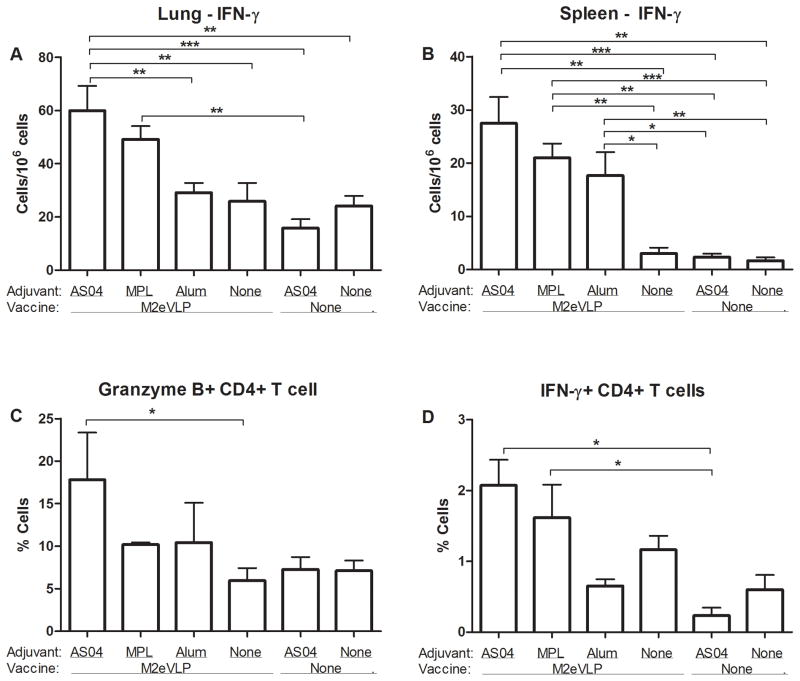
To determine effector T cell responses, we measured IFN-γ cytokine producing lung and spleen cell spots after in vitro stimulation with M2e peptide (Fig. 5). The spot numbers of IFN-γ secreting cells were detected at a significantly higher level in lungs from mice in the M2eVLP.AS04 group than those from mice in the M2eVLP (p < 0.01) or the M2eVLP.Alum (p < 0.01), the naive (p < 0.01), and the mock.AS04 group (p < 0.001), respectively (Fig. 5A). Moreover, significantly higher levels of IFN-γ–secreting cells were observed in spleens from M2eVLP.AS04, M2eVLP.MPL, or M2eVLP.Alum immune mice compared to those from M2VLP alone, naïve, and mock.AS04 control mice. In addition, M2eVLP.alum group showed higher levels of IL-4 secreting cell spots from lung samples, indicating Th2-biased immune response which might have affected the survival rate (data not shown).
Fig. 5. Vaccination with AS04-adjuvanted M2eVLP is effective in inducing recall cellular immune responses.
(A) IFN-γ–secreting cells in lung (n = 5). (B) IFN-γ–secreting cells in spleen (n = 5). Lung cells and splenocytes were isolated from mice at day 5 p.i.. Cytokine-producing cell spots were counted by ELISPOT reader. Granzyme B- (C) or IFN-γ– (D) secreting CD4+ T cells in lungs. Lung cells were harvested, stained with CD45, CD4, CD8α, IFN-γ, and granzyme B antibodies, and analyzed by flow cytometry. Data represent mean ± SEM. Groups of mice are the same as described in the Fig. 1 in terms of the presence or absence of M2eVLP vaccine and the addition of adjuvant or not (None).
Lung cells were stimulated with M2e peptide for a short time course of 5 hours prior to intracellular staining of granzyme B and IFN-γ. The level of M2e-specific granzyme B producing CD4+ T cells was significantly increased approximately 3-fold in the M2eVLP.AS04 group compared with the M2eVLP group without adjuvant (p < 0.05, Fig. 5C). Furthermore, the percentage of M2-specific IFN-γ producing CD4+ T cells was significantly increased in the M2e5VLP.AS04 and M2e5VLP.MPL groups compared with the mock.AS04 control group (p < 0.05, Fig. 5D). These results indicate that higher levels of IFN-γ and granzyme B secreting T cell responses in the M2eVLP.AS04 group are likely contributing to improved protection.
AS04 adjuvant stimulates bone-marrow dendritic cells (BMDCs) activating CD4+ T cells
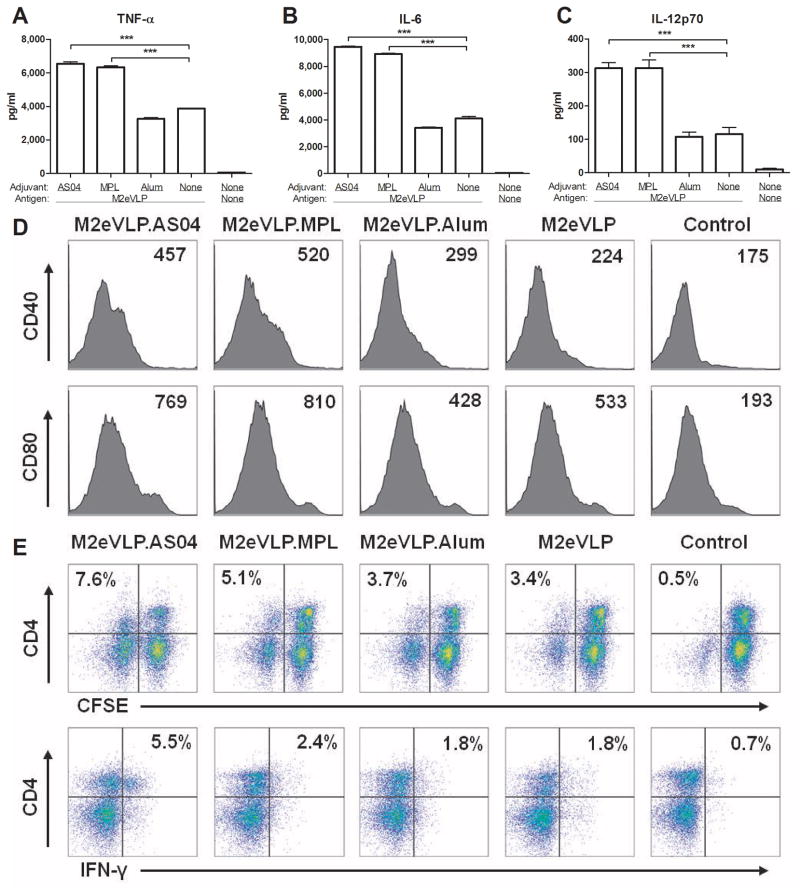
Adjuvant effects on stimulating DCs were analyzed after incubation of BMDCs in vitro with M2eVLP in the presence of different adjuvant formulations. The levels of TNF-α (Fig. 6A), IL-6 (Fig. 6B), and IL-12 (Fig. 6C) in the supernatants from BMDCs treated with M2eVLP were significantly higher than those from mock control. Inclusion of AS04 or MPL but not Alum in M2eVLP during BMDC cultures resulted in further increases in TNF-α, IL-6, and IL-12 cytokine levels. The expression of costimulatory molecules CD40 and CD80 provides essential signals to naive T cells to initiate an optimal adaptive response. BMDCs treated with MPL or AS04 induced higher levels of CD40 and CD80 activation markers as compared with BMDCs treated with M2eVLP alone (Fig. 6D).
Fig. 6. AS04-mediated stimulation of dendritic cells is responsible for T cell activation.
(A–D) Expression of cytokines and markers by activated dendritic cells. C57BL/6 BMDCs were stimulated with M2eVLP alone or in combination with AS04, MPL or Alum. TNF-α (A), IL-6 (B), and IL-12 (C) was determined by a cytokine ELISA. Data represent mean ± SEM. (D) Representative histograms of CD40 or CD80 expression of BMDCs. Numbers in the histograms indicate mean fluorescence intensity (MFI) of each marker. (E) C57BL/6 BMDCs were stimulated with M2eVLP alone or in combination with AS04, MPL or Alum. After wash, BMDC were cocultured for 5 days with allogeneic BALB/c splenocytes with the ratio of 1:10 for BMDC to splenocytes. Profiles showing CFSE profile and IFN-γ intracellular cytokine staining of CD4+ T cells are representative of two independent experiments. The numbers indicate percentages of CFSE− CD4+ cell or IFN-γ+ CD4+ cell population.
To investigate whether BMDCs with enhanced expression of co-stimulatory molecules by AS04 would translate into activating T cells, CFSE-labeled-allogeneic splenocytes were incubated for 5 days with BMDCs that had been pretreated with M2eVLP alone or in combination with AS04, MPL, or Alum. T cell proliferation was evaluated by measuring the decrease in CFSE labeling in proliferating cells. BMDCs that were stimulated with M2eVLP induced the proliferation of CD4+ T cells and also increased the level of proliferated CD4+ T cells when BMDCs were stimulated by adding MPL or AS04 adjuvant to M2eVLP (Fig. 6E). Furthermore, BMDCs when stimulated with M2eVLP plus AS04 increased the percentage of IFN-γ–secreting CD4+ T cells by approximately 3 fold compared to M2eVLP alone (Fig. 6E). The addition of Alum adjuvant to M2eVLP did not stimulate BMDCs subsequently resulting in no further proliferation and activation of T cells compared to those of M2eVLP alone. Therefore, these results suggest that AS04 adjuvant stimulates DCs to secrete more cytokines as well as to induce increased levels of CD4+ T cell proliferation and IFN-γ production.
Discussion
Most universal influenza vaccine studies targeting M2e were performed in BALB/c mice that were known to be a high responder to subunit vaccines including M2e antigen. Immune responses to M2 were reported to be highly influenced depending on the genetic background of mouse strains. Strong immune responses of antibodies and IL-4 cytokine secreting CD4+ T cells were observed in BALB/c mice (H-2d) that received M2 DNA priming and M2 recombinant adenoviral vector boosting [23]. In contrast, the same M2 DNA and adenovirus vector vaccines failed to induce detectable levels of humoral and cellular immune responses to M2 in C57BL/6 mice (H-2b) [23]. Since C57BL/6 mice were considered a low responder, we tested AS04 and its adjuvant components. Unexpectedly, this study showed that M2eVLP was immunogenic in both BALB/c and C57BL/6 mouse strains. Therefore, these results suggest that M2eVLP that contains tandem repeat of M2e anchored in the lipid membrane of VLP [14] would be more immunogenic than other forms of M2e vaccines in mice with different genetic background.
Protective efficacy was expected to be similar among immunized groups because antibody responses for M2e were found to be comparable after M2eVLP immunization in the presence or absence of adjuvant. Despite similar levels of M2e antibody responses among groups, M2eVLP adjuvanted with AS04 was more effective than M2eVLP alone in protecting vaccinated mice against weight loss and lung viral replication as well as in increasing survival rates after lethal influenza virus challenge. In particular, AS04 adjuvant increased IgG1 and IgG2c isotype antibodies, IFN-γ producing lung and spleen cells, and granzyme B positive CD4+ T cells. Addition of Alum adjuvant lowered IgG2c antibody responses and protective efficacy of M2eVLP vaccine. TLR4 ligand, MPL behaved similarly as AS04 in some immune protective correlates such as antibody isotypes, IFN-γ producing lung and spleen cells, and stimulating BMDCs in vitro to induce inflammatory cytokines. Importantly, AS04 composed of both MPL and Alum was more effective in lung viral clearance, preventing weight loss, and in enhancing survival rates as well as in increasing granzyme B positive CD4+ T cells. Classically, CD4+ T cells were believed to participate in the antiviral immune responses by providing the help for CD8+ T and B cell responses to promote viral clearance. Recently, it has been suggested that CD4+ T cell effectors are broadly multifunctional with direct roles in promoting protection against lethal influenza virus infection [35]. The upregulation of granzyme B and perforin expression in influenza-specific CD4+ T cells is restricted to the infection site, suggesting that the appropriate priming of CD4+ T cells by AS04-adjuvanted M2eVLP vaccination would promote the generation of these cytotoxic CD4+ T cells in lung microenvironment. It is likely that induction of granzyme B and IFN-γ producing CD4+ T cells might play an important role in improving protective efficacy as a result of AS04 adjuvant addition to M2eVLP.
Recent observations suggest that the addition of MPL to Alum enhances the vaccine response by rapidly triggering a local cytokine response leading to an optimal activation of antigen presenting cells (APCs) [36]. Interestingly, we found that BMDCs treated with M2eVLP in combination with MPL-containing formulations induced higher levels of cytokine and costimulatory molecules as compared with BMDCs treated with M2eVLP regardless of Alum addition. Furthermore, BMDCs stimulated with M2eVLP in combination with MPL or AS04 induced the proliferation of CD4+ T cells and increased the production of IFN-γ–secreting CD4 T cells. Therefore, it is likely that the superior protection by AS04 adjuvant might have largely resulted from antigen presenting dendritic cell-mediated activation of CD4+ T cells via MPL-mediated TLR4 stimulation. Current data also demonstrate synergism between MPL and Alum adjuvants on protective efficacy of M2eVLP vaccine, supporting evidence that AS04 complemented the ability of M2eVLP to enhance the anamnestic and protective humoral and T cell responses against lethal influenza virus infection via multiple immune effector mechanisms. Addition of Alum adjuvant to M2eVLP did not stimulate BMDCs compared to M2eVLP alone. Incapability of Alum adjuvant to stimulate dendritic cells may explain its inefficacy in exhibiting adjuvant effects on M2eVLP.
In conclusion, AS04 adjuvanted M2eVLP was found to induce improved protection and to generate immunologic memory for rapid recall responses upon viral infection in C57BL/6 mice. An objective in vaccine development is its applicability to the general population with diverse genetic backgrounds. Results in this study suggest that AS04-adjuvanted M2eVLP vaccines are immunogenic and may have the potential to improve cross-protection.
Acknowledgments
This work was supported by NIH/NIAID grants AI105170 (S.M.K.), AI093772 (S.M.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito T, Gorman OT, Kawaoka Y, Bean WJ, Webster RG. Evolutionary analysis of the influenza A virus M gene with comparison of the M1 and M2 proteins. J Virol. 1991;65:5491–8. doi: 10.1128/jvi.65.10.5491-5498.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zebedee SL, Lamb RA. Nucleotide sequences of influenza A virus RNA segment 7: a comparison of five isolates. Nucleic Acids Res. 1989;17:2870. doi: 10.1093/nar/17.7.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiers W, De Filette M, Birkett A, Neirynck S, Min Jou W. A “universal” human influenza A vaccine. Virus Res. 2004;103:173–6. doi: 10.1016/j.virusres.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Du L, Zhou Y, Jiang S. Research and development of universal influenza vaccines. Microbes Infect. 2010;12:280–6. doi: 10.1016/j.micinf.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhao G, Lin Y, Du L, Guan J, Sun S, Sui H, et al. An M2e-based multiple antigenic peptide vaccine protects mice from lethal challenge with divergent H5N1 influenza viruses. Virol J. 2010;7:9. doi: 10.1186/1743-422X-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang BZ, Gill HS, Kang SM, Wang L, Wang YC, Vassilieva EV, et al. Enhanced influenza virus-like particle vaccines containing the extracellular domain of matrix protein 2 and a Toll-like receptor ligand. Clin Vaccine Immunol. 2012;19:1119–25. doi: 10.1128/CVI.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turley CB, Rupp RE, Johnson C, Taylor DN, Wolfson J, Tussey L, et al. Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine. 2011;29:5145–52. doi: 10.1016/j.vaccine.2011.05.041. [DOI] [PubMed] [Google Scholar]
- 8.Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–35. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JM, Van Rooijen N, Bozja J, Compans RW, Kang SM. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc Natl Acad Sci U S A. 2011;108:757–61. doi: 10.1073/pnas.1012199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 11.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Fu TM, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, et al. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009;27:1440–7. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Ernst WA, Kim HJ, Tumpey TM, Jansen AD, Tai W, Cramer DV, et al. Protection against H1, H5, H6 and H9 influenza A infection with liposomal matrix 2 epitope vaccines. Vaccine. 2006;24:5158–68. doi: 10.1016/j.vaccine.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Song JM, OE, Kwon YM, Lee YJ, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2013;21:485–92. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou P, Liu W, Chen YH. The epitope recognized by a monoclonal antibody in influenza A virus M2 protein is immunogenic and confers immune protection. Int Immunopharmacol. 2005;5:631–5. doi: 10.1016/j.intimp.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Okuda K, Ihata A, Watabe S, Okada E, Yamakawa T, Hamajima K, et al. Protective immunity against influenza A virus induced by immunization with DNA plasmid containing influenza M gene. Vaccine. 2001;19:3681–91. doi: 10.1016/s0264-410x(01)00078-0. [DOI] [PubMed] [Google Scholar]
- 17.Mozdzanowska K, Feng J, Eid M, Kragol G, Cudic M, Otvos L, Jr, et al. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine. 2003;21:2616–26. doi: 10.1016/s0264-410x(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Zou P, Chen YH. Monoclonal antibodies recognizing EVETPIRN epitope of influenza A virus M2 protein could protect mice from lethal influenza A virus challenge. Immunol Lett. 2004;93:131–6. doi: 10.1016/j.imlet.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Eliasson DG, El Bakkouri K, Schon K, Ramne A, Festjens E, Lowenadler B, et al. CTA1-M2e-DD: a novel mucosal adjuvant targeted influenza vaccine. Vaccine. 2008;26:1243–52. doi: 10.1016/j.vaccine.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 21.De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–7. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Z, Yoshikawa T, Kadowaki S, Hagiwara Y, Matsuo K, Asanuma H, et al. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J Gen Virol. 1999;80 (Pt 10):2559–64. doi: 10.1099/0022-1317-80-10-2559. [DOI] [PubMed] [Google Scholar]
- 23.Misplon JA, Lo CY, Gabbard JD, Tompkins SM, Epstein SL. Genetic control of immune responses to influenza A matrix 2 protein (M2) Vaccine. 2010;28:5817–27. doi: 10.1016/j.vaccine.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 24.Scheifele DW, Ward BJ, Dionne M, Vanderkooi OG, Loeb M, Coleman BL, et al. Compatibility of ASO3-adjuvanted H1N1pdm09 and seasonal trivalent influenza vaccines in adults: results of a randomized, controlled trial. Vaccine. 2012;30:4728–32. doi: 10.1016/j.vaccine.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Beran J, Peeters M, Dewe W, Raupachova J, Hobzova L, Devaster JM. Immunogenicity and safety of quadrivalent versus trivalent inactivated influenza vaccine: a randomized, controlled trial in adults. BMC Infect Dis. 2013;13:224. doi: 10.1186/1471-2334-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, et al. M2e-based universal influenza A vaccine. Vaccine. 2009;27:6280–3. doi: 10.1016/j.vaccine.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Franco LM, Bucasas KL, Wells JM, Nino D, Wang X, Zapata GE, et al. Integrative genomic analysis of the human immune response to influenza vaccination. Elife. 2013;2:e00299. doi: 10.7554/eLife.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines. 2007;6:723–39. doi: 10.1586/14760584.6.5.723. [DOI] [PubMed] [Google Scholar]
- 29.Kim MC, Lee JS, Kwon YM, OE, Lee YJ, Choi JG, et al. Multiple heterologous M2 extracellular domains presented on virus-like particles confer broader and stronger M2 immunity than live influenza A virus infection. Antiviral Res. 2013;99:328–35. doi: 10.1016/j.antiviral.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS One. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009;4:e7152. doi: 10.1371/journal.pone.0007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jouvin-Marche E, Morgado MG, Leguern C, Voegtle D, Bonhomme F, Cazenave PA. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29:92–7. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 33.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 34.Song JM, Hossain J, Yoo DG, Lipatov AS, Davis CT, Quan FS, et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology. 2010;405:165–75. doi: 10.1016/j.virol.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown DM, Lee S, de Garcia-Hernandez ML, Swain SL. Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. J Virol. 2012;86:6792–803. doi: 10.1128/JVI.07172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–97. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]