Abstract
The tatanans are members of a novel class of complex sesquilignan natural products recently isolated from the rhizomes of Acorus tatarinowii Schott plants. Tatanans A, B and C have previously been reported to have potent glucokinase-activating properties that exceed the in vitro activity of known synthetic antidiabetic agents. Here, using a series of sequential [3,3]-sigmatropic rearrangements, we report the total synthesis of tatanan A in 13 steps and 13% overall yield. We also complete a concise enantioselective total synthesis of more complex, atropisomeric tatanans B and C via a distinct convergent strategy based on a palladium-catalysed diastereotopic aromatic group differentiation (12 steps, 4% and 8% overall yield, respectively). A plausible biosynthetic relationship between acyclic tatanan A and spirocyclic tatanans B and C is proposed and probed experimentally. With sufficient quantities of the natural products in hand, we undertake a detailed functional characterization of the biological activities of tatanans A–C. Contrary to previous reports, our assays utilizing pure recombinant human enzyme demonstrate that tatanans do not function as allosteric activators of glucokinase.
Small-molecule allosteric activators of human glucokinase have generated considerable interest as potential therapeutic agents for the treatment of type 2 diabetes1. These molecules are effective at stimulating insulin secretion from pancreatic β-cells and thereby rapidly reducing plasma glucose levels in animal models2. At least one glucokinase activator, piragliatin, has advanced to phase II clinical trials in humans3. The first glucokinase activator was described in 20034, and since that time pharmaceutical chemists have identified a variety of unique chemical scaffolds capable of stimulating glucokinase activity in vivo5. Although all glucokinase activators investigated to date are synthetic, the recent discovery of the tatanans, a novel class of naturally occurring lignan derivatives that reportedly possess potent glucokinase-activating capabilities, suggests that natural products might represent a rich resource for structurally and mechanistically new antidiabetic agents6,7. As a first step in exploring tatanans and the derivatives thereof as potential diabetes therapeutics, we undertook the total chemical synthesis and detailed mechanistic characterization of the glucokinase-activating properties of tatanans A, B and C.
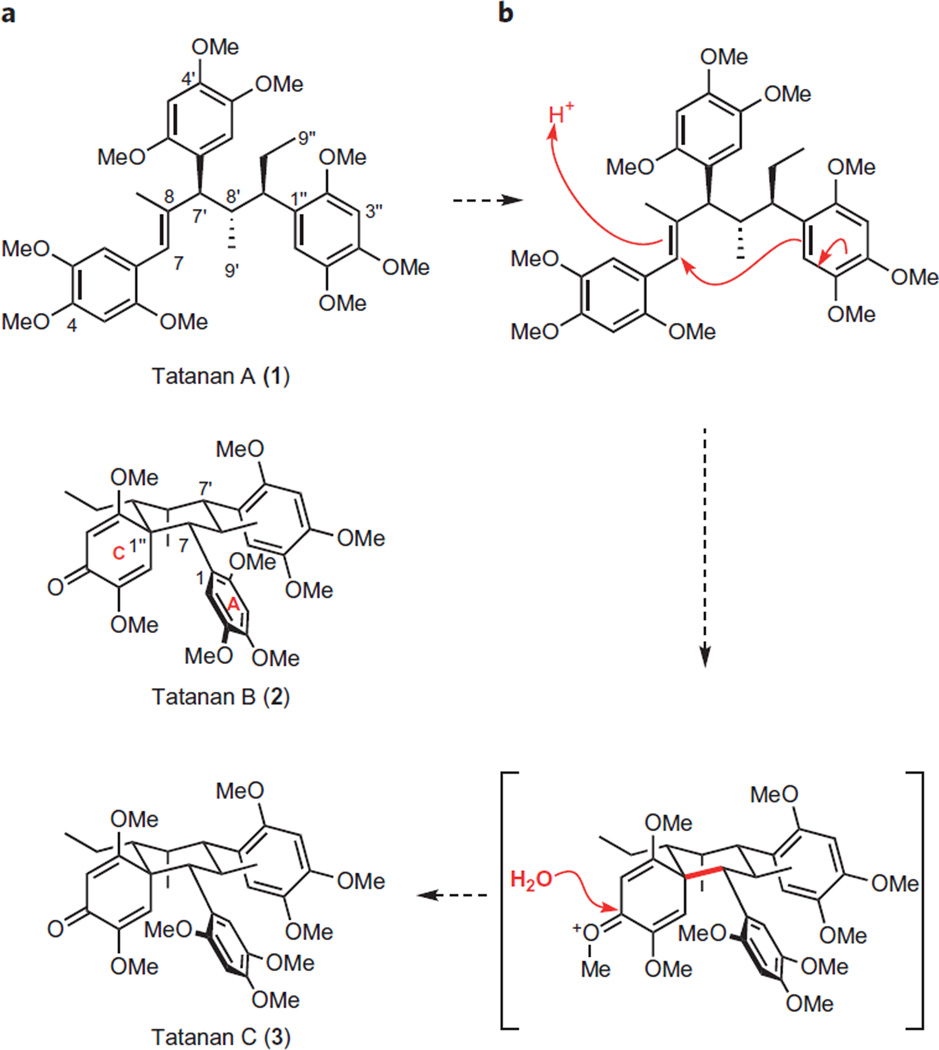
Lignans are a large class of polyphenolic natural products related to oligostilbenes8,9 but distinct in their basic monomeric unit10. Tatanans are structurally unique sesquilignans recently isolated from the ethyl acetate extracts of rhizomes of Acorus tatarinowii Schott plants6. Tatanan A incorporates three consecutive tertiary stereocentres and three aryl substituents appended to an acyclic framework. Tatanans B and C, on the other hand, are cyclic atropoisomers characterized by complex spirocyclic architectures that combine a cyclohexane ring substituted at each position with a 2,5-cyclohexadienone subunit. The existence of atropoisomerism in tatanans B and C is evidently a consequence of the restricted rotation around the C1–C7 bond that links ring A, proximal to the quaternary stereogenic centre at the spirocyclic ring junction, to the cyclohexane subunit. This type of molecular structure is unprecedented among known lignans11,12, enhancing our interest in developing a concise chemical synthesis of these natural products. We postulated that acyclic tatanan A is biosynthetically interrelated to its spirocyclic congeners through a stereospecific electrophilic ring-closing dearomatization initiated by a proton donor, as illustrated in Fig. 1b 13. Thus, an additional goal of our synthesis efforts was to investigate the facility of such a transformation.
Figure 1. Tatanans A–C are structurally unusual novel sesquilignans isolated from the rhizomes of Acorus tatarinowii plants.
These natural products have been reported to potently activate glucokinase. a, Structure of tatanans. b, Proposed biosynthetic relationship between acyclic tatanan A and spirocyclic tatanans B and C.
Results and discussion
Total synthesis of tatanans A–C
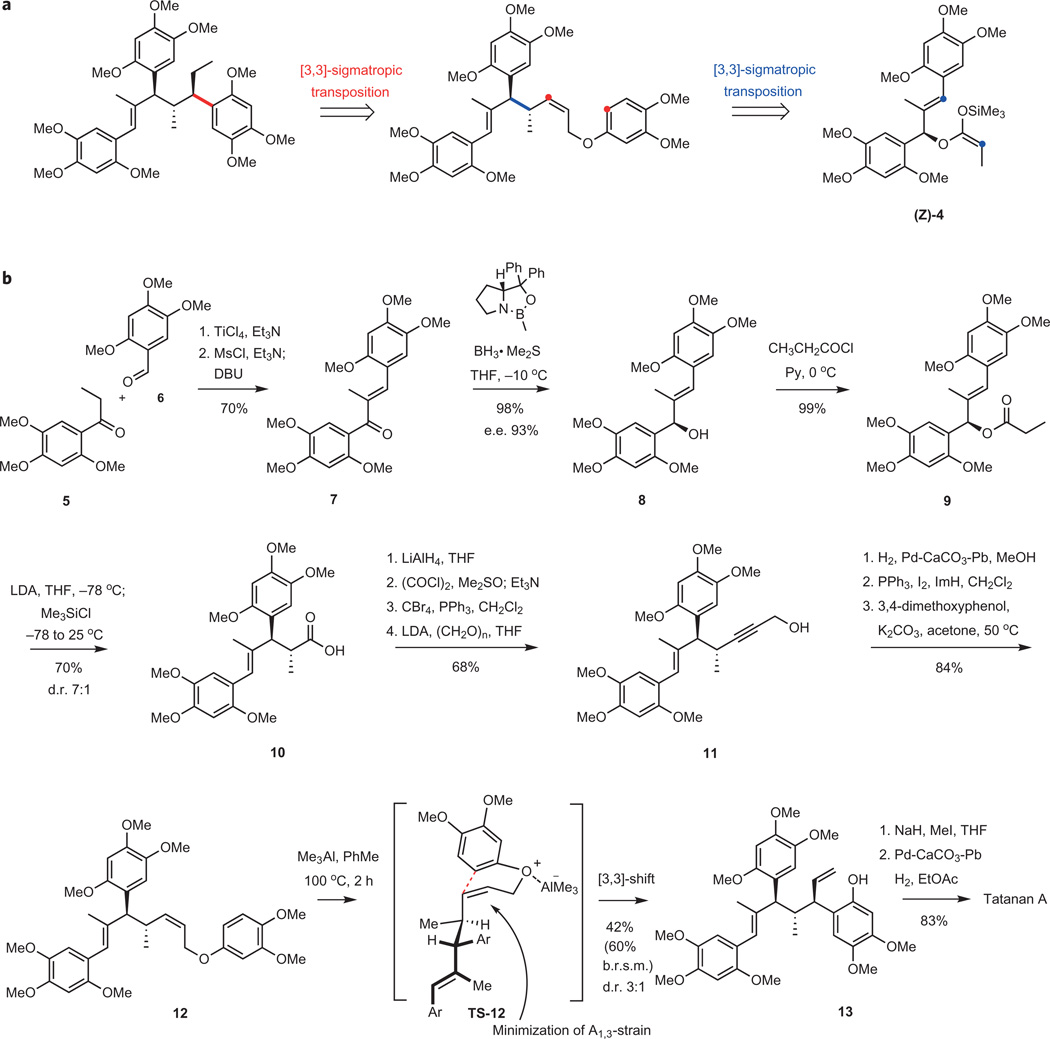
Tatanan A, with its three consecutive all-carbon tertiary stereogenic centres, became the initial goal of our synthesis efforts. Iterative application of [3,3]-sigmatropic rearrangements formed the basis of the synthesis design (Fig. 2a)14, and its concise implementation is presented in Fig. 1b. Aldol condensation of ethyl 2,4,5-trimethoxyphenyl ketone (5) with 2,4,5-trimethoxybenzaldehyde promoted by titanium tetrachloride provided ketone 7 in 70% yield15. Enantioselective reduction of this to allylic alcohol 8 was accomplished using (R)-2-methyl-CBS-oxazaborolidine (CBS = Corey-Bakshi-Shibata; 98% yield, 93% e.e.)16. The formation of the propionyl ester of 8 in nearly quantitative yield was followed by the Ireland–Claisen rearrangement17, which proceeds through an initial Z-selective enolization with lithium diisopropylamide, trapping of the lithium enolate with chlorotrimethylsilane to give the ketene acetal (Z)−4, and its relatively rapid rearrangement via a chair-like transition structure at 25 °C over the course of 1 h. This key constructive transformation18,19 establishes the requisite configuration at the C7′ and C8′ stereocentres with good stereocontrol (d.r. 7:1, 70% yield). Propargyllic alcohol 11 was accessed in four steps that included a reduction–oxidation sequence to reduce the carboxy group in 10 to the aldehyde, conversion of the aldehyde to the 1,1-dibromoalkene upon treatment with carbon tetrabromide and triphenylphosphine, and formation of the lithium acetylenide followed by quenching with paraformaldehyde, affording 11 in 68% overall yield. Selective hydrogenation with Lindlar’s catalyst and two-step aryl ether formation provided 12 in 84% overall yield.
Figure 2. Description of the total synthesis of tatanan A using consecutive sigmatropic rearrangements as a key element of the synthesis design, setting the three adjacent stereogenic centres of the natural product.
a, Outline of the synthesis plan for tatanan A, illustrating the application of the [3,3]-sigmatropic transforms. b, Full reaction sequence for the enantioselective synthesis of tatanan A, 15 steps from aldehyde 6 and ketone 5. Ms, methanesulfonyl; DBU, 1,8-diazabicycloundec-7-ene; THF, tetrahydrofuran; Py, pyridine; LDA, lithium diisopropylamide; ImH, imidazole.
Lewis-acid mediated [3,3]-sigmatropic rearrangement20 of (Z)-allylic aryl ether 12 secured the stereochemistry of the remaining chiral centre while completing the carbon framework of tatanan A. Phenol 13, isolated in 42% isolated yield (60% based on recovered starting material, b.r.s.m.), was formed in a diastereoselective process that favoured the desired stereoisomer with a 3:1 selectivity. The Z configuration of the double bond was necessary to enhance diastereocontrol. The major stereoisomer presumably arises through a reaction pathway involving transition structure TS-12, which is favoured due to minimization of allylic strain. Rearrangement of the corresponding (E)-allylic ether provided an approximately 1:1 mixture of stereoisomers. Starting from rearrangement product 13, the completion of enantioselective synthesis of tatanan A required only two additional steps. Methylation of the phenolic hydroxyl (NaH, CH3I, THF) followed by chemoselective hydrogenation of the terminal alkene in the presence of Lindlar’s catalyst delivered 26 mg of tatanan A (83% yield over two steps). Synthetic tatanan A showed identical spectroscopic data (1H and 13C NMR) to those published for the natural product; the optical rotation for the synthetic material was of the same sign but of a notably higher value ( (c 0.1, CH3OH); literature6 (c 0.1, CH3OH)).
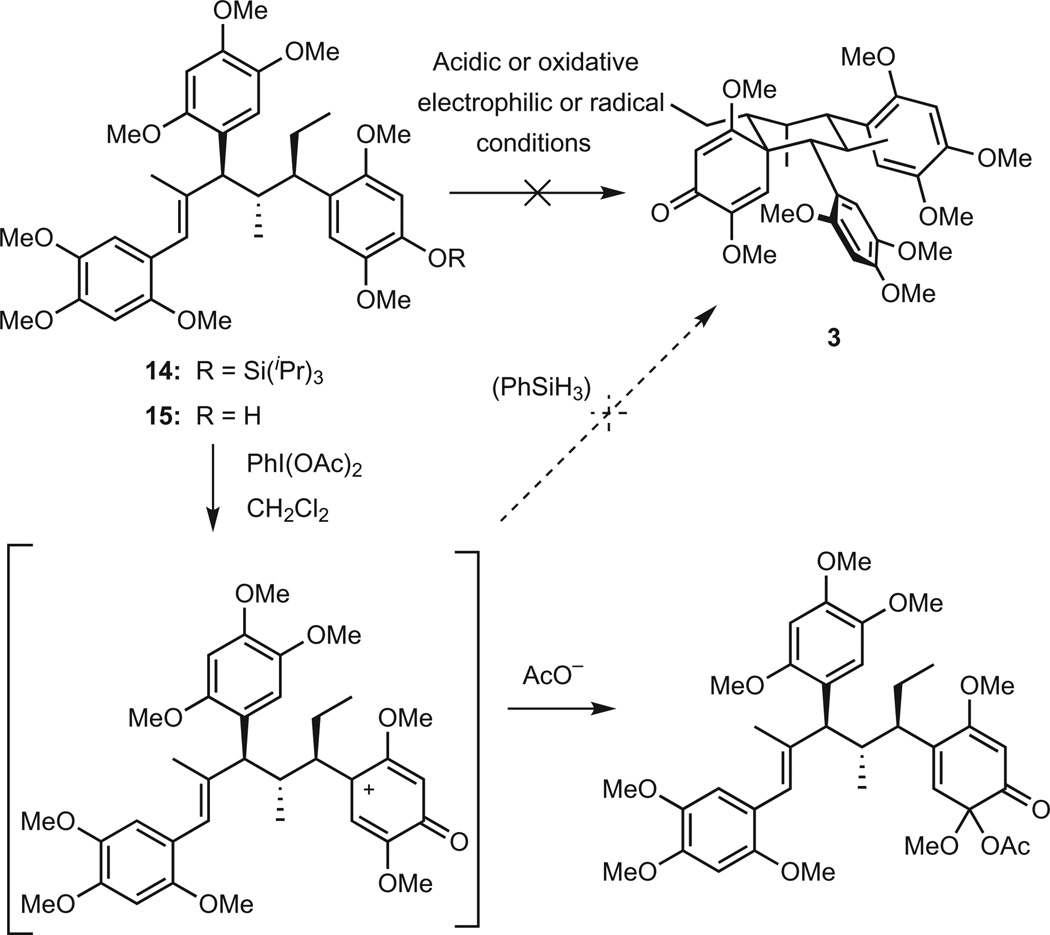
The synthesis of tatanan A on the aforementioned scale enabled a study exploring its conversion to spirocyclic tatanans B and C, emulating the proposed biosynthetic pathway depicted in Fig. 1b21,22. The C4″ triisopropylsilyl ether 15 was prepared via straightforward modification of reactions used in the total synthesis of tatanan A (Fig. 3). Exposing the C4″ phenolic hydroxyl was expected to activate ring C as a nucleophlic counterpart in the cyclization. Under a variety of acidic reaction conditions, 15 failed to undergo cyclization to tatanans B or C. For example, treatment with 2.5 mM CF3SO3H/CH2Cl2 at 0–25 8C resulted initially in no reaction, and then decomposition to an intractable mixture of products at higher temperature. No reaction was observed under milder conditions with pyridinium tosylate in CH2Cl2 or aqueous CH3OH. Next, an approach was explored based on reversed polarity in the cyclization, which again met with no success. Electrophilic or radical activation of ring C in 15 by oxidation with hypervalent iodine reagents (PhI(OAc)2, PhI(O2CCF3)2) in the presence of a hydride donor (PhSiH3) only resulted in the formation of benzoquinone products, and no spirocyclization to tatanans B or C was observed. These observations suggest that the postulated biosynthetic assembly of tatanans B and C by cyclization of tatanan A is unlikely to occur without the assistance of an enzyme.
Figure 3. Tatanans B and C can conceivably arise from cyclization of tatanan A during their biosynthesis.
The total synthesis of tatanan A enabled an exploration of biomimetic cyclization of tatanan A derivatives to tatanans B and C, which proved unsuccessful under a variety of cationic or radical reaction conditions.
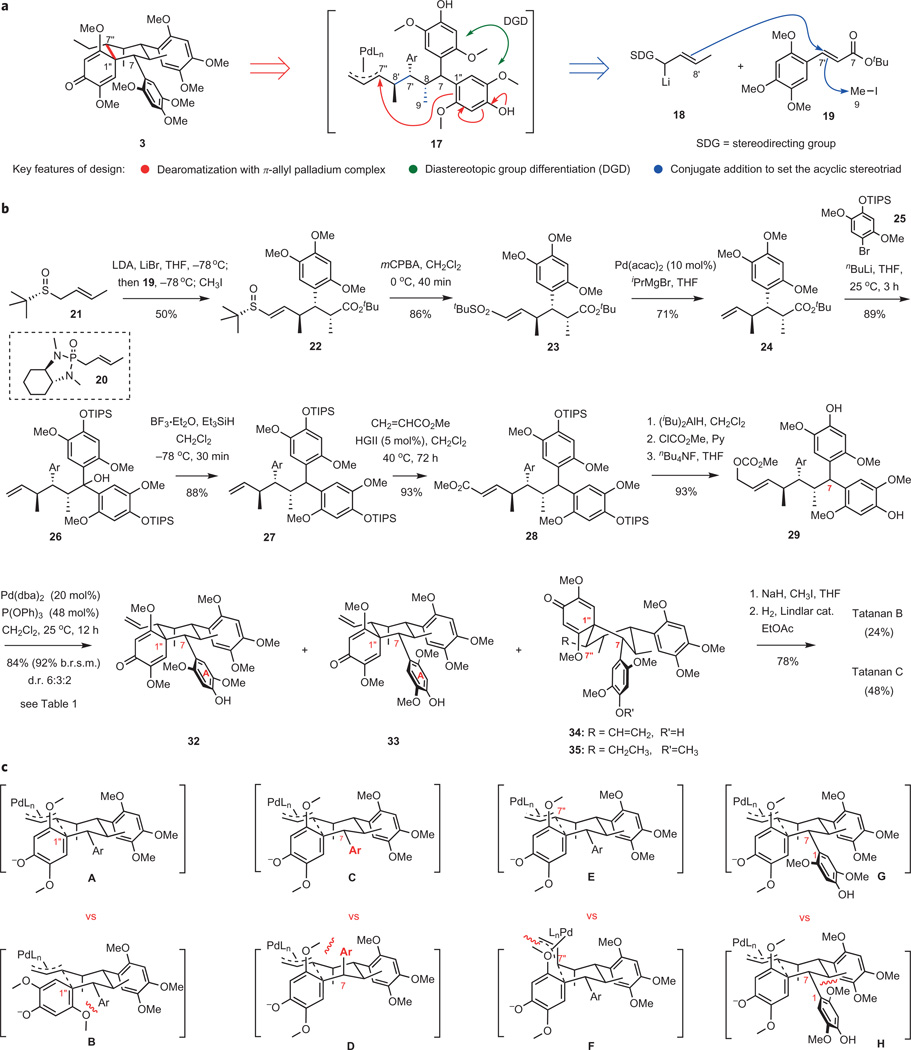
Accomplishing the synthesis of the structurally complex tatanans B and C required a reformulated approach. The key recourse in developing a new synthesis plan was to reposition the ringopening bond scission from C7–C1″ to C1″–C7″ (Fig. 4a). To enable this strategy, a phenol dearomatization through intramolecular allylation by a π-allyl palladium complex was envisioned, which was expected to occur with diastereotopic group differentiation and set three consecutive stereogenic centres of the fully substituted cyclohexane ring in one step. A precursor of acyclic intermediate 17 was to be assembled in a convergent manner by asymmetric conjugate addition of an (E)-crotyllithium reagent 18, where SDG is a stereodirecting group, to cinnamic ester 19. Alkylation of the ester enolate arising from the conjugate addition with methyl iodide was expected to build the stereotriad found in 17 in an expedient manner.
Figure 4. Total synthesis of tatanans B and C by a palladium-catalysed cyclodearomatization.
a, Reformulated synthesis plan centred on a convergent strategy enabled by a stereochemically complex palladium-catalysed cyclization featuring diastereotopic aromatic group differentiation with concomitant construction of the central quaternary centre. b, Successful implementation of the convergent synthesis plan leading to a separable mixture of atropisomeric tatanans B and C in 12 steps from sulfoxide 21. c, Four pairs of transition structures rationalizing all four stereogenic events in the complex palladium-catalysed cyclodearomatization of substrate 29. Structures A and B explain the observed selectivity for the formation of the C1″ quaternary centre. Structures C and D provide the basis for the observed high selectivity during the diastereotopic group differentiation establishing stereochemistry at C7. Structures E and F explain the preference for the observed selectivity at C7″, favouring the vinyl substituent at the equatorial position. Structures G and H rationalize the observed atropselectivity, with a moderate preference for G over H, which places the ortho-MeO group in ring A in a sterically crowded environment of the forming cyclohexane ring. LDA, lithium diisopropylamide; MCPBA, 3-chloroperbenzoic acid; acac, acetylacetonate; TIPS, triisopropylsilyl; HGII, Hoveyda– Grubbs II catalyst; dba, dibenzylideneacetone.
Implementation of the amended synthesis plan was initiated via the development of a suitable conjugate addition method (Fig. 4b). Addition of the lithiated crotyl phosphoramide to ester 19 with subsequent alkylation of the intermediate enolate with iodomethane, following the protocol developed by Hanessian and co-workers23, delivered the expected product in high yield and with outstanding diastereocontrol. However, various attempts to remove the vinylic phosphoramide substituent proved to be prohibitively inefficient, prompting us to seek a more practical alternative. Addition of the lithiated (R)-tert-butyl (E)-2-butenyl sulfoxide24 21 occurred with high diastereoselectivity, delivering ester 22 as a single diastereomer in 50% yield after in situ methylation of the transient ester enolate25. Diastereoisomeric products resulting from α-alkylation of the sulf-oxide accounted for the remainder of the mass balance. Oxidation of the sulfoxide to sulfone followed by a palladium-catalysed desulfonation (Pd(acac)2, i-PrMgBr) afforded terminal alkene 24 in good yield26. The diastereotopic aryl groups were introduced at this stage by addition of 2 equiv. of the aryllithium reagent derived from 25 through lithium–bromine exchange, followed by electrophilic reduction of 26 with Et3SiH in the presence of BF3-etherate27. The cross-metathesis reaction28 between 27 and methyl acrylate using the Hoveyda–Grubbs II catalyst effectively provided α,β-unsaturated ester 28, which was converted to allyl methyl carbonate in three steps and 86% overall yield.
For the critical assembly of the spirocyclic ring system, a transition metal-catalysed cyclization with concomitant dearomatization and diastereotopic aryl group differentiation was selected as the preferred tactical solution (Fig. 4a)29. Although intramolecular metal-catalysed allylic dearomatization is a powerful approach to the synthesis of spirocyclic cyclohexanediones, to date there are no applications of this methodology in the total synthesis of complex natural products30. Very recently, two reports describing methodological studies on direct intramolecular C-allylation of phenols have appeared in the literature, demonstrating the feasibility of this process. In 2010, Hamada and co-workers31 described a palladium-catalysed spirocyclization forming a spiro[4.5]decane ring system. In that study, a single example was reported that afforded a spirocyclic ring system containing two six-membered rings. In 2011, You and colleagues reported a related iridium-catalysed dearomatization producing a 3-azaspiro[5.5]undecadienone, together with numerous examples of intramolecular allylation giving spiro[4.5]decane ring systems32.
In the context of this total synthesis endeavour, intramolecular allylic dearomatization of complex substrate 29 was investigated concentrating on palladium(0) and iridium(I) catalysis (Table 1). We found that iridium-catalysed reactions were ineffective (entries 1 and 2)32. In contrast to Hamada’s findings, the rate of allylic dearomatization of 29 under palladium catalysis is substantially enhanced with decreasing s-donor capacity of the phosphine ligand (entries 3–7). Although little to no conversion has been observed with PPh3 or P(o-tol)3, the reaction took place with complete conversion in the presence of P(2-furyl)3, P(OPh)3 or P(OEt)3. Using palladium(II) acetate and triphenylphosphine as the source of the catalyst resulted in the formation of only a trace amount of the expected product (entry 9). Interestingly, a somewhat slower rate was observed when Pd2(dba)3 was used in place of Pd(dba)2 as the source of the active catalyst (entry 10).
Table 1.
Optimization studies for the key intramolecular allylic spiro-dearomatization.
| Entry | Catalyst | Ligand | T (°C) | Time (h) | Yield of 32 + 33 (%) | 32:33 |
|---|---|---|---|---|---|---|
| 1 | [Ir(COD)Cl]2 | P(OPh)3 | 25 | 36 | 0 | |
| 2* | [Ir(COD)Cl]2 | P(OPh)3 | 25 | 36 | 10 | 1:1 |
| 3 | Pd(dba)2 | PPh3 | 25 | 36 | 30 | 3.5:1 |
| 4 | Pd(dba)2 | P(o-tol)3 | 25 | 36 | 0 | |
| 5 | Pd(dba)2 | P(2-furyl)3 | 25 | 12 | 69 | 2.5:1 |
| 6 | Pd(dba)2 | P(OEt)3 | 25 | 12 | 75 | 2.5:1 |
| 7 | Pd(dba) | P(OPh)3 | 25 | 24 | 76 | 1.9:1 |
| 8† | Pd(dba)2 | P(OPh)3 | 25 | 12 | 84 (92% b.r.s.m.) | 2.0:1 |
| 9‡ | Pd(dba)2 | PPh3 | 50 | 24 | <1 | |
| 10 | Pd2(dba)3 | P(OPh)3 | 25 | 24 | 51 (76% b.r.s.m.) | 1.4:1 |
Typical reaction conditions: substrate (15 mg) was stirred with catalyst (20 mol%) and ligand (48 mol%) in CH2Cl2 under argon.
Cs2CO3 (2 equiv.) was used as an additive.
Performed on 100 mg scale.
Ti(OPr-i)4 (2 equiv.) was used as an additive.
COD, 1,5-cyclooctadiene; dba, dibenzylideneacetone; b.r.s.m., based on recovered starting material.
Under optimized conditions, the desired cyclization could be achieved cleanly upon treatment with Pd(dba)2 and P(OPh)3, affording an inseparable mixture of atropisomeric products 32 and 33 together with C1″,C7″-diastereomer 34 in 84% combined yield (92% based on recovered starting material, d.r. 6:3:2, respectively). During this operation, three of the six stereocentres of the target molecule, including the quaternary centre at the core of the spirocyclic ring system, are set with a high degree of stereocontrol. Only three of 16 possible stereoisomers have been observed.
The stereochemical course of the spirocyclization reaction can be understood by considering chair-like transition structures A–H (Fig. 4c), where the non-reacting aryl group resides in the equatorial position, and minimization of steric interactions between the ortho-methoxy substituents delivers the observed products. Each of the four pairs of transition structures demonstrates preferences within the four corresponding stereochemical categories operative in the palladium-catalysed cyclization, namely (i) selectivity in the formation of the quaternary centre at C1″ (A versus B); (ii) diastereo-selectivity at C7 (C versus D); (iii) selectivity at the vinyl-substituted centre C7″ (E versus F); and (iv) atropselectivity around the C1–C7 bond (G versus H). Although stereocontrol for the formation of the quaternary centre is high, control over the axial selectivity around the C1–C7 bond only reached a moderate level of ~3:1. In minor isomer 33, the 2-MeO group of ring A is situated in the sterically hindered position, eclipsing the cyclohexane subunit. Nevertheless, the formation of atropisomers 32 and 33 enabled eventual access to both natural products: tatanan B and tatanan C. A minor amount of 34 is also formed, probably by a competing pathway proceeding through a boat-like transition structure.
The synthesis of tatanans B and C was readily accomplished in two direct steps from 33 and 32, respectively: (i) phenol methylation and (ii) chemoselective hydrogenation of the vinyl group (H2, Lindlar’s catalyst, EtOAc; 78% yield over two steps). The atropisomeric tatanans B and C were separated by preparative reverse-phase HPLC. Completion of the total chemical synthesis delivered 30 mg of (−)-tatanan C and 15 mg of (−)-tatanan B. The synthetic material was spectroscopically identical to the natural products (1H and 13C NMR in two different solvents: CD3OD and (CD3)2CO); however, the data for the absolute optical rotation were substantially different (tatanan B: (c 0.10, CH3OH); literature6 0 (c 0.1, CH3OH); tatanan C: (c 0.11, CH3OH); literature6 0 (c 0.1, CH3OH)).
Biological studies
Following the initial discovery and structural characterization of tatanans by Yu and co-workers, bioactivity studies suggested that these novel natural products display antidiabetic activity through an ability to allosterically activate human pancreatic glucokinase6. This finding was intriguing, as tatanans appear to represent the first example of a biogenic glucokinase activator. Moreover, the reported bioactivities of tatanans A–C exceed that of a known, potent synthetic activator, derivatives of which are currently under investigation as therapeutic agents3. However, initial characterization of the glucokinase-activating properties of tatanans was limited, presumably due to the small quantities of tatanan A, B and C isolated from natural sources. Our successful total synthesis efforts provided substantial quantities of all three compounds, thus enabling a detailed mechanistic investigation of the glucokinase-activating characteristics of the tatanans. Previous work indicated that tatanan C was the most potent congener, with an EC1.5 value (concentration of compound required to increase enzyme activity by 50%) of 0.16 mM toward human glucokinase6. Based on this finding, our initial biochemical characterization efforts focused on tatanan C.
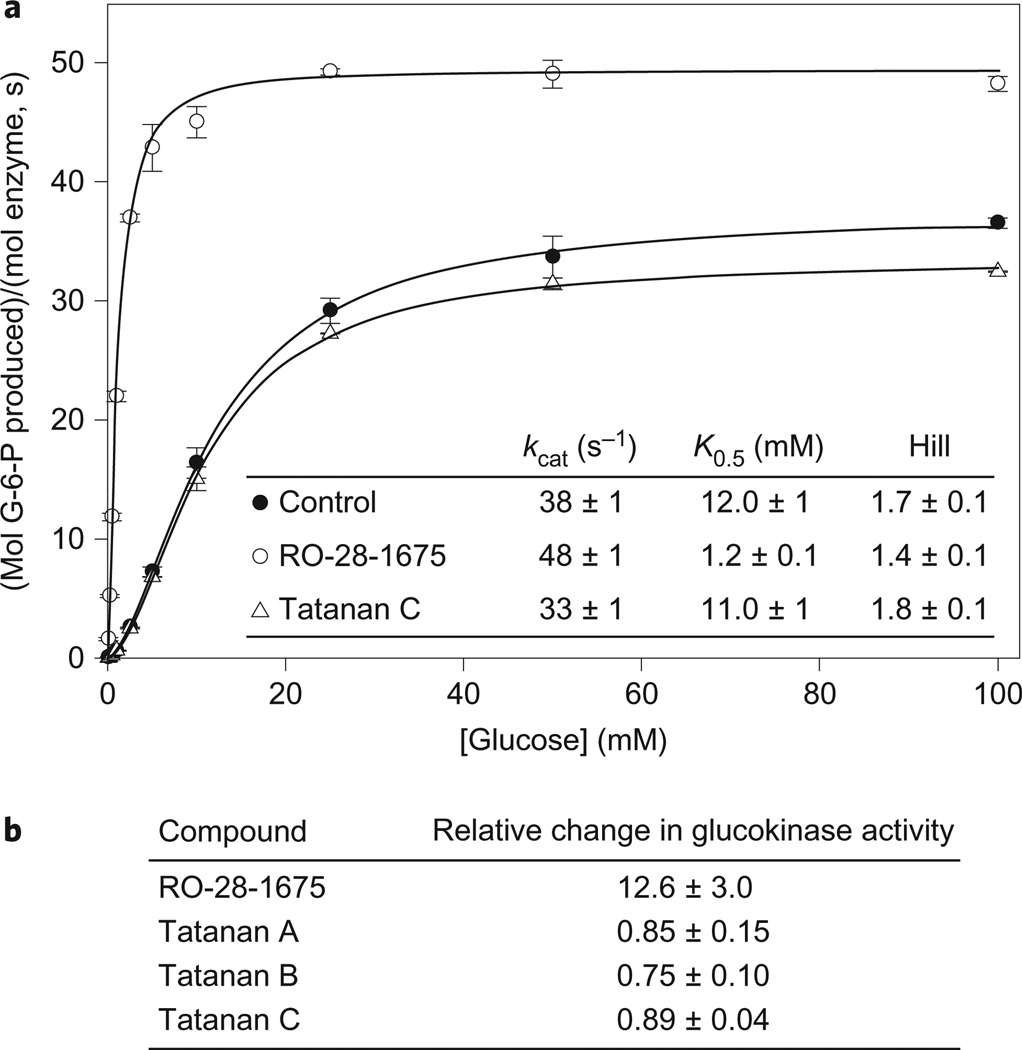
Recombinant human pancreatic β-cell glucokinase was produced and purified to greater than 95% purity using established procedures33, and the enzyme was used in assays to evaluate the impact of tatanan C upon the kinetic parameters of glucokinase. As a control, we also characterized the activation properties of RO-28-1675, a previously described synthetic glucokinase activator that has served as a lead compound for therapeutic development4. Consistent with previous reports, RO-28-1675 is a potent gluco-kinase activator that elicits a tenfold decrease in the glucose K0.5 value and a 30% increase in the kcat value (Fig. 5). Similar to other synthetic activators, RO-28-1675 also produces a reduction in the kinetic cooperativity of human glucokinase, as reflected by a lower Hill number. In contrast to RO-28-1675, the presence of tatanan C at concentrations up to 20 µM failed to produce measurable changes in the kinetic profile and properties of human glucokinase (Fig. 5). Higher concentrations of tatanan C were not explored due to the limited solubility of this compound under aqueous conditions; however, the concentrations used in our study are more than an order of magnitude higher than the EC1.5 values reported in the initial description of the tatanans.
Figure 5. In vitro enzymatic assays demonstrate that tatanans A–C do not activate human glucokinase.
a, Glucokinase activation assays conducted in the absence (control) and presence (20 µM) of tatanan C and a previously described synthetic activator, RO-28-1675 (20 µM). RO-28-1675 activates glucokinase by increasing the kcat value, decreasing the glucose K0.5 value, and reducing the Hill coefficient. Tatanan C has no effect on these catalytic constants. Experimental rate data for each glucose concentration represent the average of two or more independent assays, with the standard deviation of the mean represented by the vertical bars. Kinetic constants were obtained from the fit of the experimental data to the Hill equation and represent the average of two or more complete kinetic profiles. G-6-P, glucose-6-phosphate. b, RO-28-1675 produces a 12.6-fold increase in relative glucokinase activity, while tatanans A, B and C do not increase enzyme activity. Activity changes were calculated from the ratio of enzymatic second-order rate constants ([kcat/K0.5 activated]/[kcat/K0.5 control]) determined in the presence and absence of 20 µM activator.
We next investigated the bioactivities of the two remaining ses-quilignans, tatanan A and B. Although the reported activities for tatanan A and B towards glucokinase are somewhat lower than that of tatanan C, both compounds displayed EC1.5 values (1.85 and 0.52 µM, respectively6) that are readily accessible with enzyme activity assays. Similar to the tatanan C results, the inclusion of tatanan A and B at concentrations up to 20 µM did not produce an increase in glucokinase activity over control reactions (Fig. 5b). In contrast, the known activator RO-28-1675 produced a 12.6-fold increase in the second-order rate constant for enzyme catalysis, kcat/K0.5.
Conclusions
The concise, step-economical34,35 synthesis of novel sesquilignans tatanans B and C was accomplished by an intramolecular palladium-catalysed allylic phenol dearomatization of a complex substrate. This transformation includes a diastereotopic aryl group differentiation and effectively sets three of the six stereocentres of the densely substituted cyclohexane unit present in these molecules. The atropisomeric products are formed in ~2:1 ratio and converted to atropisomeric tatanans B and C, completing the total synthesis in 13 steps from 2,4,5-trimethoxybenzaldehyde. Tatanan A was prepared concisely by a strategy based on consecutive [3,3]-sigmatropic rearrangements, allowing us to experimentally probe its putative biosynthetic relation to spirocyclic tatanans B and C.
The observation that tatanans exhibit no glucokinase activation under all conditions investigated herein strongly supports the conclusion that tatanans are not antidiabetic, allosteric activators of glucokinase. The reason for the discrepancy between our bioactivity assays and previous reports6 is unclear, as both investigations utilized the same enzymatic assay for glucokinase activity. Notably, Ni and co-workers measured glucokinase activity at only a single glucose concentration6, whereas we determined the full kinetic profile of glucokinase in the presence and absence of potential activators. In addition, the assay conditions used by Ni and co-workers involved a non-optimal ratio of reagents; however, we also conducted assays under these non-ideal conditions and failed to observe glucokinase activation. The possibility that another natural product of unknown structure co-purified with tatanans during the initial isolation of these compounds, and is responsible for the previously observed activation of glucokinase, remains to be investigated. Our successful development of a concise and high-yielding synthetic strategy for tatanans, and derivatives thereof, will enable a broad investigation of the biological activities of these structurally unique natural products.
Supplementary Material
Acknowledgements
The authors thank Hongjun Zhou for continued assistance with NMR spectroscopy. This work was supported by the US National Institutes of Health (NIGMS GM077379 to A.Z., NIDDK DK081358 to B.G.M.) and additional kind donations from Eli Lilly and Amgen.
Footnotes
Author contributions
Q.X., A.B., J.J.J. and J.M.B. planned, conducted and analysed the experiments. A.Z. and B.G.M. designed and directed the project. A.Z. and B.G.M. wrote the manuscript. All authors contributed to discussions.
Additional information
Supplementary information and chemical compound information are available in the online version of the paper. Reprints and permissions information is available online at www.nature.com/reprints.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nature Rev. Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 2.Meininger , et al. Effects of MK–0941, a novel glucokinase activator on glycemic control in insulin-treated patients with type 2 diabetes. Diabetes Care. 2011;34:2560–2566. doi: 10.2337/dc11-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonadonna RC, et al. Piragliatin (RO4389620), a novel glucokinase activator, lowers plasma glucose both in the postabsorptive state and after a glucose challenge in patients with type 2 diabetes mellitus: a mechanistic study. J. Clin. Endocrinol. Metab. 2010;95:5028–5036. doi: 10.1210/jc.2010-1041. [DOI] [PubMed] [Google Scholar]
- 4.Grimsby J, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 5.Grimsby J, Berthel SJ, Sarabu R. Glucokinase activators for the potential treatment of type 2 diabetes. Curr. Top. Med. Chem. 2008;8:1524–1532. doi: 10.2174/156802608786413483. [DOI] [PubMed] [Google Scholar]
- 6.Ni G, et al. Glucokinase-activating sesquilignans from the rhizomes of Acorus tatarinowii Schott. J. Org. Chem. 2011;76:2056–2061. doi: 10.1021/jo1022712. [DOI] [PubMed] [Google Scholar]
- 7.Hung H-Y, Qian K, Morris-Natschke SL, Hsu C-S, Lee K-H. Recent discovery of plant-derived anti-diabetic natural products. Nat. Prod. Rep. 2012;29:580–606. doi: 10.1039/c2np00074a. [DOI] [PubMed] [Google Scholar]
- 8.Snyder SA, Gollner A, Chiriac MI. Regioselective reactions for programmable resveratrol oligomer synthesis. Nature. 2011;474:461–466. doi: 10.1038/nature10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos AL. Total synthesis of diverse carbogenic complexity within the resveratrol class from a common building block. J. Am. Chem. Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. [DOI] [PubMed] [Google Scholar]
- 10.Snyder SA, ElSohly AM, Kontes F. Synthetic approaches to oligomeric natural products. Nat. Prod. Rep. 2011;28:897–924. doi: 10.1039/c1np00001b. [DOI] [PubMed] [Google Scholar]
- 11.Whiting DA. Lignans and neolignans. Nat. Prod. Rep. 1985;2:191–211. doi: 10.1039/np9870400499. [DOI] [PubMed] [Google Scholar]
- 12.Pan JY, Chen SL, Yang MH, Wu J, Sinkkonen J, Zou K. An update on lignans: natural products and synthesis. Nat. Prod. Rep. 2009;26:1251–1292. doi: 10.1039/b910940d. [DOI] [PubMed] [Google Scholar]
- 13.Snyder SA, Kontes F. Explorations into neolignan biosynthesis: total synthesis of helictenin B, helisorin, and helisterculin A from a common intermediate. J. Am. Chem. Soc. 2009;131:1745–1752. doi: 10.1021/ja806865u. [DOI] [PubMed] [Google Scholar]
- 14.Ilardi EA, Stivala CE, Zakarian A. [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products. Chem. Soc. Rev. 2009;38:3133–3148. doi: 10.1039/b901177n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison CR. Transient titanium enolate aldol condensations. Tetrahedron Lett. 1987;28:4135–4138. [Google Scholar]
- 16.Corey EJ, Helal CJ. Reduction of carbonyl compounds with chiral oxazaborolidine catalysts: a new paradigm for enantioselective catalysis and a powerful new synthetic method. Angew. Chem. Int. Ed. 1998;37:1986–2012. doi: 10.1002/(SICI)1521-3773(19980817)37:15<1986::AID-ANIE1986>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Ireland RE, Mueller RH, Willard AK. The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation. J. Am. Chem. Soc. 1976;98:2868–2877. [Google Scholar]
- 18.Newhouse T, Baran PS, Hoffmann RW. The economies of synthesis. Chem. Soc. Rev. 2009;38:3010–3021. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young IS, Baran PS. Protecting-group-free synthesis as an opportunity for invention. Nature Chem. 2009;1:193–205. doi: 10.1038/nchem.216. [DOI] [PubMed] [Google Scholar]
- 20.Castro AMM. Claisen rearrangement over the past nine decades. Chem. Rev. 2004;104:2939–3002. doi: 10.1021/cr020703u. [DOI] [PubMed] [Google Scholar]
- 21.Stadler D, Bach T. Concise stereoselective synthesis of (−)-podophyllotoxin by an intramolecular iron(III)-catalysed Friedel–Crafts alkylation. Angew. Chem. Int. Ed. 2008;47:7557–7559. doi: 10.1002/anie.200802611. [DOI] [PubMed] [Google Scholar]
- 22.Stadler D, Bach T. Highly diastereoselective Friedel–Crafts alkylation reactions via chiral alpha-functionalized benzyllic carbocations. Chem. Asian J. 2008;3:272–284. doi: 10.1002/asia.200700241. [DOI] [PubMed] [Google Scholar]
- 23.Hanessian S, Gomtsyan A. Highly stereocontrolled sequential asymmetric Michael addition reactions with cinnamate esters—generation of three and four contiguous stereogenic centers on seven-carbon acyclic motifs. Tetrahedron Lett. 1994;35:7509–7512. [Google Scholar]
- 24.Davis FA, Thimma Reddy R, Han W, Carroll PJ. Chemistry of oxaziridines. 17. N-(Phenylsulfonyl)(3,3-dichlorocamphoryl)oxaziridine: a highly efficient reagent for the asymmetric oxidation of sulfides to sulfoxides. J. Am. Chem. Soc. 1992;114:1428–1437. [Google Scholar]
- 25.Binns MR, Haynes RK, Katsifis AG, Schober PA, Vonwiller SC. Aprotic conjugate addition of allyllithium reagents bearing polar groups to cyclic enones. 1. 3-Alkylallyl systems. J. Am. Chem. Soc. 1988;110:5411–5423. [Google Scholar]
- 26.Fabre JL, Julia M. Organic synthesis with sulfones XXIX. Stereospecific hydrogenolysis of vinylic sulfones with Grignards and transition metal catalysts. Tetrahedron Lett. 1983;24:4311–4314. [Google Scholar]
- 27.Rye CE, Barker D. Asymmetric synthesis of (+)-Galbelgin, (−)-Kadangustin J, (−)-Cyclogalgravin and (−)-Pycnanthulignenes A and B, three structurally distinct lignan classes, using a common chiral precursor. J. Org. Chem. 2011;76:6636–6648. doi: 10.1021/jo200968f. [DOI] [PubMed] [Google Scholar]
- 28.Connon SJ, Blechert S. Recent developments in olefin cross metathesis. Angew. Chem. Int. Ed. 2003;42:1900–1923. doi: 10.1002/anie.200200556. [DOI] [PubMed] [Google Scholar]
- 29.Trost BM, Crawley ML. Asymmetric transition-metal-catalyzed allylic alkylations: applications in total synthesis. Chem. Rev. 2003;103:2921–2944. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]
- 30.Qi J, Beeler AB, Zhang Q, Porco JA., Jr Catalytic enantioselective alkylative dearomatization–annulation: total synthesis and absolute configuration assignment of hyperibone. K. J. Am. Chem. Soc. 2010;132:13642–13644. doi: 10.1021/ja1057828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemoto T, Ishige Y, Yoshida M, Kohno Y, Kanematsu M, Hamada Y. Novel method for synthesizing spiro[4.5]cyclohexadienones through a Pd-catalyzed intramolecular ipso-Friedel–Crafts allylic alkylation of phenols. Org. Lett. 2010;12:5020–5023. doi: 10.1021/ol102190s. [DOI] [PubMed] [Google Scholar]
- 32.Wu QF, Liu WB, Zhuo CX, Rong ZQ, Ye KY, You SL. Iridium-catalyzed intramolecular asymmetric allylic dearomatization of phenols. Angew. Chem. Int. Ed. 2011;50:4455–4458. doi: 10.1002/anie.201100206. [DOI] [PubMed] [Google Scholar]
- 33.Larion M, Miller BG. 23-residue C-terminal alpha-helix governs kinetic cooperativity in monomeric human glucokinase. Biochemistry. 2009;48:6157–6165. doi: 10.1021/bi9007534. [DOI] [PubMed] [Google Scholar]
- 34.Wender PA, Verma VA, Paxton TJ, Pillow TH. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 2008;41:40–49. doi: 10.1021/ar700155p. [DOI] [PubMed] [Google Scholar]
- 35.Wender PA, Miller BL. Synthesis at the molecular frontier. Nature. 2009;460:197–201. doi: 10.1038/460197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.