Abstract
Genomic imprinting refers to a form of epigenetic gene regulation whereby alleles are differentially expressed in a parent-of-origin-dependent manner. Imprinting evolved independently in flowering plants and in therian mammals in association with the elaboration of viviparity and a placental habit. Despite the striking differences in plant and animal reproduction, genomic imprinting shares multiple characteristics between them. In both groups, imprinted expression is controlled, at least in part, by DNA methylation and chromatin modifications in cis-regulatory regions, and many maternally and paternally expressed genes display complementary dosage-dependent effects during embryogenesis. This suggests that genomic imprinting evolved in response to similar selective pressures in flowering plants and mammals. Nevertheless, there are important differences between plant and animal imprinting. In particular, genomic imprinting has been shown to be more flexible and evolutionarily labile in plants. In mammals, imprinted genes are organized mainly in highly conserved clusters, whereas in plants they occur in isolation throughout the genome and are affected by local gene duplications. There is a large degree of intra- and inter-specific variation in imprinted gene expression in plants. These differences likely reflect the distinct life cycles and the different evolutionary dynamics that shape plant and animal genomes.
Introduction
Genomic imprinting is a mechanism that leads to the differential expression of alleles depending on whether they are maternally or paternally inherited. Thus, mutations in imprinted genes show a non-Mendelian inheritance pattern. Unlike other parent-of-origin effects, which can be caused by cytoplasmic contributions of the egg or sperm to zygotic development, genomic imprinting is characterized by de novo differential expression from the parental alleles after fertilization [1]. Imprinting is initiated by the distinct epigenetic marking (“imprint”) of specific DNA sequences in the parental germ lines of mammals and in the gametophytes of plants. This mark is stably inherited after fertilization and eventually results in the unequal expression of the two parental alleles.
The first imprinted gene was discovered in maize (Zea mays). Through a series of elegant genetic experiments, Kermicle [2] determined that R, a gene conferring anthocyanin pigmentation to seeds, is fully expressed only when inherited from the mother. Subsequently, plant geneticists observed that the relative dosage of maternal and paternal chromosomes plays an important role in seed development, indicating that parental genomes are not equivalent [3–5]. In mammals, the non-equivalence of maternal and paternal genomes was demonstrated by nuclear transplantation experiments showing that mouse embryos with either two paternal or two maternal genomes are inviable [6,7]. Imprinting in mice was later shown to be restricted to specific loci [8], the first of which (Igf2, Igf2r, and H19) were molecularly identified in 1991 [9–11]. Here, we discuss how imprinting may have evolved in these distinct lineages but refer to other reviews that compare and contrast the molecular mechanisms of genomic imprinting in seed plants and mammals (for example, [1,12–15]).
Why did genomic imprinting evolve?
Today, hundreds of genes have been reported to be imprinted in plants and animals [1,15,16]. Nevertheless, the evolution of genomic imprinting has puzzled evolutionary biologists and has been a source of heated disputes for over two decades. Imprinting poses a fitness cost because it exposes recessive mutations, yet the imprinting status of many genes is conserved over millions of years. So why did genomic imprinting evolve? Addressing this question should take into consideration the diversity of imprinting mechanisms in flowering plants and mammals.
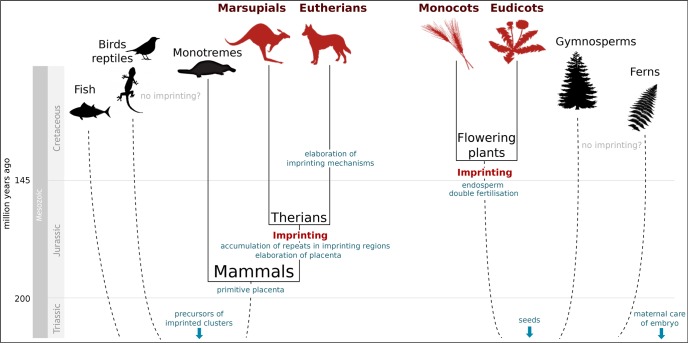
The term “imprinting” was coined to describe the selective marking and elimination of all paternal chromosomes in sciarid insects [17]. Here, however, we consider only genomic imprinting that affects individual genes or gene clusters rather than entire chromosomes or genomes. In animals, genomic imprinting of individual genes has been identified in eutherian and marsupial mammals but not in monotremes or in non-mammalian vertebrates, such as birds [18,19]. This suggests that the evolution of genomic imprinting was associated with the elaboration of viviparity in the common ancestor of all therian mammals, which lived in the Jurassic period, 160 to 200 million years ago [20,21] (Figure 1). In plants, genomic imprinting has been identified in the endosperm and embryo—the two products of double fertilization—of monocots and eudicots (reviewed in [1,15]) but has not been reported in non-flowering plants, likely due to a lack of experimental investigation. These findings suggest that imprinting evolved in association with the evolution of the endosperm (an embryo-nourishing tissue) in the Early Cretaceous, 100 to 145 million years ago [22] (Figure 1).
Figure 1. Timescale for the evolution of imprinting in animals and plants.
Imprinting and the placental habit
Clearly, the evolution of genomic imprinting was linked to the evolution of the placental habit in therian mammals and in flowering plants. In these groups, the embryo is embedded and nourished by a placenta or an endosperm, sexually derived structures that share the same set of genes as the embryo. The mammalian placenta is derived post-fertilization from the trophoblast and other extra-embryonic tissues [23]. The endosperm originates from double fertilization, where twin male gametes fuse with two female gametes that usually carry one and two copies of the same genome (1n egg and 2n central cell), giving rise to the 2n embryo and 3n endosperm, respectively (although there are variations to this theme [24,25]). The elaborated viviparity and the placental habit of mammals and flowering plants allow both maternal and paternal genes to play an active role during embryogenesis (for example, in nutrient acquisition from the mother). Accordingly, the endosperm and the trophoblast/placenta appear to be the primary sites of imprinting [1,15,26].
Despite the clear association between genomic imprinting and viviparity, not all viviparous groups evolved imprinting. Although this point is often overlooked, viviparity is widespread among many animal groups, including scorpions, seahorses, sharks, lizards, snakes, and amphibians [27]. To our knowledge, it remains to be tested whether imprinting is present in any of these groups. All land plants have an intimate maternal-offspring relationship, with multicellular sporophytic embryos being nurtured and protected by the maternal plant for an extended period of time [28]. However, there is currently no report of imprinting in any plant group outside the angiosperms. This, however, may be due simply to a sampling bias. Nevertheless, the widespread presence of parthenogenesis (development of an embryo in the absence of fertilization) in many of these taxa suggests that imprinting may indeed be absent; this is because one consequence of genomic imprinting is the failure of parthenogenetic progeny to properly develop due to the non-equivalence of maternal and paternal genomes. Parthenogenetic mice can be obtained only through the genetic engineering of imprinted genes [29,30]. Interestingly, although apomixis (asexual reproduction through seeds) is common among flowering plants, most apomicts require fertilization of the central cell for the development of functional endosperm [31]. Nevertheless, parthenogenetic embryo development (and rare cases of autonomous endosperm development) in apomicts suggests that a bypass of genomic imprinting requirements is relatively common in plants.
The kinship and maternal-offspring coadaptation theories
The evolution of imprinting in association with the placental habit strongly suggests that imprinting evolved as a regulator of maternal-offspring interactions. Many different theories have been put forward to explain the evolution of imprinting [32–35]. Although it is unlikely that any one theory can explain all cases of imprinting, two theories have gained the most popularity because they provide a general explanation for imprinting that is supported by empirical evidence: the kinship (or parental conflict) theory [36–38] and the maternal-offspring coadaptation theory [39].
The kinship theory of genomic imprinting suggests that maternal and paternal alleles of a gene have conflicting interests. This conflict arises because, in viviparous polyandrous (multiple paternity) species, paternally derived genes benefit from maximizing resource allocation at the expense of embryos from other fathers. Conversely, maternally derived genes benefit from promoting equitable growth of all embryos because all progeny are equally related to their mother. The kinship theory predicts that this conflict can result in the evolution of mechanisms that cause growth-promoting genes to be active when inherited paternally but silenced when inherited maternally. The kinship theory was later expanded to include not only parental effects on embryo growth but all other kin interactions that involve asymmetries of genetic relatedness [38]. More recent reinterpretations of the kinship theory propose that imprinting evolved as a consequence of an asymmetry generated by differences in relatedness and demography between maternally and paternally derived alleles [40,41].
Since it was proposed 25 years ago, the kinship theory has been by far the most popular theory to explain the evolution of genomic imprinting. It is supported by the dosage-dependent and opposing roles of reciprocally imprinted genes, such as Igf2 and Igf2r, during mouse fetal growth [9,10] or MEDEA and PHERES1 during plant seed development [42,43]. Yet the large number of alternative theories and the recurring misinterpretations of the kinship theory have made it controversial. One criticism that is often raised is that the kinship theory fails to predict the direction of imprinting in some loci. Two often cited examples are Ascl2/Mash2 and Meg1, imprinted genes that encode positive regulators of trophoblast development in mice and endosperm development in maize, respectively [44,45]. A naïve interpretation of the kinship theory would predict that these genes would be paternally expressed (because they promote growth), but they are maternally expressed. However, these apparent contradictions of the kinship theory can be explained if one considers the diverse roles of genes during the early and late stages of embryogenesis [32,46].
Another criticism raised against the kinship theory is the apparent predominance of maternally expressed genes (MEGs) over paternally expressed genes (PEGs) found in both mice and plants [15,16]. The maternal-offspring coadaptation theory provides an explanation for the overabundance of MEGs [39]. It proposes that, in species with extended maternal care, the offspring have higher fitness if they have a higher resemblance to their mother. Therefore, the expression of maternal alleles is favored because it facilitates the coadaptation of maternal and offspring traits. However, the maternal-offspring coadaptation theory is challenged by the occurrence of many PEGs, including an apparent dominance of PEGs in the placenta of reciprocal hybrids of horse and donkey [47]. Furthermore, the coadaptation theory is expected to lead to loss of genetic variation at imprinted loci (and consequently a loss of imprinting) [32,39]. This prediction is somehow hard to reconcile with the conservation of imprinted gene expression across millions of years.
Evolutionary origins of imprinted genes
In mice, imprinted genes are usually found in large clusters that are regulated by imprinting control regions [48,49]. Synteny analyses show that some of these clusters are also present in bird, amphibian, and fish genomes [50,51]. This means that these clusters existed long before they became imprinted. Interestingly, in chicken, these gene clusters are located predominantly in chromosomes that possess distinct chromatin properties [52]. This raises the intriguing possibility that this distinct chromatin environment might have facilitated the evolution of imprinted gene expression in early mammals [50]. Other genes became imprinted at different stages during eutherian evolution [53,54]. Plants do not have similar imprinted gene clusters. Although some predicted plant imprinted genes form microclusters [55], these lack the size and complexity of imprinted clusters in mammals. Many of the predicted plant microclusters consist of paralogous genes, suggesting that local gene duplications may play an important role in the evolution of imprinting in plant genomes [56].
Although the mechanisms differ, part of the molecular machinery leading to imprinted gene expression, including the central role played by cytosine methyltransferases, is also used by the cell to silence foreign DNA (such as retroviruses and transposable elements). This led to the suggestion that genomic imprinting evolved as a by-product of the genome's defense against foreign DNA [57–60]. Indeed, there was a significant expansion of certain types of repeat elements in the imprinted clusters of therians, compared with the same (unimprinted) clusters of monotremes [61]. This suggests that transposon insertion may have been a driving force for the origin of imprinted expression in therian mammals and lends support to the host defense hypothesis. It is important to distinguish the host defense hypothesis from adaptive theories, such as the kinship and coadaptation theories, the former is a model to explain the origin of mechanisms leading to imprinted gene expression, whereas the latter models analyze how selective pressures could drive the propagation and fixation of imprinted gene expression.
Evolutionary dynamics of imprinted genes
Since conflicts can drive fast evolution, it has been proposed based on the kinship theory that imprinted genes should evolve faster than other genes. This was confirmed by the discovery of signatures of positive selection in Igf2r in rodents [62], KLF14 in humans [63], and MEDEA in Arabidopsis [64-66]. However, explicit modeling of antagonistic evolution of imprinted loci predicts that imprinted loci should reach a stable equilibrium [67]. Evidence from empirical studies supports this conclusion: some early studies found no evidence of positive selection on imprinted genes [68], and a more recent study found that the majority of mammalian imprinted genes are not subject to positive selection [69]. When comparing mice and human imprinted genes, MEGs were found to be subject to reduced selective pressure, but a strong shift to purifying selection was found for PEGs of rodents, suggesting that MEGs and PEGs are under different evolutionary pressures [70]. Interestingly, genes that are imprinted exclusively in the placenta (as opposed to genes that are imprinted in both embryo and extraembryonic tissues) are often not conserved between mice and humans [71]. This may be a reflection of the stronger intra-litter competition that occurs during mice pregnancies, which, according to the kinship theory, would lead to an increased pressure to maintain imprinting. The mechanisms that regulate imprinting in these genes appear to be different (more dependent on histone modifications and less so on DNA methylation) than the mechanisms that regulate imprinting ubiquitously in placental, embryonic, and adult tissues, where differential DNA methylation plays a central role [72].
Around 30% to 40% of the genes imprinted in mice show conservation of imprinting in humans [73]. Fewer genes have conserved imprinting between eutherians and marsupials [27], and only two show a conservation of the associated differentially methylated regions [74,75]. This suggests that imprinting has evolved independently at individual loci in marsupials and eutherians since the two lineages separated over 160 million years ago. The increased number of imprinted genes and elaboration of imprinting mechanisms in eutherians may have been caused by their longer placental gestations.
In plants, imprinted genes are more labile. Recently, the use of allele-specific RNA sequencing greatly increased the number of imprinted candidate genes in Arabidopsis, maize, and rice (reviewed in [76]). However, there is rather limited overlap between independent studies, and it is difficult to separate inherent biological variation from technical biases: RNA sequencing has been shown to create a very large number of false positives, as high as 10 times the true number of imprinted genes in the mouse brain [77]. In addition, homology comparisons across distantly related plant species are confounded by a complex history of gene and genome duplications [78]. Nevertheless, a few homologous genes are predicted to be imprinted in both eudicots and monocots, and the E(z)-like homologs MEDEA and Mez1 have been directly confirmed to be imprinted in the endosperm of Arabidopsis and maize, respectively [64,79,80]. This suggests that imprinted expression of at least some plant genes may have been conserved for more than 100 million years (alternatively, imprinted expression evolved multiple times at these genes). Interestingly, two paralogous maize genes (FIE1 and FIE2) show imprinted gene expression, although the expression and DNA methylation dynamics of the two genes are dissimilar [81,82]. This suggests that imprinted gene expression can be maintained and modified after allo-tetraploidization or gene duplication events.
Although only a limited number of tissues have been analyzed, imprinted gene expression in mammals is thought to occur primarily during embryogenesis, but some genes are also mono-allelically expressed in other tissues, such as the brain. There is a high degree of spatial and temporal variation in imprinted expression, with some imprinted genes being monoallelically expressed in some cell types but biallelically expressed in others [83,84]. In plants, imprinted gene expression occurs primarily in the endosperm. Although some genes are also imprinted in the embryo [85,86], imprinted gene expression has not been found in adult Arabidopsis tissues [86,87].
Variation in imprinted gene expression in plants also occurs at the intra-specific level. Allelic variation for imprinting was first identified in maize [2], and recent estimates suggest that 10% to 20% of imprinted plant genes show allelic variation (some alleles are imprinted and others are biallelically expressed) [88,89]. In mammals, imprinting at any given gene appears to be much more stable across lineages (some earlier reports of allelic variation at the human insulin-like growth factor 2 receptor (IGF2R) and serotonin-2A loci [90,91] were probably due to technical issues [92,93]). Nevertheless, the effects of individual imprinted loci in mice have been shown to depend on interactions between pairs of alleles [94] and to be influenced by the genetic background [95].
Outlook
The study of genetic imprinting has been extremely valuable to understand the epigenetic mechanisms that regulate gene expression in plants and animals [1,14,15,48,96]. Yet many questions remain regarding the molecular mechanisms that cause imprinted expression and of how variation in cis and trans can modulate genomic imprinting. High-throughput cell sorting and sequencing technologies now allow an increasingly detailed profiling of genomes and epigenomes. These have revealed large differences in gene expression and imprinting between cell types. There is also evidence for a large difference in the cis-regulatory control of allelic expression [97] and even for widespread stochastic monoallelic expression in mammals [98]. A detailed profiling of allelic expression in different species and cell types will allow us to better understand the diversity and evolution of imprinting mechanisms. This will continue to offer us valuable insights into the mechanisms that regulate gene expression in animals and plants.
Abbreviations
- MEG
maternally expressed gene
- PEG
paternally expressed gene
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/63
Contributor Information
Nuno D. Pires, Email: nuno.pires@botinst.uzh.ch.
Ueli Grossniklaus, Email: grossnik@botinst.uzh.ch.
References
- 1.Raissig MT, Baroux C, Grossniklaus U. Regulation and flexibility of genomic imprinting during seed development. Plant Cell. 2011;23:16–26. doi: 10.1105/tpc.110.081018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kermicle J. Dependence of the R-mottled aleurone phenotype in maize on mode of sexual transmission. Genetics. 1970;66:69–85. doi: 10.1093/genetics/66.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718486711
- 3.Nishiyama I, Yabuno T. Triple fusion of the primary endosperm nucleus as a cause of interspecific cross-incompatibility in Avena. Euphytica. 1979;28:57–65. doi: 10.1007/BF00029173. [DOI] [Google Scholar]
- 4.Johnston S, den Nijs T, Peloquin S, Hanneman Jr., R The significance of genic balance to endosperm development in interspecific crosses. Theor Appl Genet. 1980;57:5–9. doi: 10.1007/BF00276002. [DOI] [PubMed] [Google Scholar]
- 5.Lin BY. Ploidy barrier to endosperm development in maize. Genetics. 1984;107:103–15. doi: 10.1093/genetics/107.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Surani M, Barton S, Norris M. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984;308:548–50. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- 7.McGrath J, Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984;37:179–83. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- 8.Cattanach BM, Kirk M. Differential activity of maternally and paternally derived chromosome regions in mice. Nature. 1985;315:496–98. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- 9.Barlow DP, Stoger R, Herrmann BG, Saito K, Schweifer N. The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature. 1991;349:84–7. doi: 10.1038/349084a0. [DOI] [PubMed] [Google Scholar]
- 10.DeChiara TM, Robertson EJ, Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–59. doi: 10.1016/0092-8674(91)90513-X. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomei MS, Zemel S, Tilghman SM. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–55. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 12.Grossniklaus U. Genomic imprinting in plants: a predominantly maternal affair. In: Meyer P, editor. Annu Plant Rev Plant Epigenetics. Sheffield, UK: Blackwell; 2005:. pp. 174–200. [DOI] [Google Scholar]
- 13.Feil R, Berger F. Convergent evolution of genomic imprinting in plants and mammals. Trends Genet. 2007;23:192–99. doi: 10.1016/j.tig.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Köhler C, Wolff P, Spillane C. Epigenetic Mechanisms Underlying Genomic Imprinting in Plants. Annu Rev Plant Biol. 2012;63:331–52. doi: 10.1146/annurev-arplant-042811-105514. [DOI] [PubMed] [Google Scholar]
- 15.Gehring M. Genomic imprinting: insights from plants. Annu Rev Genet. 2013;47:187–208. doi: 10.1146/annurev-genet-110711-155527. [DOI] [PubMed] [Google Scholar]
- 16.Morison IM, Paton CJ, Cleverley SD. The imprinted gene and parent-of-origin effect database. Nucleic Acids Research. 2001;291:275–276.. doi: 10.1093/nar/29.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crouse H V. The controlling element in sex chromosome behavior in Sciara. Genetics. 1960;45:1429–43. doi: 10.1093/genetics/45.10.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renfree MB, Hore TA, Shaw G, Graves JAM, Pask AJ. Evolution of genomic imprinting: insights from marsupials and monotremes. Annu Rev Genomics Hum Genet. 2009;10:241–62. doi: 10.1146/annurev-genom-082908-150026. [DOI] [PubMed] [Google Scholar]
- 19.Frésard L, Leroux S, Servin B, Gourichon D, Dehais P, Cristobal MS, Marsaud N, Vignoles F, Bed'hom B, Coville J-L, Hormozdiari F, Beaumont C, Zerjal T, Vignal A, Morisson M, Lagarrigue S, Pitel F. Transcriptome-wide investigation of genomic imprinting in chicken. Nucleic Acids Res. 2014;42:3768–82. doi: 10.1093/nar/gkt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodburne MO, Rich TH, Springer MS. The evolution of tribospheny and the antiquity of mammalian clades. Mol Phylogenet Evol. 2003;28:360–85. doi: 10.1016/S1055-7903(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 21.Luo Z-X, Yuan C-X, Meng Q-J, Ji Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature. 2011;476:442–45. doi: 10.1038/nature10291. [DOI] [PubMed] [Google Scholar]
- 22.Taylor TN, Taylor EL, Krings M. Paleobotany: The Biology and Evolution of Fossil Plants. 2. Academic Press; 2009. [Google Scholar]
- 23.Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maheshwari P. An Introduction to the Embryology of Angiosperms. New York: McGraw-Hill; 1950. [Google Scholar]
- 25.Baroux C, Spillane C, Grossniklaus U. Evolutionary origins of the endosperm in flowering plants. Genome Biol. 2002;3:reviews1026. doi: 10.1186/gb-2002-3-9-reviews1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta--a review. Placenta. 2005;26(Suppl A):S10–20. doi: 10.1016/j.placenta.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Renfree MB, Suzuki S, Kaneko-Ishino T. The origin and evolution of genomic imprinting and viviparity in mammals. Philos Trans R Soc Lond, B, Biol Sci. 2013;368:20120151. doi: 10.1098/rstb.2012.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower FO. The Origin of a Land Flora. London: Macmillan & Co.; 1908. [Google Scholar]
- 29.Kawahara M, Wu Q, Takahashi N, Morita S, Yamada K, Ito M, Ferguson-Smith AC, Kono T. High-frequency generation of viable mice from engineered bi-maternal embryos. Nat Biotechnol. 2007;25:1045–50. doi: 10.1038/nbt1331. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1097540
- 30.Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo J-S, Ogawa H. Birth of parthenogenetic mice that can develop to adulthood. Nature. 2004;428:860–64. doi: 10.1038/nature02402. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1017816
- 31.Koltunow AM, Grossniklaus U. Apomixis: a developmental perspective. Annu Rev Plant Biol. 2003;54:547–74. doi: 10.1146/annurev.arplant.54.110901.160842. [DOI] [PubMed] [Google Scholar]
- 32.Haig D. Coadaptation and conflict, misconception and muddle, in the evolution of genomic imprinting. Heredity. 2013::1–8. doi: 10.1038/hdy.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer HG, Clark AG. Non-conflict theories for the evolution of genomic imprinting. Heredity. 2014::1–7. doi: 10.1038/hdy.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore T, Mills W. Evolutionary theories of imprinting-enough already! Adv Exp Med Biol. 2008;626:116–22. doi: 10.1007/978-0-387-77576-0_9. [DOI] [PubMed] [Google Scholar]
- 35.Varmuza S, Mann M. Genomic imprinting--defusing the ovarian time bomb. Trends Genet. 1994;10:118–23. doi: 10.1016/0168-9525(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 36.Haig D, Westoby M. Parent specific gene expression and the triploid endosperm. Am Nat. 1989;134:147–55. doi: 10.1086/284971. [DOI] [Google Scholar]
- 37.Haig D, Westoby M. Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications. Philos Trans Biol Sci. 1991;333:1–13. doi: 10.1098/rstb.1991.0057. [DOI] [Google Scholar]
- 38.Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet. 2003;4:359–368. doi: 10.1038/nrg1062. [DOI] [PubMed] [Google Scholar]
- 39.Wolf JB, Hager R. A maternal-offspring coadaptation theory for the evolution of genomic imprinting. PLoS Biol. 2006;4:e380. doi: 10.1371/journal.pbio.0040380. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1052670
- 40.Brandvain Y, Van Cleve J, Ubeda F, Wilkins JF. Demography, kinship, and the evolving theory of genomic imprinting. Trends Genet. 2011;27:251–57. doi: 10.1016/j.tig.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Cleve JV, Marcus W Feldman, Laurent Lehmann. How Demography, Life History, and Kinship Shape the Evolution of Genomic Imprinting. Am Nat. 2010;176:440–55. doi: 10.1086/656277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossniklaus U, Vielle-Calzada J-P, Hoeppner MA, Gagliano WB. Maternal control of embryogenesis by MEDEA, a Polycomb group gene in Arabidopsis. Science (80-) 1998;280:446–50. doi: 10.1126/science.280.5362.446. [DOI] [PubMed] [Google Scholar]
- 43.Köhler C, Page DR, Gagliardini V, Grossniklaus U. The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet. 2005;37:28–30. doi: 10.1038/ng1495. [DOI] [PubMed] [Google Scholar]
- 44.Guillemot F, Caspary T, Tilghman SM, Copeland NG, Gilbert DJ, Jenkins NA, Anderson DJ, Joyner AL, Rossant J, Nagy A. Genomic imprinting of Mash2, a mouse gene required for trophoblast development. Nat Genet. 1995;9:235–42. doi: 10.1038/ng0395-235. [DOI] [PubMed] [Google Scholar]
- 45.Costa LM, Yuan J, Rouster J, Paul W, Dickinson H, Gutierrez-Marcos JF. Maternal control of nutrient allocation in plant seeds by genomic imprinting. Curr Biol. 2012;22:160–65. doi: 10.1016/j.cub.2011.11.059. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13854963
- 46.Iwasa Y, Mochizuki A, Takeda Y. The evolution of genomic imprinting: abortion and overshoot explain aberrations. Evol Ecol Res. 1999;1:129–50. [Google Scholar]
- 47.Wang X, Miller DC, Harman R, Antczak DF, Clark AG. Paternally expressed genes predominate in the placenta. Proc Natl Acad Sci USA. 2013;110:10705–10. doi: 10.1073/pnas.1308998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson-Smith AC. Genomic imprinting: the emergence of an epigenetic paradigm. Nat Rev Genet. 2011;12:565–75. doi: 10.1038/nrg3032. [DOI] [PubMed] [Google Scholar]
- 49.Williamson CM, Blake A, Thomas S, Beechey CV, Hancock J, Cattanach BM, Peters J. MRC Harwell, ; Oxfordshire: 2013. World Wide Web Site - Mouse Imprinting Data and References.http://www.har.mrc.ac.uk/research/genomic_imprinting/ [Google Scholar]
- 50.Dünzinger U, Nanda I, Schmid M, Haaf T, Zechner U. Chicken orthologues of mammalian imprinted genes are clustered on macrochromosomes and replicate asynchronously. Trends Genet. 2005;21:488–91. doi: 10.1016/j.tig.2005.07.004. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1027644
- 51.Dünzinger U, Haaf T, Zechner U. Conserved synteny of mammalian imprinted genes in chicken, frog, and fish genomes. Cytogenet Genome Res. 2007;117:78–85. doi: 10.1159/000103167. [DOI] [PubMed] [Google Scholar]
- 52.McQueen HA, Siriaco G, Bird AP. Chicken microchromosomes are hyperacetylated, early replicating, and gene rich. Genome Res. 1998;8:621–30. doi: 10.1101/gr.8.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguer-Dance M, Abu-Amero S, Al-Khtib M, Lefèvre A, Coullin P, Moore GE, Cavaillé J. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. 2010;19:3566–82. doi: 10.1093/hmg/ddq272. [DOI] [PubMed] [Google Scholar]
- 54.McCole RB, Loughran NB, Chahal M, Fernandes LP, Roberts RG, Fraternali F, O'Connell MJ, Oakey RJ. A case-by-case evolutionary analysis of four imprinted retrogenes. Evolution (N Y) 2011;65:1413–27. doi: 10.1111/j.1558-5646.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Zhao H, Xie S, Chen J, Xu Y, Wang K, Zhao H, Guan H, Hu X, Jiao Y, Song W, Lai J. Extensive, clustered parental imprinting of protein-coding and noncoding RNAs in developing maize endosperm. Proc Natl Acad Sci USA. 2011;108:20042–47. doi: 10.1073/pnas.1112186108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang H, Köhler C. Evolution, function, and regulation of genomic imprinting in plant seed development. J Exp Bot. 2012;63:695–709. doi: 10.1093/jxb/ers145. [DOI] [PubMed] [Google Scholar]
- 57.Barlow DP. Methylation and imprinting: from host defense to gene regulation? Science. 1993;260:309–10. doi: 10.1126/science.8469984. [DOI] [PubMed] [Google Scholar]
- 58.McDonald JF, Matzke M a, Matzke a J. Host defenses to transposable elements and the evolution of genomic imprinting. Cytogenet Genome Res. 2005;110:242–49. doi: 10.1159/000084958. [DOI] [PubMed] [Google Scholar]
- 59.Yoder JA, Bestor TH. Genetic analysis of genomic methylation patterns in plants and mammals. Biol Chem. 1996;377:605–10. [PubMed] [Google Scholar]
- 60.Yoder JA, Walsh CP, Bestor TH. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–40. doi: 10.1016/S0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]
- 61.Pask AJ, Papenfuss AT, Ager EI, McColl KA, Speed TP, Renfree MB. Analysis of the platypus genome suggests a transposon origin for mammalian imprinting. Genome Biol. 2009;10:R1. doi: 10.1186/gb-2009-10-1-r1. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718486713
- 62.Smith NGC, Hurst LD. Molecular evolution of an imprinted gene: repeatability of patterns of evolution within the mammalian insulin-like growth factor type II receptor. Genetics. 1998;150:823–33. doi: 10.1093/genetics/150.2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker-Katiraee L, Carson AR, Yamada T, Arnaud P, Feil R, Abu-Amero SN, Moore GE, Kaneda M, Perry GH, Stone AC, Lee C, Meguro-Horike M, Sasaki H, Kobayashi K, Nakabayashi K, Scherer SW. Identification of the imprinted KLF14 transcription factor undergoing human-specific accelerated evolution. PLoS Genet. 2007;3:e65. doi: 10.1371/journal.pgen.0030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spillane C, Schmid KJ, Laoueillé-Duprat S, Pien S, Escobar-Restrepo J-M, Baroux C, Gagliardini V, Page DR, Wolfe KH, Grossniklaus U. Positive darwinian selection at the imprinted MEDEA locus in plants. Nature. 2007;448:349–52. doi: 10.1038/nature05984. [DOI] [PubMed] [Google Scholar]
- 65.Kawabe A, Fujimoto R, Charlesworth D. High diversity due to balancing selection in the promoter region of the Medea gene in Arabidopsis lyrata. Curr Biol. 2007;17:1885–89. doi: 10.1016/j.cub.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 66.Miyake T, Takebayashi N, Wolf DE. Possible diversifying selection in the imprinted gene, MEDEA, in Arabidopsis. Mol Biol Evol. 2009;26:843–57. doi: 10.1093/molbev/msp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilkins JF, Haig D. Genomic imprinting of two antagonistic loci. Proc R Soc London B. 2001;268(1479):1861–67. doi: 10.1098/rspb.2001.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hurst LD, McVean GT. Do we understand the evolution of genomic imprinting? Curr Opin Genet Dev. 1998;8:701–8. doi: 10.1016/S0959-437X(98)80040-3. [DOI] [PubMed] [Google Scholar]
- 69.O'Connell MJ, Loughran NB, Walsh TA, Donoghue MTA, Schmid KJ, Spillane C. A phylogenetic approach to test for evidence of parental conflict or gene duplications associated with protein-encoding imprinted orthologous genes in placental mammals. Mamm genome. 2010;21:486–98. doi: 10.1007/s00335-010-9283-5. [DOI] [PubMed] [Google Scholar]
- 70.Hutter B, Bieg M, Helms V, Paulsen M. Divergence of imprinted genes during mammalian evolution. BMC Evol Biol. 2010;10:116. doi: 10.1186/1471-2148-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monk D, Arnaud P, Apostolidou S, Hills F a, Kelsey G, Stanier P, Feil R, Moore GE. Limited evolutionary conservation of imprinting in the human placenta. Proc Natl Acad Sci USA. 2006;103:6623–28. doi: 10.1073/pnas.0511031103. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1003365
- 72.Hudson QJ, Kulinski TM, Huetter SP, Barlow DP. Genomic imprinting mechanisms in embryonic and extraembryonic mouse tissues. Heredity. 2010;105:45–56. doi: 10.1038/hdy.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–65. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Suzuki S, Ono R, Narita T, Pask AJ, Shaw G, Wang C, Kohda T, Alsop AE, Marshall Graves JA, Kohara Y, Ishino F, Renfree MB, Kaneko-Ishino T. Retrotransposon silencing by DNA methylation can drive mammalian genomic imprinting. PLoS Genet. 2007;3:e55. doi: 10.1371/journal.pgen.0030055. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1085754
- 75.Smits G, Mungall AJ, Griffiths-Jones S, Smith P, Beury D, Matthews L, Rogers J, Pask AJ, Shaw G, VandeBerg JL, McCarrey JR, Renfree MB, Reik W, Dunham I. Conservation of the H19 noncoding RNA and H19-IGF2 imprinting mechanism in therians. Nat Genet. 2008;40:971–76. doi: 10.1038/ng.168. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1116843
- 76.Pignatta D, Gehring M. Imprinting meets genomics: new insights and new challenges. Curr Opin Plant Biol. 2012;15:530–35. doi: 10.1016/j.pbi.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 77.DeVeale B, van der Kooy D, Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: a new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963710
- 78.Pires ND, Dolan L. Morphological evolution in land plants: new designs with old genes. Phil Trans R Soc B. 2012;367:508–18. doi: 10.1098/rstb.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vielle-Calzada J-P, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U. Maintenance of genomic imprinting at the Arabidopsis medea locus requires zygotic DDM1 activity. Gene Dev. 1999;13:2971–82. doi: 10.1101/gad.13.22.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haun WJ, Laoueillé-Duprat S, O'Connell MJ, Spillane C, Grossniklaus U, Phillips AR, Kaeppler SM, Springer NM. Genomic imprinting, methylation and molecular evolution of maize Enhancer of zeste (Mez) homologs. Plant J. 2007;49:325–37. doi: 10.1111/j.1365-313X.2006.02965.x. [DOI] [PubMed] [Google Scholar]
- 81.Danilevskaya ON, Hermon P, Hantke S, Muszynski MG, Kollipara K, Ananiev E V. Duplicated fie genes in maize : expression pattern and imprinting suggest distinct functions. Plant Cell. 2003;15:425–38. doi: 10.1105/tpc.006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gutiérrez-Marcos JF, Costa LM, Dal Prà M, Scholten S, Kranz E, Perez P, Dickinson HG. Epigenetic asymmetry of imprinted genes in plant gametes. Nat Genet. 2006;38:876–78. doi: 10.1038/ng1828. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718486714
- 83.Prickett AR, Oakey RJ. A survey of tissue-specific genomic imprinting in mammals. Mol Genet Genomics. 2012;287:621–30. doi: 10.1007/s00438-012-0708-6. [DOI] [PubMed] [Google Scholar]
- 84.Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–43. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- 85.Jahnke S, Scholten S. Epigenetic resetting of a gene imprinted in plant embryos. Curr Biol. 2009;19:1677–81. doi: 10.1016/j.cub.2009.08.053. [DOI] [PubMed] [Google Scholar]
- 86.Raissig MT, Bemer M, Baroux C, Grossniklaus U. Genomic imprinting in the Arabidopsis embryo is partly regulated by PRC2. PLoS Genet. 2013;9:e1003862. doi: 10.1371/journal.pgen.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Borevitz JO. Global analysis of allele-specific expression in Arabidopsis thaliana. Genetics. 2009;182:943–54. doi: 10.1534/genetics.109.103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wolff P, Weinhofer I, Seguin J, Roszak P, Beisel C, Donoghue MT a, Spillane C, Nordborg M, Rehmsmeier M, Köhler C. High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis endosperm. PLoS Genet. 2011;7:e1002126. doi: 10.1371/journal.pgen.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waters AJ, Bilinski P, Eichten SR, Vaughn MW, Ross-Ibarra J, Gehring M, Springer NM. Comprehensive analysis of imprinted genes in maize reveals allelic variation for imprinting and limited conservation with other species. Proc Natl Acad Sci USA. 2013;110:19639–44. doi: 10.1073/pnas.1309182110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718173166
- 90.Xu YQ, Goodyer CG, Deal C, Polychronakos C. Functional polymorphism in the parental imprinting of the human IGF2R gene. Biochem Biophys Res Commun. 1993;197:747–54. doi: 10.1006/bbrc.1993.2542. [DOI] [PubMed] [Google Scholar]
- 91.Bunzel R, Blümcke I, Cichon S, Normann S, Schramm J, Propping P, Nöthen MM. Polymorphic imprinting of the serotonin-2A (5-HT2A) receptor gene in human adult brain. Mol Brain Res. 1998;59:90–92. doi: 10.1016/S0169-328X(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 92.Killian JK, Nolan CM, Wylie AA, Li T, Vu TH, Hoffman AR, Jirtle RL. Divergent evolution in M6P/IGF2R imprinting from the Jurassic to the Quaternary. Hum Mol Genet. 2001;10:1721–28. doi: 10.1093/hmg/10.17.1721. [DOI] [PubMed] [Google Scholar]
- 93.Bray NJ, Buckland PR, Hall H, Owen MJ, O'Donovan MC. The serotonin-2A receptor gene locus does not contain common polymorphism affecting mRNA levels in adult brain. Mol Psychiatry. 2004;9:109–14. doi: 10.1038/sj.mp.4001366. [DOI] [PubMed] [Google Scholar]
- 94.Wolf JB, Cheverud JM, Roseman C, Hager R. Genome-wide analysis reveals a complex pattern of genomic imprinting in mice. PLoS Genet. 2008;4:e1000091. doi: 10.1371/journal.pgen.1000091. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1115109
- 95.Wolf JB, Oakey RJ, Feil R. Imprinted gene expression in hybrids: perturbed mechanisms and evolutionary implications. Heredity. 2014: doi: 10.1038/hdy.2014.11. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koerner M V, Barlow DP. Genomic imprinting-an epigenetic gene-regulatory model. Curr Opin Genet Dev. 2010;20:164–70. doi: 10.1016/j.gde.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pastinen T. Genome-wide allele-specific analysis: insights into regulatory variation. Nat Rev Genet. 2010;11:533–38. doi: 10.1038/nrg2815. [DOI] [PubMed] [Google Scholar]
- 98.Chess A. Mechanisms and consequences of widespread random monoallelic expression. Nat Rev Genet. 2012;13:421–28. doi: 10.1038/nrg3239. [DOI] [PubMed] [Google Scholar]