Abstract
Neonatal sepsis is a common and deadly disease. It is broadly defined as a systemic inflammatory response, occurring in the first four weeks of life, as a result of a suspected or proven infection. Yet, more reliable and consistently applied diagnostic criteria would help improve our knowledge of the disease epidemiology. Several therapeutic attempts to control systemic inflammation in sepsis were unsuccessful. Immediate empirical administration of broad-spectrum anti-microbials, aggressive fluid resuscitation, and vaso-active or inotropic support (or both) are the mainstays of the therapeutic management of neonatal sepsis.
Introduction
Worldwide, 360,346 neonates died from sepsis and other infections in 2011 [1]. By definition, “neonatal” pertains to the first four weeks postnatal, whether born prematurely or at term. This article will pay particular attention to neonatal sepsis in term babies and review recent evidence published over the last two years. We will also focus our discussion on the management of neonates admitted to the pediatric intensive care unit (ICU) for a neonatal sepsis.
1. Diagnostic criteria of neonatal sepsis
In 2005, the International Pediatric Sepsis Consensus Conference defined sepsis as a “systemic inflammatory response syndrome (SIRS) in the presence of or as a result of suspected or proven infection” [2]. A SIRS is considered present if at least two of the following four criteria are observed, one of the two being abnormal temperature or leukocyte count:
Core temperature of more than 38.5°C or less than 36°C.
Tachycardia, or bradycardia for children younger than 1 year old.
Mean respiratory rate of more than 2 standard deviations (SDs) above normal for age or mechanical ventilation for an acute process not related to underlying neuromuscular disease or the need for general anesthesia.
Leukocyte count elevated or depressed for age (not secondary to chemotherapy-induced leukopenia) or more than 10% immature neutrophils.
The diagnostic criteria listed above were developed to improve the diagnosis of pediatric sepsis, from newborns to adolescents up to 18 years of age; the diagnostic value of these criteria has not been estimated in neonatal sepsis.
Other diagnostic criteria have been suggested. In 2010, a group of European experts suggested a list of seven clinical and six laboratory parameters defining late-onset neonatal sepsis. In a prospective study, Lutsar et al. [3] showed that the predictive value of these criteria to recognize cases of culture-proven late-onset neonatal sepsis was 61% (95% confidence interval (CI) 52% to 70%), which is almost equivalent to tossing a coin. Classic sepsis criteria, such as impaired peripheral perfusion, increased oxygen requirement, and mottled skin, were observed in only 40% of affected children! Hence, a more reliable list of diagnostic criteria for neonatal sepsis should be developed and validated, which should probably include not only clinical data but also laboratory parameters like cord blood level of procalcitonin or interleukin (IL)-6 or both [4,5].
2. Septic states
Experts in critical care medicine defined three septic states: sepsis, severe sepsis, and septic shock [2,6]. Does it make sense? Leclerc et al. showed that there is an added prognostic value if one differentiates these three septic states: the hazard ratios of mortality were 7.43 (95% CI 1.01 to 54.8) in critically ill children with sepsis, 27.40 (95% CI 3.26 to 230.4) in patients who contracted severe sepsis, and 61.40 (95% CI 7.8 to 486.1) in those with septic shock [7]. Clearly, the frequency of these three septic states must be taken into account in all randomized controlled trials (RCTs) where the efficacy of a treatment is studied in critically ill children, including neonates. The relationship between SIRS, sepsis, severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS) is illustrated in Figure 1.
Figure 1. Relationship between systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, septic shock, and multiple organ dysfunction syndrome (MODS).
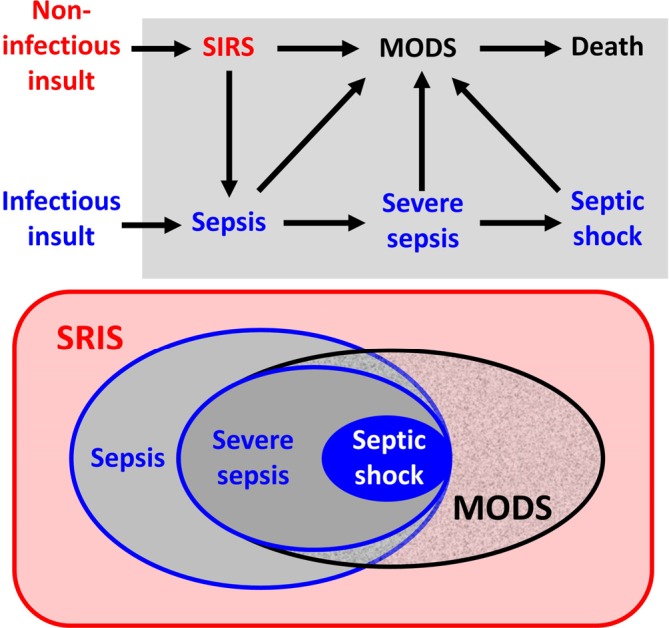
Upper panel: relationship according to infectious etiology. Lower panel: Venn diagram.
3. Epidemiology
There are good studies on the epidemiology of severe sepsis, but not on sepsis, probably because many studies were based on a public database using International Classification of Diseases, Ninth Revision (ICD-9) codes. The Centers for Medicare & Medicaid Services (www.cms.gov) do not reserve an ICD-9 code for sepsis, but do so for severe sepsis (995.92) and septic shock (785.52 and 998.02). In 1995, the incidence rate of severe neonatal sepsis and septic shock in 7 American states was 3.6 cases per 1,000 of population with a case fatality rate of 10.3%; about one third of severe neonatal sepsis was observed in term babies [8,9]. Similarly, 62 neonates were admitted to the pediatric ICU Sainte-Justine Hospital over a 1-year period (2010); frequency rates were 50% (n = 31) for SIRS alone, 29% (n = 18) for sepsis, 11% (n = 7) for severe sepsis, and 6% (n = 4) for septic shock [10].
By definition, early-onset sepsis happens within the first week after birth (some would say in the first 72 hours of life [11]). Its incidence in the UK is about 0.5 per 1,000 term births; the mortality rate is about 10%, and severe permanent disability is frequently observed in survivors [12]. Late-onset sepsis occurs between the 8th and the 89th day after birth. In a prospective study conducted in Taiwan, 17% of late-onset neonatal sepsis cases (118 out of 713) were detected in term neonates rather than premature babies.
4. Sepsis-related complications
Sepsis can cause many severe complications happening while the patient is in the ICU, like acute lung injury, cardiac dysfunction, capillary leak syndrome, coma, critical care neuromyopathy, thrombosis, coagulopathy, hemorrhage, hepatic failure, renal failure, reactive hemophagocytic syndrome, hyperglycemia, nosocomial infections, MODS, and death [13]. The epidemiology of these complications is not so well determined. These acute complications may be compounded by alteration of drug pharmacokinetics in sepsis. For example, Pettersen et al. [14] showed that clearance of pantoprazole was profoundly decreased in SIRS and sepsis. Moreover, many drugs have a large volume of distribution and decreased clearance in neonates, more so than in older patients [11]. Actually the effect of SIRS, sepsis, MODS, and age on the pharmacology of many drugs given to critically ill neonates remains to be ascertained.
After the ICU stay, numerous long-term complications are also described in the literature, such as persistent inflammation [15]; severe chronic pain [16]; neuro-developmental problems [17]; ICU-acquired weakness [16,18]; stress ulcer-related upper gastro-intestinal bleeding [19]; persistent renal dysfunction [20,21]; sleeping problems and post-traumatic stress disorder [22]; depression, acute stress disorder, and anxiety [23]; and poor quality of life of the patients or their family or both [16,24]. Experts in critical care medicine coined the terms “post-intensive care syndrome” (PICS) [25] and “post-intensive care syndrome-family” (PICS-F) [23] to raise attention to these frequently under-recognized chronic and devastating conditions. The epidemiology and the clinical impact in neonates of sepsis-related long-term sequelae — PICS and PICS-F — are unknown. The risk of long-term neuropsychological deficits and educational difficulties is higher in school-aged children admitted for septic illness and meningo-encephalitis compared with children admitted for other ICU-related illnesses [26]; it is unknown whether this holds true in newborns.
5. Management
Sepsis, by definition, is caused by an infection; not surprisingly, there is a consensus that an anti-infectious agent must be given as early as possible to all patients with sepsis [27]. Over the past 20 years, a large number of RCTs have attempted to control the systemic inflammatory storm characterizing sepsis with little success. Supportive care of dysfunctional organs is therefore the mainstay of therapy, which might include mechanical ventilation, fluids, vasopressors or inotropes (or both), and blood transfusion.
5.1. Treatment against infectious agents
There is no clear consensus on the management of term newborns with sepsis, and this probably explains the large variation in the practice pattern observed among British practitioners [12]. However, anti-microbial agents are unequivocally the cornerstone therapy of sepsis.
Most cases of early-onset sepsis in term newborns are caused by Group B Streptococcus, but Gram-negative bacteria are not rare. The most common choice of antibiotics in 125 UK hospital guidelines was a combination of benzylpenicillin and intravenous gentamicin [12].
Most cases of late-onset sepsis are attributable to Staphylococcus species and Group B Streptococcus, but about one third are caused by Gram-negative organisms (Klebsiella spp, Escherichia coli, etc.) [28]. In a prospective study of 113 consecutive newborns with late-onset neonatal sepsis, Lutsar et al. [3] found more than 18 different empiric antibiotic regimens; most included ampicillin, a third-generation cephalosporin, or meropenem, plus an aminoglycoside or vancomycin. Current management of late-onset neonatal sepsis is extremely variable. An ongoing large RCT compares meropenem versus standard care [29].
There is a consensus that antibiotics must be started as soon as possible once a neonatal sepsis is suspected, but there is a debate as to when they should be stopped [30]. Intravenous antibiotics are usually prescribed for 21 days for cases of neonatal meningitis and for 10 to 14 days in other severe neonatal infections. There is more and more concern that antibacterial agents are overused. There is indeed a risk to undertreat an infection if the course of antibiotics is too short, but keeping antibiotics for a prolonged time is associated with the emergence and spread of resistance to antibiotics. Some experts are also concerned by long-term effects on microbiota implantation and possible impact on future child health [31]. In 2013, the World Health Assembly underlined the risk of antibiotic resistance to global health security [32]. Can we stop antibiotics sooner, at least in patients for whom the source of sepsis is unclear or no germs have been found (or both)? Some investigators believe that markers like progression over time of procalcitonin levels or of neutrophil counts might be good indicators to stop antibiotics. A large multicenter RCT evaluating whether procalcitonin levels could be used to guide antibiotic therapy in adults with non-microbiologically proven sepsis was interrupted early because of failure to demonstrate benefit [33]. In the Procalcitonin and Survival Study (PASS), procalcitonin-guided anti-microbial escalation in the ICU did not improve survival and resulted in prolonged ICU stay and more organ damage [34]. No studies have been undertaken in neonates.
5.2. Treatment aiming to control the systemic inflammatory process
Sepsis is a systemic response to fight off pathogens. However, this systemic inflammatory process can become uncontrolled, resulting in MODS and death. Thus, it makes sense to try to control the SIRS in patients with sepsis.
More than $1 billion has been spent to complete RCTs aiming to find a magic bullet that would modulate the inflammation the right way and that would improve the outcome of patients with sepsis. High levels of cytokines like tumor necrosis factor-alpha (TNFα), IL-1, IL-10, and platelet-aggregating factor (PAF) are observed in patients with sepsis; however, all RCTs using molecules active against these cytokines (anti-IL-1, anti-IL-10, and anti-PAF) were negative, as were RCTs on recombinant human soluble thrombomodulin [35] and on recombinant bactericidal permeability-increasing protein (BPI) [36]. Some molecules were detrimental, like nitric oxide synthase inhibitors [37] and recombinant tissue factor pathway inhibitor [37]. However, one large RCT using activated protein C in adults with sepsis — the Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study — was positive [38]. Thereafter, an RCT conducted in 477 children reported no positive effects and more severe hemorrhage, including intracranial bleeding [39]. Actually, the PROWESS study in adults was probably a false positive: two other RCTs reported no difference at all in the outcome of adults in septic shock [40,41]. A meta-analysis recommends that activated protein C not be used to treat neonatal sepsis [42]. In any case, activated protein C is now withdrawn from the market.
At present, there is no evidence that mediator modulation therapy works. Nonetheless, is there any hope that we can find a magic bullet? In 2013, Qiu et al. published a meta-analysis reporting that anti-TNF (anti-TNFα-Ab and anti-TNFα receptors) “produces a modest decrease in the risk of mortality of patients with sepsis” (relative risk 0.93, 95% CI 0.88 to 0.98) [43]. The conclusion was that “a definitive trial demonstrating the potential benefit of such agents might require at least 10,000 patients with sepsis” [43]. Would it make sense to undertake such a large-scale RCT for such a small effect size? Maybe not. Actually, there is a lot of redundancy and interactions between all pro- and anti-inflammatory mediators; it is hard to believe that modulating only one of them will cure patients with sepsis. Many experts in critical care medicine believe that there will not be any “magic bullet” and that a more general approach against the SIRS would be better. Many non-specific treatments of sepsis have been advocated.
The immune system of patients with severe SIRS is overwhelmed. Recent studies demonstrated that an impairment of patients' immune system called immunoparalysis could be the main contributor to the late mortality of patients with sepsis. Reduced expression of human leukocyte antigen (HLA)-DR on monocytes (mHLA-DR), lymphocyte apoptosis, and attenuated TNFα production are some of the underlying evoked mechanisms. In theory, it would thus make sense to attempt to enhance the capacity of the immune system in cases of neonatal sepsis. We should discuss at least three treatments: colony-stimulating factors (CSFs), immune globulins (IgGs), and optimal nutrition. (a) CSFs can increase neutrophil and macrophage counts. In theory, they should be useful in patients with neutropenia. In practice, a meta-analysis reported that the different types of CSFs do not seem to work in neonatal sepsis [44]. Granulocyte-CSF (G-CSF) and granulocyte-macrophage CSF (GM-CSF) cannot be recommended in neonatal sepsis. (b) IgGs are effective in adults with sepsis [45] but not in low-birth weight newborns (<1,500 g) [46] or in newborns with gestational ages of 31 to 42 weeks [47]. (c) A meta-analysis on the efficacy of immuno-modulating diets reported that they had no effect on mortality or length of stay of critically ill patients [48]. On the other hand, there were data suggesting that standard nutrition with added nutriments might be useful in critically ill adults [49]. An RCT published in 2013 reported that early provision of glutamine did not improve clinical outcomes; glutamine supplementation was even associated with an increased mortality in critically ill adults with MODS [50]. The optimal nutrition strategy and the best feeding route (enteral or parenteral or both) remain to be determined in patients with sepsis, including neonates.
Non-specific “cleaning” of inflammatory mediators to remove “bad humors” by extracorporeal blood purification techniques is another avenue. A meta-analysis of RCTs reported that, overall, blood purification techniques — hemofiltration, hemoperfusion, plasma exchange (i.e., plasmapheresis), or hemodialysis — decrease the mortality rate of patients with sepsis: risk ratio (RR) 0.69, 95% CI 0.56 to 0.84. The most positive effect was attributable to 10 RCTs on polymyxin B hemoperfusion (RR 0.63, 95% CI 0.50 to 0.80) and two RCTs on plasma exchange (RR 0.63, 95% CI 0.42 to 0.96) [51]. The efficacy of blood purification techniques must be estimated by a large RCT before they can be strongly recommended [52]. This holds true also in neonatal sepsis: the cost/benefit ratio should be different in neonates and infants since the risk of mortality is much lower in this population than in adults, while the technical risks should be at least similar to, if not higher than, those in adults (for example, more difficult intravenous access) [53].
Increased mortality is associated with both high and low cortisol levels. In the ‘80s, a large RCT proved that high-dose steroids (up to 30 mg/kg per day of methyl-prednisolone) increase mortality in cases of severe sepsis and septic shock [54]. However, some intensivists still believed that normal-dose steroids can be useful. In 2002, an RCT reported that a 7-day treatment with low-dose hydrocortisone (50 mg every 6 hours) and fludrocortisone (50 μg once daily) decreased 28th-day mortality in adults with septic shock and relative adrenal insufficiency [55]. In 2008, the Corticosteroid Therapy of Septic Shock (CORTICUS) RCT reported negative results [56]. Although it is clear that high-dose corticosteroid treatment provides no benefit and possibly harm in patients with sepsis, the role of corticosteroid treatment in patients with severe sepsis and septic shock remains controversial, and results from recent trials are contradictory [57]. At present, other large RCTs are being conducted that should bring about clear responses on the question in adults —for example, the Adjunctive Corticosteroid Treatment in Critically Ill Patients with Septic Shock (ADRENAL) study [58] — and children (NCT00732277), but none of these RCTs was conducted specifically in neonates.
5.3. Supportive care
The last update of the “Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock” was published in 2012 [59]. Key recommendations included broad-spectrum empiric anti-microbial therapy within 1 hour of the recognition of sepsis, infection source control, septic work-up (at least urine, blood, cerebrospinal fluid cultures, and lung x-rays), and hemoglobin target of 7 to 9 g/dL in stable patients.
In cases of severe sepsis and septic shock, recommendations also included early initial resuscitation (<6 hours after first medical recognition of severe sepsis) with crystalloids (at least 30 mL/kg within 3 hours), ± vaso-active or inotropic agents or both (first-line norepinephrine, ± epinephrine, ± dobutamine if signs of cardiac dysfunction). The goal in adults is to maintain a mean arterial pressure of at least 65 mm Hg; in neonatal and pediatric sepsis, physical examination therapeutic endpoints such as capillary refill of not more than 2 seconds, normal peripheral pulses, urine output of more than 1 mL/kg per hour, or normal mental status might be more reliable endpoints than blood pressure to assess the adequacy of resuscitation. Albumin can be given in cases of refractory shock. Hydrocortisone is also an option in children with fluid- and catecholamine-resistant shock and with suspected or proven adrenal insufficiency, even though its efficacy remains to be determined (see above). Vasopressin and blood glucose control were also suggested, but two RCTs conducted in children failed to demonstrate any clinical benefit from the use of vasopressin [60] and tight glycemic control (72 to 126 mg/dL or 4.0 to 7.0 mmol/L versus less than 216 mg/dL or less than 12.0 mmol/L) [61]. Once shock is resolved, continuous veno-venous hemofiltration or intermittent hemodialysis can be considered in patients with refractory fluid overload (>10%). These guidelines should be implemented as soon as neonatal sepsis is suspected [27].
Guidelines are derived from expert opinions, but some key aspects of supportive care in severe sepsis have been evaluated by RCTs, such as goal-directed therapy and transfusion thresholds. The concept of “early-goal directed therapy” was suggested in 2001: Rivers et al. [62] randomly assigned 266 adults with severe sepsis or septic shock during the first 6 hours in the emergency department either to be monitored with central venous oxygen saturation (ScvO2) or not. In patients allocated to ScvO2 monitoring, a bundle of treatment was advocated in order to maintain the ScvO2 over 70%; this bundle included, in sequential order, mechanical ventilation, fluid bolus up to 80 mL/kg, vasoactive drugs (dobutamine and vasoconstrictive therapy), and red blood cell (RBC) transfusion if the ScvO2 was still under 70% after all of the other maneuvers were done. The mortality rates were 30.5% with early goal-directed therapy and 46.5% in controls. The reproducibility of these data was evaluated in the “Protocolized Care for Early Septic Shock” (ProCESS) study: protocol-based resuscitation—with or without ScvO2 monitoring—of adults treated for septic shock in the emergency department; protocol-based treatment did not improve outcomes (mortality and need for organ support) [63]. In children, De Oliveira et al. [64] completed an RCT involving 102 Brazilian children with severe sepsis or fluid refractory shock. The goal and bundle were similar to those of the study by Rivers et al. [61]. The mortality rates were 11.8% with early goal-directed therapy and 39.2% in controls. As few neonates were enrolled in the study (mean age of more than 5 years), generalizability to neonatal sepsis would need to be confirmed.
Improving oxygen delivery is one of the central goals of supportive care; this can be done by increasing the hemoglobin level. However, RBC transfusions are not perfectly safe [65]. What is the optimal RBC transfusion threshold of patients with sepsis? If less evidence for the ideal transfusion threshold in unstable patients is available, Transfusion Requirements in Pediatric Intensive Care Units (TRIPICU) explored that question in stable or stabilized children (mean arterial pressure of not less than 2 SDs below the mean for age and cardiovascular support —pressors/inotropes and fluids — not increased for at least 2 hours) [66]. The incidence rate of new and progressive MODS, including deaths, in a subgroup analysis of 137 patients with sepsis, severe sepsis, and septic shock was unchanged (13 in both arms), which suggests that a restrictive RBC transfusion strategy (hemoglobin threshold of 7 g/dL) may be safe in stable or stabilized children and neonates with sepsis, even if they are in septic shock [67]. Transfusion requirements in unstable neonates and infants with sepsis are unknown.
Conclusions
Early anti-microbial administration and supportive care are the mainstays of treatment of neonatal sepsis. More reliable diagnostic criteria are required: rapid identification of high versus low infectious risk newborns might be useful in order to expose to antibiotics only infected newborns and to stop antibiotics as soon as possible.
The treatment of neonatal sepsis is inconsistent. Many controversies in its management remain: even the duration of antibiotic therapy is a matter of debate. There is an urgent need for well-done RCTs and for the development of evidence-based guidelines for the management of neonates with sepsis.
Abbreviations
- CI
confidence interval
- CSF
colony-stimulating factor
- ICD-9
International Classification of Diseases, Ninth Revision
- ICU
intensive care unit
- IL
interleukin
- MODS
multiple organ dysfunction syndrome
- PAF
platelet-aggregating factor
- PICS
post-intensive care syndrome
- PICS-F
post-intensive care syndrome-family
- PROWESS
Protein C Worldwide Evaluation in Severe Sepsis
- RBC
red blood cell
- RCT
randomized controlled trial
- RR
risk ratio
- ScvO2
central venous oxygen saturation
- SD
standard deviation
- SIRS
systemic inflammatory response syndrome
- TNF
tumor necrosis factor
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/67
References
- 1.Geneva: World Health Organisation; 2012. The World Health Report 2012. [Google Scholar]
- 2.Goldstein B, Giroir B, Randolph A, and the International Concensus Conference on Pediatric Sepsis International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 3.Lutsar I, Chazallon C, Carducci FI, Trafojer U, Abdelkader B, de Cabre VM, Esposito S, Giaquinto C, Heath PT, Ilmoja ML, Katragkou A, Lascoux C, Metsvaht T, Mitsiakos G, Netzer E, Pugni L, Roilides E, Saidi Y, Sarafidis K, Sharland M, Usonis V, Aboulker JP, NeoMero Consortium Current management of late onset neonatal bacterial sepsis in five European countries. Eur J Pediatr. 2014;173: doi: 10.1007/s00431-014-2279-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718275274
- 4.Su H, Chang SS, Han CM, Wu KY, Li MC, Huang CY, Lee CL, Wu JY, Lee CC. Inflammatory markers in cord blood or maternal serum for early detection of neonatal sepsis — a systematic review and meta-analysis. J Perinat. 2014;34:268–74. doi: 10.1038/jp.2013.186. [DOI] [PubMed] [Google Scholar]
- 5.Cottineau M, Launay E, Branger B, Caillon J, Muller JB, Boscher C, Laurens C, Cabaret B, Roze JC, Gras-Le Guen C. Diagnostic value of suspicion criteria for early-onset neonatal bacterial infection: Report ten years after the Anaes recommendations. Arch Pediatr. 2014;21:187–93. doi: 10.1016/j.arcped.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 6.American College of Chest Physicians, Society of Critical Care Medicine American College of Chest Physicians/Society of Critical Care Medicine consensus conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Vu Nam T, Hubert P, Lacroix J. Cumulative influence of multiple organ dysfunction syndrome and “septic” state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348–53. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 8.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 9.Watson RS, C J.A. Scope and epidemiology of pediatric sepsis. Pediatr Crit Care Med. 2005;6:S3–5. doi: 10.1097/01.PCC.0000161289.22464.C3. [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve A, Lacroix J, Proulx F, Ducruet T, Poitras N. Multiple organ dysfunction syndrome in critically ill children: value of two sets of diagnostic criteria. Crit Care Med. 2011;39:A114. doi: 10.1186/s13613-016-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray JW, Ubbi H, Milner P. Antimicrobial treatment of serious Gram-negative infections in newborns. Curr Infect Dis Rep. 2014;16:400. doi: 10.1007/s11908-014-0400-6. [DOI] [PubMed] [Google Scholar]
- 12.Behjati S, Prentice P, Rennie J. Management of Group B streptococcal sepsis risk in well, term newborns. Acta Paediatr. 2012;101:128–31. doi: 10.1111/j.1651-2227.2011.02447.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718490378
- 13.Gauvin F, Toledano B, Champagne J, Lacroix J. Reactive hemophagocytic syndrome presenting as a component of multiple organ system failure. Crit Care Med. 2000;28:3341–5. doi: 10.1097/00003246-200009000-00038. [DOI] [PubMed] [Google Scholar]
- 14.Pettersen G, Mouksassi MS, Théorêt Y, Labbé L, Faure C, Nguyen B, Litalien C. Population pharmacokinetics of intravenous pantoprazole in paediatric intensive care patients. Br J Clin Pharmacol. 2009;67:216–27. doi: 10.1111/j.1365-2125.2008.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718490380
- 15.Bateman AP, McArdle F, Walsh TS. Time, course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37:1906–12. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 16.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM, Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/9789958
- 17.Sananes R, Manlhiot C, Kelly E, Hornberger LK, Williams WG, MacGregor D, Buncic R, McCrindle BW. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93:1577–83. doi: 10.1016/j.athoracsur.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 18.De Jonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand-Zaleski I, Boussarsar M, Cerf C, Renaud E, Mesrati F, Carlet J, Raphaël JC, Outin H, Bastuji-Garin S, Groupe de Réflexion et d'Etude des Neuromyopathies en Réanimation Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 19.Gauvin F, Dugas MA, Chaïbou M, Morneau S, Lebel D, Lacroix J. The impact of clinically significant upper gastrointestinal bleeding acquired in a pediatric intensive care unit. Pediatr Crit Care Med. 2001;2:294–8. doi: 10.1097/00130478-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle A, Goldstein SL. 3-5 year longitudinal follow-up of pediatric patients after acute rénal failure. Kidney Int. 2006;69:184–9. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 21.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 22.Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med. 2012;13:338–47. doi: 10.1097/PCC.0b013e3182196a8f. [DOI] [PubMed] [Google Scholar]
- 23.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40:618–24. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 24.Conlon NP, Breatnach C, O'Hare BP, Mannion DW, Lyons BJ. Health-related quality of life after prolonged pediatric intensive care unit stay. Pediatr Crit Care Med. 2009;10:41–4. doi: 10.1097/PCC.0b013e31819371f6. [DOI] [PubMed] [Google Scholar]
- 25.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–9. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 26.Als LC, Nadel S, Cooper M, Pierce CM, Sahakian BJ, Garralda ME. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013;41:1094–103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718490381
- 27.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases NCfIaRD, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 28.Tsai MH, Hsu JF, Chu SM, Lien R, Huang HR, Chiang MC, Fu RH, Lee CW, Huang YC. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J. 2014;33:e7–13. doi: 10.1097/INF.0b013e3182a72ee0. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718052445
- 29.Lutsar I, Trafojer UM, Heath PT, Metsvaht T, Standing J, Esposito S, de Cabre VM, Oeser C, Aboulker JP, NeoMero Consortium Meropenem vs standard of care for treatment of late onset sepsis in children of less than 90 days of age: study protocol for a randomised controlled trial. Trials. 2011;12:215. doi: 10.1186/1745-6215-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havey TC, Fowler RA, Daneman N. Duration of antibiotic therapy for bacteremia: A systematic review and meta-analysis. Crit Care. 2011;15:R267. doi: 10.1186/cc10545. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718490382
- 31.Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167:374–9. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- 32.Department of Health, World Health Assembly . Geneva: May 20-28, 2013. Antibiotic resistance - a threat to global health security and the case for action. Antibiotic resistance side event held at the 66th World Health Assembly. Presented at the 66th World Health Assembly: [Google Scholar]
- 33.Annane D, Maxime V, Faller JP, Mezher C, Clec'h C, Martel P, Gonzales H, Feissel M, Cohen Y, Capellier G, Gharbi M, Nardi O. Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open. 2013;3:e002186. doi: 10.1136/bmjopen-2012-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718490383
- 34.Jensen JU, Hein L, Lundgren B, Bestle MH, Mohr TT, Andersen MH, Thornberg KJ, Løken J, Steensen M, Fox Z, Tousi H, Søe-Jensen P, Lauritsen AØ, Strange D, Petersen PL, Reiter N, Hestad S, Thormar K, Fjeldborg P, Larsen KM, Drenck NE, Ostergaard C, Kjær J, Grarup J, Lundgren JD, Procalcitonin And Survival Study (PASS) Group Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med. 2011;39:2048–58. doi: 10.1097/CCM.0b013e31821e8791. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/12255956
- 35.Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, Hoste E, Levy H, Hirman J, Levi M, Daga M, Kutsogiannis DJ, Crowther M, Bernard GR, Devriendt J, Puigserver JV, Blanzaco DU, Esmon CT, Parrillo JE, Guzzi L, Henderson SJ, Pothirat C, Mehta P, Fareed J, Talwar D, Tsuruta K, Gorelick KJ, Osawa Y, Kaul I. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41:2069–79. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- 36.Levin M, Quint PA, Goldstein B, Barton P, Bradley JS, Shemie SD, Yeh T, Kim SS, Cafaro DP, Scannon PJ, Giroir BP. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. Lancet. 2000;356:961–7. doi: 10.1016/s0140-6736(00)02712-4. [DOI] [PubMed] [Google Scholar]
- 37.López A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, Silverman MS, Takala J, Donaldson J, Arneson C, Grove G, Grossman S, Grover R. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 38.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ, Jr, Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 39.Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, Macias WL, Abd-Allah SA, Levy H, Angle R, Wang D, Sundin DP, Giroir B, REsearching severe Sepsis and Organ dysfunction in children: a gLobal perspective (RESOLVE) study group Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836–43. doi: 10.1016/S0140-6736(07)60411-5. [DOI] [PubMed] [Google Scholar]
- 40.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD, PROWESS-SHOCK Study Group Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717698023
- 41.Annane D, Timsit JF, Megarbane B, Martin C, Misset B, Mourvillier B, Siami S, Chagnon JL, Constantin JM, Petitpas F, Souweine B, Amathieu R, Forceville X, Charpentier C, Tesnière A, Chastre J, Bohe J, Colin G, Cariou A, Renault A, Brun-Buisson C, Bellissant E, APROCCHSS Trial Investigators Recombinant human activated protein C for adults with septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:1091–7. doi: 10.1164/rccm.201211-2020OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717995582
- 42.Kylat RI, Ohlsson A. Recombinant human activated protein C for severe sepsis in neonates. Cochrane Database Syst Rev. 2012;4:CD005385. doi: 10.1002/14651858.CD005385.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718490385
- 43.Qiu P, Cui X, Sun J, Welsh J, Natanson C, Eichacker PQ. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Crit Care Med. 2013;41:2419–29. doi: 10.1097/CCM.0b013e3182982add. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718047735
- 44.Carr R, Modi N, Doré CJ. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Library. 2003 doi: 10.1002/14651858.CD003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med. 2007;35:2677–85. doi: 10.1097/01.CCM.0000295263.12774.97. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718490388
- 46.The INIS Collaborative Group Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med. 2011;365:1201–11. doi: 10.1056/NEJMoa1100441. [DOI] [PubMed] [Google Scholar]
- 47.Akdag A, Dilmen U, Haque K, Dilli D, Erdeve O, Goekmen T. Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis. Am J Perinatol. 2014;31: doi: 10.1055/s-0033-1363771. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Marik PE, Zaloga GP. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980–90. doi: 10.1007/s00134-008-1213-6. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718490389
- 49.Heyland DK, Heyland J, Dhaliwal R, Madden S, Cook D. Randomized trials in critical care nutrition: look how far we've come! (and where do we go from here? JPEN J Parenter Enteral Nutr. 2010;34:697–706. doi: 10.1177/0148607110362993. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718490390
- 50.Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, Canadian Critical Care Trials Group A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489–97. doi: 10.1056/NEJMoa1212722. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718001291
- 51.Zhou F, Peng Z, Murugan R, Kellum JA. Blood purification and mortality in sepsis: a meta-analysis of randomized trials. Crit Care Med. 2013;41:2209–20. doi: 10.1097/CCM.0b013e31828cf412. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718040354
- 52.Kalil AC, Florescu MC. Blood purification: can we purify our patients from sepsis. Crit Care Med. 2013;41:2244–5. doi: 10.1097/CCM.0b013e318291cad5. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen TC, Kiss JE, Goldman JR, Carcillo JA. The role of plasmapheresis in critical illness. Crit Care Clin. 2012;28:453–68. doi: 10.1016/j.ccc.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bone RC, Fisher CJ, Clemmer TP, Slotman GJ, Metz CA, Balk RA. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653–8. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- 55.Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troché G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 56.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J, CORTICUS Study Group Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1098968
- 57.Patel GP, Balk RA. Systemic steroids in severe sepsis and septic shock. Am J Respir Crit Care Med. 2012;185:133–9. doi: 10.1164/rccm.201011-1897CI. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/14021977
- 58.Venkatesh B, Myburgh J, Finfer S, Webb SA, Cohen J, Bellomo R, McArthur C, Joyce CJ, Rajbhandari D, Glass P, Harward M, ANZICS CTG investigators The ADRENAL study protocol: adjunctive corticosteroid treatment in critically ill patients with septic shock. Crit Care Resusc. 2013;15:83–8. [PubMed] [Google Scholar]
- 59.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717997908
- 60.Choong K, Bohn D, Fraser DD, Gaboury I, Hutchison JS, Joffe AR, Litalien C, Menon K, McNamara P, Ward RE, Canadian Critical Care Trials Group Vasopressin in pediatric vasodilatory shock: a multicenter randomized controlled trial. Am J Respir Crit Care Med. 2009;180:632–9. doi: 10.1164/rccm.200902-0221OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1166253
- 61.Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, Parslow R, Tasker RC, Elbourne D, CHiP Investigators A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med. 2014;370:107–18. doi: 10.1056/NEJMoa1302564. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718232164
- 62.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 63.The ProCESS Investigators A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370: doi: 10.1056/NEJMoa1401602. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718312028
- 64.de Oliveira CF, de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, Troster EJ. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–75. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1103912
- 65.Chapman CE, Stainsby D, Jones H, Love E, Massey E, Win N, Navarrete C, Lucas G, Soni N, Morgan C, Choo L, Cohen H, Williamson LM, Serious Hazards of Transfusion Steering Group Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–52. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 66.Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, Gauvin F, Collet JP, Toledano BJ, Robillard P, Joffe A, Biarent D, Meert K, Peters MJ, TRIPICU Investigators, Canadian Critical Care Trials Group. Pediatric Acute Lung Injury and Sepsis Investigators Network Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1080944
- 67.Karam O, Tucci M, Ducruet T, Hume H, Lacroix J, Gauvin F, for the Canadian Critical Care Trials Group, and the PALISI Network Red blood cell transfusion thresholds in pediatric septic patients. Pediatr Crit Care Med. 2011;12:512–8. doi: 10.1097/PCC.0b013e3181fe344b. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/13355997


