Abstract
A novel Pd-catalyzed cascade alkoxycarbonylative macrolactonization to construct THP/THF-containing bridged macrolactones in one step from alkendiols is described. Products with various ring sizes and substituents were obtained. Challenging macrolactones involving tertiary alcohols were synthesized smoothly as well. Mechanistically, experimental evidence to support a trans-oxypalladation step has been provided. The method was applied to the synthesis of potent anticancer 9-demethylneopeltolide.
Keywords: Macrolide, Palladium, Carbonylation, Neopeltolide, Macrolactonization
Macrocyclic structural motifs are widely prevalent in many approved drug molecules, natural products and other molecules with important function.[1] Among them, tetrahydropyran (THP) and tetrahydrofuran (THF)-containing macrolides are a diverse group of natural products with a wide range of biological activity.[2] For example, neopeltolide (1),[3] lyngbyaloside B (2),[4] and exiguolide (3)[5] have shown potent anticancer activity (Figure 1). Neopeltolide also exhibits potent antifungal activity against pathogenic yeast Candida albicans. Exiguolide specifically inhibits fertilization of sea urchin gametes.
Figure 1.
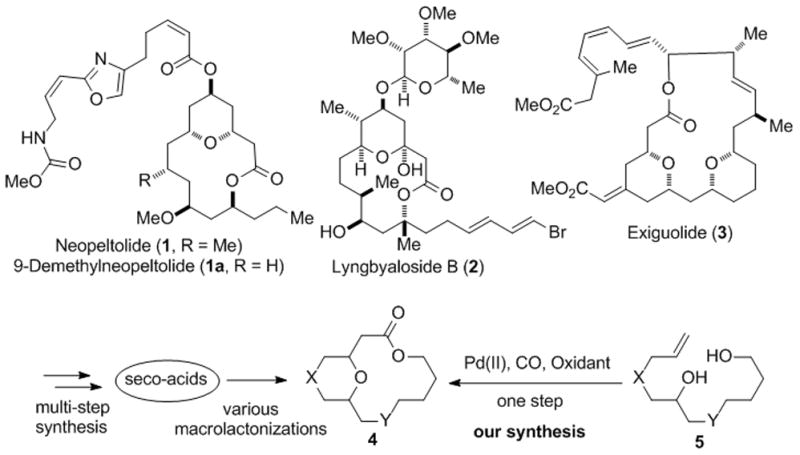
Selected THP-containing macrolides and our synthetic strategy.
The core structural skeletons of THP/THF-containing natural macrolides can be represented as generic structure 4 (Figure 1). Various strategies have been developed to prepare such structural motifs. Currently, most of the them are synthesized from seco-acid precursors via various macrolactonizations.[6] Most of the commonly used macrolactonization methods require multiple steps to prepare the corresponding seco-acids including tedious masking and unmasking of the carboxylic acid and/or alcohol, more than stoichiometric amount of reagents to activate the carboxylic acid and/or alcohol, and relatively harsh reaction conditions to promote the macrolactonization. While other methods such as ring-closing metathesis,[7] macrocyclic Prins-type cyclizations,[8] dual macrolactonization/pyran-hemiketal formation via acylketene intermediate[9] and transannular oxa-Michael cyclizations[10] have been used recently to make several THP-containing macrolides, highly efficient and catalytic methods are still needed. We envisioned the possibility of synthesizing both the THP/THF ring and the macrolactone ring in one step from relatively simple alkendiols (cf. 5→4, Figure 1) by developing Pd-catalyzed alkoxycarbonylative macrolactonizations. Pd-catalyzed alkoxycarboxylation, the venerable Semmelhack reaction, has been widely used to make THP/THF-containing esters as well as small sized lactones.[11] However, to date, Pd-catalyzed alkoxycarbonylative macrolactonization to form products such as 4 has not been reported. Pd-catalyzed carbonylative macrocyclization has been rarely studied as well.[12] We were hoping to trap the reactive acyl-palladium species derived from a sequence of Wacker-type oxypalladation and CO migratory insertion by a tethered remote alcohol to afford the desired THP/THF-containing bridged macrolactone. The proposed process requires only a catalytic amount of palladium catalyst. Since olefins are relatively inert and compatible in most of the reaction conditions, tedious masking and unmasking practices could be avoided. In addition, no carboxylic acid synthesis and its further activation are required. We were also hoping that the palladium metal center might serve as a template through coordination to bring the remote nucleophilic alcohol and the electrophilic acyl species into proximity and therefore facilitate the macrolactonization by compensating the entropic disadvantage. Herein, we report a novel Pd-catalyzed cascade alkoxycarbonylative macrolactonization to synthesize various THP/THF-containing bridged macrolactones including 9-demethylneopeltolide (1a).
Our exploration started with the commonly used Semmelhack reaction conditions and the Lambert carbonylation conditions.[13] After investigating several reaction parameters, we found that various THP/THF-containing macrolactones could be obtained using Pd(OAc)2 (0.1 equiv) as catalyst and CuCl2 (3.0 equiv) as oxidant under carbon monoxide atmosphere (balloon) in 1,2-dichloroethane (DCE, 0.002 M) at room temperature with slow addition of the starting alkendiols. As the macrolactone ring changes from 13- to 18-membered ring (7a–h), there is no significant change in the reaction yield and cis-2,6-disubstituted THP-containing macrolactones were produced as the predominant products. The reaction condition is compatible with ketal group (7g). Both internal cis and trans double bonds are tolerated as well (7j–k). Notably, para- and meta-cyclophanes were synthesized in 7l and 7m, respectively. A 23-membered macrolactone ring was formed in the case of 7l, which represents the largest ring size we have explored so far. The structure of cis-7m was unambiguously assigned by X-ray crystallography.[14] Epimeric substrates 6n and 6o underwent the cascade reaction and gave products 7n and 7o in 79% and 61% yield respectively. While cis-THP product is slightly favored in the case of 7o (dr. 2/1), trans-THP product dominates in the case of 7n (dr. 1/3.3). Product 7p was obtained in 77% yield, surprisingly, with almost no diastereoselectivity. THF-containing macrolactones were formed in good yield as well (7q–r) but with poor diastereoselectivity. The reaction tolerates sterically hindered alcohol next to an all-carbon quaternary center (7q).
Since the new stereocenter is formed in the oxypalladation step, we wondered the details of this step in our case. trans-Oxypalladation has been proposed for the Semmelhack reaction.[11,15] Recently, elegant studies from several groups[16] including those of Stoltz, Hayashi, Wolfe and Henry have shown that, in the Wacker-type reaction, both cis- and trans-oxypalladation pathways are feasible. Similar phenomena have been observed in the related aminopalladation reactions.[17] Thus, substrate 8 with a cis-double bond was prepared and subjected to the carbonylative macrolactonization conditions. Products 9 and 10 were obtained in 51% yield with 8.3/1 diastereoselectivity favoring cis product 9 (Scheme 1). The relative configuration of 9 was assigned based on NOE studies. The stereochemical outcome at the α-carbon (asterisked) of 9 supports a trans-oxypalladation process via a chair-like transition state (cf. 8→A→B→9). The minor product was tentatively assigned as 10 based on transition state C, which is less favored than A due to strong steric repulsions. Since CuCl2 was used as oxidant, at this stage, we cannot exclude the formation of acyl chlorides for the macrolactonization.[13,18]
Scheme 1.
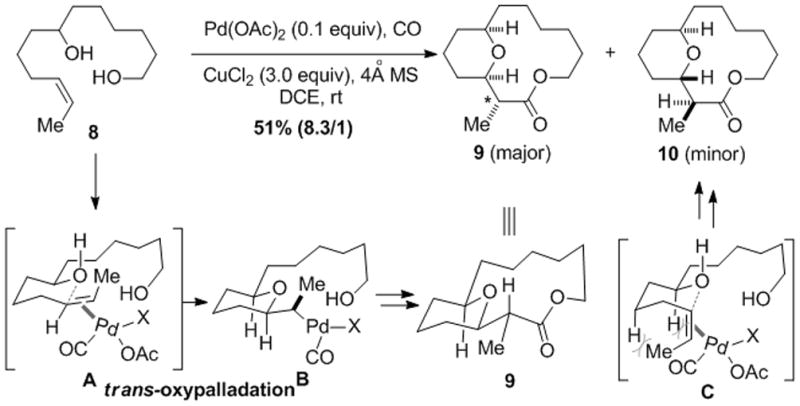
A mechanistic study of the oxypalladation step.
We then wondered whether the Pd-catalyzed alkoxycarbonylative macrolactonization would provide challenging tertiary macrolactones, which are common structural feature of many natural products such as lyngbyaloside B (2). Macrolactonization involving the OH group of a tertiary alcohol presents a great synthetic challenge and has not been well studied.[9,19] We evaluated tertiary alcohol substrates 11a–d. All of them underwent the cascade cyclization smoothly and gave the desired tertiary macrolactones in good yield and diastereoselectivity.
We then tested the effectiveness of the Pd-catalyzed alkoxycarbonylative macrolactonization for synthesizing 9-demethylneopeltolide,[20] a simplified neopeltolide [8a–b,21] analog but with similar inhibitory activity against P388 murine leukemia cells (IC50 = 0.813 nM). In order to evaluate the Pd-catalyzed alkoxycarbonylative macrolactonization in different stereo-settings and generate stereoisomers of 9-demethylneopeltolide, we prepared epimers 13a and 13b (see Supporting Information for their synthesis). Both 13a and 13b underwent the desired cyclization and products 14a and 14b were produced, respectively, in good yield and excellent cis-selectivity. Compound 14b was then converted to 9-demethylneopeltolide (1a) uneventfully through a sequence of ketal removal, reduction and Mitsunobu reaction with the known acid 16 [22] (see Supporting Information for its structure).
In summary, we have developed an efficient Pd-catalyzed cascade alkoxycarbonylative macrolactonization to construct THP/THF-containing macrolactones with different ring sizes and substituents. Challenging macrolactones involving tertiary alcohols were synthesized efficiently as well. Mechanistically, experiments have been conducted to support a Wacker-type trans-oxypalladation step. The application of this method was demonstrated in the synthesis of 9-demethylneopeltolide.
Supplementary Material
Scheme 2.
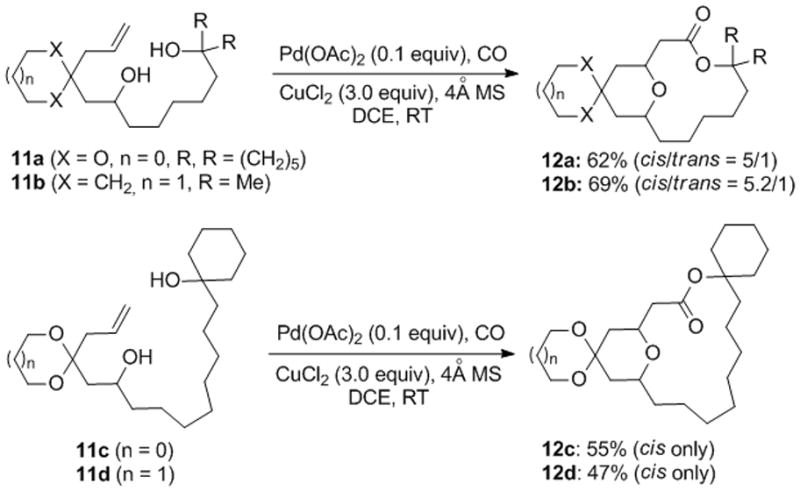
Alkoxycarbonylative macrolactonization of tertiary alcohols.
Scheme 3.
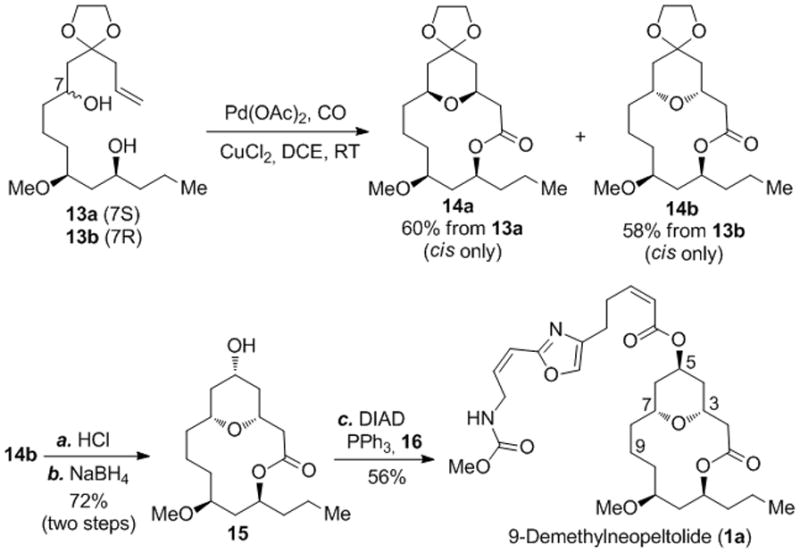
Alkoxycarbonylative macrolactonization of 13a and 13b and synthesis of 9-demethylneopeltolide (1a). Reagents and conditions: a) HCl (0.5 N), MeOH; then HCl (1 N), THF, RT; b) NaBH4 (3.9 equiv), MeOH, 0 °C, 74% from 14b; c) PPh3 (3.8 equiv), DIAD (3.8 equiv), 16 (4.0 equiv), benzene, RT, 56%; DIAD = diisopropyl azodicarboxylate.
Table 1.
Substrate scope
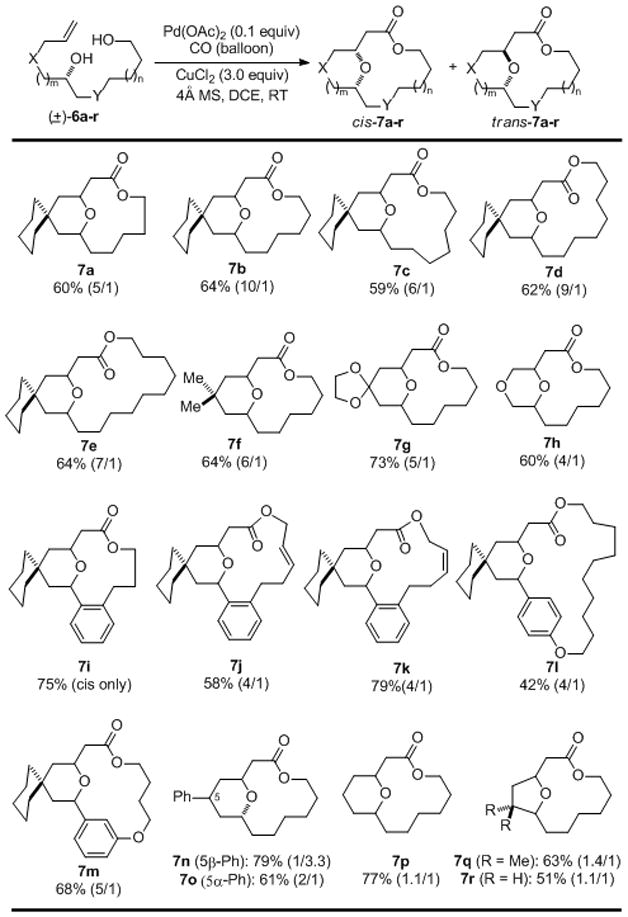
|
Isolated yield with cis/trans ratio in parenthesis.
Acknowledgments
We thank the Xia group at Purdue University for mass spectrometric assistance, the NYU Molecular Design Institute for the purchase of the Bruker SMART APEXII Diffractometer, and Dr. Chunhua Hu for his assistance with data collection and structure determination. Financial support from the Purdue University, Purdue Center for Cancer Research and the American Cancer Society, is gratefully acknowledged. Y. Bai thanks the Purdue Research Foundation for a research assistantship and D. Davis thanks the H.C. Brown Funds for a summer research support. M. Dai is a recipient of the 2013 Showalter Research Trust Award and 2013 ORAU Ralph E. Powe Junior Faculty Enhancement Award.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.a) Driggers EM, Hale SP, Lee J. Nat Rev Drug Discov. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]; b) Kopp F, Stratton CF, Akella LB, Tan DS. Nat Chem Bio. 2012;8:358–365. doi: 10.1038/nchembio.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorente A, Lamariano-Merketegi J, Albericio F, Álvarez M. Chem Rev. 2013;113:4567–4610. doi: 10.1021/cr3004778. [DOI] [PubMed] [Google Scholar]
- 3.Wright AE, Botelho JC, Guzmán E, Harmody D, Linley P, McCarthy PJ, Pitts TP, Pomponi SA, Reed JK. J Nat Prod. 2007;70:412–416. doi: 10.1021/np060597h. [DOI] [PubMed] [Google Scholar]
- 4.Luesch H, Yoshida WY, Harrigan GG, Doom JP, Moore RE, Paul VJ. J Nat Prod. 2002;65:1945–1948. doi: 10.1021/np0202879. [DOI] [PubMed] [Google Scholar]
- 5.a) Ohta S, Uy MM, Yanai M, Ohta E, Hirata T, Ikegami S. Tetrahedron Lett. 2006;47:1957–1960. [Google Scholar]; b) Crane EA, Zabawa TP, Farmer RL, Scheidt KA. Angew Chem. 2011;123:9278–9281. doi: 10.1002/anie.201102790. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:9112–9115. doi: 10.1002/anie.201102790. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Fuwa H, Suzuki T, Kubo H, Yamori T, Sasaki M. Chem Eur J. 2011;17:2678–2688. doi: 10.1002/chem.201003135. [DOI] [PubMed] [Google Scholar]
- 6.For reviews: Parenty A, Moreau X, Niel G, Campagne JM. Chem Rev. 2013;113:PR1-PR40. doi: 10.1021/cr300129n.Parenty A, Moreau X, Campagne JM. Chem Rev. 2006;106:911–939. doi: 10.1021/cr0301402.
- 7.a) Grubbs RH. Handbook of Metathesis. Vol. 2 Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]; b) Cossy J, Arseniyadis S, Meyer C. Metathesis in Natural Product Synthesis. Wiley-VCH; Weinheim, Germany: 2010. [Google Scholar]
- 8.a) Custar DW, Zabawa TP, Scheidt KA. J Am Chem Soc. 2008;130:804–805. doi: 10.1021/ja710080q. [DOI] [PubMed] [Google Scholar]; b) Woo SK, Kwon MS, Lee E. Angew Chem. 2008;120:3286–3288. [Google Scholar]; Angew Chem Int Ed. 2008;47:3242–3244. doi: 10.1002/anie.200800386. [DOI] [PubMed] [Google Scholar]; c) Wender PA, DeChristopher BA, Schrier AJ. J Am Chem Soc. 2008;130:6658–6659. doi: 10.1021/ja8015632. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Bahnck KB, Rychnovsky SD. J Am Chem Soc. 2008;130:13177–13181. doi: 10.1021/ja805187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Hoye TR, Danielson ME, May AE, Zhao H. Angew Chem. 2008;120:9889–9892. [Google Scholar]; Angew Chem Int Ed. 2008;47:9743–9746. doi: 10.1002/anie.200804049. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hoye TR, Danielson ME, May AE, Zhao H. J Org Chem. 2010;75:7052–7060. doi: 10.1021/jo101598y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Hilli F, White JM, Rizzacasa MA. Org Lett. 2004;6:1289–1292. doi: 10.1021/ol0497943. [DOI] [PubMed] [Google Scholar]; b) Kanematsu M, Yoshida M, Shishido K. Angew Chem. 2011;123:2666–2668. doi: 10.1002/anie.201007327. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:2618–2620. doi: 10.1002/anie.201007327. [DOI] [PubMed] [Google Scholar]
- 11.Semmelhack MF, Bodurow CJ. J Am Chem Soc. 1984;106:1496–1498.Semmelhack MF, Kim C, Zhang N, Sanner BM, Meier M. Pure Appl Chem. 1990;62:2035–2040.Li Z, Gao Y, Jiao Z, Wu N, Wang DZ, Yang Z. Org Lett. 2008;10:5163–5166. doi: 10.1021/ol802115u.. For examples of synthetic applications: Carpenter J, Northrup AB, Chung D, Wiener JJM, Kim S-G, MacMillan DWC. Angew Chem. 2008;120:3624–3628. doi: 10.1002/anie.200800086.Angew Chem Int Ed. 2008;47:3568–3572. doi: 10.1002/anie.200800086.Yang Z, Xie X, Jing P, Zhao G, Zheng J, Zhao C, She X. Org Biomol Chem. 2011;9:984–986. doi: 10.1039/c0ob00971g.Xiao Q, Ren WW, Chen ZX, Sun TW, Li Y, Ye QD, Gong JX, Meng FK, You L, Liu YF, Zhao MZ, Xu LM, Shan ZH, Shi Y, Tang YF, Chen JH, Yang Z. Angew Chem. 2011;123:7511–7515. doi: 10.1002/anie.201103088.Angew Chem Int Ed. 2011;50:7373–7377. doi: 10.1002/anie.201103088.
- 12.a) Wu X, Neumann H, Beller M. Chem Rev. 2013;113:1–35. doi: 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]; b) Lu SM, Alper H. J Am Chem Soc. 2005;127:14776–14784. doi: 10.1021/ja053650h. [DOI] [PubMed] [Google Scholar]; c) Lu SM, Alper H. Chem Eur J. 2007;13:5908–5916. doi: 10.1002/chem.200601724. [DOI] [PubMed] [Google Scholar]; d) Lu SM, Alper H. J Am Chem Soc. 2008;130:6451–6455. doi: 10.1021/ja7111417. [DOI] [PubMed] [Google Scholar]; e) Lenoble G, Urrutigoity M, Kalck P. Tetrahedron Lett. 2001;42:3697–3700. [Google Scholar]; f) Knight JC, Prabaharan R, Ward BD, Amoroso AJ, Edwards PG, Kariuki BM. Dalton Trans. 2010;39:10031–10033. doi: 10.1039/c0dt01205j. [DOI] [PubMed] [Google Scholar]; g) Setoh M, Yamada O, Ogasawara K. Heterocycles. 1995;40:539–542. [Google Scholar]; h) Yoneda E, Zhang SW, Onitsuka K, Takahashi S. Tetrahedron Lett. 2001;42:5459–5461. [Google Scholar]; i) Takahashi T, Kusaka S-i, Doi T, Sunazuka T, Omura S. Angew Chem. 2003;115:5388–5392. doi: 10.1002/anie.200352229. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2003;42:5230–5234. doi: 10.1002/anie.200352229. [DOI] [PubMed] [Google Scholar]
- 13.a) Cernak TA, Lambert TH. J Am Chem Soc. 2009;131:3124–3125. doi: 10.1021/ja809897f. [DOI] [PubMed] [Google Scholar]; b) Ambrosini LA, Cernak TA, Lambert TH. Synthesis. 2010:870–881. [Google Scholar]; c) Ambrosini LA, Cernak TA, Lambert TH. Tetrahedron. 2010;66:4882–4887. [Google Scholar]
- 14.CCDC 985926 contains the supplementary crystallographic data for compound cis-7m.
- 15.Stille JK, Divakaruni R. J Organomet Chem. 1979;169:239–248.. For examples of trans-oxypalladation in the Wacker reaction, see: Bäckvall JE, Akermark B, Ljunggren SO. J Am Chem Soc. 1979;101:2411–2416.Majima T, Kurosawa H. J Chem Soc Chem Commun. 1977:610–611.
- 16.a) Trend RM, Ramtohul YK, Stoltz BM. J Am Chem Soc. 2005;127:17778–17788. doi: 10.1021/ja055534k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Uozumi Y, Kato K, Hayashi T. J Am Chem Soc. 1997;119:5063–5064. [Google Scholar]; c) Hayashi T, Yamasaki K, Mimura M, Uozumi Y. J Am Chem Soc. 2004;126:3036–3037. doi: 10.1021/ja031946m. [DOI] [PubMed] [Google Scholar]; d) Hay MB, Hardin AR, Wolfe JP. J Org Chem. 2005;70:3099–3107. doi: 10.1021/jo050022+. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Francis JW, Henry PM. J Mol Catal A: Chem. 1996;112:317–326. [Google Scholar]
- 17.a) Liu G, Stahl SS. J Am Chem Soc. 2007;129:6328–6335. doi: 10.1021/ja070424u. [DOI] [PubMed] [Google Scholar]; b) Bäckvall JE. Acc Chem Res. 1983;16:335–342. [Google Scholar]
- 18.Quesnel JS, Arndtsen BA. J Am Chem Soc. 2013;135:16841–16844. doi: 10.1021/ja4098093. [DOI] [PubMed] [Google Scholar]
- 19.a) Masamune S, Hayase Y, Schilling W, Chan W, Bates GS. J Am Chem Soc. 1977;99:6756–6758. doi: 10.1021/ja00462a049. [DOI] [PubMed] [Google Scholar]; b) Booth PM, Fox CM, Ley SV. J Chem Soc Perkin Trans. 1987;1:121–129. [Google Scholar]; c) Clyne DS, Weiler L. Tetrahedron. 1999;55:13659–13682. [Google Scholar]; d) ElMarrouni A, Lebeuf R, Gebauer J, Heras M, Arseniyadis S, Cossy J. Org Lett. 2012;14:314–317. doi: 10.1021/ol203064r. [DOI] [PubMed] [Google Scholar]
- 20.For the synthesis and biological evaluation of 9-demethylneopeltolide: Fuwa H, Saito A, Naito S, Konoki K, Yotsu-Yamashita M, Sasaki M. Chem Eur J. 2009;15:12807–12818. doi: 10.1002/chem.200901675.
- 21.For total syntheses of neopeltolide: references 8a and 8b, and Youngsaye W, Lowe JT, Pohlki F, Ralifo P, Panek JS. Angew Chem. 2007;119:9371–9374. doi: 10.1002/anie.200704122.Angew Chem Int Ed. 2007;46:9211–9214. doi: 10.1002/anie.200704122.Paterson I, Miller NA. Chem Commun. 2008:4708–4710. doi: 10.1039/b812914b.Fuwa H, Naito S, Goto T, Sasaki M. Angew Chem. 2008;120:4815–4817. doi: 10.1002/anie.200801399.Angew Chem Int Ed. 2008;47:4737–4739. doi: 10.1002/anie.200801399.Ulanovskaya OA, Janjic J, Suzuki M, Sabharwal SS, Schumacker PT, Kron SJ, Kozmin SA. Nat Chem Biol. 2008;4:418–424. doi: 10.1038/nchembio.94.Guinchard X, Roulland E. Org Lett. 2009;11:4700–4703. doi: 10.1021/ol902047z.Fuwa H, Saito A, Sasaki M. Angew Chem. 2010;122:3105–3108. doi: 10.1002/anie.201000624.Angew Chem Int Ed. 2010;49:3041–3044. doi: 10.1002/anie.201000624.Ghosh AK, Shurrush KA, Dawson ZL. Org Biomol Chem. 2013;11:7768–7777. doi: 10.1039/c3ob41541d.. For formal synthesis: Vintonyak VV, Maier ME. Org Lett. 2008;10:1239–1242. doi: 10.1021/ol8001255.Kartika R, Gruffi TR, Taylor RE. Org Lett. 2008;10:5047–5050. doi: 10.1021/ol802254z.Tu W, Floreancig PE. Angew Chem. 2009;121:4637–4641.Angew Chem Int Ed. 2009;48:4567–4571. doi: 10.1002/anie.200901489.Kim H, Park Y, Hong J. Angew Chem. 2009;121:7713–7717.Angew Chem Int Ed. 2009;48:7577–7581. doi: 10.1002/anie.200903690.Yadav JS, Krishana GG, Kumar SN. Tetrahedron. 2010;66:480–487.Martinez-Solorio D, Jennings MP. J Org Chem. 2010;75:4095–4104. doi: 10.1021/jo100443h.Yang Z, Zhang B, Zhao G, Yang J, Xie X, She X. Org Lett. 2011;13:5916–5919. doi: 10.1021/ol2025718.Raghavan S, Samanta PK. Org Lett. 2012;14:2346–2349. doi: 10.1021/ol3007698.Sharma GVM, Reddy SV, Ramakrishna KVS. Org Biomol Chem. 2012;10:3689–3695. doi: 10.1039/c2ob25151e.. For analog synthesis: Vintonyak VV, Kunze B, Sasse F, Maier ME. Chem Eur J. 2008;14:11132–11140. doi: 10.1002/chem.200801398.Custar DW, Zabawa TP, Hines J, Crews CM, Scheidt KA. J Am Chem Soc. 2009;131:12406–12414. doi: 10.1021/ja904604x.Cui Y, Tu W, Floreancig PE. Tetrahedron. 2010;66:4867–4873. doi: 10.1016/j.tet.2010.03.066.Cui Y, Balachandran R, Day BW, Floreancig PE. J Org Chem. 2012;77:2225–2235. doi: 10.1021/jo2023685.Fuwa H, Kawakami M, Noto K, Muto T, Suga Y, Konoki K, Yotsu-Yamashita M, Sasaki M. Chem Eur J. 2013;19:8100–8110. doi: 10.1002/chem.201300664.
- 22.Wang Y, Janjic J, Kozmin SA. J Am Chem Soc. 2002;124:13670–13671. doi: 10.1021/ja028428g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


