Abstract
Background
The purpose of this study was to identify factors predictive of outcome in patients undergoing temporal bone resection (TBR) for head and neck cancer.
Methods
This was a retrospective study of 72 patients undergoing TBR. Factors associated with survival and recurrence were identified on multivariable regression.
Results
Most tumors were epithelial (81%), commonly (69%) involving critical structures. Cervical metastases were uncommon (6%). Squamous cell carcinoma (SCC) of the external auditory canal carried a high rate of parotid invasion (25%) and parotid nodal metastases (43%). The 5-year rate of overall survival (OS) was 62%; disease-specific survival (DSS), 70%; recurrence-free survival (RFS), 46%. Factors independently associated with outcome on multivariable analysis were margin status and extratemporal spread of disease to the parotid, mandible, or regional nodes. Recurrence was common (72%) in cT3–4 tumors.
Conclusions
Margin status and extratemporal disease spread are the strongest independent predictors of survival and recurrence. In SCC of the external auditory canal, high rates of parotid involvement support adjunctive parotidectomy. Risk of recurrence in T3–T4 tumors may support a role for adjuvant therapy.
Keywords: temporal bone, ear canal, squamous cell carcinoma, head and neck neoplasms
Malignant tumors of the temporal bone and external auditory canal are uncommon, with annual incidence estimated at between 1 and 6 per million.1,2 Cancers of the external auditory canal/temporal bone comprise <0.2% of head and neck cancers.3 The rarity and anatomic complexity of these tumors hampered the development of an en bloc ablative operation until 1954, when Parsons and Lewis4 from Memorial Hospital in New York first described the technique of subtotal temporal bone resection. Conley and Novack5 refined this technique further in 1960 with the description of lateral temporal bone resection. Subsequent developments in imaging, microsurgery, reconstruction, and neuroanesthesia have all contributed to advancements in skull base surgery. As a result, oncologic temporal bone procedures, including lateral, subtotal, and total temporal bone resection, have become the standard of care for malignant disease involving the external auditory canal/temporal bone.
Survival and recurrence outcomes in patients with cancer of the external auditory canal/temporal bone have been reported in several single-institution series. Most cohorts have been limited to squamous cell histology, and comprehensive outcomes analyses have been limited.2,6–24 Further complicating analysis, there is no recognized American Joint Committee on Cancer (AJCC) or Union Internationale Contre le Cancer (UICC) staging system for tumors of the external auditory canal/temporal bone, with multiple systems having been proposed.3,16,25,26 To date, the most widely used system is the University of Pittsburgh staging system, first described in 1990 by Arriaga and colleagues25 and modified in 2000 by Moody et al.3
Other than Pittsburgh stage and surgical margin status, no other factors have been consistently demonstrated to be associated with outcome, and multivariable analyses of factors affecting outcome have not been reported.7,16,20,21 Such a comprehensive analysis of patient and tumor characteristics that are predictive of survival outcomes would aid clinical decision making and risk stratification. The objective of this study was to identify factors predictive of survival and recurrence in a contemporary cohort of patients undergoing temporal bone resection for cancer involving the external auditory canal/temporal bone.
PATIENTS AND METHODS
The study population consisted of 72 patients. After institutional review board approval, we reviewed the records of 262 patients who underwent any form of temporal bone surgery for neoplastic disease between 1994 and 2010 at 2 affiliated medical centers in New York, Memorial Sloan–Kettering Cancer Center and New York Presbyterian Hospital–Weill Cornell Medical Center. These years were chosen to restrict the cohort to patients undergoing contemporary techniques of imaging, surgery, reconstruction, and radiation therapy. After review, we identified a cohort of patients with the inclusion criterion of malignant tumors involving the external auditory canal/temporal bone on either clinical or radiographic examination. This cohort included 10 temporal bone resections performed for periauricular cutaneous tumors closely adjacent to, but not grossly invading, the temporal bone, in which temporal bone resection was performed due to suspicion for occult bone invasion. Cases included epithelial, mesenchymal, and salivary histologies. One case of chondroblastoma, traditionally considered benign, was included due to the proclivity of this tumor to aggressive local invasion and recurrence.27 We excluded 190 cases in which temporal bone resection was performed for a benign tumor, solely to enhance access to a tumor that was not invading or adjacent to the external auditory canal/temporal bone, or for a central nervous system tumor.
Patient, clinical tumor, pathologic tumor, and treatment characteristics were recorded. Cases undergoing treatment prior to referral to our centers were categorized as either recurrent or persistent, depending on whether a disease-free interval was experienced prior to presentation. The most recent version of the University of Pittsburgh staging system3 (Table 1) was used to classify tumors, both clinically and pathologically. Periauricular cutaneous tumors invading the external auditory canal/temporal bone were classified using the Pittsburgh system23; those not grossly invading the external auditory canal/temporal bone were classified as “unstaged” in the Pittsburgh system, and also classified with the AJCC system. Parotid tumors involving the external auditory canal/temporal bone are classified T4 in either the Pittsburgh or the AJCC staging systems.
TABLE 1.
Pittsburgh staging system.
| Factor | Description/distinguishing features/characteristics |
|---|---|
| T classification | |
| T1 | Limited to the external auditory canal without bony erosion or evidence of soft tissue involvement |
| T2 | Limited to the external auditory canal with bone erosion (not full-thickness) or limited soft tissue involvement (<5 mm) |
| T3 | Erosion through the osseous external auditory canal (full-thickness) with limited soft tissue involvement (<5 mm), or tumor involving the middle ear and/or mastoid |
| T4 | Erosion of the cochlea, petrous apex, medial wall of the middle ear, carotid canal, jugular foramen, or dura; or tumor with extensive soft tissue involvement (>5 mm, such as involvement of the temporomandibular joint or styloid process);or evidence of facial paresis |
| N classification | |
| N0 | No regional nodes identified |
| N1 | Single metastatic regional node <3 cm in size |
| N2a | Single ipsilateral metastatic node 3–6 cm in size |
| N2b | Multiple ipsilateral metastatic lymph nodes |
| N2c | Contralateral metastatic lymph node |
| N3 | Metastatic lymph node >6 cm in size |
| Overall stage | |
| I | T1 N0 |
| II | T2 N0 |
| III | T3 N0 |
| IV | T4 N0 |
| IV | T1–T4 N+ |
Currently at our institutions, the extent of temporal bone resection is determined by the extent of disease, and categorized as sleeve resection of the external auditory canal, mastoidectomy, lateral temporal bone resection, subtotal temporal bone resection, or total temporal bone resection. Although the approach has evolved over time, adjunctive superficial parotidectomy is generally performed for high-stage squamous cell carcinoma (SCC) of the external auditory canal or periauricular skin. Elective dissection of the cN0 neck is generally performed for SCC classified cT3–T4, for select other cases based on histopathology and clinical extent of disease, or when vascular access for microvascular reconstruction is required.
Outcomes analyses were performed for the entire cohort of 72 patients, and for the subset of 31 patients with SCC of the external auditory canal. Patient and clinical characteristics analyzed included age, sex, race, tobacco use, alcohol use, medical comorbidity, previous treatment, tumor location, tumor histology, local extension, facial nerve status, and clinical Pittsburgh TNM classification. Treatment characteristics included the type of resection, parotidectomy, neck dissection, craniotomy, facial nerve sacrifice, type of reconstruction, and adjuvant therapy. Pathologic characteristics included tumor histology; local extension; bone-, perineural-, lymphatic-, and angioinvasion; depth of invasion; pathologic Pittsburgh TNM classification; and margin status. Margin status was defined as negative, close (<5 mm) or positive. Actuarial outcomes including overall survival (OS), disease-specific survival (DSS), recurrence-free survival (RFS), and cumulative recurrence rates were calculated from the date of presentation, using the Kaplan–Meier method. RFS was defined as the cumulative probability of remaining alive and free of recurrent disease. Univariate survival analysis was performed with the log-rank test. Variables with p < .20 were then evaluated further with multivariable Cox proportional hazards regression, using forward entry to construct a parsimonious model based on likelihood ratios. A priori power analysis performed in PS 3.0 (Dupont and Plummer, Nashville, TN28,29) estimated that our study sample size would be able to detect hazard ratios >1.7 with 0.80 power. Statistical analysis was performed in SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Patient characteristics
Seventy-two patients with tumors involving the external auditory canal or temporal bone met criteria for inclusion. Of these patients, 61 were treated at Memorial Sloan–Kettering Cancer Center and 11 at New York Pres-byterian Hospital–Cornell, between 1994 and 2010. Mean length of follow-up was 40.3 months (range, 0.3–188.3 months). Patient characteristics at the time of presentation are summarized in Table 2. Mean age was 63.3 years (range, 3.8–85.2 years), and the majority (73.6%) of patients were male. Reflecting the referral center nature of our institutions, 33 patients (45.8%) had been previously treated: 26 presented with recurrent cancer, and 7 with persistent (incompletely resected) cancer.
TABLE 2.
Patient characteristics at presentation.
| Characteristic | No. of patients (%) |
|---|---|
| Age, y | |
| <60 | 26 (36.1) |
| ≥60 | 46 (63.9) |
| Sex | |
| Male | 53 (73.6) |
| Female | 19 (26.4) |
| Race | |
| White | 39 (54.2) |
| Non-white | 4 (5.6) |
| Unknown | 29 (40.2) |
| Tobacco use | |
| Active smoker | 7 (9.7) |
| Former smoker | 29 (40.3) |
| Never-smoker | 31 (43.1) |
| Unknown | 5 (6.9) |
| Alcohol use | |
| Regular drinker | 12 (16.7) |
| Social drinker | 26 (8.3) |
| Former drinker | 6 (8.7) |
| Never-drinker | 25 (34.7) |
| Unknown | 3 (4.2) |
| Medical comorbidity | |
| None | 28 (38.9) |
| Present | 44 (61.1) |
| Previous treatment | |
| None | 39 (54.2) |
| Persistent disease | 7 (9.7) |
| Recurrent disease | 26 (36.1) |
| Previous treatment modality | |
| Surgery alone | 10 (30.3) |
| Surgery + radiation | 18 (54.5) |
| Surgery + chemoradiation | 3 (9.1) |
| Radiation | 1 (3.0) |
| Chemoradiation | 1 (3.0) |
Clinical tumor characteristics
Clinical tumor characteristics at presentation are detailed in Table 3. Of 72 cases, 31 were squamous cell cancers of the external auditory canal. The most common histologic diagnoses were squamous cell carcinoma (54.2%) and basal cell carcinoma (BCC, 19.4%), with epithelial malignancies altogether comprising 80.6% of cases. Salivary tumors comprised 11.1% of cases; mesenchymal tumors, 8.3% of cases. The most common site of tumor origin was the external auditory canal (36 cases; 50.0%), followed by skin cancers arising from the auricle, concha, or periauricular skin (23 cases; 31.9%). Seven cases (9.7%) were locally advanced parotid tumors grossly invading the temporal bone. Remaining cases originated from the infratemporal fossa, mastoid bone, petrous apex, or temporomandibular joint.
TABLE 3.
Clinical tumor characteristics.
| Factor | No. of patients (%) |
|---|---|
| Histology | |
| Epithelial | 58 (80.6) |
| Squamous cell carcinoma | 39 (54.2) |
| Basal cell carcinoma | 14 (19.4) |
| Sebaceous gland carcinoma | 1 (1.4) |
| Melanoma | 2 (2.8) |
| Cerumen gland carcinoma | 1 (1.4) |
| Eccrine adenocarcinoma | 1 (1.4) |
| Salivary | 8 (11.1) |
| Adenosquamous carcinoma | 1 (1.4) |
| Adenoid cystic carcinoma | 4 (5.6) |
| Myoepithelial carcinoma | 2 (2.8) |
| Mucoepidermoid carcinoma | 1 (1.4) |
| Mesenchymal | 6 (8.3) |
| MFH | 2 (2.8) |
| Chondrosarcoma | 2 (2.8) |
| Chondroblastoma | 1 (1.4) |
| Synovial sarcoma | 1 (1.4) |
| Tumor site | |
| Auricle | 4 (5.6) |
| Concha | 2 (2.8) |
| External auditory canal | 36 (50.0) |
| Infratemporal fossa | 1 (1.4) |
| Mastoid bone | 1 (1.4) |
| Parotid gland | 7 (9.7) |
| Petrous apex | 2 (2.8) |
| Periauricular skin | 17 (23.6) |
| Temporomandibular joint | 1 (1.4) |
| Facial nerve status (nonparotid tumors) | |
| Paretic or paralyzed | 13 (20.0) |
| Intact | 52 (80.0) |
| Pittsburgh T classification (all cases) | |
| Unstaged* | 10 (13.9) |
| cT1 | 21 (29.2) |
| cT2 | 2 (2.8) |
| cT3 | 11 (15.3) |
| cT4 | 28 (38.9) |
| AJCC T classification (periauricular skin cancers) | |
| cT1 | 2 (11.8) |
| cT2 | 4 (23.5) |
| cT3 | 1 (5.9) |
| cT4 | 8 (47.1) |
| Unknown | 2 (11.8) |
| N classification | |
| cN0 | 62 (86.1) |
| cN+ | 4 (5.6) |
| Unknown | 6 (8.3) |
| M classification | |
| cM0 | 66 (91.7) |
| cM+ | 1 (1.4) |
| Unknown | 5 (6.9) |
Abbreviations: MFH, malignant fibrous histiocytoma; AJCC, American Joint Committee on Cancer.
Those not grossly invading the external auditory canal/temporal bone were classified as “unstaged.”
The majority of cases (54.2%, 39 of 72) were of advanced cT3–4 classification in the Pittsburgh staging system, as were the majority of periauricular skin cases (60%, 9 of 15) able to be classified using the AJCC system. Local tumor extension to involve critical structures was common, with 50 of 72 cases (69.4%) invading 1 or more of the following: the middle ear, otic capsule, petrous apex, infratemporal fossa, mandible, parotid gland, or dura. Eleven tumors (15.3%) invaded the middle ear, 7 tumors (9.7%) extended intracranially, 6 nonparotid tumors (9.2%) grossly invaded the parotid gland, and 8 tumors (11.1%) grossly invaded the mandible. Cervical nodal metastasis was uncommon at presentation, occurring in 6.1% of patients (4 of 66 patients with known clinical nodal status). All 4 patients had squamous cell cancers, 2 of whom had received prior irradiation. Of SCC patients without prior radiation, 2 of 24 (8.3%) presented with clinical nodal metastasis.
At the time of presentation, the facial nerve was paretic or paralyzed in 18 cases (25%): 5 of 7 (71.4%) parotid tumors and 13 of 65 (20%) nonparotid tumors. The non-parotid tumors causing facial nerve dysfunction included 9 periauricular skin cancers (5 SCC, 1 BCC, 1 melanoma, 1 eccrine carcinoma), 2 SCC and 1 BCC arising from the external auditory canal, and 1 malignant fibrous histiocytoma (MFH) with mastoid invasion.
Mode of treatment
Details of surgery, reconstruction, and adjuvant therapy are summarized in Table 4. Lateral temporal bone resection has been the most common procedure performed, accounting for 60 cases (83.3%). Five cases (6.9%) required subtotal temporal bone resection, and 2 cases (2.8%) required total temporal bone resection due to locally advanced disease. Only 3 patients in this cohort underwent sleeve resection of the external auditory canal, for 2 cases of localized SCC of the concha in the early years of the study, and for 1 BCC of the tragus and concha. Two patients underwent simple mastoidectomy alone, in 1 case of auricular SCC and in 1 case of MFH.
TABLE 4.
Details of treatment.
| Factor | No. of patients (%) |
|---|---|
| Type of resection | |
| Sleeve resection | 3 (4.2) |
| Mastoidectomy | 2 (2.8) |
| Lateral TBR | 60 (83.3) |
| Subtotal TBR | 5 (6.9) |
| Total TBR | 2 (2.8) |
| Parotidectomy* | |
| None | 24 (36.9) |
| Performed | 41 (63.1) |
| Mandibulectomy | |
| None | 58 (80.6) |
| Performed | 14 (19.4) |
| Neck dissection | |
| None | 28 (38.9) |
| Performed | 44 (61.1) |
| Craniotomy | |
| None | 61 (84.7) |
| Performed | 11 (15.3) |
| Facial nerve management | |
| Preserved | 44 (61.1) |
| Sacrificed | 28 (38.9) |
| Reconstruction | |
| None | 14 (19.4) |
| Microvascular free flap | 36 (50.0) |
| Skin graft | 7 (9.7) |
| Local skin flap | 2 (2.8) |
| Temporalis muscle flap | 7 (9.7) |
| Pectoralis major flap | 5 (6.9) |
| Trapezius flap | 1 (1.4) |
| Adjuvant therapy | |
| None | 35 (48.6) |
| Radiation | 31 (43.1) |
| Chemoradiation | 6 (8.3) |
Abbreviation: TBR, temporal bone resection.
Excluding primary parotid tumors.
Due to the large percentage of locally advanced tumors, 55 patients (76.4%) also underwent additional surgery outside of the temporal bone. Superficial parotidectomy was performed in 41 of 65 nonparotid tumors (63.1%), including nearly all (88.9%, 16 of 18) squamous cell carcinomas of the external auditory canal classified cT2–4. Segmental mandibulectomy was performed in 14 patients (19.4%) and craniotomy in 11 patients (15.3%). Neck dissection was performed in 44 patients (61.1%), including 20 of 24 SCCs (83.3%) classified cT3–4.
Pathologic tumor characteristics
Pathologic analysis (Table 5) confirmed the advanced extent of these tumors. The majority (60.9%, 42 of 69) of tumors were classified pT3–T4 on the Pittsburgh scale. Among tumors in which pathologic extent of invasion was examined, there was a high incidence of bone invasion (44.9%, 31 of 69), perineural invasion (42.6%, 29 of 68), and angioinvasion (45.9%, 17 of 37). Of 10 temporal bone resections performed for periauricular skin cancers not grossly invading the external auditory canal/temporal bone, 2 were found to have occult bone invasion.
TABLE 5.
Pathologic extent of disease.
| Factor | No. of patients (%) |
|---|---|
| Pathologic extent of disease | |
| Bone invasion | |
| Absent | 38 (52.8) |
| Present | 31 (43.1) |
| Not examined | 3 (4.2) |
| Perineural invasion | |
| Absent | 39 (54.2) |
| Present | 29 (40.3) |
| Not examined | 4 (5.6) |
| Lymphatic invasion | |
| Absent | 14 (19.4) |
| Present | 6 (8.3) |
| Not examined | 52 (72.2) |
| Angioinvasion | |
| Absent | 20 (27.8) |
| Present | 17 (23.6) |
| Not examined | 35 (48.6) |
| Parotid invasion* | |
| Absent | 25 (61.0) |
| Present | 14 (34.1) |
| Not examined | 2 (4.9) |
| Depth of invasion | |
| <1 mm | 13 (18.1) |
| 1 mm | 9 (12.5) |
| Not examined | 50 (69.4) |
| Margin status | |
| Negative | 42 (58.3) |
| Close or positive | 30 (41.7) |
| Parotid nodal status* | |
| Negative | 21 (51.2) |
| Positive | 7 (17.1) |
| Not examined | 13 (31.7) |
| Pittsburgh T classification | |
| pT0† | 8 (11.1) |
| pT1 | 17 (23.6) |
| pT2 | 2 (2.9) |
| pT3 | 12 (16.7) |
| pT4 | 30 (41.7) |
| Not examined | 3 (4.2) |
| N classification‡ | |
| pN0 | 38 (86.4) |
| pN+ | 6 (13.6) |
Excluding primary parotid tumors.
Cutaneous primary not clinically involving external auditory canal/temporal bone.
Neck dissection specimens.
Of patients undergoing superficial parotidectomy in whom pathologic examination for tumor invasion and/or parotid nodal status was performed, 35.9% (14 of 39) had pathologically confirmed direct tumor invasion, and 25.0% (7 of 28) had metastatic disease within intraparotid lymph nodes. Within the subset of 31 cases of SCC of the external auditory canal, 25.0% (5 of 20) had pathologic evidence of direct parotid invasion, and 42.9% (6 of 14) had parotid nodal metastases.
Surgical margins were close or positive in 41.7% of all cases, and in 56.7% of cases classified T4. Limited to cases of SCC, margins were close or positive in 37.8% of cases, and in 46.7% of cases classified T4. Among patients undergoing neck dissection, there were only 2 cases of clinically occult cervical metastases, both in patients with SCC, neither of whom had received prior irradiation. The incidence of clinically occult cervical metastasis among not previously irradiated patients with SCC was 12.5% (2 of 16).
Predictors of survival
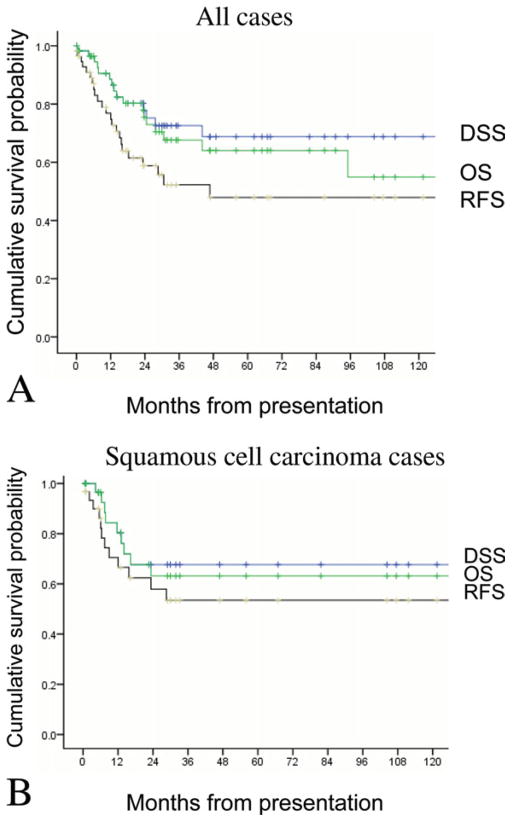
For the entire cohort of patients undergoing temporal bone resection, 5-year overall survival (OS) rate was 62.3%, disease-specific survival (DSS) rate was 70.4%, and recurrence-free survival (RFS) rate was 45.5%. When limited only to cases of SCC, 5-year OS was 63.2%, DSS was 67.7%, and RFS was 53.5% (Figure 1).
FIGURE 1.
Overall rates of overall survival (OS), disease-specific survival (DSS), and recurrence-free survival (RFS). For all cases (A), 5-year OS was 62.3%; DSS, 70.4%; RFS, 45.5%. For squamous cell carcinoma (SCC) cases (B), 5-year OS was 63.2%; DSS, 67.7%; RFS, 53.5%. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Univariate analysis to identify factors associated with OS, DSS, and RFS was then performed. Pittsburgh T classification was not a strong predictor of survival: 5-year DSS ranged from 87.5% in pT1–2 tumors to 63.4% in pT3–4 tumors (p = .20, log-rank). Similarly, histology was not a strong predictor of survival. However, pathologic regional (neck or parotid) nodal metastasis was a strong predictor of survival, with node-negative patients experiencing 5-year DSS of 74.8%, compared with 40.0% for node-positive patients (p = .003, log-rank). Limited to tumors with SCC histology, 5-year DSS was 80.8% in node-negative patients, and 18.8% in node-positive patients (p < .0001) (Figure 2). In addition, margin status was a strong predictor of 5-year DSS, among all patients (81.7% vs 50.0%; p = .03, log-rank), and among patients with SCC (90.5% vs 29.4%; p = .001, log-rank).
FIGURE 2.
Disease-specific survival among patients experiencing regional (neck or parotid) metastases was significantly poorer than those without regional metastases. For all cases (A), the difference was 74.8% versus 40.0% (p = .003). For SCC cases (B), the difference was 80.8% versus 18.8% (p < .0001).[Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Multivariable Cox regression was then used to identify factors that were independently predictive of outcome, when controlling for 2 statistically significant and clinically meaningful covariates: regional nodal metastasis and margin status. The additional factors that were independently predictive of 1 or more survival outcomes were: advanced Pittsburgh pT stage, middle ear invasion, mandible invasion, pathologic bone invasion, pathologic parotid invasion, performance of craniotomy, and need for facial nerve sacrifice. Parotid gland and mandible invasion were significant predictors of all 3 survival outcomes. These results are detailed in Table 6.
TABLE 6.
Univariate analysis of factors predictive of outcome.
| Factor | OS | DSS | RFS |
|---|---|---|---|
| Patient characteristics | |||
| Age, y | .96 | .52 | .85 |
| Sex | .85 | .78 | .36 |
| Tobacco use | .74 | .78 | .98 |
| Alcohol use | .54 | .38 | .13 |
| Medical comorbidity | .59 | .98 | .64 |
| Previously treated | .72 | .58 | .30 |
| Facial nerve function | .89 | .91 | .15 |
| Clinical tumor characteristics | |||
| Tumor location | .26 | .84 | .42 |
| Parotid invasion | .73 | .89 | .85 |
| Middle ear invasion | .04 | .06 | .04 |
| Intracranial extension | .04 | .01 | .01 |
| Infratemporal fossa invasion | .84 | .59 | .20 |
| Mandible invasion | <.0001 | <.0001 | .001 |
| Otic capsule invasion | .22 | .26 | .70 |
| Advanced cT classification* | .29 | .20 | .02 |
| Clinical nodal metastasis | .03 | .02 | .06 |
| Pathologic tumor characteristics | |||
| Histology: epithelial | .98 | .46 | .47 |
| Histology: salivary | .29 | .37 | .003 |
| Histology: mesenchymal | .97 | .69 | .82 |
| Histologic grade | .12 | .52 | .04 |
| Bone invasion | .07 | .02 | .17 |
| Parotid invasion | .03 | .06 | .02 |
| Parotid nodal metastasis | .09 | .03 | .005 |
| Perineural invasion | .98 | .59 | .15 |
| Lymphatic invasion | .93 | .81 | .38 |
| Angioinvasion | .43 | .48 | .26 |
| Depth of invasion >1 mm | .27 | .050 | .92 |
| Margin status | .47 | .02 | .01 |
| Advanced pT classification* | .30 | .12 | .008 |
| Cervical nodal metastasis | .004 | .047 | .047 |
| Regional metastasis† | .003 | <.0001 | .001 |
| Treatment characteristics | |||
| Type of TBR performed | .41 | .20 | .29 |
| Parotidectomy performed | .92 | .59 | .68 |
| Craniotomy performed | .001 | .001 | .10 |
| Facial nerve sacrificed | .10 | .12 | .12 |
| Postoperative radiation | .02 | .03 | .003 |
| Postoperative chemoradiation | .01 | .03 | <.0001 |
Abbreviations: OS, overall survival; DSS, disease-specific survival; RFS, recurrence-free survival; HR, hazard ratio; TBR, temporal bone resection.
Advanced: T3–T4.
Regional: cervical or parotid nodes.
Recurrence of disease
The 5-year cumulative rate of recurrence was 50.2%, mostly due to local and distant failure. For all cases, the 5-year rates of failure (at any time) were: local, 20.5%; regional, 5.5%; distant, 22.9%. Limited to cases with SCC histology, the 5-year rate of local failure was 33.5%; regional failure, zero; distant failure, 6.3%.
The rate of recurrence was highest for salivary tumors, all of which failed with distant metastases within 24 months. In comparison, the 5-year rate of recurrence was lower for other histologies, 41.7% for epithelial tumors, and 50.0% for mesenchymal tumors (p = .003, log-rank).
Rates of failure were significantly elevated among patients with tumors classified pT3–4, with a 5-year recurrence rate of 71.5%, compared with 22.3% in pT1–2 tumors (p = .02, log-rank). Similar results were observed in tumors with SCC histology: 63.8% in pT3–4 tumors vs 18.8% in other tumors (p = .06, log-rank). In the pT3–4 (all histologies) subgroup, the 5-year rate of local failure was 40.2%; regional failure, 14.4%; distant failure, 28.0% (Figure 3).
FIGURE 3.
For all cases (A), the cumulative incidence of recurrence was significantly higher in tumors classified pT3–4 (71.5%), as compared with other tumors (22.3%, p = .02). For SCC cases (B), recurrence was higher in pT3–4 tumors (63.8%) than in p1–2 tumors (18.8%, p = .06). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The mean time to recurrence was 13.1 months. Of patients experiencing local recurrence, surgical salvage was attempted in only 4 of 14 patients (28.6%), due to unresectable skull base involvement or synchronous distant metastases in the remaining cases. These 4 cases comprised 3 SCC and 1 basal cell carcinoma, and attempts at local salvage were successful in all cases, defined as achieving survival of at least 24 months. In unsalvageable local recurrences, 9 of 10 were SCC, and mean survival was 7.6 months. Of patients failing at distant sites, mean survival was 18.6 months. As a result of the low rate of salvageable recurrences, DSS was significantly poorer in patients experiencing recurrence (36.1% vs 96.3%, p < .0001, log-rank), as shown in Figure 4.
FIGURE 4.
Disease-specific survival was significantly poorer in patients experiencing recurrence than in those remaining recurrence-free (36.1% vs 96.3%, p < .0001). [Color figure can be viewed in the online issue, which is available at wiley onlinelibrary.com.]
DISCUSSION
Cancers involving the external auditory canal/temporal bone are generally associated with poor prognosis. The rarity, diversity, and anatomic complexity of malignant disease involving the external auditory canal/temporal bone have limited our understanding of oncologic outcomes. The extant literature consists of numerous case series that have reported a wide range of survival data, reflecting differences in the underlying characteristics of cohorts drawn from various institutions.1,6,8–10,12–15,18,19,22,24,30–41 Historical survival data can be most readily generalized to clinical practice if factors predictive of outcome can be identified. Several larger series have reported preliminary descriptions of factors influencing survival, based on univariate comparisons.2,6,7,16,20,21,24
In this study, we use multivariable analyses to identify factors independently associated with survival and recurrence in 72 patients undergoing temporal bone resection over 16 years at our 2 affiliated institutions. In all, 80% of cases were epithelial cancers, and 83% of patients underwent lateral temporal bone resection as the primary temporal bone procedure. Seventy percent of tumors involved local critical structures. Many patients underwent adjunctive procedures such as parotidectomy, mandibulectomy, facial nerve sacrifice, or formal craniotomy. The parotid gland was at high risk in squamous cell carcinomas of the external auditory canal: these tumors invaded the parotid gland in 25% of cases, and metastasized to the parotid gland in 43% of cases. Overall, the incidence of neck metastasis was low, both clinically (6%) and on pathologic analysis (13%), although these numbers should be interpreted with caution because nearly a third of patients had received prior radiation therapy.
Despite radical surgery, surgical margins were close (<5 mm) or positive in one third of cases, and in the majority of pT3–4 cases. Although 5-year DSS was 70%, the 5-year cumulative rate of recurrence was 50%. Recurrence rates were particularly high in parotid tumors (100%) and in pT3–4 tumors (72%), attributable mainly to local and distant recurrences. The majority (71%) of local recurrences were not salvageable and, accordingly, mean survival in patients recurring locally was 7.6 months. On multivariable analysis, the strongest predictors of survival and recurrence were surgical margin status and the presence of extratemporal disease: regional (neck/parotid) metastases or invasion of the parotid gland or mandible.
Before proceeding, it is worthwhile to consider some caveats to our findings. First, patients referred to our centers had a high incidence of advanced or previously treated disease, and overall statistics may not be generalizable to patient cohorts at other centers. Furthermore, this analysis was limited to patients undergoing surgery, and the outcomes of patients triaged to nonsurgical therapy due to unresectable disease would not be reflected in this study. For these reasons, multivariable analysis is valuable, by identifying factors independently associated with outcome. Second, our restriction of this cohort to contemporary patients necessarily limited sample size. This may have reduced statistical power to identify more subtle determinants of outcome, as described in the Methods section. Taking into account these limitations, we were nevertheless able to identify several strong independent predictors of survival and recurrence in this analysis. Finally, because this was a retrospective study, we were unable to evaluate how specific surgical interventions or adjuvant therapies influence outcome; these questions would be most appropriately addressed in a prospective study.
Strengths of this study were the inclusion of the largest contemporary cohort reported to date, high-quality pathologic analysis, and the use of multivariable statistics. To the best of our knowledge, no multivariable analyses of predictors of outcome in patients undergoing temporal bone resection have been reported, making this analysis the first to identify independent predictors of survival and recurrence outcomes (see Table 7). The literature on temporal bone resection includes important data that have been contributed by several large series.2,6,16,20,21,23,24 However, most of these reports have been based on patients treated over the past half-century or longer. Treatment has changed substantially during this time. Since the initial description of the en bloc temporal bone resection in 1954, therapeutic capabilities have rapidly evolved to include high-resolution cross-sectional imaging, oncologic and neurotologic microsurgery of the lateral skull base, microvascular reconstruction, and intensity-modulated radiotherapy. These advances underscore the value of basing outcomes analyses on a contemporary cohort.
TABLE 7.
Multivariable analysis of factors predictive of outcome.
| Variable | OS
|
DSS
|
RFS
|
|||
|---|---|---|---|---|---|---|
| (HR) | p value | (HR) | p value | (HR) | p value | |
| Margin status | 2.36 (0.86–6.45) | .09 | 3.23 (1.00–10.49) | .050 | 2.89 (1.12–7.52) | .21 |
| Regional nodal metastasis | 4.67 (1.42–15.36) | .01 | 6.21 (1.78–21.68) | .004 | 5.32 (1.43–19.73) | .01 |
| Previously treated | 1.20 (0.44–3.42) | .72 | 1.19 (0.39–3.66) | .75 | 1.16 (0.46–2.90) | .75 |
| Clinical parotid invasion | 1.39 (0.47–4.74) | .56 | 1.49 (0.16–19.29) | .73 | 1.10 (0.48–2.55) | .81 |
| Clinical middle ear invasion | 1.75 (0.99–3.14) | .05 | 3.43 (0.90–13.12) | .07 | 1.77 (1.04–3.00) | .03 |
| Clinical intracranial extension | 1.45 (0.80–2.62) | .23 | 2.51 (0.73–8.67) | .14 | 1.42 (0.82–2.45) | .21 |
| Clinical mandible invasion | 2.86 (1.58–5.21) | .001 | 3.25 (1.73–6.09) | <.0001 | 2.09 (1.13–3.83 | .02 |
| Advanced cT classification* | 1.14 (0.66–1.94) | .65 | 1.32 (0.68–2.15) | .41 | 1.67 (0.87–3.19) | .12 |
| Pathologic bone invasion | 2.53 (0.80–8.00) | .11 | 4.96 (1.07–23.07) | .04 | 1.55 (0.55–4.34) | .41 |
| Pathologic parotid invasion | 10.98 (1.66–72.67) | .01 | 10.11 (1.47–69.48) | .02 | 6.37 (1.71–23.72) | .006 |
| Perineural invasion | 1.15 (0.28–4.76) | .85 | 1.71 (0.33–8.81) | .52 | 2.17 (0.72–6.62) | .17 |
| Depth of invasion >1 mm | 1.17 (0.24–3.07) | .81 | 2.30 (0.49–10.85) | .29 | 1.05 (0.31–3.63) | .93 |
| Advanced pT classification* | 1.17 (0.80–1.72) | .43 | 1.32 (0.79–2.13) | .28 | 1.65 (1.01–2.73) | .049 |
| Craniotomy performed | 3.82 (1.42–10.20) | .007 | 4.34 (1.47–12.80) | .008 | 2.06 (0.73–5.60) | .16 |
| Facial nerve sacrificed | 2.67 (1.01–7.07) | .048 | 3.29 (1.04–10.42) | .04 | 2.15 (0.87–5.32) | .09 |
| Postoperative radiation | 1.64 (0.89–3.03) | .11 | 1.51 (0.76–3.13) | .25 | 1.61 (0.93–2.78) | .75 |
Abbreviations: OS, overall survival; DSS, disease-specific survival; RFS, recurrence-free survival; HR, hazard ratio.
Advanced: T3–T4.
The existing literature reports a wide range of survival outcomes in patients undergoing temporal bone resection, with 5-year OS rates ranging from 31% to 76%, and DSS rates ranging from 42% to 80%. These numbers are reflections of the underlying characteristics of the patient cohort at each institution.8–15,17–19,22,23,30–32,34,35,37–42 Attempting to improve understanding of prognostic factors, 5 groups have recently analyzed outcomes using univariate analyses. In 1997, Testa et al20 reported outcomes of 79 Brazilian patients treated between 1953 and 1995. On univariate analysis, T classification, squamous cell histology, and bone erosion were associated with poorer survival. In 1998, Manolidis et al16 reviewed 81 patients treated in Nashville between 1972 and 1996. Although no statistical analyses were performed, the data suggested that pain at presentation, soft tissue extension, and salivary histology might portend poorer survival. Yin et al21 recently reported a series of 95 Japanese patients treated between 1976 and 2003, and identified Pittsburgh stage and margin status as predictors of outcome on univariate analysis. In 2008, Madsen et al2 reviewed 68 Danish cases treated between 1992 and 2001. T classification, surgical margins, and middle ear invasion were associated with poorer survival on univariate analysis. Most recently, Gidley et al7 from MD Anderson Cancer Center updated and expanded on an earlier report from that institution,11 with a series of 124 cases of SCC of the temporal bone treated between 1945 and 2005. These authors identified T classification and prior treatment as predictors of outcome on univariate analysis.7
Building on these important studies, we have confirmed and expanded on prior findings. We identified several strong independent predictors of survival, including regional (neck/parotid) nodal status, margin status, mandible or parotid invasion, and craniotomy or facial nerve sacrifice. These data underscore the strong negative prognostic influence of positive margins or extratemporal spread of disease, whether to regional nodes, the mandible, parotid gland, or dura/brain. In partial contrast to prior reports, Pittsburgh T classification was not significantly predictive of survival on multivariable analysis, and was independently associated only with recurrence outcomes. We found that the primary challenge in patients with locally advanced cancer of the external auditory canal/temporal bone is achieving negative surgical margins and, accordingly, that the most common site of failure is local recurrence, which is usually not surgically salvageable.
Based on these outcomes data, we currently consider the minimum operation for the vast majority of malignant disease involving the external auditory canal/temporal bone to be a lateral temporal bone resection. Sleeve resection may be contemplated in conchal SCC that does not breach the external auditory canal, or in small cT1 basal cell carcinoma of the external auditory canal. In carefully selected cases of cutaneous malignancy of the postauricular skin invading a localized area of mastoid bone, a cortical or simple mastoidectomy may be sufficient. In cases of squamous cell carcinoma of the external auditory canal, we advocate a superficial parotidectomy as an en bloc adjunct to temporal bone resection due to the high rates of occult parotid invasion or parotid nodal metastasis. The deep lobe of the parotid gland should also be carefully inspected at the completion of superficial parotidectomy, but deep lobe parotidectomy will rarely be necessary.
The data in this study confirm a low rate of occult disease in the neck, and a low rate of neck recurrence, and routine elective dissection of the cN0 neck may not always be necessary. However, we do perform elective neck dissections in patients with locally advanced cases of SCC (of the external auditory canal or periauricular region), in other select high-risk cases based on histopathology and tumor location, and in patients requiring vessels for microvascular reconstruction. Finally, our data reveal a strikingly high rate of local recurrence in cT3–4 cancers involving the external auditory canal/temporal bone, especially primary parotid cancers, underscoring the need to consider adjuvant radiotherapy, even in cases lacking traditional pathologic adverse features such as perineural invasion, positive margins, or regional nodal metastasis. The strongest negative prognostic factors in this study were neck metastasis, positive surgical margins, and extratemporal spread of disease, especially to the mandible, parotid gland, or brain/dura.
Acknowledgments
Contract grant sponsor: National Cancer Institute of the National Institutes of Health; contract grant number: T32 CA009685.
References
- 1.Lodge WO, Jones HM, Smith ME. Malignant tumors of the temporal bone. AMA Arch Otolaryngol. 1955;61:535–541. doi: 10.1001/archotol.1955.00720020552003. [DOI] [PubMed] [Google Scholar]
- 2.Madsen AR, Gundgaard MG, Hoff CM, et al. Cancer of the external auditory canal and middle ear in Denmark from 1992 to 2001. Head Neck. 2008;30:1332–1338. doi: 10.1002/hed.20877. [DOI] [PubMed] [Google Scholar]
- 3.Moody SA, Hirsch BE, Myers EN. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol. 2000;21:582–588. [PubMed] [Google Scholar]
- 4.Parsons H, Lewis JS. Subtotal resection of the temporal bone for cancer of the ear. Cancer. 1954;7:995–1001. doi: 10.1002/1097-0142(195409)7:5<995::aid-cncr2820070524>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Conley JJ, Novack AJ. The surgical treatment of malignant tumors of the ear and temporal bone. Part I. AMA Arch Otolaryngol. 1960;71:635–652. doi: 10.1001/archotol.1960.03770040035006. [DOI] [PubMed] [Google Scholar]
- 6.Dean NR, White HN, Carter DS, et al. Outcomes following temporal bone resection. Laryngoscope. 2010;120:1516–1522. doi: 10.1002/lary.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidley PW, Roberts DB, Sturgis EM. Squamous cell carcinoma of the temporal bone. Laryngoscope. 2010;120:1144–1151. doi: 10.1002/lary.20937. [DOI] [PubMed] [Google Scholar]
- 8.Bibas AG, Ward V, Gleeson MJ. Squamous cell carcinoma of the temporal bone. J Laryngol Otol. 2008;122:1156–1161. doi: 10.1017/S0022215107001338. [DOI] [PubMed] [Google Scholar]
- 9.Cristalli G, Manciocco V, Pichi B, et al. Treatment and outcome of advanced external auditory canal and middle ear squamous cell carcinoma. J Craniofac Surg. 2009;20:816–821. doi: 10.1097/SCS.0b013e3181a14b99. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie MB, Francis HW, Chee N, Eisele DW. Squamous cell carcinoma of the temporal bone: a radiographic-pathologic correlation. Arch Otolaryngol Head Neck Surg. 2001;127:803–807. [PubMed] [Google Scholar]
- 11.Goodwin WJ, Jesse RH. Malignant neoplasms of the external auditory canal and temporal bone. Arch Otolaryngol. 1980;106:675–679. doi: 10.1001/archotol.1980.00790350017006. [DOI] [PubMed] [Google Scholar]
- 12.Ito M, Hatano M, Yoshizaki T. Prognostic factors for squamous cell carcinoma of the temporal bone: extensive bone involvement or extensive soft tissue involvement? Acta Otolaryngol. 2009;129:1313–1319. doi: 10.3109/00016480802642096. [DOI] [PubMed] [Google Scholar]
- 13.Knegt PP, Ah-See KW, Meeuwis CA, van der Velden LA, Kerrebijn JD, De Boer MF. Squamous carcinoma of the external auditory canal: a different approach. Clin Otolaryngol Allied Sci. 2002;27:183–187. doi: 10.1046/j.1365-2273.2002.00561.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunst H, Lavieille JP, Marres H. Squamous cell carcinoma of the temporal bone: results and management. Otol Neurotol. 2008;29:549–552. doi: 10.1097/MAO.0b013e31816c7c71. [DOI] [PubMed] [Google Scholar]
- 15.Lobo D, Llorente JL, Suarez C. Squamous cell carcinoma of the external auditory canal. Skull Base. 2008;18:167–172. doi: 10.1055/s-2007-994290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manolidis S, Pappas D, Jr, Von Doersten P, Jackson CG, Glasscock ME., III Temporal bone and lateral skull base malignancy: experience and results with 81 patients. Am J Otol. 1998;19 (Suppl 6):S1–S15. [PubMed] [Google Scholar]
- 17.Moffat DA, Wagstaff SA, Hardy DG. The outcome of radical surgery and postoperative radiotherapy for squamous carcinoma of the temporal bone. Laryngoscope. 2005;115:341–347. doi: 10.1097/01.mlg.0000154744.71184.c7. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Kumamoto Y, Natori Y, et al. Squamous cell carcinoma of the external auditory canal and middle ear: an operation combined with preoperative chemoradiotherapy and a free surgical margin. Otol Neurotol. 2006;27:242–249. doi: 10.1097/01.mao.0000190463.88873.3d. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa K, Nakamura K, Hatano K, et al. Treatment and prognosis of squamous cell carcinoma of the external auditory canal and middle ear: a multi-institutional retrospective review of 87 patients. Int J Radiat Oncol Biol Phys. 2007;68:1326–1334. doi: 10.1016/j.ijrobp.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 20.Testa JR, Fukuda Y, Kowalski LP. Prognostic factors in carcinoma of the external auditory canal. Arch Otolaryngol Head Neck Surg. 1997;123:720–724. doi: 10.1001/archotol.1997.01900070064010. [DOI] [PubMed] [Google Scholar]
- 21.Yin M, Ishikawa K, Honda K, et al. Analysis of 95 cases of squamous cell carcinoma of the external and middle ear. Auris Nasus Larynx. 2006;33:251–257. doi: 10.1016/j.anl.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu R, Hinerman RW, Indelicato DJ, et al. Squamous cell carcinoma of the external auditory canal: long-term clinical outcomes using surgery and external-beam radiotherapy. Am J Clin Oncol. 2009;32:401–404. doi: 10.1097/COC.0b013e31818f2d48. [DOI] [PubMed] [Google Scholar]
- 23.Gaudet JE, Walvekar RR, Arriaga MA, et al. Applicability of the Pittsburgh staging system for advanced cutaneous malignancy of the temporal bone. Skull Base. 2010;20:409–414. doi: 10.1055/s-0030-1253575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi FL, Gu FM, Dai CF, Chen B, Li HW. Survival outcomes in surgical treatment of 72 cases of squamous cell carcinoma of the temporal bone. Otol Neurotol. 2011;32:665–669. doi: 10.1097/MAO.0b013e318210b90f. [DOI] [PubMed] [Google Scholar]
- 25.Arriaga M, Curtin H, Takahashi H, Hirsch BE, Kamerer DB. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990;99:714–721. doi: 10.1177/000348949009900909. [DOI] [PubMed] [Google Scholar]
- 26.Begg CB, Zhang ZF, Sun M, Herr HW, Schantz SP. Methodology for evaluating the incidence of second primary cancers with application to smoking-related cancers from the Surveillance, Epidemiology, and End Results (SEER) program. Am J Epidemiol. 1995;142:653–665. doi: 10.1093/oxfordjournals.aje.a117689. [DOI] [PubMed] [Google Scholar]
- 27.Selesnick SH, Levine JM. Chondroblastoma of the temporal bone: consistent middle fossa involvement. Skull Base Surg. 1999;9:301–305. doi: 10.1055/s-2008-1058141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19:589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 29.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 30.Austin JR, Stewart KL, Fawzi N. Squamous cell carcinoma of the external auditory canal. Therapeutic prognosis based on a proposed staging system. Arch Otolaryngol Head Neck Surg. 1994;120:1228– 1232. doi: 10.1001/archotol.1994.01880350036007. [DOI] [PubMed] [Google Scholar]
- 31.Chang CH, Shu MT, Lee JC, Leu YS, Chen YC, Lee KS. Treatments and outcomes of malignant tumors of external auditory canal. Am J Otolaryngol. 2009;30:44–48. doi: 10.1016/j.amjoto.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Depondt J, Bouccara D, Enaux M, Gehanno P, Sterkers O. Malignant tumors of the petrous bone. Apropos of 25 cases. Ann Otolaryngol Chir Cervicofac. 1995;112:309–316. [PubMed] [Google Scholar]
- 33.Go KG, Annyas AA, Vermey A, Robinson PH, Belopavlovic M, Mehta DM. Evaluation of results of temporal bone resection. Acta Neurochir (Wien) 1991;110:110–115. doi: 10.1007/BF01400676. [DOI] [PubMed] [Google Scholar]
- 34.Kawahara N, Sasaki T, Asakage T, et al. Long-term outcome following radical temporal bone resection for lateral skull base malignancies: a neurosurgical perspective. J Neurosurg. 2008;108:501–510. doi: 10.3171/JNS/2008/108/3/0501. [DOI] [PubMed] [Google Scholar]
- 35.Kollert M, Draf W, Minovi A, Hofmann E, Bockmuhl U. Carcinoma of the external auditory canal and middle ear: therapeutic strategy and follow up. Laryngorhinootologie. 2004;83:818–823. doi: 10.1055/s-2004-825804. [DOI] [PubMed] [Google Scholar]
- 36.Moffat DA, Grey P, Ballagh RH, Hardy DG. Extended temporal bone resection for squamous cell carcinoma. Otolaryngol Head Neck Surg. 1997;116:617–623. doi: 10.1016/S0194-59989770237-7. [DOI] [PubMed] [Google Scholar]
- 37.Pfreundner L, Schwager K, Willner J, et al. Carcinoma of the external auditory canal and middle ear. Int J Radiat Oncol Biol Phys. 1999;44:777–788. doi: 10.1016/s0360-3016(98)00531-8. [DOI] [PubMed] [Google Scholar]
- 38.Prasad S, Janecka IP. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg. 1994;110:270–280. doi: 10.1177/019459989411000303. [DOI] [PubMed] [Google Scholar]
- 39.Spector JG. Management of temporal bone carcinomas: a therapeutic analysis of two groups of patients and long-term follow-up. Otolaryngol Head Neck Surg. 1991;104:58–66. doi: 10.1177/019459989110400112. [DOI] [PubMed] [Google Scholar]
- 40.Zanoletti E, Danesi G. The problem of nodal disease in squamous cell carcinoma of the temporal bone. Acta Otolaryngol. 2010;130:913–916. doi: 10.3109/00016480903390152. [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Tu G, Xu G, Tang P, Hu Y. Squamous cell carcinoma of temporal bone: reported on 33 patients. Head Neck. 1999;21:461–466. doi: 10.1002/(sici)1097-0347(199908)21:5<461::aid-hed13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Rinaldo A, Ferlito A, Suarez C, Kowalski LP. Nodal disease in temporal bone squamous carcinoma. Acta Otolaryngol. 2005;125:5–8. doi: 10.1080/00016480410018287. [DOI] [PubMed] [Google Scholar]