Abstract
Cancer has become the number one cause of death amongst Americans, killing approximately 1,600 people per day. Novel methods for early detection and the development of effective treatments are an eminent priority in medicine. For this reason, isolation of tumor-specific ligands is a growing area of research. Tumor-specific binding agents can be used to probe the tumor cell surface phenotype and customize treatment accordingly by conjugating the appropriate cell-targeting ligand to an anticancer drug. This refines the molecular diagnosis of the tumor and creates guided drugs that can target the tumor while sparing healthy tissues. Additionally, these targeting agents can be used as in vivo imaging agents that allow for earlier detection of tumors and micrometastasis. Phage display is a powerful technique for the isolation of peptides that bind to a particular target with high affinity and specificity. The biopanning of intact cancer cells or tumors in animals can be used to isolate peptides that bind to cancer-specific cell surface biomarkers. Over the past 10 years, unbiased biopanning of phage-displayed peptide libraries has generated a suite of cancer targeting peptidic ligands. This review discusses the recent advances in the isolation of cancer-targeting peptides by unbiased biopanning methods and highlights the use of the isolated peptides in clinical applications.
Keywords: Peptide, phage display, tumor targeting, cancer, drug delivery
INTRODUCTION
Cancer has become the number one cause of death amongst Americans, killing approximately 1,600 people per day [1]. Development of novel treatments for this disease will be dependent on the ability to modulate cellular pathways that are aberrant in cancer cells as well as the capacity to deliver therapeutics effectively to the tumor. A potential paradigm for the diagnosis and treatment of cancer is to generate a panel of tumor-specific binding agents. These agents could be used to “classify” the tumor cell surface phenotype and to customize treatment accordingly by attaching the appropriate cell-targeting ligand to an anticancer drug. This allows a more refined molecular diagnosis of the tumor and creates “smart bullet” drugs that will be more effective and have fewer side effects. Furthermore, many pharmaceutical agents have been developed, only to be abandoned as treatments due to high levels of toxicity in vivo. Reduction of the amounts required for treatment could resurrect some of these drugs from the shelves of pharmaceutical companies.
It is apparent that treatment of cancers will require some degree of customization of therapeutic regimens. The success of Herceptin® (trastuzumab) attests to the viability of personalized therapies for cancer. Herceptin® is a monoclonal antibody that binds to the HER2/neu receptor that is over-expressed on the cell surface of approximately 25% of breast cancers [2]. As such, patients are stratified by HER2 expression and therapy decisions are guided by HER2 status. Moreover, within HER2 positive patients, many do not respond to the antibody treatment or eventually develop resistance. This phenomenon appears to be the result of activation of compensative signaling pathways and not loss of HER2 expression. To overcome this, researchers at Genentech have generated a trastuzumab-cytotoxic conjugate which selectively kills HER2 positive tumor cells, even those resistant to passive immunotherapy with the naked antibody [3]. The initial studies in small animals are promising that this antibody conjugate will prove efficacious for patients who fail to show response on trastuzumab. The need for tailored treatments was also made clear in clinical trials of AstraZeneca’s drug Iressa® (Gefitinib). While highly effective in 10% of lung cancer patients, this drug failed to enhance survival in the other 90%. Patients who respond to Iressa® have a somatic mutation in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) [4, 5]. Similarly, the EGFR monoclonal antibody Cituxamab® is used for these patients as they frequently have an amplification of EGFR levels as well. These examples stress the need for a detailed molecular diagnosis and a tailored therapy regime [6]. They also indicate the heterogeneity of the disease. Even within a single class of cancer, there is considerable variability and these variations affect treatment plans and clinical outcomes. As such, agents that allow for a molecularly refined diagnosis and treatment are crucial.
The bottleneck in targeted delivery and molecular imaging has been the identification of ligands that have the ability to discriminate between closely related cell types and have the required affinity for clinical use. The difficulty of the problem is driven home when one considers that the human body contains 210 distinct cell types and is composed of roughly 1014 cells total. The surface of a cell represents an assortment of macromolecules, which provides the cell with a topographical surface that is specific to the type and state of the cell. In cancer, a variety of genetic and epigenetic changes occur, resulting in changes in the proteomic profile of the cell [7]. These genetic modifications can result in a change in the type, number, and arrangement of cell surface receptors, leading to unique cell surface topographies. This surface profile can be thought of as a molecular address for the delivery of biomolecules. Identification of ligands that discriminate between subtle differences in the cellular landscape with high specificity and affinity is necessary in order to fulfill the goals of targeted therapies and molecular diagnosis, as well as expanding in vitro diagnostic capabilities.
In some cases, information is known about the macromolecules expressed on the cell-surface, and ligands have been generated for these cellular receptors. However, this is a major challenge as not only does one have to know the receptor on the target cell type but also its expression levels in all other cells. Proteomic approaches have been used to identify biomarkers that are of value as diagnostic, prognostic, and/or drug targets [8]. Most of these studies employ two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) to compare protein expression between cancer samples and normal control samples followed by mass spectrometry (MS) to identify the differentially expressed proteins. While powerful, the 2D-PAGE/MS method for identifying biomarkers has limitations. Mainly, it is often difficult to detect low abundance proteins and certain classes of proteins are under-represented, especially membrane proteins. This is particularly problematic if the goal is to identify uniquely expressed cell surface proteins for diagnostics or targeted drug delivery. Furthermore, if internalization of the therapeutic is the goal, the isolated ligand must not only bind to the receptor but also mediate cellular uptake [9].
Most efforts in the field have focused on exploiting monoclonal antibodies [10]. There are currently 9 monoclonal antibody (MAb) treatments in the clinics for treatment of cancer. Most of these antibodies are non-conjugated antibodies for passive therapies; however, there are three therapeutic immunoconjugates available. Bexxar® (Iodine I 131 Tositumomab) and Zevalin® (Ibritumomab tiuxetan) are both radiolabeled monoclonal antibodies against CD20 used to treat Non-Hodgkin’s lymphoma. Mylotarg® (gemtuzumab ozogamicin), an anti-CD33-calicheamicin immunoconjugate, is approved for treatment of acute myelogenous leukemia (AML). The expanding use of monoclonal antibodies attests to the development of personalized therapies for cancer treatments.
While monoclonal antibodies as delivery agents can display high affinity and specificity, they suffer from certain limitations. Because of their size, immunoconjugates have difficulty penetrating tumor tissue [10-12]. Furthermore, they suffer from nonspecific uptake by the reticuloendothelial system, which can result in liver and bone toxicities when the antibody is conjugated to a toxin or radioisotope [13]. The in vivo half-life of antibodies is not well matched to the half-lives of common radioisotopes used for positron emission tomography (PET) imaging, resulting in poor tumor contrast. As such, antibodies have not found wide use as molecular imaging agents. Notably, it is challenging to chemically modify these macromolecules for downstream applications.
To overcome these limitations, peptides can employed as targeting ligands. Peptides are smaller than currently used antibody-based targeting agents and display many favorable characteristics. Specifically, peptides can be synthesized in large quantities and are amenable to regiospecific derivatization [14]. They usually avoid uptake by the reticuloendothelial system. Furthermore, peptides can display high affinities for protein receptors, making them attractive ligands. Recently progress has been made in tuning the in vivo stability of peptides by selective modifications. Known peptidyl ligands that bind to cell surface receptors over-expressed in neoplastic cells can be employed as targeting agents [15]. However, these known ligands bind to a small fraction of the cell surface proteome.
The advent of phage-displayed peptide libraries facilitated screening large peptide libraries for binding to target proteins [16-18]. Suddenly, the door was opened to being able to identify peptidic ligands for biomarkers which do not have naturally occurring ligands. This opportunity was not lost on cancer biologist, and peptides were identified for proteins that play a role in cancer [19]. Peptides that bind to the tumor antigens HER2/neu [20-22], EGFR [21, 23], Hepsin [24], Tie2 [25], Il-6 receptor [26], GRP78 [27], CD21 [28, 29], melanin [29], EphA2 [30], MMP-9 [31], and N-cadeherin [32] have been selected.
In 1996, two seminal papers were published that opened up new avenues for obtaining cancer targeting agents. Both of these papers employed phage displayed peptide libraries to isolate peptides that could bind to specific cell types. However, instead of panning on purified proteins that were anticipated to be good cellular receptors for targeted therapies, they employed a nonbiased selection on heterogeneous targets. Pasqalini and Rhouslahti reported panning phage libraries in a living animal to obtain peptides that home to the vasculature of specific organs [33]. In the same year, Johnston and co-workers panned on cells in culture to obtain peptidic ligands that bind to and trigger uptake within target cell types [34]. In both cases, the peptidic ligands display specificity for their target cell type over other cells. Importantly these approaches eliminated the need for prior identification and purification of the cellular target. Furthermore, it allows for selection of peptides that bind to their target in a native cellular context. In the case of in vivo panning, this is taken one step further in that the selected peptides must be able to reach their target within an animal, despite many biological constraints, such as serum stability, cellular access, and clearance rates, which are inherent in an animal. Additionally, cell-based biopanning allows for isolation of peptides that mediate cellular internalization, a key feature if the eventual application is drug or gene delivery. While neither of these reports was focused on cancer specific targeting, the approach is ideally suited for this application. Subsequently many papers have been published describing the isolation of tumor targeting peptides from phage displayed libraries [35, 36]. This review will focus on advances within the past 5 years in nonbiased phage display selections for the isolation of peptidic cancer targeting ligands and will highlight the novel use of these peptides in different applications.
IN VITRO BIOPANNING USING CANCER CELLS IN CULTURE
Phage display is a powerful technique for the isolation of peptides that bind to a particular target (reviewed in references [17, 18]) and is typically exploited to identify peptide ligands for purified proteins. However, whole cells in culture can be used as the bait to isolate cell binding peptides. Despite the heterogeneity of the target, this approach has been highly successful in identifying cell binding ligands for a variety of cell types utilizing different phage display formats. Depending on the downstream applications of the ligand, the selection pressures can be varied to isolate ligands that bind to the cell surface or to obtain peptides which trigger cellular uptake upon binding. A surprising feature of peptides isolated by the procedure is their cell specificity. In other words, the peptides tend to bind to the cell type for which they were selected against and not other cell types- in some cases very closely related cell types. While a negative selection is employed in some cases to remove ligands that might bind to common cell surface features, in many cases, no negative selection is required.
The general approach, often referred to as biopanning, involves incubating a phage library consisting of random peptides with a cell type of interest (Fig. 1). The cells then undergo a series of stringent washes designed to remove unbound or weakly bound phage. Phage can be eluted from the cell surface to obtain the fraction of bound phage and used to infect E. coli for phage retrieval and amplification. Alternatively, cell lysates are prepared to isolate all cell associated phage. This process is repeated until the ratio of total phage incubated with the cells compared to the total bound phage no longer increases, approximately 4-6 rounds. As bacteriophage do not exhibit tropism for mammalian cells, the phage that are amplified from the library are either bound very tightly to the cell surface or internalized. Since the only unique component of any phage in the library is the displayed peptide, this peptide should be the factor determining which phage bind to a particular cell type.
Fig. (1).
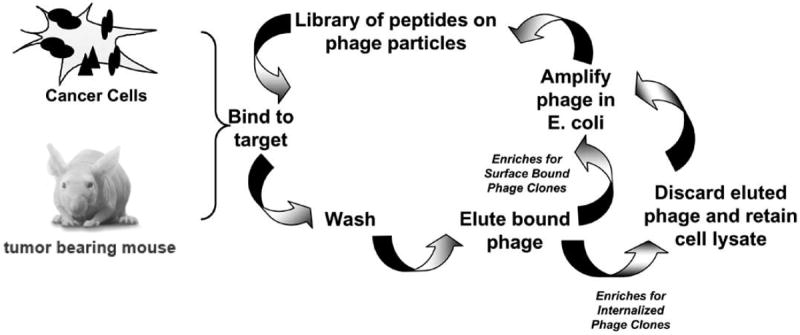
Schematic for the unbiased selection of cancer-targeting peptides from phage displayed-peptide libraries. Cancer cells in culture or tumors within animals can be used as the bait for the biopanning. Amplification of eluted phage enriches for phage clones that bind the cell surface. Amplification of phage from the cell lysates enriches for peptide that mediate cellular uptake upon binding.
Using cells as the bait for the peptide selection has several advantages. First, it keeps the cellular receptors in their native context. The topography of the cell surface is defined by expression levels of the plasma membrane bound protein as well as their arrangement within the membrane; this cannot be mimicked using purified membrane proteins. Second, by changing wash conditions, the selection can be biased towards the isolation of peptides that mediate cellular uptake. When using purified membrane proteins, the selection process is driven by binding alone. Third, this approach employs an unbiased screen in which there is no selective pressure towards binding a particular macromolecule. This has the advantage that it requires no prior knowledge of the cellular receptor. Thus, targeting peptides can be isolated for cell a type of which little is known about their cellular landscape. Furthermore, it allows for the discovery of cell surface macromolecules that may not have been considered as viable targets or have not yet been identified [37].
The number of cancer specific peptides isolated by this method has expanded as seen in Table I. As observed from the list of peptides, few sequence similarities are observed between the peptides. This most likely stems from the differences in library design, the cell lines used as bait, and panning protocols. Peptide libraries of linear and cyclized peptides have been utilized and the peptide length has varied from 6-20 amino acids. While many of these peptides were isolated by biopanning on cell lines, fresh cells used ex vivo have been employed as well. Recently, human colon cancer cells isolated by laser capture microdissection have been employed for biopanning [38]. This highlights the robustness of the system and the flexibility in the nature of the cellular bait. Although isolated on cells in vitro many of these peptides have been shown to home to tumors in vivo.
Table 1. Peptides Selected by Whole Cell Biopanning.
| Cancer Type | Cell Line used for Selection | Peptide Sequencea | Receptor Identified | In vivo Homing Validated | Used in Imaging Methods | Delivery of Bioactive Molecules |
|---|---|---|---|---|---|---|
|
| ||||||
| Hepatocarcinoma | BEL-7402 | TACHQHVRMVRP [42] | Yes | |||
|
| ||||||
| SMMC-7721 | KSLSRHDHIHHH [43] | Yes | Yes | |||
|
| ||||||
| Mahlavu | SFSIIHTPILPL [44] | Yes | Yes | |||
|
| ||||||
| Melanoma | Me6652/4 | CTVALPGGYVRVC [45] | GRP78 | Yes | ||
|
| ||||||
| B16-F10 | TRTKLPRLHLQS [46] | Yes | ||||
|
| ||||||
| Prostate | Capan-2 (irradiated) | SHGFSRHSMTLI [47] | Yes | |||
|
| ||||||
| LnCaP | DPRATPGS [48] | |||||
|
| ||||||
| DU-145 | FRPNRAQDYNTN [49] (DUP-1) | Yes | ||||
|
| ||||||
| Gastric | XGC9811-L4 | GRRTRSRRLRRS [50] | Yes | |||
|
| ||||||
| GC9811-P | SMSIASPYIALE [51] | Yes | ||||
|
| ||||||
| Colon | HT29 | CPIEDRPMC [52] | Yes | |||
|
| ||||||
| WiDr | HEWSYLAPYPWF [53] | No [54] | ||||
| QIDRWFDAVQWL [53] | ||||||
|
| ||||||
| Human Colonic adenomas | VRPMLQ [55] | Yes | Yes | |||
|
| ||||||
| Resected human colon tumors | SPTKSNS [38] | |||||
|
| ||||||
| SW480 | VHLGYAT [56] | Yes | ||||
|
| ||||||
| Head and Neck | MDA167Tu | TSPLNIHNGQKL [57] | Yes | |||
|
| ||||||
| Nasopharyngeal | NPC-TW 04 | RLLDTNRPPLLPY [58] | Yes | Yes | ||
|
| ||||||
| Breast | MDA-MB-321 | YQATPARFYTNT [53] | ||||
| CGWMGLELC [53] | ||||||
|
| ||||||
| SKBR3 | LTVSPWY [59] | Yes | ||||
| WNLPWYYSVSPT [59] | ||||||
|
| ||||||
| Neuroblastoma | WAC 2 | HLQIQPWYPQIS [60] | Yes [61] | |||
| VPWMEPAYQRFL [60] (p160) | ||||||
|
| ||||||
| Glioma | RG2 | VGLPEHTQ [62] | ||||
| ELRGDSLP [62] | ||||||
| DSTKSGNM [62] | ||||||
| DYDMTKNT [62] | ||||||
| DLTKSTAP [62] | ||||||
| ESRGDSYA [62] | ||||||
|
| ||||||
| U87-MG | MCPKHPLGC [63] | |||||
|
| ||||||
| Cervical | SiHa | CRLTGGKGVGC [64] | ||||
| CADPNSVRAMC [64] | ||||||
| CAAHYRVGPWC [64] | ||||||
|
| ||||||
| Medullary Thyroid | TT | CHTFEPVGC [65] | ||||
|
| ||||||
| Rhabdomyosarcoma | RD | CQQSNRGDRKRC [66] | αvβ3 | Yes | ||
| CMGNKRSAKRPC [66] | ||||||
|
| ||||||
| Lymphoma | A20 | SAKTAVSQRVWLPSHRGGEP [67] | ||||
| KSREHVNNSACPSKRITAAL [67] | ||||||
| WLSEAGPVVTVRALRGTGSW [67] | ||||||
| Molt-4 | CAYHRLRRC [68] | Yes | ||||
|
| ||||||
| Leukemia | Kasumi-1 | CPLDIDFYC [69] | α4β1 | |||
|
| ||||||
| Lung Cancer | H1299 (large cell) | VSQTMRQTAVPLLWFWTGSL [70] (H1299.1) | Yes | |||
| YAAWPASGAWTGTAPCSAGT [71] (H1299.2) | ||||||
| EHMALTYPFRPP [72] | ||||||
|
| ||||||
| H2009 (Adenocarcinoma) | RGDLATLRQLAQEDGVGVR [70] (H2009.1) | αvβ6 [37] | Yes | Yes | Yes [73, 74] | |
|
| ||||||
| A549 (Adenocarcinoma) | MTVCNASQRQAHAQATAVSL [70] | |||||
|
| ||||||
| CL1-5 | TDSILRSYDWTY [75] | Yes | Yes | |||
|
| ||||||
| Bladder | HT-1376 (ex vivo) | CSNRDARRC [76] | Yes | |||
|
| ||||||
| Pancreatic | Pancreatic ductal carcinomas arising from Kras/p52L/L mice (ex vivo) | KTLLPTP [77] | Plectin-1 | Yes | Yes | |
Common peptide names that have been used in the literature are indicated in parenthesis next to the peptide sequence
While most unbiased biopanning experiments have been performed with cells, unbiased panning can be employed for biological mixtures of proteins found in the tumor stroma. The extracellular matrix in tumors is enriched in fibrin which arises from clotting of fibronectin that leaks into the interstitial space of the tumor. Taking advantage of this phenomena, Ruoslahti and co-workers have performed biopanning using clotted plasma as the target [39]. Two cyclic peptides were isolated and determined to bind to the fibrin-fibronectin network. These peptides accumulate in the extracellular space of MDA-MB-435 tumors in animal xenografts and bind to frozen sections of clinical breast tumor samples.
The peptides listed in Table I were isolated from experiments in which the selections were pushed to convergence. In other words, the selections were carried out for multiple rounds until the few “best” peptides or predominant consensus sequences were identified, as defined by binding affinity. However, other selections have been performed on a large set of cell lines or patient samples in which short peptide consensus motifs were identified early in the selection process by large scale sequencing [40, 41]. While these short peptide motifs are unlikely to be high affinity ligands that are useful for cell-targeting, they can be used to identify cell surface similarities amongst the set of cells used in the experiments. This can provide information about the heterogeneity of the disease as well as an understanding of similarities between cancers arising from different organ sites.
IN VIVO PANNING TO IDENTIFY TUMOR TARGETING PEPTIDES
In vivo panning has been employed to isolate organ homing phage from peptide libraries. In this method, first published by Ruoslahti and co-workers, phage libraries are injected into the tail vein of mice [33]. After a short incubation time, the mice are sacrificed and the target organs removed. The organ-associated phage are retrieved and amplified from the homogenized tissue and the panning repeated in another mouse. After 3-5 rounds of panning, several peptide motifs are typically identified for a given organ [78-80]. Using the same technology, tumor targeting peptides can be identified [81]. As the process is dependent on the ability to retrieve infectious bacteriophage, circulation times are generally in the 5 to 15 minute time range. For this reason, in vivo biopanning generally identifies peptides that target vasculature addresses, although several of peptides are found to penetrate within the tumor. This method can be coupled with in vitro panning to enrich for likely peptide candidates before moving to in vivo panning. This approach has been applied to a number of different tumor types (Table II).
Table II. Cancer-Targeting Peptides Selected by in vivo Biopanning.
| Tumor Type | Peptide Sequence | Receptor | Free Peptide Tested | Used in Imaging Applications | Delivery of Bioactive Molecules |
|---|---|---|---|---|---|
|
| |||||
| Gastric (AZ-P7a) | SWKLPPS [91] (a) | α3β1 (b) | Yes | Yes | |
| Human gastric adenocarcinoma | CGNSNPKSC [92] | Yes | Yes [93] | ||
|
| |||||
| Lewis lung carcinoma (irradiated/SU11248) | HVGGSSV [83] | Yes | Yes | ||
|
| |||||
| Lung (CL1-5) | SVSVGMKPSPRP [88] | Yes | Yes | ||
|
| |||||
| Oral (SAS) | SVSVGMKPSPRP [88] | Yes | Yes | ||
| SNPFSKPYGLTV [94] | Yes | Yes | |||
| WDSNTYTPRPLM [94] | Yes | Yes | |||
|
| |||||
| Nasopharyngeal carcinoma (CNE-1) | EDIKPKTSLAFR [95] | Yes | |||
|
| |||||
| Prostate (PC-3) | IAGLATPGWSHWLAL [96] | Yes | |||
|
| |||||
| Breast (MDA-MB-435) | CNGRCVSGCAGRC (NGR) [81] | Amino-peptidase N | Yes | Yes | Yes [100] |
| CDCRGDCFC (RGD-4C) [81] | αvβ3, α5β1 | Yes | Yes [97-99] | Yes [81, 98, 101, 102] | |
|
| |||||
| Medullary thyroid carcinoma (RET-C634R transgenic mice) | SRESPHP [103] | ||||
|
| |||||
| K14-HPV16 mice basal cell squamous carcinoma | CGKRK [82] (c) | Yes | |||
| CDTRL [82] | Yes | ||||
|
| |||||
| RIP1-Tag2 mice Pancreatic islets | CRGRRST [104] (c) | PDGFRβ | Yes | ||
| CRSKG [104] | Yes | ||||
| CKAAKNK [104] | Yes | ||||
| CKGAKAR [104] | Yes | ||||
| FRVGVADV [104] | Yes | ||||
|
| |||||
| Human myeloid leukemia (HL-60) | KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK (F3) [105] (c) | Yes | |||
|
| |||||
| MDA-MB-435 (tumor lymphatic vessels) | CGNKRTRGC (LyP-1) [85] (c) | p32/gC1qR [106] | Yes | Yes [84] | |
|
| |||||
| C8161 Melanoma | CLSDGKRKC (LSD) [86] (c) | Yes | Yes | ||
|
| |||||
| Prostate Tumors from TRAMP mice | CREAGRKAC (REA) [86] (c) | Yes | Yes | ||
|
| |||||
| B16-F10 | TRTKLPRLHLQS [46] (c) | ||||
Intraperitoneal injections used instead of intravenous injections.
Receptor identified by similarity of the peptide to laminin but not confirmed.
Combination of ex vivo/in vivo biopanning employed.
Different peptides are isolated for different tumor types, even employing the same library, suggesting that the tumor influences the receptor profile of the endothelium. This discrimination can be quit exquisite. For example, peptides have been identified that can distinguish between neovasculature in dysplastic skin and neovasculature in the resultant tumor [82]. Recently, Hallahan and colleagues have identified a peptide that binds to tumors that are sensitive to a combination treatment of radiation and systemic administration of the VEGF receptor tyrosine kinase inhibitor SU11248 while remaining blind to nonresponsive tumors [83]. This demonstrates the specificity that can be achieved with peptides and suggests that peptidic agents can be developed to assess drug responses.
Recently Eriki Rhouslahti’s laboratory has employed a combination of ex vivo and in vivo selections to isolate peptides that bind the tumor lymphatic vessels as opposed to the primary vasculature [84-87]. As the lymphatic vessels in tumors provide a route for cancer cells to escape from the primary tumor, targeting these vessels for the destruction has the potential to inhibit metastasis. Biopanning has isolated a cyclic 9-mer peptide, LyP-1, that binds lymphatic vessels in breast, prostate, and osteosarcoma tumors [84, 85]. The peptide does not home to a melanoma or leukemia xenograft indicating that the peptide is specific for a certain lymphatic marker present in some tumors. Furthermore it does not bind to tumor vasculature or other tissues in the mouse. Surprisingly, the peptide translocates to the nucleus upon binding. Peptides for tumor lymphatic vessels have been isolated for other tumor types and are indicated in Table II [86]. Again, highly specific peptides can be isolated. For example, peptides have been isolated that can distinguish between lymphatic vessels in premalignant lesions and fully developed tumors in a TRAMP animal model of prostate cancer [86].
In vitro and in vivo biopanning can lead to the isolation of different peptides, even when the same library and cell line are employed. The 12-mer library was biopanned against the CL1-5 NSCLC cell line in vitro as well in vivo using CL1-5 tumor xenographs [75, 88]. The in vitro panning isolated the peptide TDSILRSYDWTY (SP5-2) while the in vivo experiment resulted in the selection of the peptide SVSVGMKPSPRP (SP5-52). Both experiments were preformed in the same laboratory. As anticipated, SP5-52 targets the vasculature while SP5-2 penetrates into the tumor results in a more diffuse binding pattern throughout the tumor mass. This emphasizes that these two approaches to biopanning are targeting different molecular features.
It is important to remember that the tumor vasculature arises from mouse endothelium. As such, the peptides isolated by this method may not translate to human vasculature if the peptide binding does not cross species. This limitation can be overcome by identifying the cellular target of the peptide and determining if its homolog is expressed in human tumor vasculature. The isolated peptide sequence can be used as a starting point for mutagenic optimization for binding to its human counterpart. Alternatively, the biopanning can be performed in humans. Arap and Pasqualini first demonstrated that phage could be injected into terminal human patients and retrieved from human tissues [89]. High density sequencing was performed to identify tripeptide motifs that segregate to different organ vasculature. This was followed by a report by the Krag laboratory in which serial panning/biopsies were performed in patients [90]. While promising, biopanning in humans has not yet been performed to identify high affinity tumor targeting agents that can be used clinically.
USE OF CANCER-TARGETING PEPTIDES FOR DRUG DELIVERY
Clearly one of the primary uses for these tumor targeting peptides is cell-specific delivery of chemotherapeutics. Tumor specific delivery of therapeutics can increase efficacy of the treatment while decreasing untoward side effects, thus widening the therapeutic window. There are two primary formats for targeted drug delivery. In the first, the targeting peptide is directly conjugated to the chemotherapeutic target. In the second configuration, the targeting moiety is attached to a drug carrier [107]. A variety of drug carriers have been developed including polymeric drug scaffolds [108], micelles [109], dendrimers [110, 111], and liposomes [112]. The advantages and disadvantages for each drug delivery approach are outlined in Table III.
Table III. Comparison of Different Drug Carrier Platforms as Therapeutics.
| Advantages | Disadvantages | |
|---|---|---|
| Direct-Conjugate |
|
|
| Liposomal Formulation |
|
|
| Polymeric Micelle Formulation |
|
|
| Polymeric Drug Carriers |
|
|
Chemotherapeutics can be covalently coupled to a targeting agent for drug delivery. This approach requires that chemically compatible sites exist on the drug molecule and the ligand. In most cases the drug must be released to be functional, yet the linkage must be stable until its reaches its tumor target. Most efforts have focused on utilizing acid labile linkers that are stable at pH 7 but upon internalization free drug will be released from the conjugate at the acidic pH found in the lysosome. Ester and carbamate linkages can be employed as well [100, 113, 114] as they are generally stable in serum but cleaved when internalized within the cells as a result of the dramatic increase in esterases. Doxorubicin has been attached via a hydrazone linker to two non-small cell lung cancer targeting peptides resulting is cell specific death in vitro [73]. While the therapeutic window was dramatically widened, the targeted drug was less effective that free doxorubicin. This may stem from low drug uptake, inefficient drug release from the peptide carrier, or incorrect cellular trafficking. These problems can be overcome by increasing the drug load of the conjugate, changing the linkage of the drug to the targeting agent, or employing a more potent drug. In contrast, taxol conjugated to a melanoma targeting peptide is more efficacious in inducing apoptosis than free taxol [45].
The proapoptotic peptide KLAKLAKKLAKLAK has been fused to several cell-targeting peptides to create a chimeric peptide [52, 68, 86]. This peptide is nontoxic to cells until internalized, at which point it disrupts the mitochondrial membrane resulting in cell death. The peptide can be synthesized using D-amino acids rendering it protease resistant while maintaining its ability to induce apoptosis. The chimeric peptides have been found to affect highly specific cell death of the target cell type. While it is unlikely that the proapoptotic drug will replace small molecule chemotherapeutics, it serves as a useful tool to assess the utility of the peptide.
Direct peptide-drug conjugates have the advantage of high specificity and are predicted to penetrate the tumor better than a larger nanoparticle [115]. On the other hand, drug carriers such as liposomes, micelles, and polymers can carry more drug molecules per targeting event and have longer circulation times. Peptides isolated from phage-displayed selections have been incorporated into doxorubicin loaded liposomes [44, 58, 75, 88, 91, 94, 116, 117]. In all cases, the peptide-targeted liposome was more effective in reducing tumor growth and enhancing survival when compared to a non-targeted liposome. For example, the peptide TDSILRSYDWTY was incorporated into liposomes containing doxorubicin or vinorelbine [75]. Both formulations were more effective in reducing tumor growth and increasing survival times than non-targeted liposomes or the free drug. Correspondingly, nuclear doxorubicin was greater than 2-fold higher in tumors treated with the targeted liposome compared to the non-targeted. Recently, the tumor binding F3 peptide was incorporated onto the surface of a multi-functional micelle containing fluorescent quantum dots, iron oxide nanoparticles, and doxorubicin [118]. This peptide facilitated delivery of the micelles into the targeted MDA-MB-435 cells. Polymers can also serve as drug carriers and addition of a targeting peptide can increase intracellular delivery to a targeted cancer cell [28, 97, 108]. For example, we have recently incorporated a NSCLC targeting peptide into a polyglutamic acid polymer carrying doxorubicin [74]. The targeted polymer results in 2-fold greater uptake and a corresponding reduction in cell viability.
A word of caution about targeting larger drug carrier molecules to tumors is required. Tumors possess a disordered and leaky vasculature which allows for large particles to extravasate from the vasculature. This coupled with a dysfunctional lymphatic system results in retention of nanoparticles of 50 to 400 nm in the tumor [115]. This effect, known as enhanced permeability and retention (EPR) can complicate our understanding of the active targeting by the peptide. Mathematical modeling of tumor targeting suggests that passive targeting is the driving force for tumor accumulation of nanoparticles [119, 120]. While increased efficacy of targeted liposomal drugs is observed over non-targeted liposomal formulation, the reason for this is debatable. In some cases, especially in vasculature targeting, it appears that more liposomes accumulate in the tumor when targeted [75, 117]. In others, it appears that the targeting ligand does not increase the liposomal delivery to the tumor but it facilitates cellular uptake of the drug as well as increasing distribution of the liposome throughout the tumor [121, 122]. This stresses the need for appropriate controls in these experiments. Additionally, total drug delivery as well as tumor localization should be determined for targeted therapies.
USE OF PEPTIDE LIGANDS FOR DIAGNOSTIC APPLICATIONS
The ability of these peptides to distinguish between tumor and normal tissues make them ideal as diagnostic agents. The peptides can be used for in vitro characterization of cancer cells or tumor samples. Peptides can be used as antibody replacements for immunohistochemistry on fixed tumor samples. This has the advantage that peptide binding can be directly tested on a patient’s tumor in order to determine which targeting peptide should be employed for in vivo applications. However, as the molecular target for most of these peptides is unknown, they are unlikely to replace antibodies for pathological classifications. Peptides can also serve as capture reagents. Peptides selected for binding to a B-cell lymphoma cell line can enrich for the cancerous cells out of a background of normal B-cells [67]. Although, peptides as affinity reagents have not been widely used to date, these tumor specific peptides may be able to enrich tumor cell from biological fluids.
Additionally, targeting peptides can be used for in vivo diagnostic applications. Peptides have been attached to a variety of dyes or fluorescent nanoparticles, such as quantum dots, for in vivo optical fluorescence imaging [77, 118, 123]. While progress is being made in the development of near infrared red reagents [124], whole body fluorescence imaging in humans is currently not feasible due to high background fluorescence, poor light penetration, and inherent light scattering [125]. However, it is valuable research tool in animal models. In a novel diagnostic application, a 6-mer peptide selected for binding to adenomas of the colon was fluorescently labeled and administered topically to patients undergoing colonoscopy [55]. Using a fluorescent microendoscope, dysplastic regions of the colon could be distinguished from normal tissue with sensitivity and specificity of greater than 80%. This success highlights the potential use of peptides in clinics for early diagnosis of cancer. This also points to the possibility of using labeled peptides during surgery to distinguish tumor borders in real time.
Positron Emission Tomography (PET) imaging has high sensitivity; as such it is an ideal platform for molecular imaging. The low spatial resolution of PET has been compensated for by the generation of PET/CT scanners that combine the sensitivity of PET with the anatomical resolution of CT. Guided PET agents will increase the sensitivity of detection while providing molecular information about the tumor without biopsy. However, as tumor targeting peptides exhibit defined binding profiles, it is reasonable to be skeptical about their use as early detection agents. Instead they are more likely to find utility in providing molecular information about a tumor before or during treatment as well as detecting micrometastasis. Information obtained from PET imaging would aid in determining which ligand(s) should be employed for tumor targeting for an individual patient. Of importance, PET imaging can provide whole body biodistribution which is crucial in optimizing targeted therapies [126].
Compared to antibodies, peptides are more amenable to harsh conditions for chemical modifications and labeling [127, 128]. Unlike antibodies, the in vivo half-life of the peptides is well matched to the half-life of most commonly used PET radionucleotides. Despite these advantages, few peptides isolated from phage-displayed libraries have been used for PET imaging. Instead, most peptide-based PET imaging has been performed using naturally occurring peptidyl ligands such as bombesin and somatostatin [129, 130]. Several αvβ3-binding peptides, including the RGD-4C peptide [97, 98], have been used in several applications to image angiogenic tumor vasculature [131, 132]. This peptide is in early clinical trials as a molecular PET imaging agent for angiogenesis [133]. In this early study, all tumors detected by CT were visible by PET imaging using the 18F labeled peptide. Importantly, the peptide is stable in vivo.
It should be noted that the development of molecular agents for PET imaging requires a fine balance between clearance rates, tumor retention, and nonspecific uptake in other tissues. Small changes in the peptides, linkers, chelator, and isotope can dramatically affect the biodistribution. The empirical process involved in optimizing PET agents is time consuming and costly. Additionally, the high signal resulting from kidney clearance can pose a problem for some applications. Furthermore, there are reports that accumulation can occur in the kidneys and the mechanism of this process is variable between peptides [134]. As such, studies should be performed to determine kidney clearance and kidney accumulation. None-the-less, tumor-targeting peptides isolated by phage display biopanning are likely to emerge as molecular imaging agents.
Magnetic resonance imaging yields high resolution images but current contrast agents suffer from a lack of sensitivity. It is challenging to target enough T1 contrast agent, such as gadoteridol, to a tumor to achieve a reasonable signal. For this reason, many have turned to iron oxide particles which are a highly sensitive T2 agent that darkens the signal in regions in which the nanoparticles accumulate [135]. A plectin-1 binding peptide that homes to pancreatic ductal adenocarcinomas has been coupled to fluorescent cross-linked iron oxide nanoparticles [77]. This peptide-nanoparticle homes to pancreatic ductal adenocarcinoma as determined by intravital microscopy and ex vivo MRI. Using the versatile fluorescence microscopy and ex vivo MRI. Using the versatile Huisgen cycoladdition, better known as click chemistry, the LyP-1 peptide has been attached to fluorescently labeled, dextran encapsulated iron oxide particles. Stressing the issue of “targeting” of nanoparticles, the authors found the total amount of non-targeted and targeted nanoparticle in the tumor to be the same yet the targeted-particle penetrated within the tumor while the naked nanoparticle remained localized around the tumor blood vessels. In both of these examples, the peptides home to the appropriate tumors in animals as assessed by ex vivo fluorescent imaging but in vivo MR imaging was not performed [136].
To further facilitate attachment of peptides to iron oxide nanoparticles, a one-step procedure for the surface functionalization of super paramagnetic iron oxide (SPIO) with a targeting peptide has recently been developed [137]. The hydrophobic surfactants on the SPIO nanoparticles can be displaced through ligand exchange with a peptide containing a C-terminal poly(ethylene glycol)-tethered cysteine residue. The resulting SPIO particles are biocompatible and demonstrate high T2 relaxivity. Attachment of the αvβ6–binding peptide, H2009.1, results in specific targeting of αvβ6–expressing lung cancer cells as demonstrated by in vitro MR imaging and Prussian blue staining. This surface chemistry may expand the use of SPIO for MR imaging.
USE OF PEPTIDIC LIGANDS FOR DELIVERY OF OLIGONUCLEOTIDES
Peptides can deliver oligonucleotides for gene therapy. Direct conjugation of an oligonucleotide is possible [59] but recent efforts have focused on using targeted carriers that are loaded with the oligonucleotide or gene of interest [138, 139]. For example, the RGD-4C peptide has been incorporated into lipid-protamine-DNA lipopolyplexes for transformation of αvβ3–positive cells [139]. Using a different scaffold, a quantum dot nanoparticle has been functionalized with a tumor targeting peptide (F3) and a siRNA duplex that serves to reduce expression of a reporter protein, EGFP [140]. The siRNA is attached via a disulfide so that it is released intracellularly allowing it to reach its mRNA target. Indeed, the peptide mediates cellular uptake of the functionalize nanoparticle resulting in a decrease in green fluorescence from the EGFP and an increase in red fluorescence from the Qdot.
Lessons from viral gene transfer can be used as guides in the development of effective peptide-targeted gene delivery. Phage clones that internalize into mammalian cells can be utilized for DNA transfer; however, the transfection efficiency is low [141-147]. Selected tumor targeted peptides have been grafted onto eukaryotic viral vectors which have high transfection efficiencies. However, this requires the removal of the native viral tropism in order to redirect the gene transfer to the targeted cell type [148-150]. Recently, Arap and Pasqualini took a novel approach to the problem; Instead of grafting the peptides onto the viral vector, features of the viral vectors were incorporated into a fd-tet bacteriophage [99, 151, 152]. In this method, a mammalian transgene cassette from adeno-associated virus (AAV) is inserted into a non-coding region of the bacteriophage genome. This cassette is flanked on either end by inverted terminal repeats allowing for improved expression of the transgene. When incorporated into the phage clone displaying the RGD-4C peptide, this chimeric virus mediates efficient transfection in cells expressing αv integrins. Importantly, the chimera enables functional gene transfer specifically to tumor vasculature and adjacent tumor cells. Remarkably, no significant gene expression is observed in the liver. Systemic delivery of the chimeric phage encoding the tHSVtk gene results in expression of herpes simplex virus type-1 thymidine kinase selectively in the targeted tumor. Gene expression can be imaged with PET by administration of 2’-[18F]-fluoro-2’-deoxy-1-β-D-arabino-fura-nosyl-5-thyl-uracil ([18F]FEAU) [153]. This novel approach opens up new avenues of gene therapy and molecular imaging.
PEPTIDES WITH CELLULAR EFFECTS
In the field of targeted delivery, the assumption has been that the targeting agents, be they antibodies or peptides are benign delivery vehicles having no effect on the targeted cell. Once their cargo is delivered, little thought is paid to the homing agent. Yet, cell surface receptors are known to modulate a variety of cellular behaviors including proliferation, adhesion, migration, and invasion. As such, it is possible that binding of the peptide may modulate these cellular activities. Such biological activities have been observed for several of the peptides isolated [43, 48, 50, 51]. For example, the peptide SMSIASPYIALE which was selected for binding to the GC9811-P gastric cancer cell line is able to block cell adhesion on a variety of extracellular matrix proteins and reduced cell invasiveness into matrigel [51]. Importantly, the peptide reduces the number of disseminated peritoneal nodules in an animal model. Similar affects on adhesion, migration, and metastasis were observed for the peptide GRRTRSRRLRRS that binds gastric cancer cells that form metastatic liver lesions in vivo [50]. The tumor lymphatic targeting peptide LyP-1 induces apoptosis specifically in cells which have affinity for this peptide [84]. Systemic administration in animals bearing MDA-MB-435 tumors results in a decrease in tumor lymphatic and a reduction in tumor growth rates. In sum, many of these peptides have cellular effects that may act in concert with targeted delivery of therapeutics. To date, no peptides identified by biopanning have stimulated tumor cell growth or promoted aggressive phenotypes.
INCREASING THE AFFINITY OF THE ISOLATED PEPTIDES
Retaining the affinity and activity of peptides selected from phage displayed libraries outside of the context of the phage scaffold has been an obstacle. While many reports utilized the monomeric peptide, the affinities of these peptides are typically in the micromolar range which is unsuitable for most clinical applications [45, 51, 52, 69]. In most cases, the peptides are displayed in multiple copies on the phage, thus the peptides may bind to their cellular target via a multivalent interaction. The increased affinity due to the multivalent binding is lost when the peptides are used in their monomeric forms. Additionally, many endocytotic processes are triggered by receptor multimerization on the cell surface. If internalization of the peptide is desired, the free peptide must facilitate this interaction on the cell surface.
Through empirical testing, it has found that multimerization of the cell-targeting peptides on a trilysine core is a useful scaffold for retaining the peptide activity outside of the context of the phage (Scheme I) [70, 154]. The trilysine scaffold mimics the presentation of the peptides on the pIII protein of the phage in both valency and the orientation of the displayed peptides. We have found the tetrameric trilysine framework to be a general platform for cell-targeting peptides selected from bacteriophage displayed peptide libraries [67, 71, 155, 156]. Tetramerization of the peptides increases the affinity of the peptide for its target cell 25-100 fold when compared to the monomeric peptide, indicating the importance of multivalent binding.
Scheme I.
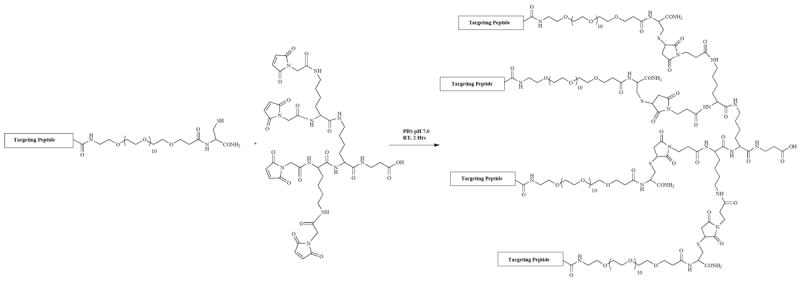
Convergent synthesis of tetrameric cell targeting peptides. Adapted from Li S, McGuire MJ, Lin M, Liu Y-H, Oyama T, Sun X, Brown KC. Mol Cancer Ther 2009; 8: 1239-1249.
To take full advantage of these multivalent peptides, a synthetic route that takes advantage of the chemoselective reaction of a cysteine with a maleimide to synthesize the tetrameric peptide has been developed (Scheme I). This chemistry is facile and is not restricted to labs with expertise in synthetic peptide chemistry. The chemistry allows for a variety of chemical moieties to be placed in the peptide in a regiospecific fashion, expanding the utility of the peptide. This route will be of utility to the many labs performing phage display selections.
BEAD-BASED PEPTIDE LIBRARIES AS SOURCES OF TUMOR TARGETING LIGANDS
While I have focused this discussion on the use of phage-displayed peptide libraries as a source of cancer specific binding ligands, it should be noted that a variety of different formats of peptide libraries exist. These methods have been reviewed previously [157] and are not the focus of this review as few of them have been utilized for unbiased biopanning. However, I would like to highlight the use of bead-based peptide libraries for the selection of cell-binding peptides. Like phage display, bead-based libraries are well-suited for selections using intact cells [36]. While bead-based libraries do not have the diversity represented in phage displayed libraries, they do have the advantage that non-natural amino acids can be incorporated into the peptides. Lam and co-workers have pioneered this approach [158, 159]. Using this method a peptide that promotes adherence and growth of the lung adenocarcinoma cell line, A549 [158] was selected from a one-bead-one compound library. This cyclic 6-mer peptide binds to two other NSCLC cell lines, Calu-1 and H178 but not to a normal human bronchoepithelial cell line. The receptor for this peptide has been identified as the integrin α3β1. Similar methodology was used to isolate peptides that bind preferentially to malignant lymphocytes [160, 161], breast cancer cells [162], glioblastoma cells [163], and ovarian adenocarcinomas [164]. Several of these peptides have been utilized for near-infrared imaging of tumors in vivo.
Moving from peptides, the Kodadek laboratory has generated peptoid libraries that can be screened for cell binding [165]. Peptoids are N-substituted oligogylcine polymers that have many features of natural occurring peptides but are protease resistant. Using a two color cell-based screening method, peptoids were isolated that bind specifically to cells expressing vascular endothelial growth factor receptor 2 (VEGFR2) but not an isogenic cell line lacking the receptor. While this screen was biased towards selecting a ligand for a particular receptor, it is clear that a similar screen can be performed using a cancerous cell type and a corresponding nonmalignant cell line.
PEPTIDES AS THERAPEUTICS
To understand the potential utility of peptides selected from phage-displayed libraries, it is important to note the current state of peptide-based drugs. Peptide drugs are under-represented within the arsenal of FDA approved drugs. In the past, pharmaceutical companies steered away from this class of molecules due to their large size (greater than the desired 500 Da limit) and in vivo biological properties. That said, there are almost 50 peptide therapeutics approved for clinical use worldwide, and the peptide therapeutic market is expanding [166-168]. The market for peptide-based therapeutics is growing at an annually rate of 7.5% and is anticipated to be worth 13 billion by next year [169].
The major drawback sited with peptide is short biological half-lives due to proteolysis and rapid renal filtration. Proteolysis can be overcome by blocking of the amino and carboxy termini, cyclization, incorporation of non-natural amino acids, and modifications of specific cleavage sites. A variety of approaches have been taken to overcome the rapid glomerular filtration including PEGylation, glycosylation, protein conjugation, and hydrophobic depoting. Methods to improve peptide circulation times in vivo have been reviewed elsewhere [167, 170, 171]. Delivery is another issue with peptide drugs that needs addressing. These molecules are not orally bioavailable and most are administered by injection. However, there have been substantial advances in the development of peptide delivery systems, including the use of controlled release polymeric formulations and osmotic pumps [167, 172]. For example, ELIGARD® and LUPRON DEPOT® are both formulations of Leuprolide in a biodegradable matrix. These formulations reduce injections from daily to monthly. Another concern has been the cost of large-scale peptide synthesis [14]. Improvements in synthetic methodology along with the clinical success of more expensive monoclonal antibodies have lessened this concern. To this end, FUZEON® (Enfuvitride), an antiviral used for treatment of HIV, is a 36 amino acid peptide that is made in multiton quantities. However, it should be noted that the cost of FUZEON® is substantially higher than other HIV antivirals and it is administered by twice daily injections. As such, FUZEON® has not found widespread use and its application is not feasible in non-industrialized nations.
Of the FDA approved peptide therapeutics, few are indicated for cancer treatment. Leuprolide, a gonadotropin hormone-releasing hormone mimic is used for the treatment of prostate cancer and the proteosome inhibitor Bortezomib is in the clinic for multiple myeloma. However, several therapeutic peptides for cancer indications are in the pipeline. For example, the cyclic RGD peptide Cilengitide (EMD121974), currently in phase III clinical trials, has shown promise for the treatment of glioblastoma [173]. Exherin (ADH-1), a cyclic pentapeptide (Ac-CHAVC) antagonist of N-cadherin, was well tolerated in phase I clinical trials [174]. Indications of anti-tumor efficacy were observed in this small patient set. It should be noted that peptide therapeutics are well suited for cancer indications; peptides can have high affinity for cell surface receptors that function in critical cellular signaling pathways and the life threatening nature of the disease makes delivery by injection more tolerable. Additionally, the cost of peptide production is anticipated to be less than production of monoclonal antibodies which are already in the clinics [168].
Peptides isolated from phage-displayed peptide libraries are now beginning to emerge from the therapeutic pipeline. Romiplostim (AMG 531, Nplate®) was approved by the FDA in August 2008 for the treatment of thrombocytopenia in patients with immune thrombocytopaenia purpura [175-177]. Amgen developed this drug from a peptide selected for binding to human thrombopoietin receptor (Mp1) [178]. Romiplostim is a fusion of 4 copies of this peptide to an Fc fragment which extends the peptide’s in vivo half-life. A similar peptibody scaffold is used in AMG-386, Amgen’s anti-angiogenic therapeutic. In this case, the peptide prevents binding of angiopoietin-1 and angiopoietin-2 to Tie2 [179]. AMG-386 is in phase II clinical trials for treatment of a variety of cancer types. Results for the phase I trial have recently been published and the drug is well tolerated [180]. Early signs of efficacy were observed. Affymax is developing Hematide™, an erythropoietin mimetic peptide selected from a phage-displayed library [181, 182]. The peptide is displayed as a dimer to increase its affinity and PEGylated to increase its biological half-life. Monthly injections are required. Phase III clinical trials for the treatment of anemia associated with chronic renal failure are in progress [183, 184]. In sum, these successes indicate that peptides isolated from phage-displayed peptide libraries can translate into useful therapeutics. It is important to note that the Romiplostim took over 10 years from selection to FDA approval. More recently isolated peptides are most likely in early stages of development.
The above peptide-based drugs were all selected by biopanning on purified protein targets. However, use of the NGR peptide as a targeting agent has recently begun phase I studies [185, 186]. This peptide, isolated by in vivo biopanning using a breast tumor model, binds to CD13 and αvβ3. The peptide was fused to human tumor necrosis factor (hTNF) [185] or truncated tissue factor (tTF) [186] in order to deprive the tumor of its blood supply. In both cases, the drug was well tolerated. Phase II and III clinical trials remain to be performed to determine efficacy.
FUTURE CHALLENGES
Despite the success of phage display for identifying peptidic tumor-targeting ligands, there are still challenges in the field. A major challenge in the field is identification of the cellular target of the selected peptides. This is driven home when one considers that of the peptides listed in Tables I and II, the receptors for these ligands have been identified for only 15%. Although these ligands can be used for drug delivery without knowledge of the cellular receptor, there are several reasons that receptor identification should remain a priority. First, receptor identification can provide information about changes in the cell surface profile during carcinogenesis, tumor maintenance, and metastasis. This opens avenues of new basic research on the role of the receptor in the disease. Second, once identified, new ligands can be generated for the receptor. While peptides might be appropriate in some applications, antibodies, peptoids, or small molecules may be better choice for others. Third, moving the ligand towards clinical use will be facilitated by an understanding of its binding partner.
Most efforts towards receptor identification have focused on biochemical affinity purification or protein cross-linking followed by mass spectrometric identification of the isolated protein species [45, 77]. The reasons for the low success rate are partly due inherent nature of membrane proteins. They are present in low abundance and solubility is an issue. This makes affinity purification and mass spectrometric identification difficult. Due to the difficulties with affinity purification of membrane protein, genetic methods are emerging as means to identify cellular targets. Subtractive hybridization cloning can be used as well to generate a set of potential receptors [69].
It is important to remember that the cell surface has topography in which proteins can multimerize with binding partners or cluster within microdomains. This surface landscape can contribute to the specificity of the peptidic ligands. In other words, the specificity may not arise from absolute protein expression levels but an arrangement of the receptors on the cell. This level of information can be lost upon the preparation of membrane protein for affinity purification and is not born out in mRNA levels. It is also important to note that while the assumption in the field has been that the peptides bind protein receptors, they may be binding sugar moieties of glycoproteins or glycolipids, or phospholipids.
Protein databases can be searched for sequence similarity to the peptide. This has yielded candidate receptors for a few isolated peptides [37, 40, 104]. For example, homology of a lung adenocarcinoma binding peptide to the GH viral coat protein of foot and mouth disease virus led to the identification of αvβ6 as the cellular receptor for this peptide [37]. However, most phage-displayed peptide libraries are chemically synthesized and do not originate from biological sources. Furthermore, the complete sequence coverage of the longer peptides is limited. As such, the probability of the peptide sequence matching a biologically derived sequence is statically low. Furthermore, many matches do not provide biological insight into the potential receptors. In sum, new techniques are needed to identify the receptor partners for the selected ligands. A combination of cell biology, proteomic and genomic approaches will be needed to tackle this difficult problem.
CONCLUSION
Over the past 10 years, phage displayed peptide-libraries have proven to be a rich source of cancer targeting ligands. The peptides can have antibody-like affinities and cell specificity. The chemistry is in place for regiospecific modification of peptides; as such they can easily be manipulated for different purposes. The goal is to now to optimize these peptides and utilize them for clinical applications. This is of high priority for cancer patients, clinical practitioners, and scientists alike. More and more, the barriers towards using peptides as drugs or drug delivery agents are dissolving. This is driven home by the increasing number of peptide pharmaceutical on the market and the number of companies focusing on peptide formulations. The recent release of Romiplostim on the market is encouraging that peptides isolated from library selections can translate to effective therapeutics. It is likely that more pre-clinical and early phase clinical trials using some of these peptides as drug delivery agents or molecular imaging probes will increase during the next few years.
Acknowledgments
KCB is supported by the NIH (1RO1CA106646) and the Welch Foundation (I1622). This manuscript is written in memory of H. Lyndon Brown and the other cancer patients for whom advances in treatment have come too late. This paper represents publication #CSCN036 from the Cell Stress and Cancer Nanomedicine Program of the Simmons Comprehensive Cancer Center.
References
References 187-189 are related articles recently published.
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Lewis Phillips G, Li G, Dugger D, Crocker L, Parsons K, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimito RA, Brannigan BA, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Sun S, Schiller J, Spinola M, Minna JD. New molecularly targeted therapies for lung cancer. J Clin Invest. 2007;117:2740–50. doi: 10.1172/JCI31809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyerson M, Carbone DP. Genomic and proteomic profiling of lung cancers: Lung cancer classification in the age of targeted therapy. J Clin Oncol. 2005;23:3219–26. doi: 10.1200/JCO.2005.15.511. [DOI] [PubMed] [Google Scholar]
- 8.Celis JE, Gromov P. Proteomics in translational cancer research: towards an integrated approach. Cancer Cell. 2003;3:9–15. doi: 10.1016/s1535-6108(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen UB, Marks JD. Internalizing antibodies and targeted cancer therapy: direct selection from phage libraries. Pharm Sci Technol Today. 2000;3:282–91. doi: 10.1016/s1461-5347(00)00280-7. [DOI] [PubMed] [Google Scholar]
- 10.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005;23:1147–57. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak HF, Nagy JA, Dvorak AM. Structure of solid tumors and their vasculature: implications for therapy with monoclonal antibodies. Cancer Cell. 1991;3:77–85. [PubMed] [Google Scholar]
- 12.Hoekman K, van der Vijgh WJ, Vermorken JB. Clinical and preclinical modulation of chemotherapy-induced toxicity in patients with cancer. Drugs. 1999;57:133–55. doi: 10.2165/00003495-199957020-00002. [DOI] [PubMed] [Google Scholar]
- 13.Neumeister P, Eibl M, Zinke-Cerwenka W, Scarpatetti M, Sill H, Linkesch W. Hepatic veno-occlusive disease in two patients with relapsed acute myeloid leukemia treated with anti-CD33 calicheamicin (CMA-676) immunoconjugates. Ann Hematol. 2001;80:119–20. doi: 10.1007/s002770000239. [DOI] [PubMed] [Google Scholar]
- 14.Bray BL. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat Rev Drug Discov. 2003;2:587–93. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 15.Schally AV, Nagy JA. New approaches to treatment of various cancers based on cytotoxic analogs of LHRH, somatosin, and bombesin. Life Sci. 2003;72:2305–20. doi: 10.1016/s0024-3205(03)00113-9. [DOI] [PubMed] [Google Scholar]
- 16.Smith G. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 17.Kehoe J, Kay B. Filamentous phage display in the new millennium. Chem Rev. 2005;105:4056–72. doi: 10.1021/cr000261r. [DOI] [PubMed] [Google Scholar]
- 18.Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- 19.Landon LA, Zou J, Deutscher SL. Is phage display technology on target for developing peptide-based cancer drugs? Curr Drug Discov Technol. 2004;1:113–32. doi: 10.2174/1570163043335108. [DOI] [PubMed] [Google Scholar]
- 20.Urbaelli L, Ronchini C, Fontana L, Menard S, Orlandi R, Monaci P. Targeted gene transduction of mammalian cells expressing the HER2/neu receptor by filamentous phage. J Mol Biol. 2001;313:965–76. doi: 10.1006/jmbi.2001.5111. [DOI] [PubMed] [Google Scholar]
- 21.Karasseva NG, Glinsky VV, Chen NX, Komatireddy R, Quinn TP. Identification and characterization of peptides that bind human ErbB-2 selected from a bacteriophage library. J Protein Chem. 2002;21:287–96. doi: 10.1023/a:1019749504418. [DOI] [PubMed] [Google Scholar]
- 22.Pero S, Shukla G, Armstrong A, Peterson D, Fuller S, Godin K, et al. Identification of a small peptide that inhibits the phosphorylation of Erb2 and proliferation of Erb2 overexpressing breast cancer cells. Int J Cancer. 2004;111:951–60. doi: 10.1002/ijc.20306. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Zhao R, Wu X, Sun Y, Yao M, Li J, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptors for targeted delivery of therapeutics. FASEB J. 2005;19:1978–85. doi: 10.1096/fj.05-4058com. [DOI] [PubMed] [Google Scholar]
- 24.Kelly K, Setlur S, Ross R, Anbazhagan R, Waterman P, Rubin W, et al. Detection of early prostate cancer using a hepsin-targeted imaging agent. Cancer Res. 2008;68:2286–91. doi: 10.1158/0008-5472.CAN-07-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Li Z, Wang H, Qu C, Chen X, Li J, et al. Identification and characterization of a novel peptide ligand of Tie2 for targeting gene therapy. Acta Biochim Biophys Sin. 2008;40:217–25. doi: 10.1111/j.1745-7270.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- 26.Su J-L, Lai K-P, Chen C-A, Yang C-Y, Chen P-S, Chang C-C, et al. A novel peptide specifically binding to interleukin-6 receptor (gp80) inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:4827–35. doi: 10.1158/0008-5472.CAN-05-0188. [DOI] [PubMed] [Google Scholar]
- 27.Blond-Elguindi S, Cwirla S, Dower W, Lipshutz R, Sprang S, Sambrook J, et al. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell. 1993;75:717–28. doi: 10.1016/0092-8674(93)90492-9. [DOI] [PubMed] [Google Scholar]
- 28.Ding H, Prodinger W, Kopecek J. Identification of CD21-binding peptides with phage display and investigation of binding properties of HPMA copolymer-peptide conjugates. Bioconjug Chem. 2006;17:514–23. doi: 10.1021/bc0503162. [DOI] [PubMed] [Google Scholar]
- 29.Howell R, Revskaya E, Pazo V, Nosanchuk J, Casadevall A, Dadachova E. Phage display library derived peptides that bind to human tumor melanin as potential vehicles for targeted radionuclide therapy of metastatic melanoma. Bioconjug Chem. 2007;18:1739–48. doi: 10.1021/bc060330u. [DOI] [PubMed] [Google Scholar]
- 30.Koolpe M, Dail M, Pasquale E. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem 227. 277:46974–9. doi: 10.1074/jbc.M208495200. [DOI] [PubMed] [Google Scholar]
- 31.Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, et al. Tumor targeting with a selective gelantinase inhibitor. Nat Biotechnol. 1999;17:768–74. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 32.Devemy E, Blaschuk O. Identification of a novel dual E- and N-cadherin antagonist. Peptides. 2009;30:1539–47. doi: 10.1016/j.peptides.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–6. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 34.Barry MA, Dower WJ, Johnston SA. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299–305. doi: 10.1038/nm0396-299. [DOI] [PubMed] [Google Scholar]
- 35.Krumpe L, Mori T. Potential of phage-displayed peptide library technology to identify functional targeting peptides. Expert Opin Drug Discov. 2007;2:525–37. doi: 10.1517/17460441.2.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aina OH, Liu R, Sutcliffe JL, Marik J, Pan C-X, Lam KS. From combinatorial chemistry to cancer targeting peptides. Mol Pharmacol. 2007;4:631–51. doi: 10.1021/mp700073y. [DOI] [PubMed] [Google Scholar]
- 37.Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, et al. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for non-small cell lung cancer. Cancer Res. 2007;67:5889–95. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 38.Kubo N, Akita N, Shimizu A, Kitahara H, Parker A, Miyagawa S. Identification of oligopeptide binding to colon cancer cells separated from patients using laser capture microdissection. J Drug Target. 2008;16:396–404. doi: 10.1080/10611860802088796. [DOI] [PubMed] [Google Scholar]
- 39.Pilch J, Brown D, Komatsu M, Jarvinen T, Yang M, Peters D, et al. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc Natl Acad Sci USA. 2006;103:2800–4. doi: 10.1073/pnas.0511219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, et al. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006;66:34–40. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 41.Shukla G, Krag D. Selection of tumor-targeting agents on freshly excise human breast tumors using a phage display library. Oncol Rep. 2005;13:757–64. [PubMed] [Google Scholar]
- 42.Du B, Qian M, Zhou Z, Wang P, Wang L, Zhang X, et al. In vitro panning of a targeting peptide to hepatocarcinoma from a phage display peptide library. Biochem Biophys Res Commun. 2006;342:956–62. doi: 10.1016/j.bbrc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Y-Q, Wang H-R, Li H-P, Hao H-J, Zheng Y-L, Gu J. Targeting of hapeatoma cell and suppression of tumor growth by a novel 12mer peptide fused to superantigen TSST-1. Mol Med. 2006;12:81–7. doi: 10.2119/2006-00011.Jiang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo A, Lin C-T, Wu H-C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther. 2008:579–89. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y, Lillo AM, Steiniger SCJ, Liu Y, Ballatore C, Anichini A, et al. Targeting heat shock proteins on cancer cells: Selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–44. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 46.Eriksson F, Culp W, Massey R, Egevad L, Garland D, Persson M, et al. Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol Immunother. 2007;56:677–87. doi: 10.1007/s00262-006-0227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang C, Liu X, Rehemtulla A, Lawrence T. Identification of peptides that bind to irradiated pancreatic tumor cells. Int J Radiat Oncol Biol Phys. 2005;62:1497–503. doi: 10.1016/j.ijrobp.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 48.Romanov VI, Durand DB, Petrenko VA. Phage-display selection of peptides that affect prostate carcinoma cells attachment and invasion. Prostate. 2001;47:239–51. doi: 10.1002/pros.1068. [DOI] [PubMed] [Google Scholar]
- 49.Zitzmann S, Mier W, Schad A, Kinscherf R, Askoxylakis V, Kramer S, et al. A new prostate carcinoma binding peptide (DUP-1) for tumor imaging and therapy. Clin Cancer Res. 2005;11:139–46. [PubMed] [Google Scholar]
- 50.Hu S, Guo X, Xie H, Du Y, Pan Y, Shi Y, et al. Phage display selection of peptides that inhibit metastasis ability of gastric cancer cells with high liver metastatic potential. Biochem Biophys Res Comp. 2006;341:964–72. doi: 10.1016/j.bbrc.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 51.Bai F, Liang J, Wang J, Shi Y, Zhang K, Liang S, et al. Inhibitory effects of a specific phage-displayed peptide on high peritoneal metastasis of gastric cancer. J Mol Med. 2007;85(2):169–80. doi: 10.1007/s00109-006-0115-8. [DOI] [PubMed] [Google Scholar]
- 52.Kelly K, Jones D. Isolation of a colon tumor specific binding peptide using phage display selection. Neoplasia. 2003;5:437–44. doi: 10.1016/s1476-5586(03)80046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen UB, Schreiber V, Schultz H, Mischler F, Schughart K. Tumor cell-targeting by phage-displayed peptides. Cancer Gene Ther. 2002;9:606–12. doi: 10.1038/sj.cgt.7700476. [DOI] [PubMed] [Google Scholar]
- 54.Rittner K, Schreiber V, Erbs P, Lusky M. Targeting of adenovirus vectors carrying a tumor cell-specific peptide: in vitro and in vivo studies. Cancer Gene Ther. 2007;14:509–18. doi: 10.1038/sj.cgt.7701036. [DOI] [PubMed] [Google Scholar]
- 55.Hsiung P-L, Hardy J, Friedland S, Soetikno R, Du C, Wu A, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454–8. doi: 10.1038/nm1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Chen J, Zhang Y, Hu Z, Hu D, Pan Y, et al. Panning and identification of a colon tumor binding peptide from a phage display peptide library. J Biomol Screen. 2007;12:429–35. doi: 10.1177/1087057106299164. [DOI] [PubMed] [Google Scholar]
- 57.Hong FD, Clayman GL. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 2000;60:6551–6. [PubMed] [Google Scholar]
- 58.Lee T-Y, Wu H-C, Tseng Y-L, Lin C-T. A novel peptide specifically binding to nasopharyngeal carcinoma for targeted drug delivery. Cancer Res. 2004;64:8002–8. doi: 10.1158/0008-5472.CAN-04-1948. [DOI] [PubMed] [Google Scholar]
- 59.Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003;17:256–8. doi: 10.1096/fj.02-0280fje. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Spring H, Schwab M. Neuroblastoma tumor cell-binding peptides identified through random peptide phage display. Cancer Lett. 2001;171:153–64. doi: 10.1016/s0304-3835(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 61.Askoxylakis V, Zitzmann S, Mier W, Graham K, Kramer S, von Wegner F, et al. Preclinical evaluation of the breast cancer cell-binding peptide, p160. Clin Cancer Res. 2005;11:6705–12. doi: 10.1158/1078-0432.CCR-05-0432. [DOI] [PubMed] [Google Scholar]
- 62.Samoylova T, Petrenko V, Morrison N, Globa L, Baker H, Cox N. Phage probes for malignant glial cells. Mol Cancer Ther. 2003;2:1129–37. [PubMed] [Google Scholar]
- 63.Spear M, Breakefield X, Beltzer J, Schuback D, Weissleder R, Pardo F, et al. Isolation, characterization, and recovery of small peptide phage display epitope selected against viable malignant glioma cells. Cancer Gene Ther. 2001;8:506–11. doi: 10.1038/sj.cgt.7700334. [DOI] [PubMed] [Google Scholar]
- 64.Robinson P, Stuber D, Deryckere F, Tedbury P, Lagrange M, Orfanoudakis G. Identification using phage display of peptides promoting targeting and internalization into HPV-transformed cell lines. J Mol Recogn. 2005;18:175–82. doi: 10.1002/jmr.723. [DOI] [PubMed] [Google Scholar]
- 65.Bockman M, Drosten M, Putzer BM. Discovery of targeting peptides for selective therapy of medullary thyroid carcinoma. J Gene Med. 2005;7:179–88. doi: 10.1002/jgm.648. [DOI] [PubMed] [Google Scholar]
- 66.Witt H, Hajdin K, Iljin K, Greiner O, Niggli F, Schafer B, et al. Identification of a rhabdomyosarcoma targeting peptide by phage display with dequence similarities to the tumor lymphatic-homing peptide LyP-1. Int J Cancer. 2009;124:2026–32. doi: 10.1002/ijc.24170. [DOI] [PubMed] [Google Scholar]
- 67.McGuire MJ, Samli KN, Chang Y, Brown KC. Novel ligands for cancer diagnosis: selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp Hematol. 2006;34:443–52. doi: 10.1016/j.exphem.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 68.Nishimura S, Takahashi S, Kamikatahira H, Kuroli Y, Jaalouk D, O’Brien S, et al. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem. 2008;283:11752–62. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jager S, Jahnke A, Wilmes T, Adebahr S, Vogtle F-N, deLima-Hahn E, et al. Leukemia targeting ligands isolated from phage displayed peptide libraries. Leukemia. 2007;21:411–20. doi: 10.1038/sj.leu.2404548. [DOI] [PubMed] [Google Scholar]
- 70.Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett. 2003;202:219–30. doi: 10.1016/j.canlet.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Oyama T, Rombel IT, Samli KN, Zhou X, Brown KC. Isolation of multiple cell-binding ligands from different phage displayed-peptide libraries. Biosens Bioelectron. 2006;21:1867–75. doi: 10.1016/j.bios.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 72.Zang L, Shi L, Guo J, Pan Q, Wu W, Pan X, et al. Screening and identification of a peptide specifically targeted to NCI-H1299 from a phage display peptide library. Cancer Lett. 2009;281:64–70. doi: 10.1016/j.canlet.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 73.Zhou X, Chang Y, Oyama T, McGuire MJ, Brown KC. Cell-specific delivery of a chemotherapeutic to lung cancer cells. J Am Chem Soc. 2004;129:15656–7. doi: 10.1021/ja0446496. [DOI] [PubMed] [Google Scholar]
- 74.Guan H, Li S, McGuire MJ, Brown KC. Peptide-targeted polyglutamic acid doxorubicin conjugates for the treatment of αvβ6-positive cancers. Bioconjug Chem. 2008;19:1813–21. doi: 10.1021/bc800154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang D-K, Lin C-T, Wu C-H, Wu H-C. A novel peptide enhances therapeutic efficacy of liposomal anti-cancer drugs in mice models of human lung cancer. PLOS Med. 2009;4:1–11. doi: 10.1371/journal.pone.0004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee S-M, Lee E-J, Hong H-Y, Kwon M-K, Kwon T-H, Choi J-Y, et al. Targeting bladder tumor cells in vivo and in the urine with a peptide identified by phage display. Mol Cancer Ther. 2007;5:11–9. doi: 10.1158/1541-7786.MCR-06-0069. [DOI] [PubMed] [Google Scholar]
- 77.Kelly K, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H, et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLOS Med. 2008;5:657–68. doi: 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rajotte D, Arap W, Hagerdorn M, Koivunen E, Pasqualini R, Ruoslahti E. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest. 1998;102:430–7. doi: 10.1172/JCI3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Essler M, Ruoslahti E. Molecular specialization of breast vasculature: a breast homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci USA. 2002;99:2252–7. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kolinin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–32. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 81.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–80. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 82.Hoffman J, Giraudo E, Sing M, Zhang L, Inoue M, Porkka K, et al. Progressive vascular changes in a transgenic mouse model of squamous cell carcinoma. Cancer Cell. 2003;4:383–91. doi: 10.1016/s1535-6108(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 83.Han Z, Fu A, Wang H-R, Diaz R, Geng L, Onishko H, et al. Noninvasive assessment of cancer response to therapy. Nat Med. 2008;14:343–9. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 84.Laakkonen P, Akerman M, Biliran H, Yang M, Ferrer F, Karpanen T, et al. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc Natl Acad Sci USA. 2004;101:9381–6. doi: 10.1073/pnas.0403317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med. 2002;8:751–5. doi: 10.1038/nm720. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Giraudo E, Hoffman J, Hanahan D, Ruoslahti E. Lymphatic zip codes in premalignant lesions and tumors. Cancer Res. 2006;66:5696–706. doi: 10.1158/0008-5472.CAN-05-3876. [DOI] [PubMed] [Google Scholar]
- 87.Laakkonen P, Zhang L, Ruoslahti E. Peptide targeting of tumor lymph vessels. Ann NY Acad Sci. 2008;1131:37–43. doi: 10.1196/annals.1413.003. [DOI] [PubMed] [Google Scholar]
- 88.Lee T-Y, Lin C-T, Kuo S-Y, Chang D-K, Wu H-C. Peptide-mediated targeting to tumor blood vessels of lung cancer for drug delivery. Cancer Res. 2007;67:10958–65. doi: 10.1158/0008-5472.CAN-07-2233. [DOI] [PubMed] [Google Scholar]
- 89.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, et al. Steps towards mapping the human vasculature. Nat Med. 2002;8:121–7. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 90.Krag D, Shukla G, Shen G-P, Pero S, Ashikaga T, Fuller S, et al. Selection of tumor-binding ligands in cancer patients with phage display libraries. Cancer Res. 2006;66:7724–33. doi: 10.1158/0008-5472.CAN-05-4441. [DOI] [PubMed] [Google Scholar]
- 91.Akita N, Maruta F, Seymour L, Kerr D, Parker A, Asai T, et al. Identification of oligopeptides binding to peritoneal tumors of gastric cancer. Cancer Sci. 2006;97:1075–81. doi: 10.1111/j.1349-7006.2006.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhi M, Wu K-C, Dong L, Hao Z-M, Deng T-Z, Hong L, et al. Characterization of a specific phage-displayed peptide binding to vasculature of human gastric cancer. Cancer Biol Ther. 2004;3:1232–5. doi: 10.4161/cbt.3.12.1223. [DOI] [PubMed] [Google Scholar]
- 93.Hui X, Han Y, Liang S, Liu Z, Liu J, Hong L, et al. Specific targeting of the vasculature of gastric cancer by a new tumor-homing peptide CGNSNPKSC. J Control Release. 2008;131:86–93. doi: 10.1016/j.jconrel.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 94.Chang D-K, Chiu C-Y, Kuo S-Y, Lin C-W, Lo A, Wang Y-P, et al. Anti-angiogenic targeting liposomes increase therapeutic efficacy of solid tumors. J Biol Chem. 2009;284(19):12905–16. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun L, Chu T, Wang Y, Wang X. Radiolabeling and biodistribution of a nasopharyngeal carcinoma-targeting peptide identified by in vivo phage display. Acta Biochim Biophys Sin. 2007;39:624–32. doi: 10.1111/j.1745-7270.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 96.Newton J, Kelly K, Mahmood U, Weissleder R, Deutscher S. In vivo selection of phage for the optical imaging of PC-3 human prostate carcinoma in mice. Neoplasia. 2006;8:772–80. doi: 10.1593/neo.06331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Line B, Mitra A, Nan A, Ghandehari H. Targeting tumor angiogenesis: comparison of peptide and polymer-peptide conjugates. J Nucl Med. 2005;46:1552–60. [PubMed] [Google Scholar]
- 98.Wang H, Chen K, Cai W, Li Z, He L, Kashefi A, et al. Integrin-targeting imaging and therapy with RGD4C-TNF fusion. Mol Cancer Ther. 2008;7:1044–53. doi: 10.1158/1535-7163.MCT-07-2084. [DOI] [PubMed] [Google Scholar]
- 99.Hajitou A, Trepel M, Lilley C, Soghomonyan S, Alauddin M, Marini F, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–98. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 100.van Hensbergen Y, Broxterman H, Elderkamp Y, Lankelma J, Beers J, Heijn M, et al. A doxorubicin-CNGRC-peptide conjugate with prodrug properties. Biochem Pharm. 2002;63:897–908. doi: 10.1016/s0006-2952(01)00928-5. [DOI] [PubMed] [Google Scholar]
- 101.de Groot F, Broxterman H, Adams H, van Vliet A, Tesser G, Elderkamp Y, et al. Design, synthesis, and biological evaluation of a dual tumor-specific motive containing integrin-targeted plasmin-cleavable doxorubicin prodrug. Mol Cancer Ther. 2002;1:901–11. [PubMed] [Google Scholar]
- 102.Kim J, Lee H. Tumor targeting by doxorubicin-RGD-4C peptide conjugate in an orthotopic mouse hepatoma model. Int J Mol Med. 2004;14:529–35. [PubMed] [Google Scholar]
- 103.Bockmann M, Hilken G, Schmidt A, Cranston A, Tannapfel A, Drosten M, et al. Novel SRESPHP peptide mediates specific binding to primary medullary thyroid carcinoma after systemic injection. Hum Gene Ther. 2005;16:1267–75. doi: 10.1089/hum.2005.16.1267. [DOI] [PubMed] [Google Scholar]
- 104.Joyce J, Laakkonen P, Bernasconi M, Bergers G, Ruoslahti E, Hanahan D. Stage-specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2003;4:393–403. doi: 10.1016/s1535-6108(03)00271-x. [DOI] [PubMed] [Google Scholar]
- 105.Porkka K, Laakkonen P, Hoffman J, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc Natl Acad Sci USA. 2002;99:7444–9. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fogal V, Zhang L, Krajewski S, Ruoslahti E. Mitochondrial/cell surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 2008;68:7210–8. doi: 10.1158/0008-5472.CAN-07-6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prokop A, Davidson J. Nanovehicular intracellular delivery systems. J Pharm Sci. 2008;97:3518–90. doi: 10.1002/jps.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vicent MJ, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24:39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 109.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 110.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–24. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 111.Lee CC, Mackay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 112.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 113.Meyer-Losic F, Quinonero J, Dubois V, Alluis B, Dechambre M, Michel M, et al. Improved therapeutic efficacy of doxorubicin through conjugation with a novel peptide drug delivery technology (Vectocell) J Med Chem. 2006;49:6908–16. doi: 10.1021/jm0606591. [DOI] [PubMed] [Google Scholar]
- 114.Li C. Poly(L-glutamic acid)- anticancer drug conjugates. Adv Drug Deliv Rev. 2002;54:695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 115.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46:149–68. doi: 10.1016/s0169-409x(00)00131-9. [DOI] [PubMed] [Google Scholar]
- 116.Pastorino F, Brignole C, Di Paolo D, Nico B, Pezzolo A, Marimpietri D, et al. Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Res. 2006;66:10073–82. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- 117.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, et al. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400–9. [PubMed] [Google Scholar]
- 118.Park J-H, von Maltzahn G, Ruoslahti E, Bhatia S, Sailor M. Micellar hybrid nanoparticles for simultaneous magnetofluorescent imaging and drug delivery. Angew Chem Int Ed Engl. 2008;47:7284–8. doi: 10.1002/anie.200801810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thurber G, Schmidt M, Wittrup K. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–32. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thurber G, Schmidt M, Wittrup K. Factors determining antibody distribution in tumors. Trends Pharmacol Sci. 2008;29:57–61. doi: 10.1016/j.tips.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kirpotin D, Drummond D, Shao Y, Shalaby R, Hong K, Nielsen U, et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase inetrnalization in animal models. Cancer Res. 2006;66:6732–40. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 122.Mamot C, Drummond D, Noble C, Kallab V, Guo Z, Hong K, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–8. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 123.Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–21. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Becker A, Hessenius C, Licha K, Ebert B, Sukowski U, Semmler W, et al. Receptor-targeted optical imaging of tumors with near-infrared fluorescent ligands. Nat Biotechnol. 2001;19(4):327–31. doi: 10.1038/86707. [DOI] [PubMed] [Google Scholar]
- 125.Pierce M, Javier D, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer. 2008;123:1979–90. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Dongen G, Visser G, Lub-De Hooge M, De Vreis E, Perk L. Immuno-PET: a navigator in monoclonal antibody development and applications. Oncologist. 2007;12:1379–89. doi: 10.1634/theoncologist.12-12-1379. [DOI] [PubMed] [Google Scholar]
- 127.Okarvi SM. Peptide-based radiopharmaceuticals and cytotoxic conjugates: potential tools against cancer. Cancer Treat Rev. 2008;34:13–26. doi: 10.1016/j.ctrv.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 128.Okarvi SM. Peptide-based radiopharmaceuticals: future tools for diagnostic imaging of cancers and other diseases. Med Res Rev. 2004;24:357–97. doi: 10.1002/med.20002. [DOI] [PubMed] [Google Scholar]
- 129.Reubi J, Maecke H. Peptide-based probes for cancer imaging. J Nucl Med. 2008;49:1735–8. doi: 10.2967/jnumed.108.053041. [DOI] [PubMed] [Google Scholar]
- 130.Wangler C, Buchmann I, Eisenhut M, Haberkorn U, Mier W. Radiolabeled peptides and proteins in cancer therapy. Protein Pept Lett. 2007;14:273–9. doi: 10.2174/092986607780090874. [DOI] [PubMed] [Google Scholar]
- 131.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin laphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–82. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 132.Dijkgraaf I, Beer AJ, Wester HJ. Application of RGD-containing peptides as imaging probes for alphavbeta3 expression. Front BioSci. 2009;14:887–99. doi: 10.2741/3284. [DOI] [PubMed] [Google Scholar]
- 133.Kenny L, Coombes R, Oulie I, Contractor K, Miller M, Spinks T, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–86. doi: 10.2967/jnumed.107.049452. [DOI] [PubMed] [Google Scholar]
- 134.Gotthardt M, Eerd-Vismale J, Oyen W, de Jong M, Zhang H, Rolleman E, et al. Indication for different mechanisms of kidney uptake of radiolabeled peptides. J Nuc Med. 2007;48(4):596–601. doi: 10.2967/jnumed.106.036020. [DOI] [PubMed] [Google Scholar]
- 135.Laurent S, Forge D, Port M, Roch A, Robic C, Elst L, et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterization, and biological applications. Chem Rev. 2008;108:2064–110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]
- 136.Von Maltzahn G, Ren Y, Park J-H, Min D-H, Kotamraju V, Jayakumar J, et al. In vivo tumor cell-targeting with “click” nanoparticles. Bioconjug Chem. 2008;19:1570–8. doi: 10.1021/bc800077y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huang G, Zhang C, Li S, Khemtong C, Yang S-G, Tian R, et al. A novel strategy for surface modification of superparamagnetic iron oxide nanoparticles for lung cancer. J Mater Chem. 2009;19:6367–72. doi: 10.1039/b902358e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wood K, Azarin S, Arap W, Pasqualini R, Langer R, Hammond P. Tumor-targeted gene delivery using molecularly engineered hybrid polymers functionalized with a tumor-homing peptide. Bioconjug Chem. 2008;19:403–5. doi: 10.1021/bc700408r. [DOI] [PubMed] [Google Scholar]
- 139.Harvie P, Dutzar B, Galbraith T, Cudmore S, O’Mahony D, Anklesaria P, et al. Targeting of lipid-protamine-DNA (LPD) lipopolyplexes using RGD motifs. J Liposome Res. 2003;13:231–47. doi: 10.1081/lpr-120026389. [DOI] [PubMed] [Google Scholar]
- 140.Derfus A, Chen A, Min D-H, Ruoslahti E, Bhatia SN. Targeted quantum dot conjugates for siRNA delivery. Bioconjug Chem. 2007;18:1391–6. doi: 10.1021/bc060367e. [DOI] [PubMed] [Google Scholar]
- 141.Larocca D, Burg MA, Jensen-Pergakes K, Ravey EP, Gonzalez AM, Baird A. Evolving phage vectors for cell targeted gene delivery. Curr Pharm Biotechnol. 2002;3:45–57. doi: 10.2174/1389201023378490. [DOI] [PubMed] [Google Scholar]
- 142.Larocca D, Jensen-Pergakes K, Burg MA, Baird A. Receptor-targeted gene delivery using multivalent phagemid particles. Mol Ther. 2001;3:476–84. doi: 10.1006/mthe.2001.0284. [DOI] [PubMed] [Google Scholar]
- 143.Larocca D, Jensen-Pergakes K, Burg MA, Baird A. Gene transfer using targeted filamentous bacteriophage. Methods Mol Biol. 2002;185:393–401. doi: 10.1385/1-59259-241-4:393. [DOI] [PubMed] [Google Scholar]
- 144.Larocca D, Kassner PD, Witte A, Ladner RC, Pierce GF, Baird A. Gene transfer to mammalian cells using genetically targeted filamentous bacteriophage. FASEB J. 1999;13:727–34. doi: 10.1096/fasebj.13.6.727. [DOI] [PubMed] [Google Scholar]
- 145.Larocca D, Witte A, Johnson W, Pierce GF, Baird A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum Gene Ther. 1998;9:2393–9. doi: 10.1089/hum.1998.9.16-2393. [DOI] [PubMed] [Google Scholar]
- 146.Poul M-A, Marks JD. Targeted gene delivery to mammalian cells by filamentous bacteriophage. J Mol Biol. 1999;288:203–11. doi: 10.1006/jmbi.1999.2678. [DOI] [PubMed] [Google Scholar]
- 147.Ivanenkov VV, Felici F, Menon AG. Targeted delivery of multivalent phage display vectors into mammalian cells. Biochim Biophys Acta. 1999;1448:463–72. doi: 10.1016/s0167-4889(98)00163-3. [DOI] [PubMed] [Google Scholar]
- 148.Trepel M, Grifman M, Weitzman M, Pasqualini R. Molecular adaptors for vascular-targeted adenoviral gene delivery. Hum Gene Ther. 2000;11:1971–81. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 149.Makela A, Matilainen H, White D, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–11. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mizuguchi H, Hayakawa Y. Targeted adenovirus vectors. Hum Gene Ther. 2004;15:1034–44. doi: 10.1089/hum.2004.15.1034. [DOI] [PubMed] [Google Scholar]
- 151.Hajitou A, Rangel R, Trepel M, Soghomonyan S, Gelovani J, Alauddin M, et al. Desig and construction of targted AAVP vectors for mammalian cell transduction. Nat Protoc. 2007;2:523–31. doi: 10.1038/nprot.2007.51. [DOI] [PubMed] [Google Scholar]
- 152.Hajitou A, Lev D, Hannay J, Korchin B, Staquicini F, Soghomonyan S, et al. A preclinical model for predicting drug response in soft-tissue sarcoma with targeted AAVP molecular imaging. Proc Natl Acad Sci USA. 2008;105:4471–6. doi: 10.1073/pnas.0712184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soghomonyan S, Hajitou A, Rangel R, Trepel M, Pasqualini R, Arap W, et al. Molecular PET imaging of HSV1-tk reporter gene expression using [18F]FEAU. Nat Protoc. 2007;2:416–23. doi: 10.1038/nprot.2007.49. [DOI] [PubMed] [Google Scholar]
- 154.Landon LA, Deutscher SL. Combinatorial discovery of tumor targeting peptides using phage display. J Cell Biochem. 2003;90:509–17. doi: 10.1002/jcb.10634. [DOI] [PubMed] [Google Scholar]
- 155.McGuire MJ, Sykes KF, Samli KN, Barry MA, Stemke-Hale KA, Tagliaferri F, et al. A library selected Langerhans cell-targeting peptide enhances an immune response. DNA Cell Biol. 2004;23:742–52. doi: 10.1089/dna.2004.23.742. [DOI] [PubMed] [Google Scholar]
- 156.De J, Chang Y, Samli KN, Schisler JC, Newgard CB, Johnston SA, et al. Isolation of a Mycoplasma-specific binding peptide from an unbiased phage-displayed peptide library. Mol BioSyst. 2005;1:149–57. doi: 10.1039/b504572j. [DOI] [PubMed] [Google Scholar]
- 157.Sergeeva A, Kolonin M, Molldrem J, Pasqualini R, Arap W. Display technologies: applications for the discovery of drug and gene delivery systems. Adv Drug Deliv Rev. 2006;58:1622–54. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lau DH, Guo L, Liu R, Song A, Shao C, Lam KS. Identifying peptide ligands for cell surface receptors using cell-growth-on-bead assay and one-bead one-compound combinatorial library. Biotechnol Lett. 2002;24:497–500. [Google Scholar]
- 159.Mikawa M, Wang H, Guo L, Liu R, Marik J, Takada Y, et al. Novel peptide ligands for integrin α4β1 overexpressed in cancer cells. Mol Cancer Ther. 2004;3:1329–34. [PubMed] [Google Scholar]
- 160.Aina OH, Sroka TC, Chen M-L, Lam KS. Therapeutic cancer targeting peptides. Biopolymers. 2002;66:184–99. doi: 10.1002/bip.10257. [DOI] [PubMed] [Google Scholar]
- 161.Park SI, Renil M, Vikstrom B, Amro N, Song L, Xu B, et al. The use of one-bead one-compound combinatorial library method to identify peptide ligands for α4β1 integrin receptor in no-Hodgkin’s lymphoma. Lett Pept Sci. 2002;8:171–78. [Google Scholar]
- 162.Yao N, Xiao W, Wang X, Marik J, Park S-H, Takada Y, et al. Discovery of targeting ligands for breast cancer cells using the one-bead one-compound combinatorial method. J Med Chem. 2009;52:126–33. doi: 10.1021/jm801062d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xiao W, Yao N, Peng L, Liu R, Lam KS. Near-infrared optical imaging in glioblastoma xenograft with ligand-targeting α3 integrin. Eur J Nucl Med Mol Imaging. 2009;36:94–103. doi: 10.1007/s00259-008-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aina OH, Marik J, Liu R, Lau DH, Lam KS. Identification of novel targeting peptides for human ovarian cancer cells using “one-bead-one-compound” combinatorial libraries. Mol Cancer Ther. 2005;4:806–13. doi: 10.1158/1535-7163.MCT-05-0029. [DOI] [PubMed] [Google Scholar]
- 165.Udugamasooriya D, Dineen S, Brekken R, Kodadek T. A peptoid “antibody surrogate” that antagonizes VEGF receptor 2 activity. J Am Chem Soc. 2008;130:5744–52. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 166.Otvos L. Peptide-based drug design: Here and now. In: Otvos L, editor. Peptide-Based Drug Design. New York: Humana Press; 2008. pp. 1–8. [DOI] [PubMed] [Google Scholar]
- 167.Nestor J. Peptide and Protein Drugs: Issues and Solutions. In: Taylor J, Triggle D, editors. Comprehensive Medicinal Chemistry II. Oxford: Elsevier Ltd; 2007. pp. 573–601. [Google Scholar]
- 168.Ladner R, Sato A, Gorzelany J, de Souza M. Phage display-derived peptides as therapeutic alternatives to antibodies. Drug Discov Today. 2004;9:525–9. doi: 10.1016/S1359-6446(04)03104-6. [DOI] [PubMed] [Google Scholar]
- 169.Glaser V. Competetion mounting in peptide market. Gen Eng Biotechnol News. 2009:29. [Google Scholar]
- 170.McGregor D. Discovering and improving novel peptide therapeutics. Curr Opin Pharmacol. 2008;8:616–9. doi: 10.1016/j.coph.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 171.Lu Y, Yang J, Sega E. Issues related to targeted delivery of proteins and peptides. AAPS J. 2006;8:E466–E78. doi: 10.1208/aapsj080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Malik D, Baboota S, Ahuja A, Hasan S, Ali J. Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv. 2007;4:141–51. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 173.Reardon D, Nabors L, Stupp R, Mikkelsen T. Cilengitide: an integrin-targeting arginine-glycine-aspartic acid peptide with promising activity for glioblastoma multiforme. Expert Opin Investig Drugs. 2008;17:1225–35. doi: 10.1517/13543784.17.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Perotti A, Sessa C, Mancuso A, Noberasco C, Cresta S, Locatelli A, et al. Clinical and pharmacological phase I evaluation of Exherin™ (ADH-1), a selective anti-N-cadherin peptide in patients with N-cadherin-expressing solid tumours. Ann Oncol. 2009;20:741–5. doi: 10.1093/annonc/mdn695. [DOI] [PubMed] [Google Scholar]
- 175.Cines D, Yasothan U, Kirkpatrick P. Romiplostim. Nat Rev Drug Discov. 2008;7:887–8. doi: 10.1038/nrd2741. [DOI] [PubMed] [Google Scholar]
- 176.Frampton J, Lyseng-Williamson K. Romiplostim. Drugs. 2009;69:307–17. doi: 10.2165/00003495-200969030-00006. [DOI] [PubMed] [Google Scholar]
- 177.Bussel J, Kuter D, George J, McMillan R, Aledort L, Conklin G, et al. AMG 531, a thrombpoiesis-stimulation protein, for chronic ITP. N Engl J Med. 2006;355:1672–81. doi: 10.1056/NEJMoa054626. [DOI] [PubMed] [Google Scholar]
- 178.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276:1696–9. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 179.Oliner J, Min H, Leal J, Yu D, Rao S, You E, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 180.Hebst R, Hong D, Chap L, Kurzrock R, Jackson E, Silverman J, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 181.Wrighton NC, Farrell F, CChang R, Kashyap A, Barbone F, Mulcahy L, et al. Small peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458–64. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 182.Wrighton NC, Balasubramanian P, Barbone F, Kashyap A, Farrell F, Jolliffe L, et al. Increases potency of an erythropoietin peptide mimetic through covalent dimerization. Nat Biotechnol. 1997;15:1261–5. doi: 10.1038/nbt1197-1261. [DOI] [PubMed] [Google Scholar]
- 183.Perugini M, Varelias A, Sadlon T, D’ Andrea R. Hematopoietic growth factor mimetic: From concept to clinc. Cytokine Growth Factor Rev. 2009;20:87–94. doi: 10.1016/j.cytogfr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 184.Macdougall I. Hematide, a novel peptide-based erythropoiesis-stimulating agent for the treatment of anemia. Curr Opin Invest Drugs. 2008;9:1034–47. [PubMed] [Google Scholar]
- 185.Gregorc V, Santoro A, Bennicelli E, Punt C, Citterio G, Timmer-Bonte J, et al. Phase Ib study of NGR-hTNF, a selective vascular targeting agent, administered at low doses in combination with doxorubicin to patients with advanced solid tumours. Br J Cancer. 2009;101:219–24. doi: 10.1038/sj.bjc.6605162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bieker R, Kessler T, Schwoppe C, Padro T, Persigehi T, Bremer C, et al. Infraction of tumor vessels by NGR-peptide-directed targeting of tissue factor: experimental results and first-in-man experience. Blood. 2009;113:5019–27. doi: 10.1182/blood-2008-04-150318. [DOI] [PubMed] [Google Scholar]
- 187.Crombez L, Morris MC, Deshayes S, Heitz F, Divita G. Peptide-based nanoparticle for ex vivo and in vivo drug delivery. Curr Pharm Des. 2008;14(34):3656–65. doi: 10.2174/138161208786898842. [DOI] [PubMed] [Google Scholar]
- 188.Shrivastava A, Nunn AD, Tweedle MF. Designer peptides: learning from nature. Curr Pharm Des. 2009;15(6):675–81. doi: 10.2174/138161209787315620. [DOI] [PubMed] [Google Scholar]
- 189.Zwanziger D, Beck-Sickinger AG. Radiometal targeted tumor diagnosis and therapy with peptide hormones. Curr Pharm Des. 2008;14(24):2385–400. doi: 10.2174/138161208785777397. [DOI] [PubMed] [Google Scholar]


