Abstract
Mild traumatic brain injury (mTBI) is considered the ‘signature injury’ of combat veterans that have served during the wars in Iraq and Afghanistan. This prevalence of mTBI is due in part to the common exposure to high explosive blasts in combat zones. In addition to the threats of blunt impact trauma caused by flying objects and the head itself being propelled against objects, the primary blast overpressure (BOP) generated by high explosives is capable of injuring the brain. Compared to other means of causing TBI, the pathophysiology of mild-to-moderate BOP is less well understood. To study the consequences of BOP exposure in mice, we employed a well-established approach using a compressed gas-driven shock tube that recapitulates battlefield-relevant open-field BOP. We found that 24 hours post-blast a single mild BOP provoked elevation of multiple phosphor- and cleaved-tau species in neurons, as well as elevating manganese superoxide-dismutase (MnSOD or SOD2) levels, a cellular response to oxidative stress. In hippocampus, aberrant tau species persisted for at least 30 days post-exposure, while SOD2 levels returned to sham control levels. These findings suggest that elevated phospho- and cleaved-tau species may be among the initiating pathologic processes induced by mild blast exposure. These findings may have important implications for efforts to prevent blast-induced insults to the brain from progressing into long-term neurodegenerative disease processes.
Keywords: Blast-induced neurotrauma, brain trauma, cerebellum, mitochondrial oxidative stress, neurodegeneration, tauopathy
INTRODUCTION
The wars waged by the US and allied military forces in Iraq and Afghanistan—frequently referred to as Operation Enduring Freedom (OEF, the war in Afghanistan), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND, military operations in Iraq after August 2010)—span more than a decade starting in 2001.Over this period, more than 2.4 million US and coalition military personnel have deployed to Afghanistan and Iraq [1] for whom combat exposure to high explosive blasts has been a common occurrence. Estimates of the prevalence of blast exposure among Iraq-deployed Veterans can be garnered from epidemiologic studies that found approximately 15% of active duty soldiers returning from Iraq had experienced at least one mild or greater TBI; and of these, the majority (approximately 75%) were associated with blast exposure [2]
It has long been appreciated that mild traumatic brain injury (mTBI), common in the sport of boxing, can lead to a dementia syndrome that includes Parkinson’s disease-like motor signs and cognitive symptoms that include bradyphrenia (slowed thinking), confusion, and memory impairment [3]. It is becoming increasingly evident that mTBI experienced by football players is associated with chronic traumatic encephalopathy (CTE), a mid-life dementing disorder evidenced upon autopsy as prominent diffuse neurofibrillary tangles—a hallmark pathologic brain lesion observed in several other neurodegenerative diseases [3–6]. Importantly, moderate and severe head injuries significantly increase the risk of developing Alzheimer’s disease (AD) [7]. Recent findings indicate that as with sports-related mTBI, blast exposure is also associated with pathologic phospho-tau expression in the brain [6, 8–10].
Detonation of high explosives can inflict brain injury by at least three means: (i) ‘primary’ blast effects that are linked to overpressure (BOP) events; (ii) ‘secondary’ blast effects caused by fragmented objects or shrapnel that can inflict blunt or penetrating trauma when hitting the head; and (iii) ‘tertiary’ effects caused by acceleration/deceleration as the head is propelled through the air [11]. Even in the absence of direct blunt impacts or appreciable acceleration/deceleration (coup-contrecoup) arising from secondary and tertiary blast effects, primary BOP is capable of injuring the brain [11–16]. Indeed, exposure to the intense energy imparted by BOPs is sufficient to induce temporary disorientation, confusion, neuronal swelling, reactive gliosis, myelin disruption, loss of consciousness, subdural hematomas, cerebral hemorrhage, and edema [11–14, 17].
Compared to secondary and/or tertiary blast-induced TBI, much less is understood about the pathophysiology of primary blast-induced mTBI, which only recently has been appreciated as a significant source of brain injury [16, 18]. It is therefore crucial to better understand the underlying pathogenesis of BOP-induced mTBI. Investigating early post-blast pathology is particularly important because it is during this period that well-conceived interventions may have the best chance to prevent progression of latent and presumably more labile central nervous system (CNS) insults, into more chronic forms of CTE-like pathology. Working toward this goal, we have modeled battlefield-relevant BOP-induced mTBI in mice using a shock tube that recapitulates the blast forces generated by high explosives in the open field. We have found that at 24 hours, and persisting for at least 30 days, a single mild BOP exposure that produces only minor transient acute injury in mice, nonetheless significantly increases expression of multi-phosphorylated tau species that have been associated with the pathogenesis of numerous neurodegenerative diseases, including CTE, frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17), and AD.
MATERIALS AND METHODS
Shock tube
The shock tube shown in Fig. 1 was used to generate shock waves that replicate BOP produced by open-field high-explosive detonations (Baker Engineering and Risk Consultants, San Antonio, TX). The shock tube construction followed designs derived from computation fluid dynamic modeling carried out by BakerRisk using their proprietary “Blast Wave Target Interaction” (BWTI) software. The instrument is approximately 5.2m in length and comparable in output performance to other shock tubes used to expose rats and mice to blast [19]. The shock tube consists of a variable volume driver that permits control of the primary positive peak duration over a range of 4–10 ms, independent of the selected peak pressure. A dual diaphragm spool distributes the pressure difference between the driver and driven section of the tube across two membranes (5 mil Mylar, Tapp Plastics, Dublin, CA). To induce ‘detonation’, the spool pressure between the two diaphragms is rapidly released via remotely controlled high-speed electronic valves that cause both diaphragms to rupture simultaneously. In this study, pressurized helium was used for all experiments. Static pressure measurements were recorded for each exposure via three side-mounted pressure sensors (PCB piezoelectronics, Depew, NY) positioned 89 cm upstream and 89 cm downstream from the animal harness. All sensor data was collected at 20 kHz (AtoD National instruments) and processed using Lab- View2011 software (Austin, TX). The end of the shock tube is fitted with a side-vented attenuator/diffuser (Fig. 1) that reduces ambient transient blast noise to less than 100 decibels and suppresses reflected shock waves. Thus, the shock tube is capable of presenting blast forces at the animal exposure site that replicate open field BOP associated with as much as 99.8 kg of TNT detonated at 12.5m distance, while permitting safe operation in a standard biomedical research facility without disturbing adjoining laboratories. As discussed below, much lower exposures were used for the experiments reported herein.
Fig. 1.

Shock tube: Photograph denotes the four main functional components of the shock tube: (i) the driver in which compressed helium is loaded. Helium was used rather than air because it is more easily adjusted to replicate the extremely short time course of the initial positive pressure pulse that is characteristic of high explosive detonations in the open-field; (ii) the spool assembly which is controlled by high-speed electronic valves that regulate simultaneous rupture of two mylar diaphragms at a pre-determined, highly reproducible psi (see Methods for description); (iii) the exposure area containing the animal restraint harness and anesthesia delivery system (see Methods for more details); and (iv) the attenuator which reduces reflections/rarefactions to improve correspondence of the shock waves with idealized open-field BOP characteristics (see Fig. 2) and which suppresses ambient noise to levels suitable for use in a biomedical research facility. Arrows denote placement of pressure gauges.
Dynamic and total applied pressure determination
The shock tube generates a shockwave that loads objects placed at the test location. The pressure or load applied to the object is a function of the shock pressure moving down the tube and the interactions of the shock with the object. The applied pressure on a surface of an object varies with time and is generically given by:
where:
Po = Free field or incident overpressure (pressure above atmospheric), also commonly referred to as the static pressure.
CR = Reflection factor—the value of which varies with the pressure and angle at which the shockwave strikes the object.
Q= Dynamic pressure, which is the pressure exerted by the flow of air behind the shock wave.
CD = Drag coefficient, which is a function of the geometry of the object. The drag coefficient can be less than 0, indicating that the dynamic pressure results in a pressure below atmospheric pressure on the surface.
A practical estimate of the dynamic pressure as a function of overpressure is given by:
where:
Q= peak dynamic pressure
Po = peak incident overpressure
Pa = atmospheric pressure.
These relationships were used to derive the applied loads that would be acting on the rostral, ventral, and dorsal surfaces of the mouse and are shown in Fig. 2. The relationships are not exact since the body of the mouse responds to the applied pressure, resulting in a change in geometry. The geometrical changes can result in changes in the reflection factor as well as the drag coefficient.
Fig. 2.
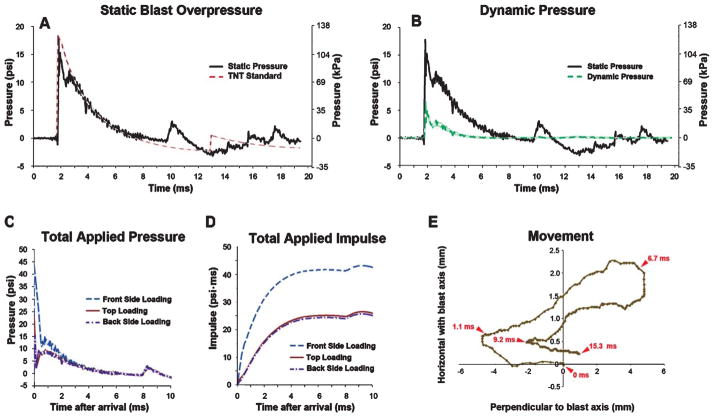
Blast overpressure characteristics and blast-induced head displacement. A) The time (ms) versus pressure (psi, left y-axis; kPa, right y-axis) plot shows an example of the static BOP used in this study as measured 5 cm directly above the animal (black trace) and illustrates the close correspondence of the shock tube-generated BOP with the Friedlander waveform expected from an open-field detonation of 15.9 kg TNT at 7.6m (dashed red trace). See Results for details. B) Green (dashed) trace indicates the intensity of the calculated dynamic pressure (blast wind) was approximately 3-fold less intense than the static BOP (solid black trace) is replotted from panel A. C) Illustrates total calculated applied pressure and (D) calculated total applied impulse (the impulse is the integration of pressure over the time of application) for BOP used in this study (A and B). E) Illustrates a representative mouse head excursion (determined from high speed video frame-by-frame analysis of a mark 2mm below eye).
Blast exposure
Mice (C57/BL6; male; 3–4 months; Jackson Laboratories) had access to food and water ad libitum. All animals were housed and handled in accordance with protocols approved by the Veterans Affairs Puget Sound Health Care System’s Institutional Animal Care and Use Committee (IACUC). All mice were allowed to acclimatize to the animal facility for at least one week prior to blast exposure. In preparation for blast exposure, animals were anesthetized with 2% isoflurane delivered with non-rebreathing anesthesia machine at a flow rate of 1 Lpm oxygen (5% isoflurane was used for initial induction). Mice were warmed with a heating pad (Gaymar, Orchard Park, NY) while anesthetized, except for the approximate 2–5 minutes spent in the shock tube (the time required to place the animal, pressurize the driver, deliver BOP, and remove the animal). To minimize blast-induced head and body motion the mice were securely mounted using plastic cable ties that attached each limb to a steel frame restraint harness supporting an open¼inch rigid mesh. Aiming to reproduce an in-theater scenario where the soldier is facing the incoming shock wave with the torso turned toward the shock wave front, the animals’ ventral body surface was oriented perpendicular with respect to the oncoming blast wave in accordance with well-established methods [19, 20]. Each BOP-exposed animal was yoked with a non-blasted sham control animal that was mounted in the shock tube and held under anesthesia for the identical amount of time as its paired BOP-exposed mouse. At the conclusion of experiments, animals were humanely euthanized via pentobarbital injection per IACUC approved methods.
While enclosed in the shock tube, the animals were monitored with a video camera (AOS Technologies XPRI, Baden, Switzerland) through polycarbonate view ports positioned above and to the side of the animal restraint apparatus. During some experiments, a Photron APX-RS high-speed camera (monochrome, Photron, San Diego, CA) operating at 20,000 frames per second, was used to record the amount of BOP-induced head movement. Frame-by-frame video analysis was performed using Logger Pro software (Vernier Software & Technology, Beaverton, OR).
Following blast exposure, the mice were immediately removed from the shock tube, anesthesia was discontinued, and the mice were placed in a partially heated observation enclosure. Animals were observed for one hour to identify evidence of distress. Mice in the blasted group demonstrated transient evidence of discomfort including piloerection and partially closed eyelids that resolved within the one hour observation period. Mice were monitored in an activity chamber (PAS-home cage, San Diego Instruments, San Diego, CA). Full body necropsies of mice 24 hours after blast exposure demonstrated evidence of lung injury consisting primarily of scattered petechial hemorrhages most prominent at the lung periphery. In some cases, small areas of hemorrhage involving less than 10% of the lungs were observed. Overall, the lung injury fell within the slight score based on the Yelverton blast injury scoring system [21]. At the 24-hours post-blast time point, the solid organs showed no gross evidence of injury such as hematoma or laceration. The respiratory tract and gastrointestinal tract showed no evidence of rupture or hemorrhage.
Western blots
Brains of BOP-exposed and sham control mice were acutely dissected in ice cold PBS; each brain was then sub-dissected into anatomical regions of hippocampus, cortex (comprising predominantly cortex, but also including underlying subcortical thalamic regions), and cerebellum. Tissue lysates for western blot analyses were then prepared as previously described [22] with the following minor modifications: phosphatase inhibitor cocktail sets 2 and 3 (Sigma, St. Louis, MO) were added (10 μ/ml) to the lysis buffer and tissues were homogenized twice by hand using an Eppendorf tube-fitting pestle (Eppendorf, Hauppauge, NY) before centrifuge clarification. Criterion 4–20% TGX gels (Bio-Rad, Hercules, CA) were loaded with 20 μg/lane and probed only once after overnight incubation (4 C) with the following antibodies: AT8, AT100, AT270 (from Thermo Scientific, Waltham, MA); Tau-5, TauC3, anti-phospho-tau 396 (from Life Technologies, Grand Island, NY); CP13, and PHF-1 (gifts from Peter Davies); rabbit polyclonal pan-tau antibodies (gift from Virginia Lee); SOD2, AβPP, and GFAP (from EMD Millipore, Billerica, MA). Probes for total tau levels and/or pyruvate kinase (Rockland, Gilbertsville, PA) were conducted similarly after stripping. Target bands were densitometrically analyzed with ImageQuant TL (GE, Piscataway, NJ). Tau epitope-specific levels were then standardized by total tau levels, and the resulting blast condition-values were normalized against identically calculated controls.
Immunofluorescence and immunohistochemistry
Mice exposed to BOP and sham treated controls were euthanized with pentobarbital (86 mg/kg) and transcardially perfused with 4% paraformaldehyde in PBS after cardiac arrest. Each brain was postfixed overnight in 4% paraformaldehyde in PBS. Cryoprotection was accomplished by washing and then transferring brains to PBS with 30% sucrose at 4 degrees and allowing the brain to equilibrate with the solution. Floating sections were made from brains that were bisected sagittally, embedded in OCT (Tissue-Tek, Torrance, CA) and cut to 40 μm sections on a cryostat (CM1850UV, Leica, Buffalo Grove, IL). The sections were placed into a 6 well culture dishes containing PBS and 0.05% sodium azide. Sections were either immediately processed or were stored at 4 C until needed (<1 month). Antigen retrieval was performed by washing the sections in PBS (3×5 min), transferring them to 50mM sodium citrate (pH 8.5–9.0) and then heating at 80 C for 30 minutes. Sections cooled to room temperature were washed (3×5 min) in PBS and then blocked (PBS, 10% normal goat serum (NGS), 0.05% Triton X-100) for 3 hours at 37 C, with gentle agitation on an orbital shaker (10 rpm). Primary antibody solution (PBS, 5% NGS, 0.05% Triton X-100) was added to each well (500 μl per well) and incubated at 4 C overnight. Floating sections were washed (3×15 min, PBS, 1% NGS, 0.05% Triton X-100) and incubated in secondary antibody solution (PBS, 5% NGS, 0.05% Triton X- 100) overnight at 4 C. Sections were transferred to slides after additional washes (2×15 min, PBS, 1% NGS, 0.05%Triton X-100; 2×15 min, PBS) and cover slipped with a drop of Prolong gold antifade solution with DAPI (Life Technologies, Grand Island, NY). The sections were imaged using a TCS SP5 confocal microscope (Leica, Buffalo Grove, IL).
Statistical analysis
Data were analyzed using standard T-tests comparing each blast-exposed group to their corresponding yoked-control sham group with reported p values indicating results of two-tailed T-tests, or where appropriate, analysis of variance (ANOVA) was used and indicated by F scores and corresponding p values.
RESULTS
Blast exposure characteristics
Wild-type C57BL/6 mice were exposed to a single BOP with the following characteristics: 15.8 psi (108.9 kPa) peak static pressure, 5.87 ms positive phase duration, 15.3 psi*ms resulting blast impulse, and 1.4 Mach shock wave velocity (black trace). Figure 2A illustrates the close correspondence between the characteristics of the BOP generated by the shock tube and the Friedlander free-field waveform expected from a surface TNT detonation in the open field (dashed red trace). The second minor peak in the Friedlander waveform at 13 ms is due to a phenomenon often referred to as repeat, and is observed in blast measurements of uncased explosives where the detonation products create a near vacuum at the explosive center and then slam back in on themselves, causing a minor second shock. The small peak in the shock tube-generated BOP at 10 ms, while similar in duration and intensity to a repeat event, was more likely due to minor BOP reflections from the attenuator. The extremely low intensity (<2 psi) of the Friedlander repeat and reflected BOP peaks are insignificant with respect to the primary positive waveform lasting approximately 5 ms, which was responsible for nearly all the force imparted by the BOP. Corresponding calculated dynamic pressure, total applied pressure, and total applied impulse are illustrated in Figs. 2B, 2C, and 2D, respectively. These exposure parameters correspond to an open-field BOP generated by detonation of approximately 16 kg TNT 7.6m away and are comparable to established mild-to moderate BOP-exposure parameters used with mice [8, 19]. In keeping with estimates that mild blast exposures in humans often produce limited head movement compared to other forms of head injury [23], we found that these parameters produced head movements of <10mm parallel to the direction of propagation of the oncoming blast wave and <2.5mm perpendicular to the direction (Fig. 2E). Maximum head displacement in the vertical axis perpendicular to the oncoming blast wave was negligible (<1 mm; data not shown).
Systemic and gross behavioral effects of blast exposure
One-hundred percent of the BOP-exposed mice survived 30 days post exposure. Unlike blunt impacts to the head, BOP induces multiple organ and systemic responses in parallel with changes in the brain, even when body armor is worn [16, 24]. Indeed, the importance of systemic responses to blast exposure in the pathobiology of blast TBI has been recently demonstrated [25]. Thus, in addition to examining the brains of blast-exposed mice, in a subset of animals necropsies were performed 24 hours post-BOP exposure for assessment of acute blast-induced changes in multiple organs and scoring the severity of the pathological alterations using the Yelverton blast injury scoring system [21] as applied to mice [19]. Gross pathologic examination of the skulls and brains of BOP-exposed mice (n = 15) revealed no instances of skull fractures, no overt CNS contusions or hemorrhages, and no apparent injury or disruption of the subarachnoid vasculature compared to sham control animals. By gross pathologic inspection, no injuries were found in liver, spleen, pancreas, adrenal glands, kidneys, stomach, small intestines, or urinary bladder (n = 15). BOP-induced mild lung injury was observed in 12/15 animals, consisting of petechiael subpleural hemorrhages (12/15 animals) with occasional ecchymosis (7/15 animals) that in all instances involved less than 10% of the lungs. Based on the Yelverton blast injury scale [21], the injury scores among the blast-exposed mice ranged from 0–18 (mean = 10.4, sem±1.8), which corresponds to a respiratory tract injury severity score rated as mild [19] (Table 1). Oxygen saturation measured 10 minutes post-blast and again at 24 hours post-blast were within normal %O2 range and not statistically significantly different from pre-blast%O2 levels (n = 6; mean%O2 (±s.e.m.) pre-, 10 min post-, and 24 h post-blast = 96±0.5, 94±1.4, and 97±0.38, respectively; F [2, 10] = 3.42, n.s.).
Table 1.
Number of BOP exposed animals (total = 15) partitioned into observed injury categories
| Organ1 | Negative (0) | Trace (3–4) | Slight (5–21) | Moderate (22–36) | Extensive (37–64) |
|---|---|---|---|---|---|
| Lung | 3 | 0 | 12 | 0 | 0 |
| Pharynx/Larynx | 15 | 0 | 0 | 0 | 0 |
| Trachea | 15 | 0 | 0 | 0 | 0 |
| HollowAbdominal Organs2 | 15 | 0 | 0 | 0 | 0 |
| Solid Abdominal Organs3 | 15 | 0 | 0 | 0 | 0 |
Number in parenthesis indicates Yelverton blast injury score for corresponding level of injury.
Liver, spleen, pancreas, adrenal glands, kidneys.
Small intestines, stomach, urinary bladder.
Each BOP-exposed animal was yoked to a sham control animal that did not receive BOP but was anesthetized using isofluorane (approximately 5 minutes per animal, see Methods for details) and then mounted in the shock tube identically to its paired BOP-exposed animal; including the same amount of time under anesthesia and the same amount of time held in the temperature-controlled post-exposure recovery area. Immediately after blast, the exposed mice appeared different from yoked sham animals, displaying slowed movement, piloerection, and reduced grooming behavior. By the end of the one-hour observation period in the recovery cage, by qualitative experimenter inspection blasted mice appeared indistinguishable from shams. Figure 3 shows quantification of changes in exploratory behavior measured for 120 minutes starting at 1 hour after blast in a beam-break activity cage. The results reveal a significant decrease in beam breaks by BOP-exposed mice compared to sham controls (p < 0.014). By 24 post-exposure, the activity levels among blast-exposed animals returned to normal sham levels.
Fig. 3.
Exploratory activity returned to normal yoked sham control levels within 24 hours after blast exposure. Histogram indicates mean beam crossings per min in activity cages normalized to shams measured 1 and 24 hours post exposure. One hour post-treatment BOP-exposed mice displayed significantly less exploratory behavior (p < 0.01) compared to sham controls. At 24 hours post-exposure activity among BOP-treated animals was comparable to sham controls (n = 5 BOP and n = 5 sham controls). Error bars indicate standard error of the mean (SEM).
Taken together, the blast exposure used in this study models the acute effects expected of a mild intensity, open-field BOP exposure producing mild and transient symptoms and are consistent with key aspects of mild blast exposure commonly experienced by OEF/OIF/OND personnel in combat zones [26].
Effects of a single mild BOP exposure on tau phosphorylation
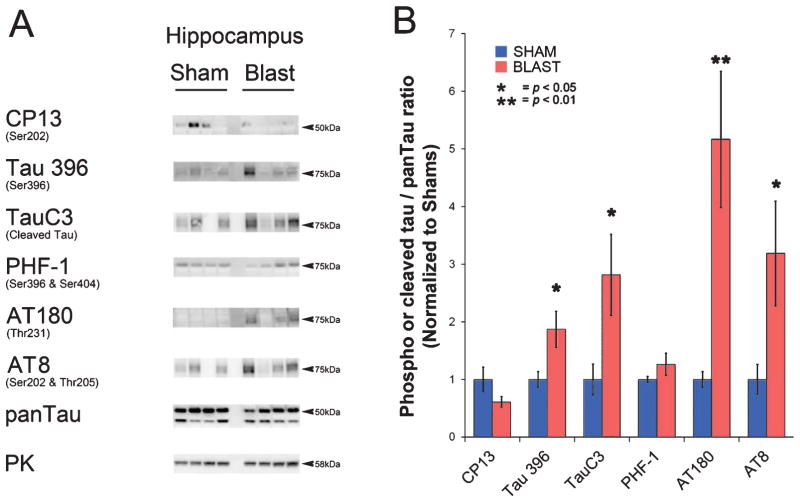
At 24 hours following a single blast exposure (15.8 psi), brains from BOP and yoked sham controls were examined using of a panel of well-characterized tau antibodies: CP13 (Ser202), Tau396 (Ser396), AT270 (Thr181), PHF-1 (Ser396 & Ser404), AT8 (Ser202 &Thr205), AT100 (Ser212 & Thr214), Tau5 (pan-specific total tau), as well as TauC3, an antibody recognizing pathologic cleaved tau [27, 28]. Figure 4 shows representative western blots (Fig. 4A) and summary histograms (Fig. 4B) indicating that, while total pan-tau levels among BOP and sham-treated mice were not significantly different, the ratios of phospho-tau to total pan-tau were significantly elevated for the tau epitopes detected by CP13 (cortex, hippocampus, cerebellum; p < 0.01, p < 0.05, p < 0.01, respectively), Tau396 (cortex, cerebellum; p < 0.05, p < 0.01, respectively), AT270 (cortex, cerebellum; p < 0.05, p < 0.01, respectively), AT100 (hippocampus, cerebellum; p < 0.05, p < 0.05, respectively), and TauC3 (cortex, hippocampus, cerebellum; p < 0.05, p < 0.05, p < 0.05, respectively).
Fig. 4.
Multiple pathologic phospho- and cleaved-tau species are increased 24 h after BOP. A) Representative western blots illustrate blast-induced elevation of phospho- and cleaved tau species in cortex, hippocampus, and cerebellum. Each lane corresponds to one sham or blast-exposed animal. B) Histograms indicate quantification of mean densitometric ratios for each tau epitope with respect to total pan-Tau immunoreactivity. The epitopes recognized by specific antibodies are denoted with reference to homologous phospho-amino acid sequences in human tau. Pyruvate kinase (PK) served as a gel protein load control. Indicated p-values are t-test results (n = 5 for blast and n = 5 for shams). Error bars indicate standard error of the means (SEM).
As discussed above, we focused on the ~16 psi exposure regime because it is in keeping with established mild exposures in mice and because it caused only mild acute distress/injury with 100% survival. We also tested the effects of a lower intensity BOP exposure (11.9 psi [82.0 kPa]±0.24 psi (s.e.m.), with 4.8 ms positive phase duration) on tau phosphorylation by western blotting whole cell protein lysates from cortex, hippocampus, and cerebellum 24 hours posttreatment using PHF-1, AT180, Tau396, AT270, and CP13 (n = 5 BOP-exposed and 5 Sham controls for each brain region and tau antibody tested). Under these still milder conditions, only in cortex did we detect a statistically significant increase in the phosphotau/ total tau ratio recognized by AT270 (p < 0.02) compared to controls (data not shown). These data suggest that under these experimental conditions, this lower BOP exposure may demark a threshold for blast-induced disturbances in phospho-tau expression. BOP intensities greater than 16 psi began to cause occasional lethality (data not shown). Thus, in order to focus on the consequences of mild BOP exposure, except where indicated above, all results in this report correspond to the 16 psi BOP parameters characterized in Fig. 2.
To corroborate the biochemical findings above and to investigate the morphologic expression pattern of blast-induced phospho-tau (pTau) expression, we carried out immunofluorescence staining on BOP and sham-treated brain sections (Fig. 5). In hippocampus, CP13 immunoreactivity was markedly increased in peri-somal domains of CA4 pyramidal neurons (Figs. 5A, B). Apparent cerebellar basket cell processes of the molecular layer were prominently immunostained with pTau AT270 monoclonal antibody (Figs. 5C, D) and cleaved tau expression, recognized by TauC3, was induced by BOP in Purkinje cells (Figs. 5E, F). These findings indicate that a single mild blast exposure is sufficient to provoke early-occurring processes involving increased phospho-tau species that were most readily observed in specific neuronal populations of the hippocampus and cerebellum. Further supporting the conclusion that BOP elicited injury responses in these brain regions, we also found markedly increased SOD2 immunoreactivity throughout the hippocampus (Fig. 5G, H) and in the cerebellar molecular layer, including Purkinje cell bodies (Fig. 5I, J). This finding suggests that aberrant early-occurring changes in tau expression may be initiated within specific brain regions and cell types concordantly with elevated oxidative stress as evidenced by strong presumed compensatory upregulation of SOD2 expression.
Fig. 5.

Blast exposure provokes increased pathologic tau and elevated SOD2 expression. A-B) Confocal microscopy revealed elevated CP13 immunoreactivity (red) in hippocampal CA4 pyramidal neurons of blast-exposed but not sham-treated mice. GFAP immunoreactivity in CA4 region (green) appeared comparable between BOP and sham animals. C, D) In cerebellum phospho-tau AT270 immunoreactivity was markedly increased in apparent basket cells of blast-exposed mice. Cell bodies stained with DAPI appear blue. E, F) Cleaved-tau immunostained by TauC3 (red) was evident in cerebellar Purkinje cell bodies of blast-exposed animals. G, H) SOD2 (green), a marker of mitochondrial oxidative stress, was markedly elevated in hippocampus of blast exposed animals. Overall hippocampal GFAP staining (purple) appeared comparable between sham and blast exposed animals. I, J) In cerebellum, Purkinje cells also displayed increasedSOD2expression. Blast-induced changes in GFAP levels (red) were less pronounced. K, L) GFAP immunoreactivity (green) in the glial limitans of parietal cortex and M, N) AT270 immunostaining (red) in cerebellar white matter tracks underlying the DAPI-stained granule cell layer was elevated in blast exposed animals. Scale bars indicate 40 m for panels A-F, I-L; 100 μm for G-H, M-N.
In contrast to the widespread blast-induced increases in SOD2 expression, BOP provoked more focal elevations in GFAP expression that appeared restricted to the glial limitans at the surface of the cortex (Fig. 5K, L). In keeping with the highly restricted domains of elevated GFAP observed by confocal microscopy, overall GFAP protein levels were not significantly elevated in western blots of total hippocampal and cortical protein lysates of blast-exposed animals compared to sham controls 24 hours post-treatment. Finally, Figs. 5M and 5N show that BOP provoked elevated phospho-tau expression in cerebellum, evidenced by increasedAT270 immunoreactivity (red) in the myelinated medullar white matter tracks adjacent to the overlying granule cell layers that stained blue with DAPI. We did not observe obvious changes in phospho-tau immunostaining of white matter outside the cerebellum.
To investigate the persistence of aberrantly elevated SOD2 and tau we examined the hippocampus 30 days following a single BOP exposure identical to the exposure used for the 24 hour experiments. Western blot analysis revealed SOD2 levels were significantly elevated at 24 hours post-blast (p < 0.002), but returned to sham control levels after 30 days (Fig. 6). We also measured total hippocampal amyloid-β protein precursor (AβPP) expression via western blotting (n = 5 BOP and 5 control subjects) using 22C11 monoclonal antibodies which detect membrane bound full-length and secreted AβPP and found that blast produced no significant changes in AβPP expression either at 24 hours or 30 days post treatment compared to controls (data not shown). In contrast to this, western blots (Fig. 7A) and summary histograms (Fig. 7B) show that in hippocampus the ratios of phospho-tau or cleaved tau to total tau were significantly elevated for the tau species recognized byTau396 (p < 0.05),TauC3 (p < 0.05), and AT180 (p < 0.01), and AT8 (p < 0.05) in blast-exposed mice compared to yoked sham controls. At 30 days post exposure, hippocampal pan-Tau levels in blast-exposed mice were not significantly different from sham controls (data not shown).
Fig. 6.

Increased SOD2 expression in hippocampus at 24 hours post-blast returned to sham levels with 30 days. A) At 24 hours post-exposure western blots revealed increased SOD2 levels in hippocampus of blast-exposed mice and by 30 days appeared comparable to control sham-treated animals. Each lane represents one BOP or sham animal. B) Histograms show quantification of mean densitometric SOD2 western blot immunoreactivty at 24 hours and 30 days post-treatment normalized with respect to yoked shams controls. Indicated p values are t-tests results (n = 5 BOP and n = 5 sham animals). Error bars indicate standard error of the means (SEM).
Fig. 7.
In hippocampus pathologic tau species were elevated 30 days following a single mild BOP. A) Representative western blots show that phospho-tau epitopes recognized by Tau396, AT180, and AT8 appear elevated in blast-exposed mice while total pan-tau immunoreactivity was comparable to sham animals. Cleaved tau recognized by TauC3 also appeared elevated. Pyruvate kinase (PK) served as a gel protein load control. B) Histograms indicated quantification of the ratios of phospho and cleaved tau with respect to total pan tau. Indicated p values are t-tests results (n = 5 BOP and n = 5 sham animals). Error bars indicate standard error of the means (SEM).
DISCUSSION
Tau pathology is a significant feature of traumatic brain injury
Aberrant accumulation of neurofibrillary tangles mainly composed of phosphorylated tau is a common pathological feature of many neurodegenerative disorders including AD, frontotemporal dementia, FTDP-17, corticobasal degeneration, Pick’s disease, progressive supranuclear palsy, spinocerebellar ataxia type 11, and CTE where tau-positive inclusions are a predominant neuropathologic signature [29]. The prominence of tau-related pathology in such etiologically and genetically diverse neurodegenerative disorders argues strongly for the significance of recently described tau pathology in both severe brain injuries and in CTE associated with repetitive mild concussive and sub-concussive impacts to the head, such as those frequently seen in boxers and football players [3, 5, 6, 8, 30–32]. Bearing in mind the complex injurious environment generated by explosions, concussive and sub-concussive impacts often occur as a result of secondary blast effects generated from flying objects that strike the head, and/or tertiary blast effects where the force of an explosion propels the body into other objects causing abrupt acceleration/ deceleration of the head. Indeed, Goldstein and colleagues, using a blast-induced TBI animal model designed to accentuate the consequences of blast-induced head acceleration/deceleration, demonstrated elevated levels of phospho-tau up to two weeks after blast exposure [8].
Systemic nature of primary BOP
An important distinction between blast exposure and other methods of imparting trauma to the head is that blast can simultaneously expose multiple organ systems to possible blast-induced physiologic reactions and injury [15]. The lungs are particularly vulnerable to injury from BOP and in cases of severe exposure, pulmonary trauma is a common cause of lethality [33]. The current form of the interceptive body armor worn by the OEF/OIF/OND deployed combat troops greatly decreases the injuries of the torso due to effective protection from shrapnel and other types of projectiles. Recent information also implies that the protective equipment the US military uses attenuate blast pressure behind the vest [24]. In spite of the widespread use of body armor, there have been a growing number of reported blast-induced lung injuries among Iraq/Afghanistan deployed combat personnel [34–36] suggesting that body armor does not obviate the potential for adverse short-term pulmonary responses. Importantly, a large body of data indicates that thoracic blast effects can interact with the effects of BOP in the CNS [12, 25, 37]. Among the peripheral organs examined in this report, mild insults were found only in the lungs, but did not alter oxygen saturation. Overall, our findings are in keeping with previous reports characterizing mild BOP-induced systemic responses in mice [19, 20]. In those reports a similar blast regime (~26 psi, 6ms positive phase duration), that while approximately 20% greater in impulse intensity, nonetheless displayed similar acute injuries that were restricted primarily to the lungs among surviving mice [19, 20]. Compared to our BOP regimen, this relatively small increase in BOP intensity was sufficient to induce 5% lethality [19, 20]. Under our experimental conditions we also found that BOPs above 16–18 psi crossed a threshold where we began to observe instances of acute BOP injury that would require veterinary interventions to prevent potential lethality (data not shown). It is for this reason that we focused the experiments in this study on a BOP exposure regimen that was compatible with 100% survival without medical intervention, and hence well within a domain that can be characterized as a mild blast exposure.
The effects of primary BOP
As discussed earlier, BOPs generated by detonating high explosives are capable of injuring the brain even in the absence of appreciable head acceleration/ deceleration. Importantly, exposure to such blast events is a common occurrence among OIF/OIF/OND service members [38]. Moss and colleagues [23] have estimated that survivable BOPs with a peak static pressure as low as 1 bar above ambient (1 bar corresponds to approximately 14.5 psi) present mechanical loads within the brain capable of injuring the CNS without invoking acceleration/deceleration as the major source of injury. Supporting such computational results, a large number of blast-induced TBI animal model studies have been carried out in which head movement has been constrained with the aim of identifying the consequences of mild-to-moderate primary BOP exposures, without significant involvement of other blast effects-induced mechanical factors. This body of work, carried out by multiple research groups using rats, mice, pigs, monkeys, and other mammals (reviewed in [15]), have reported a wide-range of BOP-induced pathology including microglial/macrophage activation, aberrant neurofilament phosphorylation, neuron loss, axonal damage, reactive gliosis, and increased oxidative stress, among many others. These neuropathological changes were often accompanied by significant behavioral and neurological impairments measured by a wide range of behavioral and functional tasks that include the Barnes maze, rotarod, novel object recognition, Morris water maze, active avoidance, beam walking, and elevated-plus maze tests [11–13, 17, 20, 37, 39–44].
The head movements of mice in our experiments ranged from approximately 2 to 10mm within an axis, compared to those carried out by Goldstein and colleagues [8], in which the mice were oriented so as to maximize head movement and accordingly produced blast-induced head displacements that were approximately 4-fold greater than those observed in this report. In those studies when head movement was constrained, blast-induced behavioral impairment on a Barnes maze task was ameliorated. One possibility is that the primary BOP (the shock wave, itself), when added with the mechanical forces imparted by significant head acceleration/deceleration, could combine to produce a compound CNS insult that reaches a level where specific cognitive functions become vulnerable to disruption and are associated with aberrant tau expression [8]. Taken collectively with our findings, such data are compatible with the idea that multiple aspects of blast exposure—both primary and secondary/tertiary events [8]—can provoke aberrant expression of tau species in the brain.
Mild primary BOP induces early-occurring and persistently elevated phospho-tau levels
We found that multiple species of phosphorylated tau, including phospho-tau epitopes recognized by the CP13, AT180, Tau396, AT270, and AT100 antibodies, were significantly elevated within 24 hours following a single mild primary BOP (Fig. 4). Our findings that phospho-tau levels measured by the Tau396, AT180, and AT8 antibodies were significantly elevated at 30 days post-BOP clearly implies that even a single mild BOP event is capable of provoking early and persistent elevations of tau species associated with neurodegeneration. Importantly, aberrant phospho-tau levels nonetheless remained elevated 30 days post-blast, even after SOD2 levels, a marker of mitochondrial oxidative stress, had returned to sham control levels (Fig. 6).
Confocal immunofluorescence analyses (Fig. 5) indicated that phosphorylated tau epitopes elevated at 24 hours post-blast were expressed by neurons—chiefly pyramidal neurons of the hippocampus and Purkinje and basket cells of the cerebellum. Studies of neurofibrillary tangle formation have elucidated a stepwise process that involves hyperphosphorylation [45, 46], tau cleavage [27], and conformational changes that eventually led to the paired helical filament conformation and tau aggregation—the cardinal end-stage pathologic feature of CTE and other tauopathies. An important step in this pathway is tau cleavage that both precedes and promotes formation of neurofibrillary tangles [27, 28]. We found significantly elevated levels of cleaved tau both at 24 hours and 30 days post-blast (Figs. 4 and 7). This finding suggests the possibility that a single blast exposure is, in principle, capable of eliciting neuronal injury responses that may set the stage for later neurofibrillary tangle formation.
Blast-induced oxidative stress
Upregulation of CNS antioxidant defense systems, which include Mn-superoxide dismutase (SOD2), has been reported previously in TBI [41]. SOD2 levels were markedly increased at 24 hours post-blast in neurons of the hippocampus and cerebellum (Fig. 5), suggesting that blast elicited an early-occurring presumably compensatory upregulation of mitochondrial antioxidant defenses. This finding is consistent with other reports of SOD2 upregulation in blast exposure [47]. Interestingly, there is evidence that SOD2 loss can cause aberrant tau phosphorylation [48]. How these early occurring changes in blast-induced SOD2 expression relate to the development of persistent disturbances in phospho-tau expression is currently unknown and warrants further in depth study. Importantly, we observed coordinately elevated SOD2 and aberrant tau species in hippocampal neurons and cerebellar Purkinje cells 24 hours after blast (Fig. 5). This finding suggests that mild blast evoked an early-occurring pathogenic process that involves both oxidative stress responses and aberrant tau processing. Interestingly, by 30 days post-blast, SOD2 levels had returned to normal in the hippocampus, though aberrant tau pathology remained. These findings raise the possibility that while the hippocampus is capable of mounting an effective compensatory response to early blast-induced oxidative insults, it may be more difficult for neurons to restore tau regulation to normal.
Summary
We have found that multiple species of phospho and cleaved tau became aberrantly elevated within 24 hours after a single mild BOP exposure and remained elevated at least 30 days after blast—a protracted interval in the lifespan of a mouse. These findings suggest that a mild blast exposure, which is a common occurrence among OEF/OIF/OND service members, might elicit CNS disturbances capable of setting the stage for long-term tau pathology. Much further work is needed to understand the pathogenic significance of phospho- and cleaved-tau following blast. However, should aberrant tau expression ultimately prove to be a critical early step in the pathway(s) leading to CTE-like neurodegeneration, these findings suggest that appropriate tau-focused interventions might prove effective when delivered shortly after exposure.
Acknowledgments
This work was supported by the Department of Veterans Affairs Office of Research and Development Medical Research Service (DGC, ERP, BCK), University of Washington Friends of Alzheimer’s Research (DGC, ERP), University of Washington Royalty Research Fund (DGC); Northwest Network Mental Illness Research, Education and Clinical Center (ERP), Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs (BRH); NIH T32 AG000258 (JSM); None of the authors have financial or other potential conflicts of interest regarding this report.
We thank Dr. Pam McMillan for advice on tau neuropathology in mice. We thank Drs. Virginia Lee, Peter Seubert, and Peter Davies for tau antibodies, Dr. Michael Bailey (Applied Physics Laboratory, University of Washington) for the use of his high speed camera, Dr. Adam Maxwell, Brian MacConaghy, Julianna Simon (all of APL/UW) for their assistance in the high speed photography, and Quentin Baker of Baker Engineering and Risk Consultants for his important input and support of this work.
We also wish to thank Command Sergeant Major (CSM) (ret) Thomas Adams, CSM (ret) Robert Prosser, and Sergeant First Class (SFC) (ret) Creed McCaslin for their valuable input which greatly helped in designing these studies so as to make them as relevant as possible to the circumstances faced by military service members in combat.
References
- 1.Hosek B. How is deployment to Iraq and Afghanistan affecting U.S. service members and their families? An overview of early RAND research on the topic. RAND Corporation; Santa Monica California, USA: 2011. [PMC free article] [PubMed] [Google Scholar]
- 2.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 3.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlanger DM, Kutner KC, Barth JT, Barnes R. Neuropsychology of sports-related head injury: Dementia pugilistica to post concussion syndrome. Clin Neuropsychol. 1999;13:193–209. doi: 10.1076/clin.13.2.193.1963. [DOI] [PubMed] [Google Scholar]
- 5.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57 :128–134. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 6.McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omalu BI, Hamilton RL, Kamboh MI, DeKosky ST, Bailes J. Chronic traumatic encephalopathy (CTE) in a National Football League player: Case report and emerging medicolegal practice questions. J Forensic Nurs. 2010;6:40–46. doi: 10.1111/j.1939-3938.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- 10.Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G, Fitzsimmons RP. Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 11.Mayorga MA. The pathology of primary blast overpressure injury. Toxicology. 1997;121:17–28. doi: 10.1016/s0300-483x(97)03652-4. [DOI] [PubMed] [Google Scholar]
- 12.Cernak I, Savic J, Malicevic Z, Zunic G, Radosevic P, Ivanovic I, Davidovic L. Involvement of the central nervous system in the general response to pulmonary blast injury. J Trauma. 1996;40:S100–S104. doi: 10.1097/00005373-199603001-00023. [DOI] [PubMed] [Google Scholar]
- 13.Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: Possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- 14.Murthy JM, Chopra JS, Gulati DR. Subdural hematoma in an adult following a blast injury. Case report. J Neurosurg. 1979;50:260–261. doi: 10.3171/jns.1979.50.2.0260. [DOI] [PubMed] [Google Scholar]
- 15.Cernak I, Noble-Haeusslein LJ. Traumatic brain injury: An overview of pathobiology with emphasis on military populations. J Cereb Blood Flow Metab. 2010;30:255–266. doi: 10.1038/jcbfm.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warden DL, French LM, Shupenko L, Fargus J, Riedy G, Erickson ME, Jaffee MS, Moore DF. Case report of a soldier with primary blast brain injury. Neuroimage. 2009;47 (Suppl 2):T152–T153. doi: 10.1016/j.neuroimage.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 17.Bauman RA, Ling GS, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim J, Ritzel D, Bell R, Ecklund JM, Armonda R, Bandak F, Parks S. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- 18.Cernak I, Savic J, Ignjatovic D, Jevtic M. Blast injury from explosive munitions. J Trauma. 1999;47:96–103. doi: 10.1097/00005373-199907000-00021. discussion 103–104. [DOI] [PubMed] [Google Scholar]
- 19.Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu L, Slack N, Windle D, Ahmed FA. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a newexperimental model of injury in mice. Neurobiol Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Koliatsos VE, Cernak I, Xu L, Song Y, Savonenko A, Crain BJ, Eberhart CG, Frangakis CE, Melnikova T, Kim H, Lee D. A mouse model of blast injury to brain: Initial pathological, neuropathological, and behavioral characterization. J Neuropathol Exp Neurol. 2011;70:399–416. doi: 10.1097/NEN.0b013e3182189f06. [DOI] [PubMed] [Google Scholar]
- 21.Yelverton JT. Pathology scoring system for blast injuries. J Trauma. 1996;40(Suppl):s111–s115. doi: 10.1097/00005373-199603001-00025. [DOI] [PubMed] [Google Scholar]
- 22.Meabon JS, Lee A, Meeker KD, Bekris LM, Fujimura RK, Yu CE, Watson GS, Pow DV, Sweet IR, Cook DG. Differential expression of the glutamate transporter GLT-1 in pancreas. J Histochem Cytochem. 2012;60:139–151. doi: 10.1369/0022155411430095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss WC, King MJ, Blackman EG. Skull flexure from blast waves: A mechanism for brain injury with implications for helmet design. Phys Rev Lett. 2009;103:108702. doi: 10.1103/PhysRevLett.103.108702. [DOI] [PubMed] [Google Scholar]
- 24.Wood GW, Panzer MB, Shridharani JK, Matthews KA, Capehart BP, Myers BS, Bass CR. Attenuation of blast pressure behind ballistic protective vests. Inj Prev. 2013;19:19–25. doi: 10.1136/injuryprev-2011-040277. [DOI] [PubMed] [Google Scholar]
- 25.Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front Neurol. 2010;1:151. doi: 10.3389/fneur.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: Preliminary findings in a US Army Brigade Combat Team. J Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 27.de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201– 1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spires-Jones TL, de Calignon A, Matsui T, Zehr C, Pitstick R, Wu HY, Osetek JD, Jones PB, Bacskai BJ, Feany MB, Carlson GA, Ashe KH, Lewis J, Hyman BT. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. J Neurosci. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 30.Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J. CSF-biomarkers in Olympic boxing: Diagnosis and effects of repetitive head trauma. PLoS ONE. 2012;7:e33606. doi: 10.1371/journal.pone.0033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M, Budson AE. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol. 2010;69:918–929. doi: 10.1097/NEN.0b013e3181ee7d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: The role of the forensic pathologist. Am J Forensic Med Pathol. 2010;31:130–132. doi: 10.1097/PAF.0b013e3181ca7f35. [DOI] [PubMed] [Google Scholar]
- 33.Bowen IG, Fletcher ER, Richmond DR, Hirsch FG, White CS. Biophysical mechanisms and scaling procedures applicable in assessing responses of the thorax energized by air-blast overpressures or by non-penetrating missiles. Techn Progr Rep DASA 1857. Fission Prod Inhal Proj. 1967:1–46. doi: 10.2172/4412263. [DOI] [PubMed] [Google Scholar]
- 34.Smith JE. The epidemiology of blast lung injury during recent military conflicts: A retrospective database review of cases presenting to deployed military hospitals, 2003–2009. Philos Trans R Soc Lond B Biol Sci. 2011;366:291–294. doi: 10.1098/rstb.2010.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaffe DH, Peleg K, Israel Trauma G. Terror explosive injuries: A comparison of children, adolescents, and adults. Ann Surg. 2010;251:138–143. doi: 10.1097/SLA.0b013e3181b5d7ab. [DOI] [PubMed] [Google Scholar]
- 36.Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: Recreating a battlefield injury in the laboratory. J Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- 38.Belmont PJ, Jr, McCriskin BJ, Sieg RN, Burks R, Schoenfeld AJ. Combatwounds in Iraq and Afghanistan from 2005 to 2009. J Trauma Acute Care Surg. 2012;73:3–12. doi: 10.1097/TA.0b013e318250bfb4. [DOI] [PubMed] [Google Scholar]
- 39.Saljo A, Bao F, Haglid KG, Hansson HA. Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J Neurotrauma. 2000;17 :719–726. doi: 10.1089/089771500415454. [DOI] [PubMed] [Google Scholar]
- 40.Cernak I, Savic V, Kotur J, Prokic V, Kuljic B, Grbovic D, Veljovic M. Alterations in magnesium and oxidative status during chronic emotional stress. Magnes Res. 2000;13:29–36. [PubMed] [Google Scholar]
- 41.Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- 42.Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: Toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol. 2012;3:32. doi: 10.3389/fneur.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y, Wei Y, Oguntayo S, Wilkins W, Arun P, Valiyaveettil M, Song J, Long JB, Nambiar MP. Tightly coupled repetitive blast-induced traumatic brain injury: Development and characterization in mice. J Neurotrauma. 2011;28:2171–2183. doi: 10.1089/neu.2011.1990. [DOI] [PubMed] [Google Scholar]
- 44.Rostami E, Davidsson J, Ng KC, Lu J, Gyorgy A, Walker J, Wingo D, Plantman S, Bellander BM, Agoston DV, Risling M. A model for mild traumatic brain injury that induces limited transient memory impairment and increased levels of axon related serum biomarkers. Front Neurol. 2012;3:115. doi: 10.3389/fneur.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso A, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A. 2001;98:6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubovitch V, Ten-Bosch M, Zohar O, Harrison CR, Tempel-Brami C, Stein E, Hoffer BJ, Balaban CD, Schreiber S, Chiu WT, Pick CG. A mouse model of blast-induced mild traumatic brain injury. Exp Neurol. 2011;232:280–289. doi: 10.1016/j.expneurol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melov S, Adlard PA, Morten K, Johnson F, Golden TR, Hinerfeld D, Schilling B, Mavros C, Masters CL, Volitakis I, Li QX, Laughton K, Hubbard A, Cherny RA, Gibson B, Bush AI. Mitochondrial oxidative stress causes hyperphosphorylation of tau. PLoS ONE. 2007;2:e536. doi: 10.1371/journal.pone.0000536. [DOI] [PMC free article] [PubMed] [Google Scholar]