Abstract
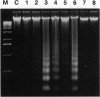
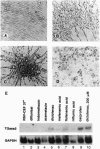
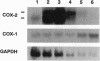
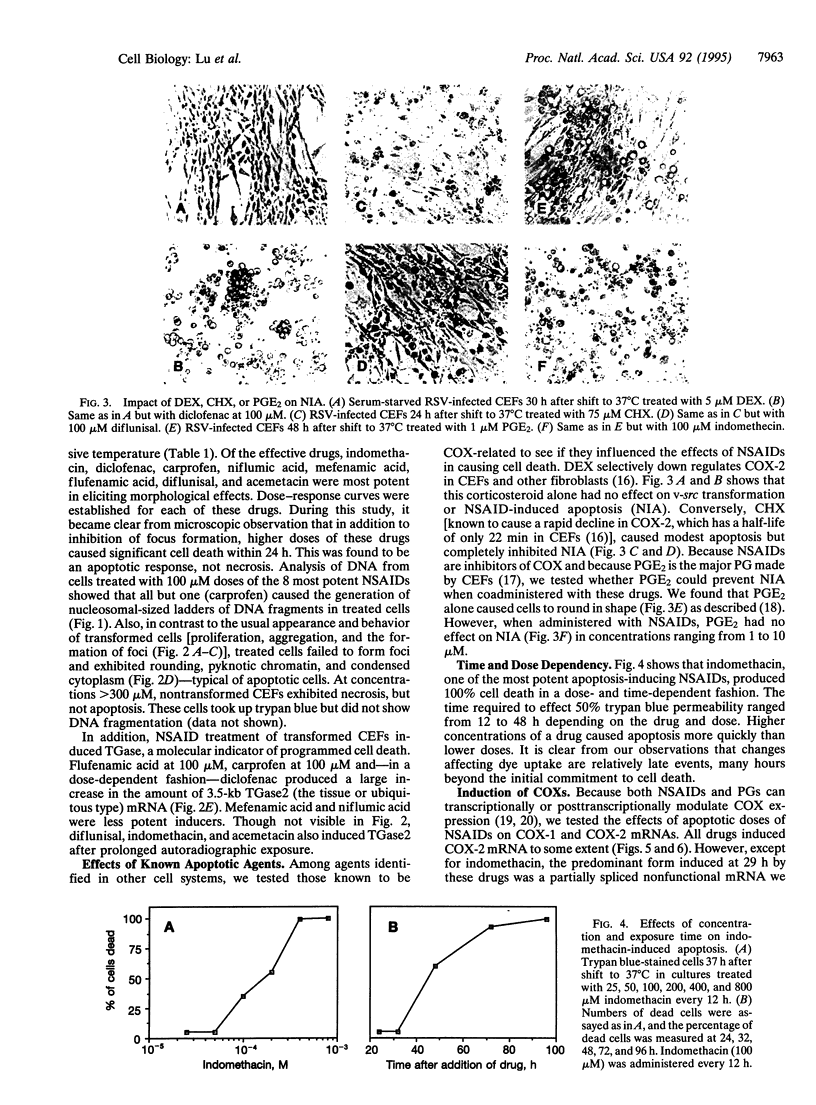
Programmed cell death (apoptosis) is an intrinsic part of organismal development and aging. Here we report that many nonsteroidal antiinflammatory drugs (NSAIDs) cause apoptosis when applied to v-src-transformed chicken embryo fibroblasts (CEFs). Cell death was characterized by morphological changes, the induction of tissue transglutaminase, and autodigestion of DNA. Dexamethasone, a repressor of cyclooxygenase (COX) 2, neither induced apoptosis nor altered the NSAID effect. Prostaglandin E2, the primary eicosanoid made by CEFs, also failed to inhibit apoptosis. Expression of the protooncogene bcl-2 is very low in CEFs and is not altered by NSAID treatment. In contrast, p20, a protein that may protect against apoptosis when fibroblasts enter G0 phase, was strongly repressed. The NSAID concentrations used here transiently inhibit COXs. Nevertheless, COX-1 and COX-2 mRNAs and COX-2 protein were induced. In some cell types, then, chronic NSAID treatment may lead to increased, rather than decreased, COX activity and, thus, exacerbate prostaglandin-mediated inflammatory effects. The COX-2 transcript is a partially spliced and nonfunctional form previously described. Thus, these findings suggest that COXs and their products play key roles in preventing apoptosis in CEFs and perhaps other cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsohn P. A., Zorn T. M. Implantation and decidualization in rodents. J Exp Zool. 1993 Sep 1;266(6):603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- Ackerman R. C., Murdoch W. J. Prostaglandin-induced apoptosis of ovarian surface epithelial cells. Prostaglandins. 1993 May;45(5):475–485. doi: 10.1016/0090-6980(93)90123-o. [DOI] [PubMed] [Google Scholar]
- Aroian R. V., Levy A. D., Koga M., Ohshima Y., Kramer J. M., Sternberg P. W. Splicing in Caenorhabditis elegans does not require an AG at the 3' splice acceptor site. Mol Cell Biol. 1993 Jan;13(1):626–637. doi: 10.1128/mcb.13.1.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker K., Aderem A., Hanafusa H. Modulation of arachidonic acid metabolism by Rous sarcoma virus. J Virol. 1989 Jul;63(7):2929–2935. doi: 10.1128/jvi.63.7.2929-2935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard P. A., Yannoni Y., Simmons D. L., Erikson R. L. Rapid repression of quiescence-specific gene expression by epidermal growth factor, insulin, and pp60v-src. Mol Cell Biol. 1989 Mar;9(3):1371–1375. doi: 10.1128/mcb.9.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune K., Rainsford K. D., Wagner K., Peskar B. A. Inhibition of anti-inflammatory drugs of prostaglandin production in cultured macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jan;315(3):269–276. doi: 10.1007/BF00499844. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cazals-Hatem D. L., Louie D. C., Tanaka S., Reed J. C. Molecular cloning and DNA sequence analysis of cDNA encoding chicken homologue of the Bcl-2 oncoprotein. Biochim Biophys Acta. 1992 Aug 17;1132(1):109–113. doi: 10.1016/0167-4781(92)90064-7. [DOI] [PubMed] [Google Scholar]
- Chiccarelli F. S., Eisner H. J., Van Lear G. E. Disposition and metabolism of fenbufen in several laboratory animals. Arzneimittelforschung. 1980;30(4A):707–715. [PubMed] [Google Scholar]
- Chiou S. K., Rao L., White E. Bcl-2 blocks p53-dependent apoptosis. Mol Cell Biol. 1994 Apr;14(4):2556–2563. doi: 10.1128/mcb.14.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evett G. E., Xie W., Chipman J. G., Robertson D. L., Simmons D. L. Prostaglandin G/H synthase isoenzyme 2 expression in fibroblasts: regulation by dexamethasone, mitogens, and oncogenes. Arch Biochem Biophys. 1993 Oct;306(1):169–177. doi: 10.1006/abbi.1993.1496. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Marnett L. J. Aspirin and the potential role of prostaglandins in colon cancer. Cancer Res. 1992 Oct 15;52(20):5575–5589. [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastino A., Grelli S., Piacentini M., Oliverio S., Favalli C., Perno C. F., Garci E. Correlation between induction of lymphocyte apoptosis and prostaglandin E2 production by macrophages infected with HIV. Cell Immunol. 1993 Nov;152(1):120–130. doi: 10.1006/cimm.1993.1272. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Jove R., Krane J. F., Poirier F., Calothy G., Hanafusa H. Genetic lesions involved in temperature sensitivity of the src gene products of four Rous sarcoma virus mutants. J Virol. 1986 Dec;60(3):858–867. doi: 10.1128/jvi.60.3.858-867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch M. P., Tertoolen L. G., Hage W. J., de Laat S. W. Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell. 1993 Aug 13;74(3):565–575. doi: 10.1016/0092-8674(93)80057-l. [DOI] [PubMed] [Google Scholar]
- Pilbeam C. C., Kawaguchi H., Hakeda Y., Voznesensky O., Alander C. B., Raisz L. G. Differential regulation of inducible and constitutive prostaglandin endoperoxide synthase in osteoblastic MC3T3-E1 cells. J Biol Chem. 1993 Dec 5;268(34):25643–25649. [PubMed] [Google Scholar]
- Simmons D. L., Levy D. B., Yannoni Y., Erikson R. L. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1178–1182. doi: 10.1073/pnas.86.4.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamonti M. S., Roh M. S., Curley S. A., Gallick G. E. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993 Jan;91(1):53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. Towards a better aspirin. Nature. 1994 Jan 20;367(6460):215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]
- Weraarchakul-Boonmark N., Jeong J. M., Murthy S. N., Engel J. D., Lorand L. Cloning and expression of chicken erythrocyte transglutaminase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9804–9808. doi: 10.1073/pnas.89.20.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Sanduja R., Tsai A. L., Ferhanoglu B., Loose-Mitchell D. S. Aspirin inhibits interleukin 1-induced prostaglandin H synthase expression in cultured endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2384–2387. doi: 10.1073/pnas.88.6.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]