Abstract
Depigmentation in vitiligo occurs by progressive loss of melanocytes from the basal layer of the skin, and can be psychologically devastating to patients. T cell-mediated autoimmunity explains the progressive nature of this disease. Rather than being confronted with periods of rapid depigmentation and bouts of repigmentation, patients with long-standing, treatment-resistant vitiligo can undergo depigmentation treatment. The objective is to remove residual pigmentation in order to achieve a cosmetically acceptable result- that of skin with a uniform appearance. In the USA, only the use of mono-benzyl ether of hydroquinone (MBEH) is approved for this purpose. However, satisfactory results can take time to appear, and there is a risk of repigmentation. MBEH induces necrotic melanocyte death followed by a cytotoxic T cell response to remaining, distant melanocytes. As cytotoxic T cell responses are instrumental to depigmentation, we propose that combining MBEH with immune adjuvant therapies will accelerate immune-mediated melanocyte destruction to achieve faster, more definitive depigmentation than with MBEH alone. Since Toll-like Receptor (TLR) agonists-imiquimod, CpG, and Heat Shock Protein 70 (HSP 70)-all support powerful Th1 responses, we propose that using MBEH in combination with these agents can achieve superior depigmentation results for vitiligo patients.
Keywords: vitiligo, monobenzone, bleaching phenols, T cells, imiquimod, CpG, HSP70
Introduction
Vitiligo is a pigmentary disorder resulting from a loss of melanocytes (1), which varies in its extent of skin involvement and has an unpredictable course (2). Depigmentation can be devastating, having a negative impact on mental health and quality of life (3). Although vitiligo is a multifactorial disease, autoimmunity is the predominant etiologic factor. This is supported by multiple findings (4), including its common association with other autoimmune diseases (5). Strong support exists for T cell-mediated immunity in vitiligo. Increased CD8+/CD4+ infiltrating T cell ratios are observed within perilesional areas of the skin in vitiligo patients (6), and cytotoxic T cells are capable of recognizing melanocytes, leading to apoptosis (7).
Despite its discovery decades ago and its noteworthy prevalence worldwide, no single treatment works effectively in all patients (3). When repigmentation treatments fail and patients have extensive skin involvement, depigmentation therapy can serve to restore a uniform appearance to the skin (8). Currently, mono-benzyl ether of hydroquinone (MBEH) is the sole USFDA approved agent for this purpose (9) and has been widely used as a topical depigmenting agent for vitiligo patients (8,10). The gradual effects, typically notable after four to twelve months of use, are undesirably slow for patients (11). Furthermore, although depigmentation is known to spread beyond the application site, MBEH treatment may not eliminate all melanocytes beyond the application site and carries a risk of repigmentation (12). Here, we propose to combine two synergistic mechanisms: the acceleration of autoimmunity and melanocyte death initiated by MBEH by amplifying T cell responses through immune adjuvants to induce effective, long-lasting, and universal depigmentation.
Monobenzone Treatment
MBEH is the active ingredient in benoquin cream and is typically formulated in a concentration of 20%. It is a slow-acting depigmenting agent inducing a type IV delayed hypersensitivity response (13). MBEH induces necrotic cell death in epidermal melanocytes (12,14). Similar to findings in mice (15), areas of human skin exposed to MBEH showed cytotoxic CD8+ T cell infiltrates, supporting that MBEH induces a cytotoxic T-cell immune response that further contributes to depigmentation (16). MBEH exposure leads to production of reactive oxygen species in pigmented cells and increases the release of CD63+, tyrosinase+, and MART-1+ exosomes, which can induce specific immunity (17). TLR agonists cytosine-guanine oligodeoxynucleotides (CpG) and TLR7-agonist imiquimod, and inducible heat shock protein 70 (HSP70i) all effectively stimulate the autoimmune response to melanocytes (18,19). Here, we follow the autoimmune concept of vitiligo pathogenesis to address the opportunity of adjuvant-enhanced bleaching treatment. We propose that the combined use of adjuvants, and MBEH, will accelerate the immune-mediated destruction of melanocytes and restore a lasting uniform appearance to the skin.
Melanization
Melanocytes differentiate from neural crest-derived stem cells, which migrate to the epidermis, hair follicles, choroid of the eye, iris, leptomeninges of the brain and stria vascularis of the cochlea (20). Within melanosomes, melanocytes produce melanin, the pigment responsible for skin and hair color (21). Melanosomes can synthesize two types of pigments: eumelanin, a dark brown-to-black insoluble polymer, and pheomelanin, a light red-yellow sulfur-containing soluble polymer (22). Both are synthesized through a series of oxidative steps from the precursor molecule, tyrosine. The first step involves conversion of tyrosine to L-DOPA, catalyzed by the enzyme tyrosinase, which is the rate-limiting step in pigment production (23). L-DOPA is then oxidized to dopaquinone, and after this the pathways diverge (24). In eumelanogenesis, dopaquinone is sequentially converted to leukodopachrome, to dihydroxyindole (DHI), and DHI carboxylic acid (DHICA), which polymerizes to form eumelanin (24). In pheomelanogenesis, however, dopaquinone reacts with cysteine or glutathione to form cysteinyldopa or glutathionyldopa, polymerizing into pheomelanin (24). The ratio of these two pigments in any given cell is determined by the availability of substrates and enzymes responsible for later steps in eumelanogenesis, including tyrosinase-related protein 2 (TRP2) and gp100 (20,25,26).
Fully melanized melanosomes are transferred to adjacent keratinocytes, where they may be arranged in a supranuclear cap to protect nuclear DNA from ultraviolet irradiation (27). Though both pigments absorb UV, eumelanin is more suited for this. Pheomelanin can predispose cells to UV damage by its tendency to produce reactive oxygen species upon UV exposure (28). Hence, melanomas occur more commonly in pheomelanized individuals (29), although the absolute number of melanocytes in healthy individuals is similar among skin types (20).
Autoimmune pathogenesis of vitiligo
Autoimmunity drives progressive depigmentation in vitiligo. Melanocyte-reactive CD8+ T cells may be more abundant in peripheral blood of patients with progressive vitiligo than in healthy individuals (30,31), with significant amounts of T cells recognizing melanocyte differentiation antigens MART-1, tyrosinase, and gp100 (7,30,32). Perilesional skin infiltrating CD8+ T cells also recognize melanocyte antigens and displayed cytotoxicity towards autologous melanocytes (7,33). Reduced local immunosuppression also plays a role in vitiligo. Tregs are less effectively chemo-attracted to the skin in vitiligo patients (34). Because Tregs will suppress self-reactive T cells that escape negative selection in the thymus (35), their decreased presence in vitiligo skin allows for continued activation of cytotoxic T cells, causing widespread melanocyte destruction (36). Th17 cells have also been implicated in vitiligo pathogenesis. Increased serum IL-17 levels in patients correlated with the body surface area (BSA) involvement of vitiligo (37).
Bleaching treatment is likewise associated with inflammatory cytokine expression. Both 4-tertiary butylphenol (4-TBP) and MBEH increase the expression of transcription factor X-box binding protein 1 (XBP1), which activates the unfolded protein response in melanocytes (38). This results in increased production of IL-6 and IL-8 by melanocytes (38). Increased IL-8 expression was likewise found in skin of patients with active disease (39). Overproduction of pro-inflammatory cytokines and decreased availability of immunosuppressive regulatory T cells, combined with increased abundance of cytotoxic T cells, all contribute to depigmentation.
Mechanism of action of topical depigmenting agents
Topical bleaching phenols, such as MBEH, 4-TBP, and 4-methoxyphenol (also known as 4-MP, mequinol, 4-hydroxyanisole), were found to cause skin depigmentation and are melanocytotoxic (40–42). Other phenolic melanocytotoxic depigmenting agents include 4-tertiary amyl-phenol, 4-tertiary butylcatechol (4-TBC), and 2,4 di-tertiary butylphenol (DTBP) (43–46). Phenolic compounds have structural similarities to tyrosine and can bind to the enzyme’s active site (47). Phenols have maximal depigmenting activity with a non-polar side chain in para-position (48). These phenolic compounds serve as alternate substrates for the enzyme tyrosinase to be enzymatically converted into cytotoxic quinones (49), which subsequently bind to cysteine residues in proteins (17,50,51). For example, 4-hydroxyanisole (since renamed 4-methoxyphenol, 4MP) is selectively incorporated into melanosomes, coming in contact with tyrosinase (40). This results in formation of an orthoquinone, which can bind tyrosinase to generate a new compound (52,53). Covalent binding of the newly generated orthoquinone to tyrosinase may generate a neoantigen (17). This haptenization and neoantigen generation process characterizes all aforementioned topical bleaching phenols. Neoantigens can induce autoimmunity by activating immune reactivity to both the modified and unadulterated autoantigens, resulting in CD8+ cytotoxic T cell migration to the skin and progressive destruction of drug-exposed and unexposed melanocytes (17). Melanocytes are absent from depigmented lesions exposed to 4-TBP (54), as 4-TBP induces melanocyte apoptosis (42). 4-TBP is preferentially cytotoxic to melanocytes (42). In patients exposed to 4-methoxyphenol, melanocytes were likewise decreased in number, histologically resembling vitiligo, and 4-MP cream achieved complete depigmentation in most patients (8).
MBEH was used in the 1930s to prevent the oxidation of rubber, but workers wearing the MBEH-containing rubber gloves soon noticed depigmentation of skin in contact with the gloves (55). The interaction of MBEH with melanocytes triggers a series of cellular effects (oxidative stress, alteration of cellular proteins and increased release of melanoma and melanocyte antigens) that selectively induce immunity against melanocytes and melanoma cells, sparing other cell types. Treatment was found to activate dendritic cells and recruit melanocyte-reactive cytotoxic T-cells to the skin, leading to local and distant melanocyte destruction, even within areas not initially exposed to the MBEH (17,56). Indeed, manufacturers noticed that prolonged exposure to the gloves led to depigmentation in areas of the body not originally exposed to the gloves (55), supporting ongoing autoimmune reactivity upon MBEH exposure (57). T cell clones reactive with MBEH-exposed melanocytes also react with unexposed melanocytes (17), indicating that T cells cross-react with normal melanosomal antigens. These findings suggest that MBEH exposure lowers the threshold for immune reactivity to melanocytes, leading to CD8+ T cell-mediated depigmentation at distant sites. An inverse relationship was found between sensitivity of melanocytes to MBEH and pigmentation levels, suggesting reduced efficacy in patients with ethnic skin (14). Other skin-whitening agents include hydroquinone, arbutin, tretinoin, alpha-hydroxy acids, kojic acid, azelaic acid, vitamin C, and flavonols (58). These are less relevant to the current strategy, as these agents primarily target tyrosinase activity and interrupt melanization without toxicity to melanocytes (58–64). Thus, permanent depigmentation cannot be established, and these agents are unlikely to affect melanocyte immune recognition.
Immune activation by adjuvants
Immune adjuvants imiquimod, CpG, and Heat Shock Proteins have been used to stimulate desirable anti-tumor immune responses (18,65,66). These agents can amplify existing immune mechanisms that mediate melanocyte destruction. Toll-Like Receptors (TLRs) are transmembrane intracellular receptors that bind pathogens and consist of glycoproteins that function as members of the innate immune system, and offer a primitive means to distinguish self from non-self (67). TLRs are found on various cell types, including antigen presenting cells (68). Different TLR subfamilies bind different ligands (69). This leads to the production of inflammatory cytokines (70), promoting lymphocyte activation, and contributing to melanocyte destruction. TLRs induce host cell apoptosis (71) and enhance adaptive immune responses (72–74).
Imiquimod is an immune response modifier approved for topical treatment of anogenital warts, which is also beneficial for treatment of basal cell carcinomas and actinic keratoses (75,76). Upon topical application, imiquimod treatment can induce local vitiligo-like hypopigmentation (77). When applied to lesions such as warts and actinic keratoses, imiquimod acts as a TLR7 agonist (75,78), inducing production of inflammatory cytokines in macrophages. As these inflammatory cytokines can destroy healthy tissue, imiquimod is applied only to the active lesion to minimize healthy tissue destruction. Activation of proinflammatory cytokines promotes proliferation and maturation of naïve T cells and their differentiation into Th1 lymphocytes (67). Thus, imiquimod indirectly promotes cytotoxic T cell responses. Imiquimod also promotes the maturation of epidermal Langerhans cells, which leads to increased antigen presentation and immune activation (79). Focally, imiquimod induces melanocyte apoptosis (80) by downregulating microphthalmia-associated transcription factor (MITF), a gene regulating melanocyte survival and transcription of melanocytic proteins (81–84). MITF downregulation results in decreased Bcl-2 transcription and a predisposition to apoptosis.
CpG oligonucleotides (CpG-ODNs) are TLR9 agonists (66). TLR9 recognizes unmethylated CpG DNA segments, which was the premise for their use in anti-tumor immunotherapy (66). In mice, CpG oligodeoxynucleotide combined with tumor-specific peptide effectively induced a Th1 tumor immune response (85,86). CpG-ODNs cause potent Th1-type cytokine responses and increased expression of major histocompatibility complex (MHC) molecules and costimulatory molecules on antigen-presenting cells (87).
Heat shock proteins (HSPs) are upregulated in response to stress (88) and serve to chaperone existing peptides and nucleotides to prevent misfolding and apoptosis, when overall protein synthesis is halted (88). When HSPs are released from cells, antigen presenting cells receive this danger signal to promote an inflammatory response (89), in part by increasing the synthesis of Th-1 cytokines (90). Inducible HSP70 can be secreted by live cells (91) and is expressed by melanocytes under stress (88). HSP70 activates the innate immune system, functioning as an endogenous ligand of TLR2 and TLR4 (92,93). Secretion of HSP70i by stressed melanocytes causes dendritic cell activation to mount autoimmune responses against melanocytes (94). Indeed, vaccination with HSP70i encoding DNA resulted in significant and continued depigmentation in vitiligo prone mice (18).
Enhanced Bleaching Treatment for Vitiligo
MBEH produces suboptimal skin bleaching results. Given the strong evidence supporting autoimmunity in vitiligo pathogenesis, we propose that limitations to MBEH treatment may be overcome with supplementary immune adjuvant therapy to enhance the depigmentation process. Combination therapy including two bleaching agents was previously tested in guinea pigs, namely, MBEH and all-trans retinoic acid (ATRA) (95). Here, we propose the synergistic use of immune adjuvants in conjunction with MBEH. Th1 cytokines activate cytotoxic processes (96), and Th1 lymphocytes mediate delayed-type hypersensitivity responses (97). Imiquimod, CpG, and HSP70 are adjuvants known to promote Th1 responses and can thus be used as immune adjuvants for enhanced depigmentation therapy. The principal mechanism is illustrated in Fig. 1. In mice, monobenzone therapy, combined with imiquimod and CpG, in “MIC” therapy, induced a potent and lasting immune response against melanoma antigens and eradicated melanoma tumors (19). The combination of MBEH and imiquimod without CpG likewise effectively induced anti-melanocyte immunity, and may constitute a more affordable option to accelerate depigmentation in vitiligo patients. Though such combination therapy was effective in mice, its clinical treatment potential for human skin has yet to be demonstrated (19). In light of the continuum of melanocytes in human skin, whereas mouse melanocytes locate to hair follicles, an equal or better treatment efficacy may be predicted for patients.
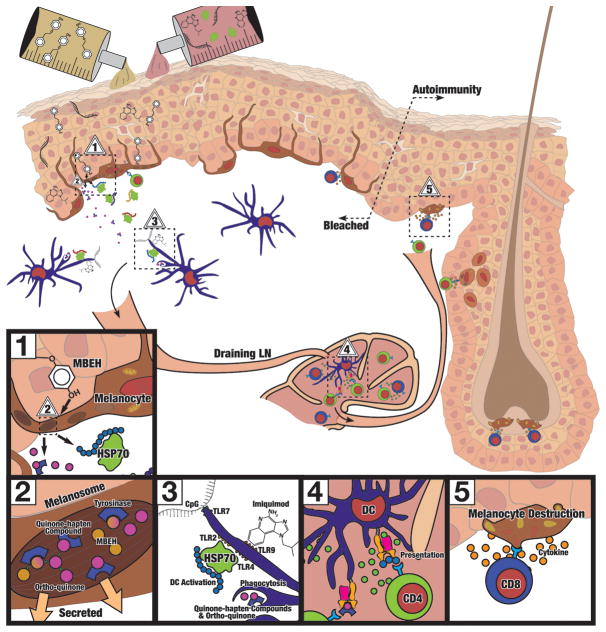
Fig. 1. Principle of adjuvant enhanced skin bleaching.
We propose that enhanced depigmentation can be achieved by (1) combined application of bleaching phenols such as MBEH and immune adjuvants such as imiquimod, CpG or HSP70 introduced to the skin. The phenolic agents will (2) traffic to the melanosomes within melanocytes found in the basal layer of the epidermis, where they are converted to toxic ortho-quinones that haptenize the tyrosinase enzyme. The modified antigenic peptides are then released by cells under stress or dying melanocytes to (3) come in contact with dermal dendritic cells in combination with co-applied adjuvants to (4) activate melanocyte-reactive T cells that (5) are recruited to the skin to eliminate remaining pigment cells and establish lasting depigmentation.
Importantly, in employing immune adjuvants for the current application, agents should be applicable topically, to avert systemic immune activation with unnecessary destruction of healthy tissues. Currently, imiquimod is the only adjuvant listed above available in a topical formulation. As adjuvant therapy is meant to enhance existing immune responses, and vitiligo patients have heightened anti-melanocyte immune reactivity, imiquimod-assisted MBEH responses are likely to be selectively amplified in vitiligo patients. HSP70i protein has been applied by gene gun vaccination in mice, and passive transdermal migration of full length protein is unlikely to occur. Finally, CpG is not available in a topical formulation for human use and has mostly been studied when administered as an intravenous or subcutaneous injectable in humans. Thus, MBEH plus imiquimod is currently the preferred option for combination treatment.
Note that the expense of common imiquimod formulations is limiting to patients when not covered by insurance companies, providing immediate incentive for further (pre)clinical testing. Fortified MBEH therapy may inadvertently affect melanocyte viability in extracutaneous sites, which could affect hearing or visual acuity; however, melanocytes in immune-privileged sites are relatively protected from such responses. Indeed, there is minimal evidence for hearing or visual impairment in vitiligo (98–100). By contrast, a significantly advantageous effect of this therapy could include targeting of occult melanomas, as supported by previous studies (15,19).
In conclusion, combining MBEH with imiquimod and/or CpG or HSP70 should be considered for enhanced depigmentation therapy of vitiligo patients. We propose that MBEH will work synergistically with the aforementioned immune adjuvants to achieve faster and longer-lasting depigmentation, to produce cosmetic results far superior to those achieved with currently available therapies.
Acknowledgments
These studies were supported in part by NIH RO3CA128068 and the Loyola RFC to CLP and support from the Netherlands Organization for Scientific Research (NWO) and Dutch Cancer Society (KWF Kankerbestrijding).
Footnotes
Conflicts of Interest
Authors of this manuscript have no conflicts of interest to declare.
KCW wrote the manuscript, JME designed the figure and provided edits, VH generated proof of concept and gathered references, CH provided critical clinical insight, RML and ICLP designed the hypothesis, contributed research, and edited the manuscript.
References
- 1.Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, et al. Presence or Absence of Melanocytes in Vitiligo Lesions: An Immunohistochemical Investigation. J Invest Dermatol. 1993;100:816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- 2.Alikhan A, Felsten LM, Daly M, et al. Vitiligo: A Comprehensive Review Part I: Introduction, epidemiology, quality of life, diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Felsten LM, Alikhan A, Petronic-Rosie V. Vitiligo: A Comprehensive Review Part II: Treatment options and approach to treatment. J Am Acad Dermatol. 2011;65:493–514. doi: 10.1016/j.jaad.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Laddha NC, Dwivedi M, Mansuri MS, et al. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22:245–250. doi: 10.1111/exd.12103. [DOI] [PubMed] [Google Scholar]
- 5.Kemp EH, Waterman EA, Weetman AP. Immunological pathomechanisms in vitiligo. Exp Rev in Molec Med. 2001;3:1–22. doi: 10.1017/S1462399401003362. [DOI] [PubMed] [Google Scholar]
- 6.Le Poole IC, van den Wijngaard RMJGJ, Westerhof W. Presence of T cells and Macrophages in Inflammatory Vitiligo Skin Parallels Melanocyte Disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Boorn JG, Konijnenberg D, Dellemijn TAM, et al. Autoimmune Destruction of Skin Melanocytes by Perilesional T cells from Vitiligo Patients. J Invest Dermatol. 2009;129:2220–2232. doi: 10.1038/jid.2009.32. [DOI] [PubMed] [Google Scholar]
- 8.Njoo MD, Vodegel RM, Westerhof W. Depigmentation therapy in vitiligo universalis with topical 4-methoxyphenol and the Q-switched ruby laser. J Am Acad Dermatol. 2000;42:760–769. doi: 10.1067/mjd.2000.103813. [DOI] [PubMed] [Google Scholar]
- 9.Alghamdi KM, Kumar A. Depigmentation therapies for normal skin in vitiligo universalis. J Europ Acad Dermatol Venereol. 2011;25:749–757. doi: 10.1111/j.1468-3083.2010.03876.x. [DOI] [PubMed] [Google Scholar]
- 10.Njoo MD, Westerhof W, Bos JD, et al. The development of guideline for the treatment of vitiligo. Arch Dermatol. 1999;135:1514–1521. doi: 10.1001/archderm.135.12.1514. [DOI] [PubMed] [Google Scholar]
- 11.Bolognia JL, Lapia BK, Somma S. Depigmentation Therapy. Dermatol and Ther. 2001;14:29–34. [Google Scholar]
- 12.Peck SM, Sobotka H. Effect of monobenzyl hydroquinone on oxidase systems in vivo and in vitro. J Invest Dermatol. 1941;4:325–329. [Google Scholar]
- 13.Gupta D, Kumari R, Thappa DM. Depigmentation therapies in vitiligo. Indian J Dermatol Venereol and Leprol. 2012;78:49–58. doi: 10.4103/0378-6323.90946. [DOI] [PubMed] [Google Scholar]
- 14.Hariharan V, Klarquist J, Reust MJ, et al. Monobenzyl ether of hydroquinone and 4-tertiary butyl phenol activate markedly different physiological responses in melanocytes: relevance to skin depigmentation. J Invest Dermatol. 2010;130:211–220. doi: 10.1038/jid.2009.214. [DOI] [PubMed] [Google Scholar]
- 15.Hariharan V, Toole T, Klarquist J, et al. Topical Application of Bleaching Phenols; in vivo studies and mechanism of action relevant to melanoma treatment. Melanoma Res. 2011;21:115–126. doi: 10.1097/CMR.0b013e328343f542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann A, Bedenk C, Keikavoussi P, et al. (2008) Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. German Soc of Dermatol. 2008;6:1053–1059. doi: 10.1111/j.1610-0387.2008.06755.x. [DOI] [PubMed] [Google Scholar]
- 17.Van den Boorn JG, Picavet DI, van Swieten PF, et al. Skin-Depigmenting Agent Monobenzone Induces Potent T-Cell Autoimmunity toward Pigmented Cells by Tyrosinase Haptenization and Melanosome Autophagy. J Invest Dermatol. 2011;131:1240–1251. doi: 10.1038/jid.2011.16. [DOI] [PubMed] [Google Scholar]
- 18.Denman CJ, McCracken J, Hariharan V, et al. HSP70i Accelerates Depigmentation in a Mouse Model of Autoimmune Vitiligo. J Invest Dermatol. 2008;128:2041–2048. doi: 10.1038/jid.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Boorn JG, Konijnenberg D, Tjin EPM, et al. Effective Melanoma Immunotherapy in Mice by the Skin- Depigmenting Agent Monobenzone and the Adjuvants Imiquimod and CpG. Plos ONE. 2010;5:1–12. doi: 10.1371/journal.pone.0010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 21.Riley PA. Melanogenesis and Melanoma. Pigment Cell Res. 2003;16:548–552. doi: 10.1034/j.1600-0749.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 22.Prota G. Recent advances in the chemistry of melanogenesis in mammals. J Invest Dermatol. 1980;75:122–127. doi: 10.1111/1523-1747.ep12521344. [DOI] [PubMed] [Google Scholar]
- 23.Hearing VJ, Jimenez M. Mammalian tyrosinase–the critical regulatory control point in melanocyte pigmentation. Int J Biochem. 1987;19:1141–1147. doi: 10.1016/0020-711x(87)90095-4. [DOI] [PubMed] [Google Scholar]
- 24.Slominski A, Tobin DJ, Shibahara S, et al. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nordlund JJ, Boissy RE, Hearing VJ, et al. The Pigmentary System: Physiology and Pathophysiology. New York NY: Oxford University Press; 1998. p. 406. [Google Scholar]
- 26.Solano FM, Martinez-Esparza, Jimenez-Cervantes C, et al. New insights on the structure of the mouse silver locus and on the function of silver protein. Pigment Cell Res. 2000;13:118–124. doi: 10.1111/j.0893-5785.2000.130821.x. [DOI] [PubMed] [Google Scholar]
- 27.Park HY, Pongpudpunth M, Lee J, et al. Biology of Melanocytes. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw Hill; 2007. pp. 591–608. [Google Scholar]
- 28.Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000;13:140–144. doi: 10.1034/j.1600-0749.13.s8.25.x. [DOI] [PubMed] [Google Scholar]
- 29.Rees J. Why are redheads so susceptible to melanoma? In: Newton-Bishop J, Gore M, editors. Melanoma: Critical Debates. Oxford: Blackwell Science; 2002. pp. 49–60. [Google Scholar]
- 30.Ogg GS, Rod Dunbar P, Romero P, et al. High frequency of skin-homing melanocytes-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittet MJ, Valmori D, Dunbar PR, et al. High Frequencies of Naive Melan-A/MART-1–specific CD8+ T Cells in a Large Proportion of Human Histocompatibility Leukocyte Antigen (HLA)-A2 Individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/Mart1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: The role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–332. doi: 10.1046/j.1523-1747.2001.01408.x. [DOI] [PubMed] [Google Scholar]
- 33.Wankowicz-Kalinska A, van den Wijngaard RM, Tigges BJ, et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Invest. 2003;83:683–695. doi: 10.1097/01.lab.0000069521.42488.1b. [DOI] [PubMed] [Google Scholar]
- 34.Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–286. doi: 10.1111/j.1755-148X.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 36.Dwivedi M, Laddha NC, Arora P, et al. Decreased regulatory T cells and CD4+/CD8+ ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell & Melanoma Research. 2013;26:586–591. doi: 10.1111/pcmr.12105. [DOI] [PubMed] [Google Scholar]
- 37.Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2010;36:292–297. doi: 10.1111/j.1365-2230.2010.03972.x. [DOI] [PubMed] [Google Scholar]
- 38.Toosi S, Orlow SJ, Manga P. Vitiligo inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132:2601–2609. doi: 10.1038/jid.2012.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miniati A, Weng Z, Zhang B, et al. Stimulated human melanocytes express and release interleukin–8, which is inhibited by luteolin: relevance to early vitiligo. Clin Exp Dermatol. 2014;39:54–57. doi: 10.1111/ced.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley PA. Mechanism of pigment cell toxicity produced by hydroxyanisole. J Pathol. 1970;101:163–169. doi: 10.1002/path.1711010211. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds JEF. Dermatological agents: mequinol. In: Reynolds JEF, editor. The Extra Pharmacopoeia. Martindale. 29. London: The Pharmaceutical Press; 1989. p. 923. [Google Scholar]
- 42.Yang F, Sarangarajan R, Le Poole IC, et al. The cytotoxicity and apoptosis induced by 4-tertiary butylphenol in human melanocytes are independent of tyrosinase activity. J Invest Dermatol. 2000;114:157–164. doi: 10.1046/j.1523-1747.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 43.Kahn G. Depigmentation caused by phenolic detergent germicides. Arch Dermatol. 1970;102:177–187. [PubMed] [Google Scholar]
- 44.James O, Mayes RW, Stevenson CT. Occupational vitiligo induced by p-tertiary butylphenol, a system disease? Lancet. 1977;310:1217–1218. doi: 10.1016/s0140-6736(77)90451-2. [DOI] [PubMed] [Google Scholar]
- 45.Fisher AA. Contact Dermatitis. 3. Philadelphia: Lea & Febiger; 1986. pp. 675–685. [Google Scholar]
- 46.O’Malley MA, Mathias CGT, Priddy M, et al. Occupational vitiligo due to unsuspected presence of phenolic antioxidant by products in commercial bulk rubber. Occupat Med. 1988;30:512–516. [PubMed] [Google Scholar]
- 47.d’Ischia M, Napolitano A, Pezzella A, et al. 5,6-Dihydroxyindoles and indole-5,6-diones. Heterocyclic Chem. 2005;89:1–63. [Google Scholar]
- 48.Calnan CD, Cooke MA. Leukoderma in industry. Occupational Med. 1974;24:59–61. [Google Scholar]
- 49.Land EJ, Ramsden CA, Riley PA. Tyrosinase autoactivation and the chemistry of ortho-quinone amines. Acc Chem Res. 2003;36:300–308. doi: 10.1021/ar020062p. [DOI] [PubMed] [Google Scholar]
- 50.Douat-Casassus C, Marchand-Geneste N, Diez E, et al. Covalent modification of a melanoma-derived antigenic peptide with a natural quinone methide. Mol Biosyst. 2006;2:240–249. doi: 10.1039/b518044a. [DOI] [PubMed] [Google Scholar]
- 51.Manini P, Napolitano A, Westerhof W, et al. A reactive ortho-quinone generated by tyrosinase-catalyzed oxidation of the skin depigmenting agent monobenzone: self-coupling and thiol-conjugation reactionsand possible implications for melanocyte toxicity. Chem Res Toxicol. 2009;22:1398–1405. doi: 10.1021/tx900018q. [DOI] [PubMed] [Google Scholar]
- 52.Naish-Byfield S, Riley PA. Tyrosinase autoactivation and the problem of the lag period. Pigment Cell Res. 1998;11:127–133. doi: 10.1111/j.1600-0749.1998.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 53.Borovansky J, Riley PA. Physiological and Pathological Functions of Melanosomes. In: Borovansky J, Riley PA, editors. Melanins and Melanosomes. Wienheim: Wiley-VCH Verlag; 2011. pp. 343–381. [Google Scholar]
- 54.Malten KE, Seutter E, Hara I, et al. Occupational vitiligo due to paratertiary butylphenol and homologues. Transactions of the St Johns Hospital Dermatological Society. 1971;57:115–131. [PubMed] [Google Scholar]
- 55.Oliver EA, Schwartz L, Warren LH. Occupational leukoderma. JAMA. 1939;113:927–928. [Google Scholar]
- 56.Zhu Y, Wang S, Xu A. A mouse model of vitiligo induced by monobenzone. Exp Dermatol. 2013;22:499–501. doi: 10.1111/exd.12184. [DOI] [PubMed] [Google Scholar]
- 57.Mosher DB, Parrish JA, Fitzpatrick TB. Monobenzylether of hydroquinone: a retrospective study of treatment of 18 vitiligo patients and a review of the literature. Br J Dermatol. 1977;97:669–679. doi: 10.1111/j.1365-2133.1977.tb14275.x. [DOI] [PubMed] [Google Scholar]
- 58.Jennifer C, Stephie CM, Abhishri SB. A Review on skin whitening property of plant extracts. Internat J Pharmacol Biolo Sci. 2012;3:332–347. [Google Scholar]
- 59.Wilcox DE, Porras AG, Hwang YT, et al. Substrate analog binding to the coupled binuclear copper active site in tyrosinase. J Am Chem Soc. 1985;107:4015–4027. [Google Scholar]
- 60.Chen JS, Wei CI, Marshall MR. Inhibition mechanism of kojic acid on polyphenol oxidase. J Agricult Food Chem. 1991;39:1897–1901. [Google Scholar]
- 61.Bubacco L, Vijenboom E, Gobin C, et al. Kinetic and paramagnetic NMR investigations of the inhibition of Streptomyces antibioticus tyrosinase. J Molec Catalysis B: Enzymatic. 2000;8:27–35. [Google Scholar]
- 62.Burdock GA, Soni MG, Carabin IG. Evaluation of health aspects of kojic acid in food. Regul Toxicol Pharmacol. 2001;33:80–101. doi: 10.1006/rtph.2000.1442. [DOI] [PubMed] [Google Scholar]
- 63.Xie LP, Chen QX, Huang H, et al. Inhibitory Effects of Some Flavonoids on the Activity of Mushroom Tyrosinase. Biochem. 2003;68:487–491. doi: 10.1023/a:1023620501702. [DOI] [PubMed] [Google Scholar]
- 64.Schurink M, Van Berkel WJH, Wichers HJ, et al. Novel peptides with tyrosinase inhibitory activity. Peptides. 2007;28:485–495. doi: 10.1016/j.peptides.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 65.Kang HY, Park TJ, Jin SH. Imiquimod, a toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J Invest Dermatol. 2009;129:243–246. doi: 10.1038/jid.2008.184. [DOI] [PubMed] [Google Scholar]
- 66.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 67.Valins W, Amini S, Berman B. The expression of Toll-like receptors in dermatological diseases and the therapeutic effect of current and newer topical Toll-like receptor modulators. J Clin Aesth Dermatol. 2010;3:20–29. [PMC free article] [PubMed] [Google Scholar]
- 68.Kadowaki N, Ho S, Antonenko S, et al. Subsets of Human Dendritic Cell Precursors Express Different Toll-like Receptors and Respond to Different Microbial Antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janeway CA, Medzhitov R. Innate Immune Recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 70.Miller LS, Modlin RL. Toll-like receptors in the skin. Semin Immunopathol. 2007;29:15–26. doi: 10.1007/s00281-007-0061-8. [DOI] [PubMed] [Google Scholar]
- 71.Choi KB, Wong F, Harlan JM, et al. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem. 1998;273:20185–20188. doi: 10.1074/jbc.273.32.20185. [DOI] [PubMed] [Google Scholar]
- 72.Tsuji S, Matsumoto, Takeuchi O, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette Guerin: Involvement of toll-like receptors. Infect Immun. 2000;68:6883–6890. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Michelsen KS, Aicher A, Mohaupt M, et al. The tole of toll-like receptors (TLRs) in bacteria-induced maturation of murine dendritic cells (DCS). Peptidoglycan and lipoteichoic acid are inducers of DC maturation and require TLR2. J Biol Chem. 2001;276:25680–25686. doi: 10.1074/jbc.M011615200. [DOI] [PubMed] [Google Scholar]
- 74.Thoma-Uszynski S, Kiertscher SM, Ochoa MT, et al. Activation of toll-like receptor 2 on human dendritic cells triggers induction of IL-12, but not IL-10. J Immunol. 2000;165:3804–3810. doi: 10.4049/jimmunol.165.7.3804. [DOI] [PubMed] [Google Scholar]
- 75.Beutner KR, Geisse JK, Helman D, et al. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol. 1999;41:1002–1007. doi: 10.1016/s0190-9622(99)70261-6. [DOI] [PubMed] [Google Scholar]
- 76.Ulrich C, Busch JO, Meyer T, et al. Successful treatment of multiple actinic keratosis in organ transplant patients with topical 5% imiquimod: a report of six cases. Br J Dermatol. 2006;155:451–454. doi: 10.1111/j.1365-2133.2006.07233.x. [DOI] [PubMed] [Google Scholar]
- 77.Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715–716. doi: 10.1016/j.jaad.2004.10.861. [DOI] [PubMed] [Google Scholar]
- 78.Sauder DN. Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol. 2000;43:S6–S11. doi: 10.1067/mjd.2000.107808. [DOI] [PubMed] [Google Scholar]
- 79.Skinner RB., Jr Imiquimod. Dermatol Clin. 2003;21:291–300. doi: 10.1016/s0733-8635(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 80.Kim CH, Ahn JH, Kang SU. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301–306. doi: 10.1007/s00403-009-1012-0. [DOI] [PubMed] [Google Scholar]
- 81.Goding CR. Mitf from neural crest to melanoma: signal transduction and transcription in the melanocyte lineage. Genes Dev. 2000;14:1712–1728. [PubMed] [Google Scholar]
- 82.McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;14:707–718. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- 83.Du J, Miller AJ, Widlund HR, et al. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333–343. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park HY, Wu C, Yonemoto L, et al. MITF mediates cAMP-induced protein kinase C-beta expression in human melanocytes. Biochem J. 2006;395:571–578. doi: 10.1042/BJ20051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uhlmann E, Vollmer J. Recent advances in the development of immunostimulatory oligonucleotides. Current Opinion in Drug Discovery and Development. 2003;6:204–217. [PubMed] [Google Scholar]
- 86.Vasilakos JP, Smith RM, Gibson SJ, et al. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cellular Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 87.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 88.Bivik C, Rosdahl I, Ollinger K. Hsp70 protects against UVB induced apoptosis by preventing release of cathepsins and cytochrome c in human melanocytes. Carcinogenesis. 2007;28:537–544. doi: 10.1093/carcin/bgl152. [DOI] [PubMed] [Google Scholar]
- 89.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 90.Todryk SM, Gough MJ, Pockley AG. Facets of heat shock protein 70 show immunotherapeutic potential. Immunol. 2003;110:1–9. doi: 10.1046/j.1365-2567.2003.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mambula SS, Stevenson MA, Ogawa K, et al. Mechanisms for Hsp70 secretion: crossing membranes without a leader. Methods. 2007;43:168–175. doi: 10.1016/j.ymeth.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pavlik A, Aneja IS. Cerebral neurons and glial cell types inducing heat shock protein HSP70 following heat stress in the rat. Prog Brain Res. 2007;162:417–431. doi: 10.1016/S0079-6123(06)62020-7. [DOI] [PubMed] [Google Scholar]
- 93.Arumugam TV, Okun E, Tang SC, et al. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 94.Kroll TM, Bommiasamy H, Boissy RE. 4 Tertiary Butyl Phenol Exposure Sensitizes Human Melanocytes to Dendritic Cell-Mediated Killing: Relevance to Vitiligo. J Invest Dermatol. 2005;124:798–806. doi: 10.1111/j.0022-202X.2005.23653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasraee B, Fallahi MR, Ardekani GS, et al. Retinoic acid synergistically enhances the melanocytotoxic and depigmenting effects of MBEH in black guinea pigs skin. Exp Dermatol. 2006;15:509–514. doi: 10.1111/j.1600-0625.2006.00441.x. [DOI] [PubMed] [Google Scholar]
- 96.Mosmann TR, Coffman RL. TH1 and TH2 Cells: Different Patterns of Lymphokine Secretion Lead to Different Functional Properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 97.Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed Type Hypersensitivity is mediated by Th1 clones. J Immunol. 1987;138:3688–3694. [PubMed] [Google Scholar]
- 98.Biswas G. Clinical Pattern of Ocular Manifestations in Vitiligo. J Indian Med Assoc. 2003;101:478–480. [PubMed] [Google Scholar]
- 99.Aydogan K, Turan OF, Onart S, et al. Audiological abnormalities in patients with vitiligo. Clin Exp Dermatol. 2006;31:110–113. doi: 10.1111/j.1365-2230.2005.02004.x. [DOI] [PubMed] [Google Scholar]
- 100.Hong CK, Lee MH, Jeong KH, et al. Clinical analysis of hearing levels in vitiligo patients. Eur J Dermatol. 2009;19:50–56. doi: 10.1684/ejd.2008.0563. [DOI] [PubMed] [Google Scholar]