Abstract
Cancer prevention has been associated with decreased rates of cancer incidence and increased survival. Cancer prevention, however, can have a greater impact if barriers to implementing cancer prevention into practice are removed and opportunities are both fostered and seized. The purpose of this article is to identify barriers and opportunities to cancer prevention in clinical practice and provide recommendations for the future. A multidisciplinary team participated in “The Future Directions Cancer Prevention and Control: Workforce Implications for Training, Practice and Policy” workshop on October 17-18, 2009 at The University of Texas MD Anderson Cancer Center in Houston, TX. During the meeting, the team discussed barriers and opportunities for the implementation of cancer prevention into clinical practice. Further data were collected from peer-reviewed journals and published government and cancer agencies reports. Several issues were identified: 1) The funding allocated to basic cancer prevention research and application is not optimal and less than that for cancer treatment; 2) Participation in cancer prevention behaviors and screening practices are lower than desired, especially among the uninsured; 3) A shortage in healthcare professionals is a major challenge in meeting the future needs of cancer prevention; 4) Demands on medical schools to balance increased enrollment, incorporate cancer prevention in an already crowded curriculum, and develop faculty are daunting; and 5) Healthcare reforms in 2010 provide both opportunities and additional challenges for cancer prevention. Based on the current state of cancer prevention, we formed six recommendations: 1) Additional funding for cancer prevention research with a focus on implementation into practice; 2) Improved tracking of cancer prevention research funding and the outcomes associated with it; 3) Continued monitoring of cancer prevention services participation with emphasis on closing the gap in health disparities; 4) Financial and technical assistance to healthcare professional schools for incorporating cancer prevention into curricula; 5) Assessment of the current state of technology in cancer prevention care; and 6) The use of effective multidisciplinary teams in cancer prevention care. Improved delivery of cancer prevention services can have a tremendous impact on cancer incidence and survival rates.
Keywords: Prevention, Control, Workforce, Cancer
II. Introduction
The great and articulate observer of medicine, Dr. Lewis Thomas, has called medicine “the youngest science,” postulating that the real renaissance for medicine began in the middle part of the last century [1]. Prior to this, medicine was not founded on evidence-based scientific principles. The influx of science into medicine led to remarkable progress in the development of new therapies and the understanding of disease pathophysiology. Despite these advancements, Dr. Thomas remarked that the control of most diseases still used “halfway technologies”, that is technologies that are not curative but compensate for the disease and thus prolong life. Therefore, Dr. Thomas advocated that future progress should focus on understanding the underlying pathogenesis of disease and developing new technologies that would prevent or treat diseases.
Our understanding of the physiological mechanisms of disease has increased with momentous scientific events such as the mapping of the human genome. Despite these advancements, progress against killer diseases historically has come mostly through public health measures like improved sanitation, food preservation, and behavior changes. The recent integration of public health measures and scientific discoveries has yielded further progress against disease as evidenced by the decline of age-adjusted death rates from major killer diseases in industrialized and developing countries. Cancer, however, still remains the second leading cause of death in the United States [2]. The key to cancer control is cancer prevention; the understanding and treatment of later stages cancer, however, has been the primary focus of research efforts with cancer prevention as a secondary focus.
Cancer prevention has been classified into three categories: primary, secondary, and tertiary cancer prevention. Behavior changes that decrease the risk of cancer fall into primary cancer prevention. Secondary prevention strategies focus on early detection of existing cancer when treatment and cure are more likely to be achieved. In tertiary cancer prevention, the focus is to prevent and control the symptoms and morbidity caused by established cancer and cancer therapy and to prevent development of secondary cancers or other disease. Primary cancer prevention has targeted behaviors such as tobacco use, poor diet, physical inactivity, and exposure to certain infectious agents, UV radiation, and occupational and environmental toxins that are associated with increased cancer risk [2]. Despite the known link between these behaviors and cancer, 744,550 cancer deaths in 2011 are projected to be attributed to tobacco use, poor nutrition, physical inactivity, overweight, and obesity [2]. Thus, more effort is needed to make primary cancer prevention a priority among the general population and to aid individuals in making these changes by providing effective resources and removing existing barriers. For individuals with inherited susceptibility to cancer, it is particularly important to provide tools and resources to identify these behaviors and to make appropriate lifestyle changes. In addition, best practices must continue to be developed and applied to eliminate tobacco use and occupational and environmental toxins, to improve diet, weight management, and physical activity participation, to control or immunize against infectious agents like hepatitis B and C, the Human Papilloma Virus (HPV) and other pathogens, and to protect against DNA damage from ultraviolet light exposure.
Besides targeting behavioral changes, other opportunities exist for reducing cancer risk such as secondary cancer prevention or early detection, which is associated with improved cancer survival. Unfortunately, secondary cancer prevention has been in a state of turmoil. Guidelines for use of screening mammography are in flux, and the PSA test for screening for prostate cancer has not lived up to its promise. Despite the effectiveness of colorectal cancer screening, many individuals at risk do not adhere to guidelines for screening. In 2011, an effective screening method for lung cancer was introduced [3]; this technology, however, may prove costly to implement, and screening guidelines still need to be developed. For some cancers, including those that are highly aggressive such as ovarian and pancreatic cancer, no effective secondary prevention strategies exist. Thus, several opportunities exist to improve secondary cancer prevention such as the development of new technology for detecting these cancers at early stages and more effective guidelines to implement secondary cancer prevention into clinical and community practices.
The focus of this article is on implementing cancer prevention into clinical practice. Our primary purpose is to identify opportunities and barriers to implementing cancer prevention into practice. Our secondary aim is to provide suggestions for advancing cancer prevention both inside and outside the clinical practice. Roadblocks and opportunities in cancer prevention are examined at several levels, including medical education and incentives, community outreach, legislative issues, and integration across health professionals. Recommendations based on those issues deemed most crucial to successful implantation of cancer prevention into clinical practice are presented.
III. Methods and Materials
The “Future Directions in Cancer Prevention and Control: Workforce Implications for Training, Practice and Policy” workshop took place October 17-18, 2009 at The University of Texas MD Anderson Cancer Center in Houston, TX.
At the workshop, which included five working groups, our group, Implementing Cancer Prevention into Clinical Practice, was composed of eleven people. After initial overview presentations, each group spent roughly six hours brainstorming about the current state of affairs, the most pressing issues, the desired outcomes, the methods already in existence to increase, to become more proficient at, or to do differently, and the methods that should be developed. At the end of our time together, our group developed a projected timeline of research and paper compositions, as well as made a presentation to the other four working groups about our ideas, topics and plans for further research and development of our paper.
Since the symposium, we gathered additional information on the topic and drafted this paper on the direction of cancer prevention training implementation into the clinical practice. Data on research funding, cancer prevention practices, workforce status, and healthcare reform were collected from published articles in peer-reviewed journals and published governmental and cancer agencies reports; these were used to support the issues discussed in this article.
IV. Results/Synthesis
Research in clinical cancer prevention, traditionally, has flowed in a unidirectional manner: 1) concepts were first tested in the laboratory, 2) knowledge gained was used to establish guidelines for patient care, and 3) then community-based guidelines, programs, and policies were developed. As a result of this unidirectional flow, valuable knowledge gained in the laboratory has been lost or misinterpreted resulting in confusing cancer prevention recommendations and delayed integration of screening practices in the community. In recognition of these problems, initiatives have been established to facilitate the multidirectional flow of cancer prevention knowledge among researchers, clinicians, and the community. During this cancer prevention workforce meeting, the group identified barriers and opportunities that influence information flow and cancer prevention delivery to both individuals and communities. Specifically, we focused on four specific areas: 1) funding available for cancer prevention research; 2) funding available for application of cancer prevention strategies into clinical practice; 3) successes and barriers to implementing cancer prevention recommendations; and 4) the anticipated impact of the New Healthcare Reform.
1. Funding Available for Cancer Prevention Research
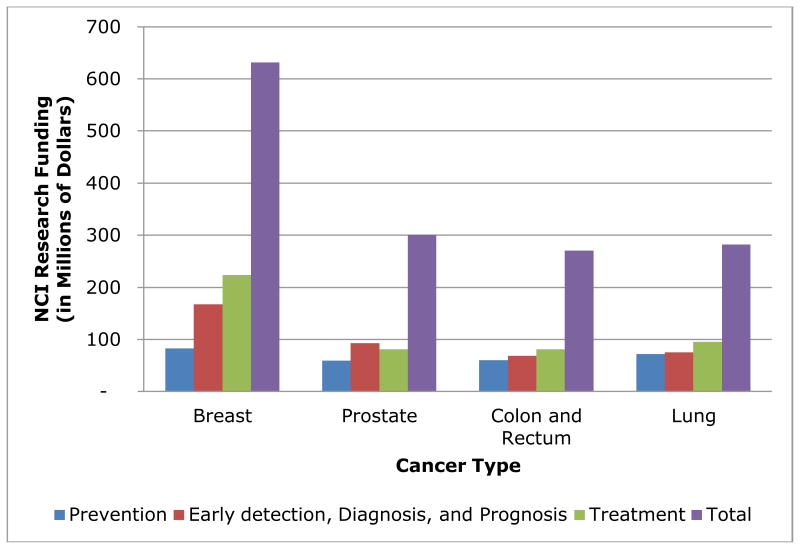
Cancer prevention compared to other cancer research areas is not well-funded. In 2010, cancer prevention studies received 32% less funding than early detection, diagnosis, and prognosis (early detection) studies and 43% less funding than cancer treatment for the four leading cancer sites (i.e., breast, prostate, lung, and colon and rectum) from the National Cancer Institute (NCI) [4]. Of the $275M classified as cancer prevention funding, more than half ($144M) was classified as “Resources and Infrastructure Related to Prevention” [4]. Projects under this category generally are not specific to cancer prevention but span all seven categories of cancer research (i.e., “Biology”, “Etiology”, “Prevention”, “Early detection, Diagnosis, and Prognosis”, “Treatment”, “Cancer Control, Survivorship, and Outcomes Research”, and “Scientific Model Systems”). In comparison, early detection and cancer prevention had $201M (50%) and $152M (32%), respectively, in a related research and infrastructure category [4]. After exclusion of this broad category, cancer prevention research for breast, prostate, lung, and colon and rectum cancers accounted for only $132M compared to $204M for early detection studies and $329M for cancer treatment studies.
2. Funding Available for Applying Cancer Prevention Strategies into Clinical Practice
Compared to cancer prevention, cancer treatment research received more funding for clinical application of findings. In 2004, approximately one-third ($1.3 billion) of the total NCI budget was awarded to research studies that were classified as “translation research” by NCI Translational Research Working Group (TRWG) [5]. A research study was classified as translational if one of the specific aims was to move findings into clinical application or products to use clinical findings to guide research [5]. While a specific breakout by research type (i.e., cancer prevention, early detection, and treatment) is not available, 2004 research projects by NCI divisions are summarized in Table 1. The Division of Cancer Treatment and Diagnosis (DCTD) received the largest proportion (39%) of 2004 translational research projects fund. DCTD's translation funding in 2004 exceeded the combined funds apportioned to the Division of Cancer Prevention (DCP), the Division of Cancer Control and Population Sciences (DCCPS), and the Division of Cancer Epidemiology and Genetics (DCEG), the last of which conducts intramural research at the NCI.
Table 1. 2004 NCI Translation Research Projects by Division (5).
| NCI Division | Focus | % of Projects (Estimated Projects) | % of Funding (Estimated $ in millions) |
|---|---|---|---|
| Division of Cancer Treatment and Diagnosis (DCTD) | Diagnosis, Assessment, Treatment, and Cure | 39% (1,090) | 39% ($520) |
| Division of Cancer Control and Population Sciences (DCCPS) | Public health research, practice, and policy | 22% (615) | 19% ($250) |
| Division of Cancer Biology (DCB) | Basic cancer research | 11% (305) | 8% ($110) |
| Office of the Deputy Director for Extramural Science (DDES) | Includes the Office of Centers, Training and Resources, the Office of Cancer Complementary and Alternative Medicine, and the Office of Grant Program Coordination | 9% (250) | 13% ($170) |
| Division of Cancer Prevention (DCP) | Cancer prevention research | 9% (250) | 9% ($120) |
| Centers for Cancer Research (CCR) | Multidisciplinary, translational research | 8% (225) | 7% ($95) |
| Division of Cancer Epidemiology and Genetics (DCEG) | Genetic and environmental causes of cancer and cancer prevention. | 2% (55) | 5% ($65) |
Additional funding for translational research in cancer is available under the National Center for Research Resources (NCRR) Clinical Science and Translation Award (CSTA) program. The aim of this program, which is not specific to cancer, is to facilitate the translation of laboratory knowledge into treatment and strategies for patient care by developing national research capabilities and by providing research training and collaboration across fields [6]. In the 2006-2008 CSTA progress report, NCI was reported to have the third largest number of grants (423 grants) of NIH Institute and Centers [6], which used CTSA resources. No data were presented on the research focus of these grants.
3. Cancer Prevention Recommendations: Successes and Barriers
Research has demonstrated that smoking, obesity, physical inactivity, and sun exposure are associated with increased risk of cancer [2]. Despite this knowledge, many individuals continue to engage in these risky lifestyle behaviors (Table 2) [2]. While cancer screening rates are increasing, individuals without health insurance or are recent immigrants are less likely to take part in cancer screening than those who are insured (Table 3) [2]. In 2011, approximately 46.6 million people in the U.S. (or 15.3%) were uninsured [7]. The prevalence of individuals lacking health insurance is higher in minority populations and those individuals classified as poor [8]. In general, these populations have higher rates of obesity and engage in lifestyle behaviors such as physical inactivity and smoking, which are known risk factors for cancer [9]. As a result, uninsured individuals experience higher cancer incidence have later stage at initial diagnosis, and are less likely to have preventive screening than their insured counterparts [10-12]. The New Healthcare Reform may help provide access to much-needed cancer prevention services for uninsured individuals, resulting in lower cancer incidence and improved disease outcomes [11, 12].
Table 2. Trends in Individual Choices (2).
| Individual Choices | Prevalence |
|---|---|
| Smoking | 20.6% of Adults 19.5% of High School Students |
| Obesity | 18.1 % in 12-19 y (2007-2008) 34.3% in 20-74 y (2007-2008) |
| Physical Activity | 37% of adolescents were physically active for ≥ 60 min on 6-7 days per week (2009) 24.4% of adults reported no physical activity (2009) 23.8% of adults participated in vigorous physical activity (2009) 49.5% of adults participated in moderate physical activity (2009) |
| Consumed 5 or more servings of Fruit and Vegetables a day | 23.5% of adults (2007) 22.3% of high school students (2009) |
| Apply Sunscreen | 9.3% of high school students (2009) 32.6% of adults (2008) |
Table 3. Early Detection Screening and HPV Vaccination Prevalence (2).
| Screening | Prevalence |
|---|---|
| Mammography in Past 2 Year, Women 40 and Older, U.S., 2008 | 67.1% of total 35.6% of total with no health insurance coverage 49.7% of total recent immigrants |
| Pap Test with past 3 years, Women 18 and Older, U.S., 2008 | 78.3 % of total 60.6% of total with no health insurance coverage 60.1% of total recent immigrants |
| HPV vaccination, Female Adolescents (13 to 17 y), U.S., 2009 | 44.3% had at least one of the three shot series |
| Colorectal Screening (Fecal occult blood test (FOBT) in past year, Sigmoidoscopy within past 5 years, or Colonoscopy within past 10 year), Adults 50 and older, 2008 | 53.2% of total 19.5% of total with no health insurance coverage |
| Prostate Cancer Screening (PSA Test) in past year, Men 50 and Older, U.S., 2008 | 44.1% of total 9.1% of total with no health insurance |
A major barrier to implementing cancer prevention into clinical practice is the state of the current cancer prevention workforce. A primary concern is that projected workforce shortages are expected across most healthcare professions, including but not limited to oncologists for whom a shortfall is predicted of 2,550-4,080 by 2020 [13], primary care physicians for whom a shortage of 45,880-58,941is expected by 2025 [14], and registered nurses who are expected to number 285,000 FTEs by 2020, a number smaller than believed to be adequate for providing care for anticipated numbers of cancer patients and survivors in the future [15]. Moreover, Universal Healthcare Coverage is expected to increase the demand for healthcare professionals by 25% [14], further straining the insufficiently sized future workforce. The geographic distribution of qualified medically trained individuals is also a concern [14]. As of September 1, 2011, shortages were identified by U.S. Department of Health's Human Resources and Service Administration for primary care, dental, and mental health providers [16]. To address the healthcare shortage, several solutions have been proposed including: 1) increasing productivity of the workforce by using electronic medical records and harnessing technology; 2) delaying retirement for existing medical professionals while increasing the number of fellowship spots; and 3) changing the work schedules of existing medical professionals while increasing the usage of NPs and PAs in place of physicians [13, 14, 17]. These changes will help bridge some of the gap between supply and demand of healthcare services, but will still leave a significant healthcare provider shortage, especially involving clinicians most involved in cancer prevention.
Another barrier to clinical cancer prevention is education. A primary issue is that cancer prevention education is missing from curricula of many professional programs [18, 19]. For example, many dental professionals lack knowledge of prevention and diagnosis of oral and pharyngeal cancers, which occur in more than 35,000 individuals each year in the United States and which have less than a 60% 5-year relative survival rate [20]. The significant morbidity and mortality associated with head and neck cancers can have severe impact on the quality-of-life of patients and their family and friends, and can create great financial strain. In addition to these cancers, patients develop literally hundreds of thousands of benign oral and pharyngeal lesions that have a potential to transform into malignancy. Many cancers can be prevented if healthcare providers can learn to recognize early signs and symptoms and recommend appropriate treatment. Primary cancer prevention through the control of tobacco, a major risk factor for oral and pharyngeal cancers and ‘precancerous’ mucosal changes, makes knowledge of tobacco cessation strategies a cornerstone for the educational preparation of healthcare providers [21], particularly those who have regular contact with healthy individuals. Dentists and dental hygienists comprise an important cadre of over 250,000 professionals with routine contact who serve as a resource to bolster cancer control through prevention, diagnosis, rehabilitation and appropriate and timely referrals. Therefore, increasing undergraduate programs, continuing professional education programs, and validated reports of effective interventions in the literature are all necessary components for implementing cancer prevention into clinical practice [20].
Another barrier to implementing clinical cancer prevention successfully is the lack of diversity among students training for careers in healthcare professions. For example, Black or African American, Hispanic or Latino, American Indian and Alaska Native, and Native Hawaiian and Other Pacific Islander graduates accounted for only 14.8% (22) of U.S. medical students graduates in 2007 while these groups accounted for 32.9% of the U.S. Population in 2010 [23]. Insufficient diversity among students perpetuates lack of diversity among the practicing professionals, such that attention to recruiting more diverse students earlier in their careers and educational preparation can have important later impact on healthcare practice. Finally, in order to alleviate future physician shortages, higher enrollment in medical schools is expected. This increased demand coupled with the increasing average age of medical faculty will create additional challenges for medical schools as they seek to recruit and integrate new faculty, replacing senior faculty members as they retire [24]. The future impact on oncology and cancer prevention efforts will be no less than for other specialty areas; however, greater numbers of Baby Boomer individuals and greater diversity within the U.S. population at large who are at risk for cancer, who have longer life expectancies, and who have access to a growing arsenal of prevention and therapeutic approaches against cancer comprise a more complex challenge to the future oncology workforce than has been faced in past. The New Healthcare Reform, which is reviewed in the next section, addresses some of the cancer prevention barriers; however, the increased demand on medical professionals and their oncology colleagues remains of concern.
4. Impact of the New Healthcare Reform
The New Healthcare Reform addresses some of the most common barriers to cancer prevention in clinical practice (Table 4). A major positive change associated with cancer prevention is the provision of affordable insurance to uninsured individuals. This change should yield increased participation in cancer screening and immunization programs. A major drawback of the reform, however, is the increased demand on healthcare professionals. The increase in educational loan repayments and training program grants for healthcare professionals may help bridge the supply-demand gap. Other opportunities in cancer prevention, that occur as a result of the New Healthcare Reform, include additional funding for research and a focus on diversity in medical education.
Table 4. Changes Proposed by New Healthcare Reform by Area.
| Areas | New Healthcare Reform Changes |
|---|---|
| Preventive Services |
|
| Research | Cures Acceleration Network (CAN) within the Office of the Director of NIH to award grants and contracts “to accelerate the development of high need cures, including through the development of medical products and behavioral therapies. |
| Education | Federally Supported Student Loans for:
|
V. Discussion
Cancer prevention strategies have been associated with reduced cancer incidence rates and increased survival, but opportunities exist to further this success. Based on available data, basic cancer prevention research and clinical application of cancer prevention strategies received less NCI funding than efforts in cancer treatment [4, 6]. Better tracking, however, on research funding allocated to cancer prevention and the outcomes of these projects are needed. Furthermore, although successful cancer prevention practices exist, many individuals, particularly those lacking health insurance, engage in behaviors that increase cancer risk and participate at lower rates in cancer prevention screening or immunizations. The New Healthcare Reform is expected to improve participation in cancer prevention by providing access to cancer prevention services for uninsured and by funding community-based prevention programs. In addition, further implementation of cancer prevention into healthcare professional curricula and increased diversity of medical students would enhance these efforts by preparing the next generation for health care already knowledgeable and committed to cancer prevention in clinical practice.
A major challenge for cancer prevention is the number of projected healthcare professional workforce shortages. Solutions have been proposed such as increasing medical school enrollment and expanding exposure to cancer prevention through educational curricula during training [25], as well as providing educational funding to both students and educational institutions. This would facilitate paths to careers in clinical cancer prevention which are difficult to navigate without guidance. Medical schools, however, are faced with the daunting task of managing increased enrollment, integration of cancer prevention into educational curriculum, and high faculty turnover as senior faculty members retire. For post-graduate medical education, strategies to strengthen physicians in preventive medicine residencies are also urgently needed [26]. Other oncology workforce strategies include recruiting physician extenders trained for care of cancer patients and survivors [13]; such professionals would be ideal agents for clinical cancer prevention practice as well.
Other opportunities exist that are not specifically explored in this article. These include harnessing technology and healthcare professionals working as multidisciplinary teams in clinical cancer prevention care. Technology can facilitate clinical cancer prevention activities (e.g., electronic reminders for routine screenings) and also could play a role in cancer prevention by providing a means to share best practices across healthcare disciplines. This could be accomplished by providing online cancer prevention training for healthcare professionals, and by creating virtual multidisciplinary cancer prevention teams both in research and clinical practice. The view that clinical cancer prevention activities are solely within the scope of oncologists, oncology nurses, and primary care physicians also needs to change and include dental care providers, dieticians, social workers, pharmacists, researchers, nurses, physician assistants, and others. If the current barriers for cancer prevention can be overcome with the advent of the New Healthcare Reform and other initiatives, then the impact of cancer prevention on decreasing cancer incidence and mortality and increasing survival would be tremendous.
VI. Recommendations
Recommendations to further integrate cancer prevention into clinical practice are as follows:
More research funding should be allocated to cancer prevention research with a focus on implementation into clinical practice.
Better tracking system of cancer prevention research and the outcome of these research projects is needed.
Participation in cancer preventative services among the uninsured should be monitored to determine the success of the new healthcare reform to provide wider access to cancer prevention services and to reduce cancer health disparities between subpopulations.
Funding and other assistance to schools for healthcare professionals should be provided as schools are faced with trying to successfully manage increasing enrollment, retirement of senior faculty, and integration of diversity and cancer prevention into their curricula.
Technology offers an opportunity to overcome some of the barriers to clinical cancer prevention. Thus, the current state of technology use in cancer prevention should be assessed, and suggestions should be made to maximize technology in implementing cancer prevention into practice.
The use of multidisciplinary healthcare professional teams is another opportunity for clinical cancer prevention. Identification of barriers and opportunities for integrating multidisciplinary teams in cancer prevention care is needed.
Figure 1. 2010 National Cancer Institute (NCI) Research Funding by Cancer Type and Research Type (4).
Acknowledgments
We would like to acknowledge all of the members of our group who participated actively during the symposium, including Dr. Elizabeth Grubbs and Dr. Shannon Silkensen.
Contributor Information
Lynn Cialdella-Kam, Email: lynn.ciadella@gmail.com, Human Performance Laboratory, Appalachian State University – NCRC, 600 Laureate Way, Kannapolis NC28081; Phone: 704-250-5354; Fax: 704-250-5409.
Parichart Sabado, Community Health Science, University of California, Los Angeles, P.O. Box 951772, Los Angeles, CA 90095-1772
Leslie Bernstein, Division of Cancer Etiology Department of Population Sciences, Dean for Faculty Affairs, Beckman Research Institute and City of Hope National Medical Center, City of Hope, 1500 E Duarte Rd., Duarte, CA, 91010.
M. Katherine Bispeck, Department of Behavioral Science, Unit 1330, The University of Texas MD Anderson Cancer Center, Houston, Texas 77230-1439.
Ernest Hawk, Division of Cancer Prevention & Population Sciences, Boone Pickens Distinguished Chair for Early Prevention of Cancer, P O Box 301439 – Unit 1370, The University of Texas MD Anderson Cancer Center, Houston, Texas 77230-1439.
Virginia Krawiec, Health Professional Training in Cancer Control and Institutional Research Grants, Extramural Grants Department, American Cancer Society, 250 Williams Street NW, 6th Floor, Atlanta, GA 30303-1002.
Joseph F. O'Donnell, HB 7010, Student Affairs Office, Remsen 331, Dartmouth Medical School, Hanover, NH 03755.
Sol Silverman, Department of Oral Medicine, University of California San Francisco School of Dentistry, 521 Parnassus Ave., Suite C-646, San Francisco, CA 94143-0658.
References
- 1.Thomas L. The youngest science : notes of a medicine-watcher. New York: Viking Press; 1983. [Google Scholar]
- 2.Society, AC, editor. Cancer Prevention & Early Detection Facts & Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 3.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institues's Division of Extramural Activities; 2010. The National Cancer Institute Funded Research Portfolio. database on the Internet. [cited 1/10/2012]. Available from: http://fundedresearch.cancer.gov/ [Google Scholar]
- 5.NCI Translational Research Working Group of the National Cancer Advisory Board. Transforming Translation- Harnessing Discovery for Patient and Public Benefit. 2007 [Google Scholar]
- 6.National Center for Research Resources. Clinical and Translational Science Awards: Advancing scientific discoveries nationwide to improve health. Progress report 2006–2008. 2009 [Google Scholar]
- 7.Martinez ME, Cohen RA. Health insurance coverage: Early release of estimates from the National Health Interview Survey, January–June 2011. National Center for Health Statistics; Dec, 2011. [Google Scholar]
- 8.Halle M, Lewis CB, Seshamani M. HHS Web Communications and New Media Division, HHS Office of Health Reform; Jan 10, 2012. Health Disparities: A case for closing the gap. serial on the Internet. Available from: http://www.healthreform.gov/reports/healthdisparities/ [Google Scholar]
- 9.U.S. Department of Health and Human Services. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat. 2010;10(252) [PubMed] [Google Scholar]
- 10.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–31. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 11.Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D. American society of clinical oncology policy statement: disparities in cancer care. J Clin Oncol. 2009;27(17):2881–5. doi: 10.1200/JCO.2008.21.1680. [DOI] [PubMed] [Google Scholar]
- 12.Ward E, Halpern M, Schrag N, Cokkinides V, DeSantis C, Bandi P, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9–31. doi: 10.3322/CA.2007.0011. [DOI] [PubMed] [Google Scholar]
- 13.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists : challenges to assuring access to oncology services. J Oncol Pract. 2007;3(2):79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dill MJ, Salsberg ES. The complexties of physician supply and demand: Projections through 2005. Association of American Medial Colleges, Center for Workforce Studies; 2008. [Google Scholar]
- 15.Buerhaus PI. Current and future state of the US nursing workforce. JAMA. 2008;300(20):2422–4. doi: 10.1001/jama.2008.729. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services: Health Resources and Services Administration. Shortage Designation: Health Professional Shortage Areas & Medically Underserved Areas/Populations. 2011 [January 10, 2012]; Available from: http://bhpr.hrsa.gov/shortage/
- 17.Kane GC, Grever MR, Kennedy JI, Kuzma MA, Saltzman AR, Wiernik PH, et al. The anticipated physician shortage: meeting the nation's need for physician services. Am J Med. 2009;122(12):1156–62. doi: 10.1016/j.amjmed.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Nekhlyudov L, Braddock CH., 3rd An approach to enhance communication about screening mammography in primary care. J Womens Health (Larchmt) 2009;18(9):1403–12. doi: 10.1089/jwh.2008.1184. [DOI] [PubMed] [Google Scholar]
- 19.Applebaum E, Ruhlen TN, Kronenberg FR, Hayes C, Peters ES. Oral cancer knowledge, attitudes and practices: a survey of dentists and primary care physicians in Massachusetts. J Am Dent Assoc. 2009;140(4):461–7. doi: 10.14219/jada.archive.2009.0196. [DOI] [PubMed] [Google Scholar]
- 20.Silverman S, Jr, Kerr AR, Epstein JB. Oral and pharyngeal cancer control and early detection. J Cancer Educ. 2010;25(3):279–81. doi: 10.1007/s13187-010-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroeder KL, Heisel DE. Role of Dentists in Cessation Counseling: Survey Findings. Smoking and Tobacco Control Monographs. 1992:297–306. [Google Scholar]
- 22.AAMC. Diversity in Medical Edication: Facts and Figures, 2008. Association of American Medial Colleges, Diversity Policy and Programs. 2008:1–146. [Google Scholar]
- 23.Burea USC. U.S. Data: State and County Quick Facts. 2010 [January 10, 2011]; Available from: http://quickfacts.census.gov/qfd/states/00000.html.
- 24.Alexander H, Liu CQ. The Aging of Full-time U.S. Medical School Faculty: 1967-2007. AAMC Analysis in Brief. 2009;9(4):1–12. [Google Scholar]
- 25.Dajani Z, Geller AC. Cancer prevention education in United States medical schools: how far have we come? J Cancer Educ. 2008;23(4):204–8. doi: 10.1080/08858190802188552. [DOI] [PubMed] [Google Scholar]
- 26.Brenner S, Siu K. Preventive medicine and public health residency training: federal policy and advocacy opportunities. J Public Health Management Practice. 2009 Nov;(Suppl):S33–S39. doi: 10.1097/PHH.0b013e3181bdfd46. [DOI] [PubMed] [Google Scholar]