Abstract
Background
The prevalence and perceived effectiveness of marijuana use has not been well studied in inflammatory bowel disease (IBD) despite increasing legal permission for its use in Crohn's disease. Health care providers have little guidance about the IBD symptoms that may improve with marijuana use. The aim of this study was to assess the prevalence, sociodemographic characteristics, and perceived benefits of marijuana use among patients with IBD.
Methods
Prospective cohort survey study of marijuana use patterns in patients with IBD at an academic medical center.
Results
A total of 292 patients completed the survey (response rate = 94%); 12.3% of patients were active marijuana users, 39.0% were past users, and 48.6% were never users. Among current and past users, 16.4% of patients used marijuana for disease symptoms, the majority of whom felt that marijuana was “very helpful” for relief of abdominal pain, nausea, and diarrhea. On multivariate analysis, age and chronic abdominal pain were associated with current marijuana use (odds ratio [OR], 0.93; 95% confidence interval [CI], 0.89–0.97; P < 0.001 and OR, 3.5; 95% CI, 1.24-9.82; P = 0.02). Age and chronic abdominal pain were also multivariate predictors of medicinal use of marijuana (OR, 0.93; 95% CI, 0.89–0.97; P < 0.001 and OR, 4.7; 95% CI, 1.8–12.2; P = 0.001). Half of the never users expressed an interest in using marijuana for abdominal pain, were it legally available.
Conclusions
A significant number of patients with IBD currently use marijuana. Most patients find it very helpful for symptom control, including patients with ulcerative colitis, who are currently excluded from medical marijuana laws. Clinical trials are needed to determine marijuana's potential as an IBD therapy and to guide prescribing decisions.
Keywords: cannabis, chronic abdominal pain, Crohn's disease, inflammatory bowel disease, marijuana
In November 2012, Massachusetts joined 10 other states in explicitly including Crohn's disease (CD), but not ulcerative colitis (UC), as an indication for legally prescribed medical marijuana. These laws reflect a growing trend toward using marijuana to treat the sequelae of chronic disease including anxiety, depression, spasticity, and chronic pain in multiple sclerosis, and poor appetite, nausea, and parasthesias in human immunodeficiency virus.1–3 Despite the inclusion of CD in medical marijuana laws, few studies have explored its use patterns or its perceived benefit in this disease, limiting our understanding of what symptoms health care providers might target with marijuana.
Worldwide, cannabis use, whether medicinal or recreational, is relatively common with 2.8% to 4.5% of people aged 15 to 64 years reporting cannabis consumption in 2009.4 In North America, this number is higher, approximately 10.7% of people are 15 to 64 years old.4 Few studies, however, have assessed the prevalence of marijuana use specifically in the inflammatory bowel disease (IBD) population. An Israeli study found that 10% of patients with IBD were using recreational drugs for disease-related purposes, the majority of which was cannabis.5 Similarly, a Canadian study found that 11.4% of patients with IBD had used cannabis in the previous year and 43.9% had used it at some point in their lifetime.6 Patients with IBD were primarily using cannabis to alleviate abdominal pain. This perceived clinical benefit was recently supported by a small placebo controlled trial that suggested marijuana may induce remission in a sizeable proportion of patients with CD who have failed medical therapy.7
Marijuana is thought to have these effects partly through its anti-inflammatory properties, which may be particularly relevant to IBD. Two cannabinoid receptors have been identified, CB1 and CB2, which make up part of the endocannabinoid system and are activated by marijuana and one of its active component, delta-9-tetrahydrocannabinol.8,9 The endocannabinoid system is a regulatory system in the gastrointestinal tract and is thought to control aspects of intestinal inflammation. The endocannabinoid system has been shown, in experimental models, to have some impact on intestinal inflammation.10
Despite these potential therapeutic benefits, there has been no study investigating marijuana use in the United States IBD population. As a consequence, little is known about the prevalence, reason for use, and perceived benefit of marijuana in this group. There is little guidance for health care providers who may want to prescribe marijuana to target specific IBD symptoms. We investigated the prevalence of marijuana use among patients with IBD at our center to understand the symptoms for which patients were treated with marijuana and how effective they perceived marijuana to be of symptom relief.
Methods
We conducted a prospective cohort survey study of patients with IBD seen at the Brigham and Women's Hospital Crohn's and Colitis Center, a single clinic site that primarily sees patients with IBD. Patients with a diagnosis of IBD (CD, UC, or indeterminate colitis) were eligible to participate. Potential subjects were identified by individual physicians during a scheduled clinic visit. Subjects were then asked to fill out surveys while in the clinic either before or after a scheduled visit. Surveys were assigned consecutive numbers and were anonymous. Patients were recruited over a 3-month period. All patients with IBD were offered enrollments and consecutive patients were enrolled, as possible.
The survey contained a total of 46 questions (see Appendix, Supplemental Digital Content 1, http://links.lww.com/IBD/A340) including basic demographic and clinical data and the short inflammatory bowel disease questionnaire (SIBDQ).11 The SIBDQ is a validated disease-specific questionnaire for assessing health-related quality of life. Its scores range from 1 to 7, with 1 being poor control and 7 being optimum control. The remaining survey questions were about the patient's experience with marijuana use. Patients identified themselves as either current, past, or never users. Those who identified themselves as either current or past marijuana users were then prompted to answer questions about both medicinal use of marijuana (defined as use to control symptoms of IBD) and recreational use of marijuana. If they answered “yes” to medicinal use, they were asked specifically if they used marijuana (yes/no) to treat abdominal pain, diarrhea, nausea, and/or loss of appetite. Patients who responded affirmatively were then asked how effective marijuana was in treating that symptom on the following scale: ineffective, slightly helpful, moderately helpful, very helpful, or complete relief. Never users answered questions about their attitudes toward medicinal use of marijuana. Surveys that were incomplete were excluded from the analysis.
The primary outcome of interest was identification of IBD symptoms for which marijuana was used and its perceived clinical benefit. Secondary outcomes included clinical and demographic characteristics associated with marijuana use. We used simple descriptive statistics to identify means and SDs for variables of interest. We used 2-tailed Student's t tests to compare continuous variables to identify predictors of marijuana use. We used chi-square tests to compare categorical variables to identify predictors of marijuana use. For categorical variables where n < 15, we used Fisher's exact tests. We constructed a logistic regression model to estimate the adjusted association between statistically significant bivariate predictors and medicinal use of marijuana, current marijuana use, and past marijuana use, respectively. The Brigham and Women's Hospital Institutional Review Board approved this study.
Results
The survey was distributed to 310 patients and 292 completed surveys were returned (response rate = 94%). The clinical and demographic characteristics of the study population are listed in Table 1. The mean age of respondents was 39.3 ± 14.1 years, 67.8% were female, and 60.6% had CD. The mean SIBDQ score of the population was 3.9 ± 1.4. Two-thirds of patients (66.1%) had been hospitalized at least once since diagnosis, 33.2% described their disease to be in remission, and 36.3% had chronic abdominal pain related to their disease. Among patients with chronic abdominal pain, 22 (20.7%) were currently using narcotics for this symptom. About half of the study population (48.6%) reported having never used marijuana, 12.3% reported current marijuana use, and 39.0% reported earlier but not current use. Among the 22 patients using narcotics, 8 were current marijuana users, 4 were past users, and 10 were never users.
Table 1. Clinical and Demographic Characteristics (n = 292).
| Age, yr | 39.3 ± 14.1 |
| Female, % | 198 (67.8) |
| White (not of Hispanic origin), n (%) | 273 (93.4) |
| Diagnosis, n (%) | |
| UC | 102 (34.9) |
| CD | 177 (60.6) |
| Indeterminate colitis | 13 (4.4) |
| SIBDQ score | 3.9 ± 1.4 |
| History of surgery, n (%) | 107 (36.6) |
| Any hospitalization since diagnosis, n (%) | 193 (66.1) |
| Current therapy with biologics, n (%) | 119 (50.7) |
| In remission, n (%) | 96 (33.2) |
| Chronic abdominal pain, n (%) | 106 (36.3) |
| Current narcotic use for abdominal pain, n (%) | 22 (20.7) |
Medicinal Use of Marijuana
Among all marijuana users (past and current), 48 patients (32%) had used it medicinally (Table 2). The majority (61.1%) reported smoking it in cigarettes as their primary method of consumption. Abdominal pain was the most frequently reported symptom treated with marijuana (89.5%) followed by poor appetite and nausea (72.9% for each) and, lastly, diarrhea (41.6%). Most patients reported that marijuana was “very helpful” or “completely relieving” in treating the symptoms for which they were using it (Fig. 1).
Table 2. Characteristics of Marijuana Use Patterns.
| Marijuana use (n = 292), n (%) | |
| Never | 142 (48.6) |
| Past | 114 (39.0) |
| Current | 36 (12.3) |
| Current users (n = 36), n (%) | |
| Recreational use | 26 (72.2) |
| Medicinal use | 30 (83.3) |
| Method | |
| Smoking | 35 (97.2) |
| Baking/eating | 1 (2.7) |
| Duration | |
| Days | 1 (2.7) |
| Weeks | 4 (11.1) |
| Years | 31 (86.1) |
| Past users (n = 114), n (%) | |
| Recreational use | 92 (80.7) |
| Medicinal use | 18 (16.1) |
| Method | |
| Smoking | 110 (96.4) |
| Baking/eating | 4 (3.5) |
| Reason for discontinuation among medicinal users | |
| No longer effective for symptom relief | 1 (5.5) |
| No longer needed for symptom relief | 2 (11.1) |
| Too risky | 12 (66.0) |
| Too difficult to obtain | 3 (16.6) |
Figure 1.
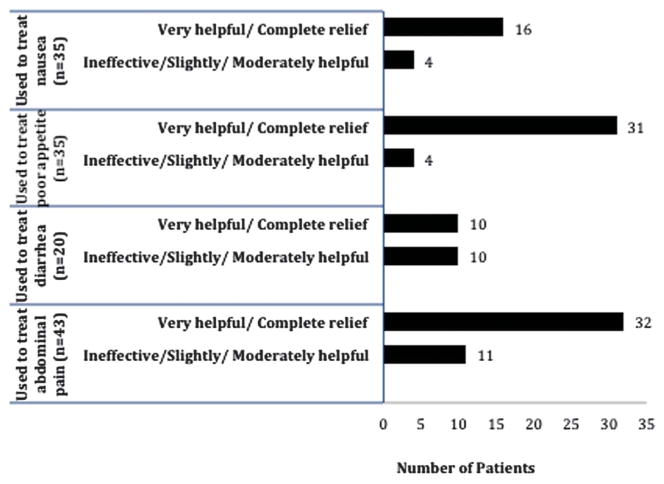
Perceived efficacy of medicinal marijuana among current and past users (n = 48).
Among past users, 80.7% had used marijuana recreationally and 16.1% had used it medicinally to control symptoms of their disease. More than half of past users (58%) reported an ongoing interest in using marijuana medicinally, primarily for abdominal pain and nausea. Of those past users who had quit using marijuana medicinally, 66.0% did so because of concerns about risk, 16.6% because it was too difficult to obtain, 11% because their symptoms had improved to the point that they no longer needed it, and 5.5% because it was no longer effective for symptom relief.
Almost half the study population (48.6%) reported having never used marijuana. Among never users, 51.7% said they would be interested in using medical marijuana once it became legally available, with the majority citing abdominal pain as the symptom for which they would want to use marijuanato treat. Only 36.6% of nonusers reported interest in future participation in clinical trial testing marijuana for symptom control.
Predictors of Current Marijuana Use
Bivariate predictors of current marijuana use are listed in Table 3. Current marijuana users were younger than nonusers (31.3 ± 11.7 yr versus 41.4 ± 14.7 yr, P < 0.001), had lower SIBDQ scores (4.4 ± 1.4 versus 5.2 ± 1.2, P = 0.001), and were more likely to have chronic abdominal pain (66.7% versus 31.7%, P = 0.003). Current narcotic use was not associated with current marijuana use (22.2% versus 7.0%, P = 0.39). There was a trend toward patients with Crohn's disease being more likely to be current marijuana users compared with those with UC and IC (69.4% versus 57.7%, P = 0.09). On multivariate analysis of the statistically significant bivariate predictors of current marijuana use, we found that age (OR, 0.93, 95% CI, 0.89–0.97, P < 0.001) and chronic abdominal pain (OR, 3.5; 95% CI, 1.24–9.82, P = 0.02) predicted current marijuana use (Table 4).
Table 3. Clinical and Disease Characteristics of Current Marijuana Users Versus Nonusers.
| Current Users (n = 36) | Nonusers (n = 142) | P | |
|---|---|---|---|
| Age, yr | 31.3 ± 11.7 | 41.4 ± 14.7 | <0.001 |
| SIBDQ score | 4.4 ± 1.4 | 5.2 ± 1.2 | 0.001 |
| CD, n (%) | 25 (69.4) | 82 (57.7) | 0.09 |
| Prior surgery, n (%) | 18 (50.0) | 52 (36.6) | 0.20 |
| Prior hospitalization, n (%) | 23 (63.8) | 95 (66.9) | 0.88 |
| Biological therapy, n (%) | 15 (41.7) | 56 (39.4) | 0.96 |
| Chronic abdominal pain, n (%) | 24 (66.7) | 45 (31.7) | 0.003 |
| Current narcotics, n (%) | 8 (22.2) | 10 (7.04) | 0.39 |
Table 4. Multivariate Predictors of Current Marijuana Use.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | 0.93 | 0.89–0.97 | <0.001 |
| Chronic abdominal pain | 3.5 | 1.24–9.82 | 0.02 |
| SIBDQ score | 0.98 | 0.94–1.02 | 0.44 |
Predictors of Continued Marijuana Use
Patients who had tried marijuana in the past but were no longer using were older than current users (39.2 ± 13.2 yr versus 31 ± 11.7 yr, P = 0.002) (Table 5). Current users had lower SIBDQ scores (4.4 ± 1.4 versus 5.2 ± 1.2, P < 0.001) and a higher prevalence of chronic abdominal pain (66.7% versus 32.4%, P < 0.001). Current marijuana users were also more likely to be using narcotics to treat their abdominal pain than patients who had quit using marijuana (22.2% versus 3.5%, P = 0.05). Diagnosis, previous surgery, previous hospitalization, and current use of biologics did not predict continuing marijuana use. On multivariate analysis (Table 6) of the significant bivariate predictors of ongoing marijuana use, only age (OR, 0.94, 95% CI, 0.90–0.98, P = 0.005) and the presence of chronic abdominal pain (OR, 2.8, 95% CI, 1.10–7.36, P = 0.03) predicted continued use.
Table 5. Clinical and Disease Characteristics of Current Versus Past Marijuana Users.
| Current Users (n = 36) | Past Users (n = 114) | P | |
|---|---|---|---|
| Age, yr | 31 ± 11.7 | 39.2 ± 13.2 | 0.002 |
| SIBDQ score | 4.4 ± 1.4 | 5.2 ± 1.2 | <0.001 |
| CD, n (%) | 25 (69.4) | 70 (61.4) | 0.16 |
| Prior surgery, n (%) | 18 (50.0) | 37 (32.4) | 0.09 |
| Prior hospitalization, n (%) | 23 (63.8) | 75 (65.7) | 0.99 |
| Biological therapy, n (%) | 15 (41.7) | 48 (42.1) | 0.96 |
| Chronic abdominal pain, n (%) | 24 (66.7) | 37 (32.4) | <0.001 |
| Current narcotics, n (%) | 8 (22.2) | 4 (3.5) | 0.05 |
Table 6. Multivariate Predictors of Continued Marijuana Use.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | 0.94 | 0.90–0.98 | 0.005 |
| Chronic abdominal pain | 2.8 | 1.10–7.36 | 0.03 |
| SIBDQ score | 0.97 | 0.93–1.01 | 0.10 |
Predictors of Current or Past Medicinal Use of Marijuana
On bivariate analysis, younger age (31.4 ± 10.2 yr versus 41.4 ± 14.7 yr, P < 0.001) predicted any current or past medicinal use of marijuana (Table 7). SIBDQ score (4.4 ± 1.3 versus 5.2 ± 1.2, P = 0.003), chronic abdominal pain (70.8% versus 31.7%, P < 0.001), previous surgery (56.3% versus 36.6%, P = 0.02), and Crohn's disease (70.8% versus 57.7%, P = 0.03) were predictors of medicinal use of marijuana. On multivariate analysis (Table 8) of the significant bivariate predictors of medicinal use of marijuana, only age (OR, 0.93; 95% CI, 0.89–0.96; P < 0.001) and the presence of chronic abdominal pain (OR, 4.7; 95% CI, 1.8–12.2; P = 0.001) predicted medical marijuana use.
Table 7. Clinical and Disease Characteristics of All Medicinal Marijuana Users Versus Nonusers.
| Medicinal Users (n = 48) | Nonusers (n = 142) | P | |
|---|---|---|---|
| Age, yr | 31.4 ± 10.2 | 41.4 ± 14.7 | <0.001 |
| SIBDQ score | 4.4 ± 1.3 | 5.2 ± 1.2 | <0.001 |
| CD, n (%) | 34 (70.8) | 82 (57.7) | 0.03 |
| Prior surgery, n (%) | 27 (56.3) | 52 (36.6) | 0.02 |
| Prior hospitalization, n (%) | 38 (79.1) | 95 (66.9) | 0.14 |
| Biological therapy, n (%) | 21 (43.7) | 56 (39.4) | 0.72 |
| Chronic abdominal pain, n (%) | 34 (70.8) | 45 (31.7) | <0.001 |
| Current narcotics, n (%) | 10 (20.8) | 10 (7.04) | 0.45 |
Table 8. Multivariate Predictors of Medical Marijuana Use.
| Variable | OR | 95% CI | P |
|---|---|---|---|
| Age | 0.93 | 0.89–0.96 | <0.001 |
| CD | 0.86 | 0.36–2.04 | 0.74 |
| Prior surgery | 1.81 | 0.79–4.13 | 0.15 |
| Chronic abdominal pain | 4.7 | 1.8–12.2 | 0.001 |
| SIBDQ score | 0.99 | 0.96–1.03 | 0.84 |
Discussion
With the passage of a referendum in November 2012, Massachusetts became the 11th state to legalize medical marijuana for use in Crohn's disease. Despite increasing legal permission, there are little data to inform physician understanding of medicinal use of marijuana, particularly in IBD. A few retrospective studies have suggested that patients with IBD are using marijuana to treat their symptoms, but the prevalence and perceived benefit of marijuana in this population, particularly in the United States, remains under described.
We found that approximately 16.4% of patients with IBD cared for at a tertiary referral center have used marijuana to treat their IBD symptoms since their diagnosis. This is consistent but slightly higher than the 10% prevalence rates previously reported in Canada and Israel.5,6 We found that patients perceive that medical marijuana is helpful for treating abdominal pain, poor appetite, and nausea associated with IBD. Patients felt it was less helpful in treating diarrhea related to their disease. The mechanism behind these benefits is unclear given the lack of data in humans. Storr et al10 found that, in a murine model of trinitrobenzenesulfonic acid–induced colitis, there was a significant upregulation of CB2 receptors in the colon with a subsequent reduction in colitis after treatment with a CB2 agonist. This argues for a possible marijuana-mediated anti-inflammatory effect. However, the fact that patients in our study felt that marijuana improved abdominal pain, nausea, and poor appetite but not diarrhea, suggests that the primary mechanism may be central nervous system–mediated and not an improvement in mucosal inflammation.
Not surprisingly, given the symptoms that patients were targeting, current marijuana users and all medicinal marijuana users were more likely to have chronic abdominal pain than nonusers. However, SIBDQ score, a more objective measure of disease activity, did not predict marijuana use in any of these populations on multivariate analysis. Current marijuana users were more likely to be using narcotics to treat their abdominal pain compared with past marijuana users and never users. It is possible that current marijuana use reflected an underlying substance use phenotype rather than a separate strategy for controlling IBD symptoms. For example, patients who used narcotics to treat their chronic abdominal pain may merely have been more willing to use other substances such as marijuana to treat their disease symptoms. On multivariate analysis, however, there was no significant difference in narcotic use between current and past users. In addition, the majority of patients who used marijuana for symptom relief were not also using narcotics, suggesting that marijuana may have a separate therapeutic benefit.
Across all categories—current use compared with never used, current use compared with past use, and any medicinal marijuana use compared with never used—age was a strong bivariate and multivariate predictor such that older patients were less likely to use or have used marijuana. There are a number of possible explanations for this finding; most obviously that marijuana use is more common, more acceptable, and more available in general for younger adults.12
Many past marijuana users who experienced a clinical benefit reported discontinuation because of concerns about its risk, suggesting that the prevalence of medicinal marijuana use may rise within the IBD population as legal barriers are removed. Other barriers such as drug screening in the workplace may still impact use. Half of never users expressed an interest in using marijuana for symptom control, when it is legally available. Marijuana may, therefore, be expected to become a common adjunct to patients' therapeutic regimens if current legislative trends continue.
We did not specifically ask about side effects/adverse events in our survey and there are currently no long-term safety data among patients with IBD who use marijuana. Marijuana can, however, have acute effects on cognition and reaction time.13 Chronic use, defined as daily use for years, has been found to have both respiratory and cardiovascular effects.14 For example, regular cannabis smokers report more respiratory symptoms including wheeze, sputum production, and chronic cough than do nonsmokers.15 Marijuana also has chronotropic cardiac effects,16 and Mittleman et al17 found that marijuana use was associated with increased risk of myocardial infarction, particularly in the 60 minutes after consumption.
There were several limitations to this study. First, patients whose IBD symptoms improved may be more likely to search their memories for a causal event, specifically marijuana use, raising the possibility of recall bias. Because we did not have objective measures of disease activity before and after marijuana use, the perceived benefit of marijuana may be overexaggerated in our population. It is important to note that our study population may not be representative of the national IBD population. More than 90% of the patients surveyed were white, which is likely a reflection of the Brigham and Women's Hospital referral base. More then half of the patients had been hospitalized at least once since diagnosis and were also currently on a biological agent, which reflects a tertiary referral center population. In addition, the 12.3% rate of current marijuana use was higher than the general North American adult population rate of 10.7%.13 It is unclear whether this difference reflects the urban nature of Boston, if higher rates are specific to our patients with IBD, or if this represents increased use because of publicity and public debate about the issue. Because we did not survey non-IBD patients seen in our clinic, it is difficult to discriminate between these possibilities. Higher-than-average marijuana use rates are unlikely, however, to be a consequence of sampling bias given the high response rate.
There are several important clinical implications of our study. Physicians should be asking their patients, particularly younger ones, about marijuana use. Marijuana self-medication for IBD symptoms may give clinicians a misleading impression of disease response to prescribed therapy, particularly when assessing symptoms such as abdominal pain, poor appetite, and nausea. Although we did not specifically ask study participants whether they discussed recreational or medicinal drug use with their physicians, our clinical impression is that this is rarely addressed during office visits.
Our findings suggest that patients with UC may also benefit from the use of medicinal marijuana although the 11 states that have legalized medical marijuana have only approved its use for only patients with CD. Lawmakers should consider adding this condition to the list of acceptable diseases that may be treated with medicinal marijuana.
Several questions remain unanswered. Because the number of patients who baked/ate marijuana was too small to analyze separately, we do not know whether this route of administration is also effective. In addition, we do not know the optimal dose or frequency of use necessary to achieve adequate symptom relief. Lastly, we do not know if it is the potential anti-inflammatory effects of marijuana that lead to symptom relief or if there is another mechanism at play. These questions would be most effectively addressed through a clinical trial. Our data suggest that at least one-third of patients with IBD would consider participating in such a trial, again emphasizing the growing interest in using marijuana in this disease.
Supplementary Material
Footnotes
The authors have no conflicts of interest to disclose.
Author contributions: Study concept and design, acquisition of data, and analysis and drafting of manuscript, J. E. Ravikoff; study design and data analysis, A. Courtwright; acquisition of data, M. Lucci; study concept and design and critical revision of manuscript, J. R. Korzenik, Josh Korzenik; study concept and design, J. Levine.
References
- 1.Baker D, Pryce G, Giovannoni G, et al. The therapeutic potential of cannabis. Lancet Neurol. 2003;2:291–298. doi: 10.1016/s1474-4422(03)00381-8. [DOI] [PubMed] [Google Scholar]
- 2.Page SA, Verhoef MJ, Stebbins RA, et al. Cannabis use as described by people with multiple sclerosis. Can J Neurol Sci. 2003;30:201–205. doi: 10.1017/s0317167100002584. [DOI] [PubMed] [Google Scholar]
- 3.Woolridge E, Barton S, Samuel J, et al. Cannabis use in HIV for pain and other medical symptoms. J Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. NSDUH Series H-44, HHS Publication No (SMA) 12-4713. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 5.Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion. 2012;85:1–8. doi: 10.1159/000332079. [DOI] [PubMed] [Google Scholar]
- 6.Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2011;23:891–896. doi: 10.1097/MEG.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- 7.Naftali T, Bar Lev L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol. 2013;11:1276–1280. doi: 10.1016/j.cgh.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Massa F, Monory K. Endocannabinoids and the gastrointestinal tract. J Endocrinol Invest. 2006;29:47–57. [PubMed] [Google Scholar]
- 9.Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med (Berl) 2005;83:944–954. doi: 10.1007/s00109-005-0698-5. [DOI] [PubMed] [Google Scholar]
- 10.Storr MA, Keenan CM, Zhang H, et al. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jowett SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96:2921–2928. doi: 10.1111/j.1572-0241.2001.04682.x. [DOI] [PubMed] [Google Scholar]
- 12.Kandel D, Chen K, Warner LA, et al. Prevalence and demographic correlates of symptoms of last year dependence on alcohol, nicotine, marijuana and cocaine in the U.S. population. Drug Alcohol Depend. 1997;44:11–29. doi: 10.1016/s0376-8716(96)01315-4. [DOI] [PubMed] [Google Scholar]
- 13.Li MC, Brady JE, DiMaggio CJ, et al. Marijuana use and motor vehicle crashes. Epidemiol Rev. 2012;34:65–72. doi: 10.1093/epirev/mxr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 15.Tetrault JM, Crothers K, Moore BA, et al. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–228. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 17.Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


