Abstract
Protection against HIV-1 infection in exposed seronegative (ESN) individuals likely involves natural resistance mechanisms that have not been fully elucidated. Human beta defensins (HBD) are antimicrobial peptides found primarily in mucosae, the main ports of HIV entry. HBD-2 and 3 mRNA are induced by HIV-1 in human oral epithelial cells and exhibit strong anti-HIV-1 activity; in addition, polymorphisms in the DEFB1 gene, which encodes HBD-1, have been associated with resistance/susceptibility to different infections, including HIV-1. Here, we have assessed the association of HBD expression with the ESN phenotype. Peripheral blood and vaginal/endocervical and oral mucosal samples were taken from 47 ESN, 44 seropositive (SP) and 39 healthy controls (HC). HBD-1, 2 and 3 mRNA copy numbers were quantified by real time RT-PCR and A692G/G1654A/A1836G polymorphisms in the DEFB1 gene were detected by restriction fragment length polymorphisms and confirmed by nucleotide sequencing. ESN expressed significantly greater mRNA copy numbers of HBD-2 and 3 in oral mucosa than HC; p=0.0002 and p=0.007, respectively. mRNA copy numbers of HBD-1, 2 and 3 in vaginal/endocervical mucosa from ESN and HC were similar. Homozygosity for the A692G polymorphism was significantly more frequent in ESN (0.39) than in SP (0.05) (p=0.0002). In summary, ESN exhibited enhanced mucosal expression of the innate defense genes HBD-2 and 3; however, additional studies are required to verify these results and the potential association of the A692G polymorphism to the relative resistance of ESN to HIV-1 infection.
Keywords: HIV-1 (Human immunodeficiency virus type 1), human beta defensins, natural resistance, HIV-1-exposed sero-negatives, polymorphism, mucosa
Introduction
Since recognition of the HIV-1 epidemic in 1981, the Acquired Immunodeficiency Syndrome (AIDS) has killed 25 million persons and more than 33 million are currently infected [1]. Despite the rapid spread of this virus, some individuals, known as exposed seronegatives (ESN), have an apparent resistance to HIV-1 infection despite multiple and repeated exposures [2]. Numerous factors have been proposed as potentially protective mechanisms including the rapid development of adaptive cellular [3] and humoral defenses [4], as well as certain elements of the innate immune response [5-7]. These findings have not been consistently reproduced from one study to another and among persons at high risk for HIV infection by the parenteral route [8]; only a 32 base pair deletion in the CCR5 gene was demonstrably associated with protection against infection [9].
Even though HIV-1 infection can be acquired perinatally, parenterally and sexually [10] and the efficiency of sexual transmission of HIV-1 is very low (0.0001 to 0.0040 for each sexual contact) [11], 75 to 85% of infections worldwide have been acquired through unprotected sexual intercourse [12, 13]. On the other hand, oral transmission of HIV-1 is uncommon [14], regardless of the demonstrable presence of HIV-1 particles in saliva [15], and oral mucosal cells [16]. The differences in the risk of oral versus vaginal/anal transmission of HIV-1 might reflect the differential presence of target cells and protective factors at these mucosal surfaces. In fact, differential expression of several soluble factors with anti-HIV-1 activity, including defensins, has been reported in mucosae [17].
Defensins are cysteine-rich cationic peptides that exhibit antimicrobial activity against a broad spectrum of microorganisms [18]; they are divided into three groups: α, β and θ, based on their structure [18-20]. Thus far, six human β-defensin (HBD) have been identified and characterized [21]. HBD-1 is constitutively expressed by epithelial cells, while HBD-2 and 3 are induced by different stimuli including viruses [22]. Exposure of oral epithelial cells to HIV-1 induces mRNA expression for HBD-2 and 3, both of which inhibit HIV-1 replication through both direct HIV-1 inactivation and by down-regulation of CXCR4 expression [23]. Furthermore, HBD-2 and 3 inhibit R5 and X4 HIV-1 infection in a dose-dependent manner at concentrations that are comparable to those found in the oral cavity (100 μg/ml) [24]. Thus, we decided to examine a possible role of HBDs in protecting against HIV-1 infection.
Thirty single-nucleotide polymorphisms (SNPs) associated with different ethnic groups have been reported in HBD genes, particularly in DEFB1 and DEFB2 that code for HBD-1 and 2 respectively [25]. As is the case with other genes, some of these SNPs may influence the biological function of the antimicrobial peptides. In fact, previous studies suggest that some SNPs in DEFB1, have been associated with differential risks of HIV-1 infection [26-28]. However, HBD polymorphisms and their relationships to the expression levels at mucosal sites have not been systematically studied as potential modifiers of the risk of HIV infection. In this report, we found an increased expression of mRNA of HBD-2 and 3 in oral mucosa from ESN; in addition, our results suggest that the polymorphism A692G in DEFB1 might be associated with resistance to HIV-1 infection.
Materials and Methods
Subjects
HIV-1 ESN and seropositive (SP) individuals were recruited from HIV-1 comprehensive care programs in the Colombian cities of Medellín and Santa Marta. The inclusion criteria for ESN subjects were similar to those in previous reports [29]. Briefly, these included unprotected sexual intercourse, anal/vaginal, with a SP individual more than five times in the previous 6 months or an average of two times weekly over 4 months within 2 years of enrollment and a negative HIV-1/2 ELISA test within one month of sample taking. None of the ESN individuals had history of intravenous drug use. When possible, the SP partner was also recruited and HIV-1 infection was confirmed by western blot test. Healthy Control (HC) individuals were adult volunteers with similar ethnic background to the ESN and SP individuals who have had fewer than 2 partners in the last 2 years, consistent (over 50% of sex acts) use of condoms and no history of piercing, tattoos or transfusions. Subjects with current oral or vaginal bleeding or infections were excluded. None of the women included in the study were using a hormone-based contraceptive method. A questionnaire for risk behavior was filled out at the time of sampling and an informed consent approved by the Bioethical Board for Human Research and prepared according to the Colombian Government Legislation, Resolution 008430 of 1993, was signed.
Mucosal Samples
The oral mucosa samples were obtained by means of a cytobrush. As many cells as possible were collected by rubbing the brush against the buccal mucosa. The vaginal samples were taken using a cervical cytobrush that was inserted and rotated 360° in all four quadrants of the vagina. Another cytobrush was gently inserted 1cm into the endocervix and rotated 360°. All samples were stored in RNA later (QIAgen, Valencia, CA) at -70°C.
Real Time RT-PCR Assay to Quantify HBD mRNA
Total RNA was extracted from epithelial cells with Trizol Reagent (Invitrogen), following the manufacturer's instructions. The amount and purity of the RNA were determined by spectrometry at 260/280nm. Isolated total RNA was treated with DNase I (Fermentas) to eliminate genomic DNA. cDNA was synthesized using the SuperScript III (Invitrogen) in accordance with the manufacturer's instructions. Each 25μl-real-time PCR mixture consisted of 2μL of cDNA, 1× reaction buffer, 5mM MgCl2, 0.2mM dNTP, 2U of platinum Taq DNA polymerase (Invitrogen), primers (0.4μM each) and SYBR green dye diluted 1:2500 (Sigma, St. Louis. USA). Cytokeratin-19 (CK19) RNA was used to normalize the RNA content in each preparation. The primer sequences and product size for HBD-1, 2 and CK19 were previously reported [31, 32]. For HBD-3 we used the following primers: forward 5′ ATCTTCTGTTTGCTTTGCTCTTCCTGTTTT 3′, reverse 5′ AGCACTTGCCGATCTGTTCCTCCTT 3′ and the product was 153bp in length. The cycling profiles were: 95°C for 10min followed by 45 cycles of 95°C for 15sec, 55°C for 30sec for HBD-1 (60°C for 30sec for HBD-2, 3 and CK19) and 72°C for 30sec. We included a melting curve to confirm the specificity of the PCR product. All real-time RT–PCR amplifications and data acquisition were performed using the Chromo 4TM detector, software 2.03 (MJ Research).
Restriction Fragment Long-Polymorphism (RFLPs) for SNPs A692G, G1654A and A1836G in DEFB1 Gene
Polymorphisms were determined by PCR-RFLPs. The primer sequences and protocols were described previously [25]. PCR products were digested in a final volume of 15μL containing 1× buffer, 0.2μL of the corresponding restriction enzyme and 4μL of PCR product. The mixture was incubated for 16h at 37°C. The enzymes used were: Bme1390I for A692G SNP, Bst11071 for A1836G SNP, HpyCH4V for G1654A SNP. The SNPs were verified by sequencing using a commercial service from Macrogen (Macrogen, Seoul, Korea). The sequences were analyzed with MEGA software version 3.1. DEFB1 exon 1 and 2 DNA sequences provided by Genebank NCBI (access number: U50930 and U50931, respectively) were used as reference.
Statistical Analysis
Fisher's-exact tests or Chi-square tests were used to compare categorical variables. The Kruskal-Wallis test was used to compare continuous variables across groups. Correlations were evaluated using Pearson correlation coefficient. All tests are two-sided, and a p<00.5 was considered statistically significant. Analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, CA, USA). Allele and genotype frequencies were calculated with GENEPOP software version 3.4. [33]. Additionally, ARLEQUIN version 3.1 [34] was used to test for differences in haplotype frequencies.
Results
Demographic Data
Thirty-nine HC, 44 SP and 48 ESN individuals were evaluated; 36 ESN and SP correspond to 18 discordant couples. Median age was 27 years (IQR= 25-40) for HC, 33 years (IQR= 30-37) for SP and 35 years (IQR= 27-40) for ESN individuals. Females comprised 74, 60 and 56% of HC, ESN and SP respectively. There were no significant differences in age or gender among groups (p=0.152 and 0.210, respectively). The day of the menstrual cycle (MC) at which the vaginal and endocervical samples were taken was similar among females in all three groups (p=0.390). Six (13%) SP individuals were not receiving antiretroviral treatment at the time of sampling (Table 1). No other sexually transmitted disease or opportunistic infections was reported in any of the study groups. We excluded ESN individuals with the homozygous CCR5 Δ32 mutation from participation.
Table 1. Demographic Profile.
| HC (n=39) | ESN (n=47) | SP (n=44) | ||
|---|---|---|---|---|
| Age | 27 | 35 | 33 | |
| Median (Range) | (20-44) | (18-61) | (18-50) | |
|
| ||||
| Gender n (%) | ||||
| Male | 10 (26%) | 19 (40%) | 19 (44%) | |
| Female | 29 (74%) | 28 (60%) | 25 (56%) | |
|
| ||||
| Sexual Orientation n (%) | Hta | 39 (100%) | 41 (87%) | 30 (68%) |
| Hmb | 0 (0%) | 5 (11%) | 3 (7%) | |
| Bisc | 0 (0%) | 1 (2%) | 11 (25%) | |
|
| ||||
| MCd Day | 14 | 20 | 18 | |
| Median (Range) | (10-18) | (9-28) | (12-24) | |
|
| ||||
| Viral Load | NDe | ND | 400 copies/mL | |
| Median (Range) | (50-139000) | |||
|
| ||||
| Lymphocytes T CD4+ | ND | ND | 333 cells/mL | |
| Median (Range) | (17-909) | |||
Heterosexual
Homosexual
Bisexual
Menstrual Cycle
No data.
ESN Exhibit Similar HBD mRNA Copy Numbers than HC and SP Individuals in Endocervical and Vaginal Mucosa
In order to determine if there were differences in the expression of HBD-1, 2 or 3 mRNA among the studied populations, the number of mRNA copies was calculated by real time-PCR from vaginal/endocervical mucosal brushings. ESN individuals had higher copy numbers of HBD-1 mRNA in vaginal (Median: 25 vs 2.6) and endocervical (82.25 vs 39.6) mucosa than did HC, although the difference was not statistically significant (p=0.428 and 0.703, respectively) (Fig. 1A). ESN also had greater copy numbers of HBD-1 than did SP in vaginal mucosa (25 vs 10), but slightly lower in endocervical mucosa (82.25 vs 117), but these differences did not reach statistical significance. In addition, higher mRNA copy numbers for HBD-2 were found in ESNs in vaginal (Median: 42 vs 16.4) and endocervical (39. vs 26.5) mucosa than were found in HC, although the differences were not statistically significant (Fig. 1B). The HBD-3 mRNA copy numbers were similar in all groups (Fig. 1C).
Fig. (1). Copy numbers of HBD mRNAs in vaginal and endocervical mucosa from HC, ESN and SP individuals.
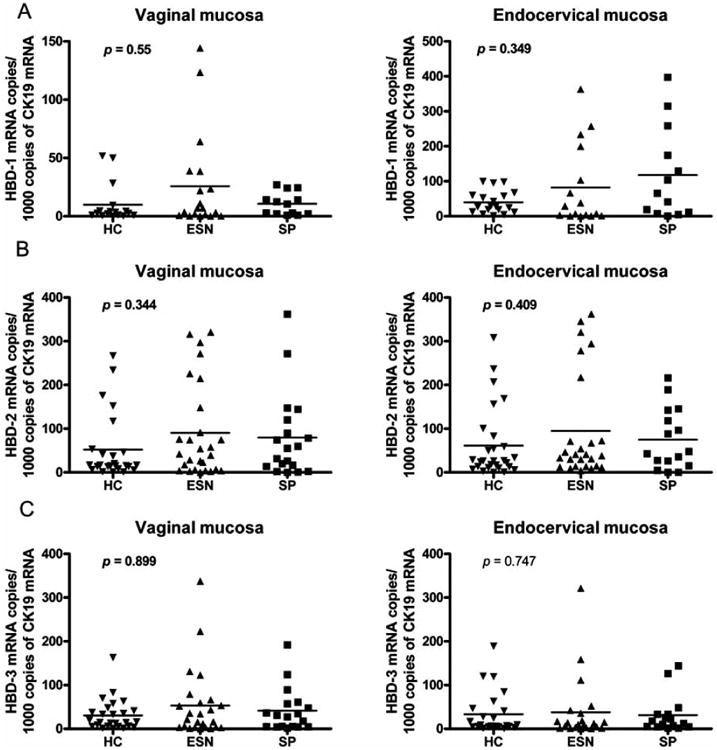
Standard curves were constructed using plasmids diluted (101-109) containing segments of the HBD-1, 2, 3 and CK19 genes. The corresponding copy numbers were calculated using the equation: 1 μg of 1000-bp DNA × 9.1 × 1011 molecules [50]. Standard curves were generated using the relationship of known number of input templates to the cycle threshold. The real time PCR generated a different cycle threshold (CT) for each dilution; the CT was defined as the PCR cycle number at which the mean fluorescence increased 10 SD above baseline. CT is inversely proportional to the log of the input copy equivalent. The number of HBD mRNA copies was expressed by each 1000 CK19 mRNA copies observed in vaginal and endocervical mucosa. (A) Number of HBD-1 mRNA copies. (B and C) Copy numbers of mRNA of HBD-2 and 3 respectively.
Determining correlations of HBD-1, -2 and -3 within groups, we found a positive correlation between HBD-2 and HBD-3 mRNAs in vaginal mucosa from ESN (r=0.573, p=0.0034) and HC (r=0.662, p=0.0004) individuals. There was also a positive correlation between HBD-2 and HBD-1 mRNAs in vaginal mucosa from ESN (r=0.541, p=0.02) individuals. Additionally, we found a positive correlation between HBD-1 and HBD-3 mRNAs in vaginal mucosa from ESN individuals (r=0.784, p=0.0002) and between HBD-2 and HBD-3 mRNA in endocervical mucosa from ESN (r=0.506, p=0.016) individuals (data not shown).
ESN Individuals Exhibit Higher Copy Number of HBD-2 and 3 mRNA in Oral Mucosa Compared with HC Individuals
In order to evaluate the expression of these genes at more than one mucosal site, we studied the presence of their mRNAs in oral mucosal tissue. Interestingly, we found that ESN and SP individuals expressed significantly higher mRNA copies for HBD-2 (Median: 42.15 for ESN, 46.2 for SP) and HBD-3 (Median 56.5 for ESN and 207 for SP) in oral mucosa than did HC (Median 2.0 for HBD-2 and 7.5 for HBD-3) individuals, p=0.0002 and p=0.007, respectively (Fig. 2). No differences were found in the mRNA copy numbers for HBD-2 and 3 between ESN and SP individuals (p=0.762 and 0.06 respectively) (Fig. 2). mRNA for HBD-1 was uncommonly detected in oral mucosal samples: in 4 (8.5%) ESN, in 4 (9%) SP, and in 3 (7.7%) HC individuals. There was a positive correlation between levels of mRNA for HBD-2 and 3 in oral mucosa from SP (r=0.617, p=0.0082), ESN (r=0.4649, p=0.044) and HC (r=0.886, p=0.0001) individuals.
Fig. (2). Copy numbers of HBDs mRNA in oral mucosa from HC, ESN and SP individuals.
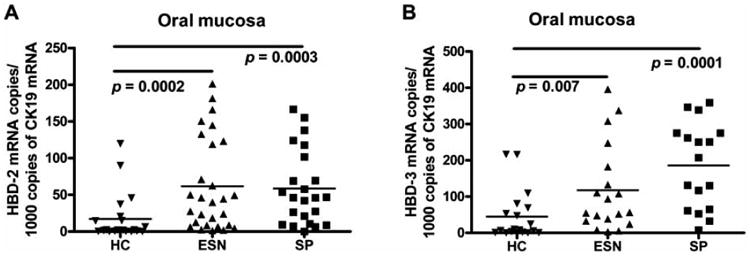
(A) Number of HBD-2 mRNA copies per 1000 CK19 mRNA copies observed in oral mucosa. (B) Copy numbers of HBD-3 mRNA.
Studying the association of HBD mRNAs between the 2 mucosal sites we found a negative correlation between vaginal and oral mucosal levels of HBD-2 mRNA (r=-0.575, p=0.01), and a positive correlation between vaginal and endocervical mucosal levels of HBD-1 mRNA (r=0.588, p=0.016), in healthy controls. In addition, we found a positive correlation between vaginal and endocervical mucosal HBD-3 mRNAs in ESN individuals (r=0.456, p=0.049).
Finally, no significant associations were found between plasma viral loads or CD4 T cell counts among SP individuals and the mRNA copy numbers of HBD-1, 2 or 3 from oral, vaginal or endocervical mucosa in ESNs (data not shown).
The A692G SNP in DEFB1 is More Common Among ESN
Earlier reports failed to demonstrate anti-HIV activity for HBD-1 [23, 24]; nevertheless, we observed that ESN individuals exhibited a slight trend towards a higher HBD-1 mRNA copy number in vaginal/endocervical mucosae than had HC individuals (Fig. 1A). In addition, there are epidemiologic data linking SNPs in DEFB1 with a higher risk for HIV infection [26-28]. In order to determine if the DEFB1 SNPs, A692G in exon 1, A1836G and G1654A in exon 2 are associated with risk for acquisition of HIV-1 infection, the corresponding segment for each exon was amplified by PCR and the SNPs were identified by RFLPs.
The heterozygous genotype (A/G) for the A692G SNP was the most frequent in all groups, seen in 56.3% of HC, 47.8% of ESN and 84.2% of SP. There were significant differences in these genotypic frequencies between the SP and ESN (p=0.0005) and between SP and HC individuals (p=0.014). Significant differences were also found in the frequencies of the homozygous genotype (G/G) for this SNP in ESN compared to SP individuals (39.1% vs 5.3%; p=0.0002) and between SP and HC (5.3% vs 28.1%; p=0.008). In contrast, the frequencies of the A/A genotype were similar in all groups, with 5 (15.6%) HC, 6 (13.1%) ESN and 4 (10.5%) SP individuals homozygous for this SNP. Pairwise comparisons including all genotypes (A/G, G/G and A/A) only indicated a significant difference between the ESN and SP individuals (p=0.016) (Table 2).
Table 2. Genotypic and Allelic Frequencies of DEFB1 SNPs.
| SNP | Population | Genotype Frequencies n (Frequencies) | Allele Frequencies n (Frequencies) | |||
|---|---|---|---|---|---|---|
| A/A | A/G | G/G | A | G | ||
| A692Ga | HC | 5 (0.156) | 18 (0.563) | 9 (0.281) | 28 (0.437) | 36 (0.563) |
| ESNa | 6 (0.131) | 22 (0.478)b | 18 (0.391)c | 34 (0.370)d | 58 (0.630)e | |
| SPa | 4 (0.105) | 32 (0.842)b | 2 (0.053)c | 40 (0.526)d | 36 (0.474)e | |
| A1836G | HC | 30 (0.938) | 2 (0.062) | 0 (0.000) | 62 (0.969) | 2 (0.031) |
| ESN | 43 (0.935) | 3 (0.065) | 0 (0.000) | 89 (0.967) | 3 (0.033) | |
| SP | 32 (0.842) | 6 (0.158) | 0 (0.000) | 70 (0.921) | 6 (0.079) | |
| G1654A | HC | 0 (0.000) | 0 (0.000) | 32 (1.000) | 64 (1.000) | 0 (0.000) |
| ESN | 0 (0.000) | 0 (0.000) | 46 (1.000) | 92 (1.000) | 0 (0.000) | |
| SP | 0 (0.000) | 0 (0.000) | 38 (1.000) | 76 (1.000) | 0 (0.000) | |
| Population | Haplotypic Frequencies | |||||
| AAG | GAG | AGG | GGG | |||
| HC | 0.406 | 0.563g | 0.031 | 0.000 | ||
| ESN | 0.370f | 0.597h | 0.000 | 0.033 | ||
| SP | 0.526f | 0.395gh | 0.000 | 0.079 | ||
p= 0.016 value between ESN and SP groups comparing all the genotypes for A692G SNP.
p= 0.0005
p= 0.0002.
p= 0.044 value between ESN and SP groups comparing all the allelic frequencies for A692G SNP.
p=0.041
p=0.042.
p=0.041
p=0.047
p=0.008.
No differences were found in the genotypic frequencies between the groups for the A1836G SNP. The wild genotype (A/A) was the most frequent in all groups (>84%), while the heterozygous genotype (A/G) was observed in low frequency: 6.2% for HC, 6.5% for ESN and 15.8% for SP individuals. The homozygous genotype for this SNP (G/G) was not detected in any individual (Table 2). Only the wild genotype (G/G) for the G1654A SNP was found in all populations. Crossing all loci (A692G, A1836G and G1654A) a statistical differences between ESN and SP individuals remained (p=0.029), due to the difference found in the homozygous (G/G) and heterozygous (A/G) genotype for the A692G SNP (Table 2). The allelic frequencies for the A692G SNP showed significant differences between the ESN and SP groups for the A allele, 37% vs 53% (p=0.041) and G allele, 63% vs 47% respectively (p=0.042) and between total allelic frequencies of ESN compared to SP individuals (p=0.044) (Table 2).
According to the allelic frequencies, 4 different combined haplotypes (AAG/GAG/AGG/GGG) were found in the population, where A692G correspond to the first, A1836G second and G1654A to the third position (Table 2). The frequency of the GAG haplotype was significantly different between the SP and ESN (39.5% vs 59.7%; p=0.008) groups and also between the SP and HC (39.5% vs 56.3%; p=0.047) individuals. In addition, in the SP group, the haplotype AAG was the most frequent (52.6%) a difference that was significant between the ESN and SP (p=0.041) (Table 2). Although there is a general tendency to produce higher levels of HBD-1 in heterozygous (A/G) and homozygous individuals for the SNP A692G (G/G) than wild type individuals, the differences were not statistically significant (data no shown).
Discussion
Recent studies have shown that HBDs have antiretroviral activity and that these peptides are induced by the exposure of oral mucosal epithelial cells to HIV-1 [23, 24]. In addition, there are a number of individuals who, despite the fact that they are serially exposed to HIV-1 through unprotected sexual intercourse with infected partners, remain healthy and HIV-1 seronegative (ESN) [29]. To date, the most important identified mechanism of natural resistance is the Δ32 mutation in the CCR5 gene [9, 30, 35, 36]. The importance of studying protective mechanisms at mucosal surfaces is underlined by the fact that more than 90% of worldwide HIV-1 cases have been transmitted across these surfaces, mainly during sexual intercourse [37].
In order to define whether our ESN individuals were resistant to HIV-1 infection, due to the presence of Δ32 mutation in the CCR5 gene, this mutation was screened by PCR. Although the heterozygous genotype, previously associated with a decreased rate of disease progression and modest resistance to HIV infection [38, 39], was more frequent in ESN, none of these individuals had the homozygous CCR5 Δ32 genotype that could account for high level resistance to HIV acquisition.
To examine the possible contribution of HBDs in protecting against HIV-1 infection, HBD mRNA from genital and oral mucosa was quantified by real time PCR in ESN, HC and SP individuals. The results indicate that the mRNA copy numbers of HBD-1 are slightly increased in vaginal and endocervical mucosa of ESN individuals compared to levels in HC; however, this difference was not significant. These results are in agreement with previous reports that have not indicated an anti-HIV activity of HBD-1 [23]. Similarly, the HBD-2 mRNA copy numbers were higher, albeit not significantly, in vaginal and endocervical mucosa from ESN compared to levels in HC individuals. Considering that the highest HBD-2 expression in the MC occurs during the menstruation phase [40], and based on the reported evidence indicating the anti-HIV-1 activity of HBD-2 [23], further studies are required to explore if during this phase, the higher expression of this HBD in ESN could provide some level of protection during HIV-1 exposure. Finally, low levels of HBD-3 mRNA have been detected in uterus [41] and the highest levels of HBD-3 have been observed during the luteal phase of the MC [42]. However, the variable range in the day of the MC of the women studied may have complicated our analyses.
The expression of mRNA for HBD-2 and 3 from oral mucosa was significantly higher in the ESN compared to the HC group. It has been previously shown that HBD-2 and 3 are expressed in oral tissues and secreted in saliva where they exert their antimicrobial activity [43, 44]. In fact, in vitro exposure of oral human epithelial cells to HIV-1 induces HBD-2 and 3 mRNA expressions, and both of these peptides can inhibit HIV-1 replication [23]. The fact that there was a significantly higher expression of HBD-2 and 3 in ESN than among HC in oral but not in genital mucosa might indicate that the HBD levels detected in the oral cavity correspond to the production of these peptides in response to the microbial challenge to which this surface is constantly exposed [45]. In contrast, the genital mucosa could be in contact with lower levels of microbes capable of inducing epithelial defensins expression [46]; however, that will need to be verified. Thus, the HBD levels detected in these surfaces might be an indication of basal levels rather than levels produced in response to a microbial exposure. In addition, it is possible that the lack of significant differences in the levels of HBDs in genital mucosa among groups could also be due to the smaller sample size analyzed for genital mucosa compared to oral mucosa.
The fact that we found similar levels of HBD-2 and 3 in mucosae, particularly in the oral cavity in ESN and SP individuals might be an indication that the chronic immune stimulation induced by exposure to HIV-1 or other pathogens results in HBD production. Thus, the increased HBD expression exhibited by ESNs compared to HC might reflect a genetic trait associated with resistance to HIV-1 infection. Further studies using in vitro stimulation of cultured epithelial cells from oral and genital mucosa obtained from ESNs, SP and HC will help to elucidate this phenomenon. The exact mechanism(s) through which HBDs might prevent the establishment of HIV-1 infection is not clear. However, it is possible that factors involved in protecting the oral mucosa might also exert an effect at vaginal and endocervical sites.
Previous reports suggest an association between SNPs in DEFB1 and the risk of acquiring HIV-1 infection [26-28]. Therefore, we studied the presence of 3 SNPs in the DEFB1 gene, previously reported in high frequencies in different ethnic populations [25]. These SNPs have characteristics that might plausibly link them to differential gene expression or gene product function; for example: (i) SNP A692G sequence is recognized by the transcription factor NFkB, which might affect the production of several molecules involved in the natural resistance to HIV infection, (ii) SNP A1836G is within a probable polyadenylation site, and the resultant nucleotide change might affect the transcription or the translation of the gene, and (iii) SNP G1654A is adjacent to the first of six conserved cysteine residues potentially having an effect on the folding of the peptide and, thus, influence peptide function [25]. However, the relationship between these SNPs and gene expression or function during HIV-1 exposure had not been explored.
The presence of SNPs in DEFB1, DEFB4 and DEFB103 genes, which code for HBD-1, 2 and 3, respectively might also be associated with resistance/susceptibility to different infections. However, these genes display broad population variability in copy numbers, with individuals exhibiting from 2 to 12 copies per diploid genome [51], making the search for SNPs very difficult and most likely the allelic distribution will not be in HWE [26, 28].
The genotypic frequency of the homozygous A692G SNP was significantly higher in ESN than in SP individuals (39.1% vs 5.3% respectively). Interestingly, the heterozygous genotype frequencies (47.8% vs 84.2%), segregate in the opposite direction; thus if this SNP is associated with any protection from HIV infection, homozygosity might be protective but the heterozygous state would not appear to be. This SNP is located in position -20 of the 5′UTR of the DEFB1 gene [47] and it does not induce an amino acid change but it may generate a binding site for the nuclear factor NF-κB [25]. If this is the case, the A692G SNP could lead to the up-regulation of several immune genes potentiating the innate defence response to different pathogens.
The haplotype frequencies indicate that ESN individuals exhibit a higher frequency of the GAG haplotype, containing the G allele than do members of the SP group, suggesting that the homozygous genotype for the A692G SNP found in lower frequency in the SP group than in the ESN group might be associated with resistance. In contrast, the A692G SNP has been previously associated with susceptibility to asthma, atopic dermatitis, and HIV-1 infection in Brazilian children [26, 47, 48]. These contradictory results might indicate that this SNP could be interacting with other genetic polymorphisms; thus, the haplotypic information might be more informative than analyses of any single SNP.
Although the anti-HIV-1 role of HBD-1 has not been previously reported, the results of this study indicate a possible role of polymorphisms in this gene in the phenomenon of natural resistance to HIV infection. So far it has been well established that monocytes, macrophages and monocyte-derived DC constitutively express HBD-1 mRNA, that is upregulated when these cells are stimulated with LPS or IFN-γ [49], suggesting that it may be induced during exposure to several pathogens, including HIV-1.
Taken together, these results suggest that a higher expression of mRNAs for HBD-2 and 3 found in oral mucosa from ESN individuals might contribute to apparent resistance to HIV-1 infection exhibited by these individuals. In addition, our data suggest that the A692G SNP in DEFB1 also is associated with this relative resistance phenotype. Additional studies in larger cohorts will be needed to confirm these observations.
Acknowledgments
This work was supported by The Committee for Research Development from the University of Antioquia; the Colombian government institute created to support scientific research (COLCIENCIAS 111534319143) and the Center for AIDS Research, Case Western Reserve University and University Hospitals of Cleveland (Grant number AI-36219). We thank to the staff from HERES Health, from Santa Marta, and Clinical Laboratory from University of Antioquia, Medellin, Colombia for all their collaboration.
Abbreviations
- HIV-1
Human immunodeficiency virus type 1
- ESN
Exposed seronegative
- HBD
Human beta defensin
- SP
Seropositive
- HC
Healthy control
- AIDS
Acquired Immunodeficiency Syndrome
- SNP
Single-nucleotide polymorphisms
- mRNA
Messenger ribonucleic acid
- PCR
Polymerase chain reaction
- cDNA
Complementary deoxyribonucleic acid
- DNA
Deoxyribonucleic acid
- RT-PCR
Reverse transcriptase- polymerase chain reaction
- RFLP
Restriction fragment length polymorphisms
- MC
Menstrual cycle
References
- 1.UNAIDS. Report on the global AIDS epidemic. Joint United Nations Programme on HIV/AIDS; Geneva: 2007. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2007/default.asp. [Google Scholar]
- 2.Shearer GM, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–4. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 3.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–7. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 4.Sierra S, Kupfer B, Kaiser R. Basics of the virology of HIV-1 and its replication. J Clin Virol. 2005;34:233–44. doi: 10.1016/j.jcv.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Montoya CJ, Velilla PA, Chougnet C, Landay AL, Rugeles MT. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin Immunol. 2006;120(2):138–46. doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Scott-Algara D, Truong LX, Versmisse P, et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Viet-namese intravascular drug users. J Immunol. 2003;171:5663–7. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 7.Velilla PA, Hoyos A, Rojas M, Patino PJ, Velez LA, Rugeles MT. Apoptosis as a mechanism of natural resistance to HIV-1 infection in an exposed but uninfected population. J Clin Virol. 2005;32:329–35. doi: 10.1016/j.jcv.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Salkowitz JR, Purvis SF, Meyerson H, et al. Characterization of high-risk HIV-1 seronegative hemophiliacs. Clin Immunol. 2001;98:200–11. doi: 10.1006/clim.2000.4969. [DOI] [PubMed] [Google Scholar]
- 9.Huang Y, Paxton WA, Wolinsky SM, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–3. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control & Prevention NCfH, STD, and TB Prevention, Divisions of HIV/AIDS Prevention. Fact Sheet - HIV and Its Transmission - CDC-NCHSTP-Divisions of HIV/AIDS Prevention. Centers for Disease Control & Prevention. 2003 Available at: http://www.cdc.gov/hiv/resources/factsheets/PDF/transmission.pdf.
- 11.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 12.Gao JJ, Filla MB, Fultz MJ, Vogel SN, Russell SW, Murphy WJ. Autocrine/paracrine IFN-alphabeta mediates the lipopolysaccharide-induced activation of transcription factor Stat1alpha in mouse macrophages: pivotal role of Stat1alpha in induction of the inducible nitric oxide synthase gene. J Immunol. 1998;161:4803–10. [PubMed] [Google Scholar]
- 13.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg A, Quinones-Mateu ME, Lederman MM. Role of Human {beta}-defensins in HIV Infection. Adv Dent Res. 2006;19:42–8. doi: 10.1177/154407370601900109. [DOI] [PubMed] [Google Scholar]
- 15.Yeung SC, Kazazi F, Randle CG, et al. Patients infected with human immunodeficiency virus type 1 have low levels of virus in saliva even in the presence of periodontal disease. J Infect Dis. 1993;167:803–9. doi: 10.1093/infdis/167.4.803. [DOI] [PubMed] [Google Scholar]
- 16.Qureshi MN, Barr CE, Hewlitt I, et al. Detection of HIV in oral mucosal cells. Oral Dis. 1997;3(Suppl 1):S73–8. doi: 10.1111/j.1601-0825.1997.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 17.DeVico AL, Gallo RC. Control of HIV-1 infection by soluble factors of the immune response. Nat Rev Microbiol. 2004;2:401–13. doi: 10.1038/nrmicro878. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–6. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 19.Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Microbiol. 2005;7:1387–97. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14:96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 21.Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 22.Ganz T. Defensins: antimicrobial peptides of vertebrates. C R Biol. 2004;327:539–49. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Quinones-Mateu ME, Lederman MM, Feng Z, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003;17:F39–48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Finnegan CM, Kish-Catalone T, et al. Human beta-defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005;79:14318–29. doi: 10.1128/JVI.79.22.14318-14329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jurevic RJ, Chrisman P, Mancl L, Livingston R, Dale BA. Single-nucleotide polymorphisms and haplotype analysis in beta-defensin genes in different ethnic populations. Genet Test. 2002;6:261–9. doi: 10.1089/10906570260471787. [DOI] [PubMed] [Google Scholar]
- 26.Braida L, Boniotto M, Pontillo A, Tovo PA, Amoroso A, Crovella S. A single-nucleotide polymorphism in the human beta-defensin 1 gene is associated with HIV-1 infection in Italian children. AIDS. 2004;18:1598–600. doi: 10.1097/01.aids.0000131363.82951.fb. [DOI] [PubMed] [Google Scholar]
- 27.Milanese M, Segat L, Pontillo A, Arraes LC, de Lima Filho JL, Crovella S. DEFB1 gene polymorphisms and increased risk of HIV-1 infection in Brazilian children. AIDS. 2006;20:1673–5. doi: 10.1097/01.aids.0000238417.05819.40. [DOI] [PubMed] [Google Scholar]
- 28.Segat L, Milanese M, Boniotto M, et al. DEFB-1 genetic polymorphism screening in HIV-1 positive pregnant women and their children. J Matern Fetal Neonatal Med. 2006;19:13–6. doi: 10.1080/14767050500381123. [DOI] [PubMed] [Google Scholar]
- 29.Goh WC, Markee J, Akridge RE, et al. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–57. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]
- 30.Diaz FJ, Vega JA, Patino PJ, et al. Frequency of CCR5 delta-32 mutation in human immunodeficiency virus (HIV)-seropositive and HIV-exposed seronegative individuals and in general population of Medellin, Colombia. Mem Inst Oswaldo Cruz. 2000;95:237–42. doi: 10.1590/s0074-02762000000200018. [DOI] [PubMed] [Google Scholar]
- 31.Aerts J, Wynendaele W, Paridaens R, et al. A real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) to detect breast carcinoma cells in peripheral blood. Ann Oncol. 2001;12:39–46. doi: 10.1023/a:1008317512253. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann J, Retz M, Harder J, et al. Expression of human beta-defensins 1 and 2 in kidneys with chronic bacterial infection. BMC Infect Dis. 2002;2:20. doi: 10.1186/1471-2334-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousset F, Ra M. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Heredity. 1995;86:248–9. [Google Scholar]
- 34.Excoffier L, L G, Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol Bioinform Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 35.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 37.Rothenberg RB, Scarlett M, del Rio C, Reznik D, O'Daniels C. Oral transmission of HIV. AIDS. 1998;12:2095–105. doi: 10.1097/00002030-199816000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Marmor M, Sheppard HW, Donnell D, et al. Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr. 2001;27:472–81. doi: 10.1097/00126334-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 39.Smith MW, Dean M, Carrington M, et al. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC), ALIVE Study. Science. 1997;277:959–65. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 40.Fleming DC, King AE, Williams AR, Critchley HO, Kelly RW. Hormonal contraception can suppress natural antimicrobial gene transcription in human endometrium. Fertil Steril. 2003;79:856–63. doi: 10.1016/s0015-0282(02)04930-0. [DOI] [PubMed] [Google Scholar]
- 41.Garcia JR, Krause A, Schulz S, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819–21. [PubMed] [Google Scholar]
- 42.King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 43.Mathews M, Jia HP, Guthmiller JM, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999;67:2740–5. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitoh M, Abiko Y, Shimabukuro S, et al. Correlated expression of human beta defensin-1, -2 and -3 mRNAs in gingival tissues of young children. Arch Oral Biol. 2004;49:799–803. doi: 10.1016/j.archoralbio.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proc Natl Acad Sci USA. 2005;102:7952–7. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prado-Montes de Oca E, Garcia-Vargas A, Lozano-Inocencio R, et al. Association of beta-defensin 1 single nucleotide polymorphisms with atopic dermatitis. Int Arch Allergy Immunol. 2006;142:211–8. doi: 10.1159/000097023. [DOI] [PubMed] [Google Scholar]
- 48.Levy H, Raby BA, Lake S, et al. Association of defensin beta-1 gene polymorphisms with asthma. J Allergy Clin Immunol. 2005;115:252–8. doi: 10.1016/j.jaci.2004.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duits LA, Ravensbergen B, Rademaker M, Hiemstra PS, Nibbering PH. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–25. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- 51.Hollox EJ, Armour JA, Barber JC. Extensive normal copy number variation of a beta-defensin antimicrobial-gene cluster. Am J Hum Genet. 2003;73(3):591–600. doi: 10.1086/378157. [DOI] [PMC free article] [PubMed] [Google Scholar]


