Abstract
Provisioning of abundant food resources in human-altered landscapes can have profound effects on wildlife ecology, with important implications for pathogen transmission. While empirical studies have quantified the effects of provisioning on host behaviour and immunology, the net interactive effect of these components on host–pathogen dynamics is unknown. We use simple compartmental models to investigate how provisioning-induced changes to host demography, contact behaviour and immune defence influence pathogen invasion and persistence. We show that pathogen invasion success and equilibrium prevalence depend critically on how provisioning affects host immune defence and that moderate levels of provisioning can lead to drastically different outcomes of pathogen extinction or maximizing prevalence. These results highlight the need for further empirical studies to fully understand how provisioning affects pathogen transmission in urbanized environments.
Keywords: supplemental feeding, compartmental models, host–pathogen interactions, urbanization, feline leukaemia virus
1. Introduction
The acquisition of food resources is a central driver of animal ecology [1]. Human activities can present wildlife with novel food resources that are abundant and spatio-temporally predictable. This resource provisioning can be accidental, as when wildlife capitalize on refuse or agricultural byproducts, or intentional, through supplemental feeding for management or recreation [2–4]. By increasing individual foraging success and thus fitness, provisioning can result in greater population size, and these subsidized populations can exert disproportionate influence on ecological processes, including pathogen transmission [5,6]. Given that provisioning often results in sustained contact between humans, wildlife, and domestic animals, understanding how provisioning influences host–pathogen interactions is a question of concern to public health, livestock well-being, and conservation [3,6,7].
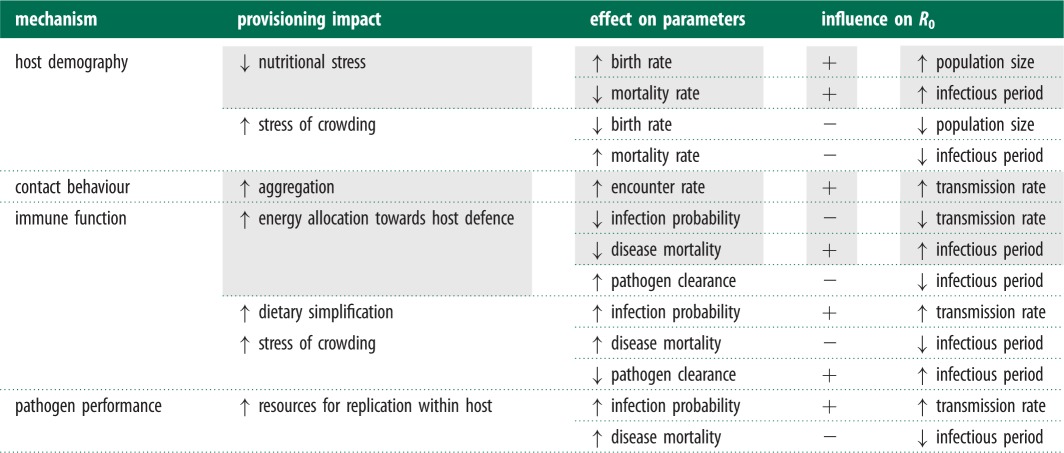
Provisioning can modify multiple processes influencing pathogen transmission, potentially in opposing directions (table 1). These tensions can be understood by considering their effects on the pathogen basic reproductive number (R0), a threshold quantity determining pathogen invasion success and outbreak size [8]. For a close-contact pathogen with density-dependent transmission, R0 is the product of the host population size, infectious period, and transmission rate. Provisioning can elevate R0 by increasing host population size through improved survival and reproduction and contact rates via aggregation around resources [7,9]. Supplemental feeding can also improve host immune response by reducing nutritional stress and increasing body condition [10,11], which can have contrasting effects on R0. Provisioning can decrease host susceptibility [12], thereby lowering the transmission rate, but improved nutrition can also increase tolerance to infection, prolonging the infectious period [13]. Practical limitations mean that the net interactive effects of provisioning on disease processes have rarely been addressed in empirical studies. For example, an observational study of songbirds found highest measures of body condition at sites with intermediate urbanization, possibly owing to high availability of bird feeders. These provisioned populations also showed the highest prevalence of antibodies to West Nile virus, but authors were unable to distinguish whether higher seroprevalence was owing to increased exposure to urban vectors or recovery from disease via abundant resources [14]. Furthermore, although conceptual and mechanistic models have explored the influence of host resources on individual infection outcomes [15,16] and asked how provisioning will alter select components of R0 (e.g. contact rate and population size [7,12]), the interactions between individual- and population-level effects and their net epidemiological outcomes have not been fully explored. Here, we develop a general modelling framework explicitly incorporating provisioning into host demography, contact behaviour, and immune defence and ask how their interaction influences resource-dependent thresholds for pathogen invasion and long-term prevalence.
Table 1.
Potential effects of provisioning on parameters reflecting host demography, contact behaviour, and immune defence and the predicted effect on R0. (Shading indicates assumptions used in the modelling framework.)
 |
2. Material and methods
To explore mechanisms by which provisioning can influence pathogen transmission, we modify simple compartmental models of disease dynamics [8] and allow parameters reflecting host demography, contact behaviour, and immune defence to depend on resource availability. Hosts are categorized according to infection status (susceptible, S, and infected, I) and we consider two scenarios reflecting differing assumptions about pathogen clearance. For non-immunizing infections where recovered individuals can be immediately reinfected, disease dynamics are described by the susceptible–infected–susceptible (SIS) framework:
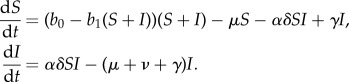 |
If pathogen clearance confers lifelong immunity, we introduce a third variable denoting recovered individuals (R) and use the susceptible–infected–recovered (SIR) framework:
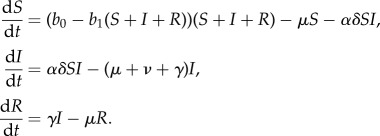 |
Host demography is described by a natural mortality rate μ and a density-dependent birth rate b0−b1N, where N is the population size and b0 and b1 are constants. We assume density-dependent pathogen transmission, but to account for opposing effects of resource-altered aggregation and resistance on transmission, we write the transmission parameter as the product of terms describing contact rate (α) and probability of infection upon encounter (δ). Pathogen clearance and disease-induced mortality occur at rates γ and ν, respectively.
We incorporate provisioning through the parameter ρ, where increasing values reflect improved resource abundance and predictability. The parameter takes values between 0 and 1, where ρ = 0 corresponds to no provisioning (and parameters take baseline values), while ρ = 1 reflects intensive supplemental feeding. The functional dependence of parameters on provisioning is assumed to be monotonic and saturating. If parameter x increases with provisioning, the functional form used is
whereas if x decreases with provisioning, we use
where xmin and xmax are the minimum and maximum values attained and θx describes the strength of the response to provisioning, allowing parameters to scale with resource availability in forms that range from a near-linear to quickly saturating effect.
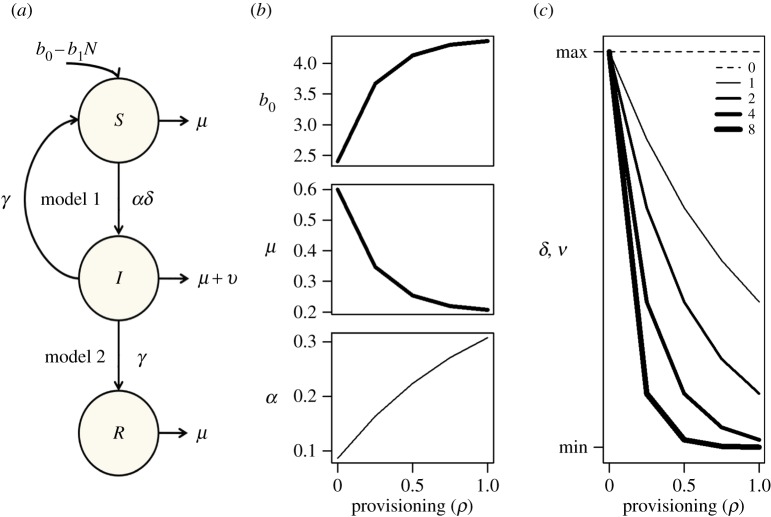
The model was parametrized from published data on feral cats (Felis catus) infected with feline leukaemia virus, a retrovirus transmitted primarily during aggressive encounters and sharing of food resources [17]. As feral cats attain high densities in urban centres through supplemental feeding and foraging on human refuse, there is concern that increased aggregation will amplify viral transmission [4]. Following evidence from the published literature and this host–pathogen system, we assumed provisioning simultaneously influences host demography by increasing fecundity (b0) and decreasing mortality (μ); contact behaviour by increasing encounter rates (α); and immune defence through reducing susceptibility and infectivity (δ) as well as disease-induced mortality (ν). As reduced susceptibility and infectivity lowers transmission and acts antagonistically with demographic, behavioural, and tolerance effects on R0 (table 1), we investigate the net effect of this interaction by covarying the strength of the immune response to provisioning (θδ = θν = θδν). Parametrization is detailed in the electronic supplementary material, and model schematics and parameter dependence on provisioning are visualized in figure 1.
Figure 1.
(a) Schematic of SIS and SIR models. (b,c) Effects of provisioning (ρ) on host fecundity (b0), mortality (μ), contact (α), transmissibility (δ), and disease-induced mortality (ν). Line width depicts how strongly provisioning affects immune defence (θδν values shown in legend).
We quantified the net effect of provisioning on pathogen invasion and long-term infection burdens through calculation of R0 and equilibrium prevalence. Expressions for R0 and prevalence were determined analytically (see the electronic supplementary material) and plotted as functions of ρ under varying assumptions about the response of host immunity to provisioning.
3. Results
Effects of provisioning on isolated mechanisms influenced R0 and equilibrium prevalence predictably (electronic supplementary material, figures S2 and S3). Provisioning-increased survival and fecundity made pathogen invasion more likely by increasing the susceptible population size and infectious period. Provisioning increased equilibrium prevalence above baseline levels, but while SIS prevalence increased dramatically, SIR prevalence attained its maximum at intermediate provisioning but decreased at intensive supplementation owing to the increased proportion of immune individuals. Increasing contact rates also increased R0 and prevalence. By contrast, changes to immunity reduced R0 below the invasion threshold (R0 < 1) and resulted in pathogen extinction.
When provisioning simultaneously affected demographic rates, contact behaviour, and immune defence, various epidemiological outcomes were observed, contingent on how strongly provisioning influenced immunity (figure 2). When provisioning had no influence on transmissibility and virulence, changes in demography and contact exerted a synergistic increase in R0 eightfold above baseline values (θδν = 0; dashed line) and increased prevalence above maxima observed when provisioning influenced these mechanisms in isolation. Weak effects of provisioning on immunity (θδν = 1; thin line) maximized R0 at intermediate resource levels and dampened the prior increase in prevalence at intensive supplemental feeding. When provisioning more strongly affected immunity (θδν = 2; medium line), the observed maxima for R0 and prevalence in both models decreased.
Figure 2.
Effects of provisioning on (a) pathogen invasion success (R0) and (b) equilibrium SIS and SIR prevalence. Line widths depict effects of provisioning on immunity as described in figure 1, and the vertical arrow indicates an increasingly saturating effect on immunity. The grey line shows the pathogen invasion threshold, R0 = 1. R0 is displayed on a log scale.
When provisioning induced a strong saturating immune response, equal to the response of demographic parameters (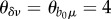 ; thick line), R0 remained above one but attained a smaller maximum at lower resource levels, while prevalence patterns diverged between models. SIS prevalence was reduced at intensive provisioning but remained above baseline levels, whereas SIR prevalence was lower than prevalence in unsupplemented populations.
; thick line), R0 remained above one but attained a smaller maximum at lower resource levels, while prevalence patterns diverged between models. SIS prevalence was reduced at intensive provisioning but remained above baseline levels, whereas SIR prevalence was lower than prevalence in unsupplemented populations.
Inducing a quickly saturating effect of provisioning on immunity (θδν = 8; thickest line) reduced R0 below one at intermediate resource levels, meaning that pathogen invasion is only possible in unprovisioned or heavily provisioned populations. Equilibrium SIS prevalence at high provisioning exceeded baseline values, whereas SIR prevalence was lower in heavily subsidized populations.
4. Discussion
Provisioning has gained growing attention from ecologists and epidemiologists to better understand infectious disease dynamics in response to environmental change and to improve wildlife, domestic animal, and human health [6,7,12]. Theoretical and empirical studies have demonstrated that provisioning can increase pathogen prevalence and outbreak intensity through increases in aggregation and population size [7,9], whereas others suggest provisioning improves nutrition and immune defence in ways that lower infection burdens [2,14,18]. We demonstrate through simple, mechanistic models that interactions between provisioning-altered host demography, contact behaviour, and immune defence can produce a range of disease outcomes in urban-foraging wildlife, thereby providing a unifying framework for understanding opposing prevalence patterns from prior studies.
In agreement with previous work, our models suggest provisioning dramatically increases pathogen invasion success and long-term prevalence when primarily influencing demography or aggregation, and that synergistic interactions between them can amplify transmission. Importantly, our study is, to our knowledge, the first to reveal that this amplifying effect can be modulated or reversed when provisioning substantially reduces susceptibility through improved immune defence. Our model found R0 to be maximized at intermediate provisioning when effects on resistance were weak, mirroring results from a modelling study where resource quality improved host resistance, fecundity, and pathogen shedding [12]. However, when host susceptibility dropped sharply in response to provisioning, prevalence was minimized or pathogen eradication occurred at intermediate resource levels. This pattern runs opposite of that predicted in other theoretical models [7,12,16] and suggests an important role of immune defence in resolving observed outcomes in which supplemental resources have decreased prevalence or catalysed epidemics.
Understanding whether pathogen invasion increases with provisioning or attains its maximum or minimum at intermediate provisioning can be explained by the response strength of immune defence to provisioning relative to behavioural and demographic processes. Reduced susceptibility through provisioning initially drives down R0 relative to unprovisioned populations, but this effect is overcome by greater susceptible density and encounter rates at high provisioning [8]. This has important implications for provisioning as a disease management strategy. First, provisioning will only reduce prevalence if resources strongly improve host condition and immunity. Second, even if provisioning is observed to reduce prevalence, further supplemental feeding can result in pathogen resurgence and increased prevalence relative to unsubsidized populations.
Our results illuminate areas of empirical study necessary to improve our understanding of host–pathogen dynamics in provisioned environments. Because our model highlighted the key role of resource-mediated immunity for determining disease outcomes, experimental manipulations are needed to determine how provisioning influences susceptibility and infectivity. Additionally, although host recovery was not a function of provisioning in our model (owing to stronger evidence for virulence-related effects of improved nutrition), field studies suggest provisioning may decrease prevalence through improved pathogen clearance [14,18]. Thus, laboratory food supplementation experiments quantifying immune defence could incorporate pathogen challenge to better elucidate this relationship [19].
Although we present simple models for directly transmitted pathogens, our framework can be expanded to include alternative transmission pathways, foraging behaviour, and within-host infection dynamics. We have examined the case where the probability of infection given contact decreases with provisioning through improved immune defence; however, provisioned animals in better condition may enhance within-host pathogen replication [20], resulting in an increasing or convex relationship between infectivity and resources [16]. Spatial dynamics of disease may also be affected by provisioning; for example, a model of Hendra virus transmission in flying foxes proposed that resources from urban gardens could increase aggregation and reduce population connectivity, resulting in explosive outbreaks [7]. Further modelling of interactions between subpopulations experiencing different resource levels and thus altered immunity could have profound implications for pathogen spread and persistence. We also assumed provisioned animals experience resources nutritionally equivalent or superior to naturally occurring resources. Although realistic in cases of intentional provisioning for management and recreation, this assumption may not hold in situations where wildlife experience dietary simplification, as when foraging at rubbish dumps. Poor-quality diets deficient in protein or micronutrients could limit or reverse provisioning-mediated improvements to immune defence [11]. Future work incorporating these ideas is a vital step towards predicting and managing disease outbreaks in humans, wildlife, and domestic animals in provisioned environments.
Supplementary Material
Supplementary Material
Acknowledgements
This work was improved through constructive feedback from Sonia Altizer, Nicole Gottdenker, Wes Flynn, Altizer laboratory members and three anonymous reviewers.
Data accessibility
R code simulating effects of provisioning on disease dynamics is provided online.
Funding statement
Funding was provided by a UGA Graduate Research Assistantship and NSF Graduate Research Fellowship to D.J.B.
References
- 1.Morales JM, Moorcroft PR, Matthiopoulos J, Frair JL, Kie JG, Powell RA, Merrill EH, Haydon DT. 2010. Building the bridge between animal movement and population dynamics. Phil. Trans. R. Soc. B 365, 2289–2301. ( 10.1098/rstb.2010.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.French SS, Fokidis HB, Moore MC. 2008. Variation in stress and innate immunity in the tree lizard (Urosaurus ornatus) across an urban–rural gradient. J. Comp. Physiol. B 178, 997–1005. ( 10.1007/s00360-008-0290-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robb GN, McDonald RA, Chamberlain DE, Bearhop S. 2008. Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. ( 10.1890/060152) [DOI] [Google Scholar]
- 4.Patronek GJ. 1998. Free-roaming and feral cats: their impact on wildlife and human beings. J. Am. Vet. Med. Assoc. 212, 218–226. [PubMed] [Google Scholar]
- 5.Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A. 2013. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501–1514. ( 10.1111/ele.12187) [DOI] [PubMed] [Google Scholar]
- 6.Bradley CA, Altizer S. 2007. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 22, 95–102. ( 10.1016/j.tree.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson RM. 1982. Population dynamics of infectious diseases: theory and applications. London, UK; Chapman and Hall. [Google Scholar]
- 9.Wright AN, Gompper ME. 2005. Altered parasite assemblages in raccoons in response to manipulated resource availability. Oecologia 144, 148–156. ( 10.1007/s00442-005-0018-3) [DOI] [PubMed] [Google Scholar]
- 10.Lochmiller RL, Vestey MR, Boren JC. 1993. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. The Auk 110, 503–510. ( 10.2307/4088414) [DOI] [Google Scholar]
- 11.Chandra RK. 1999. Nutrition and immunology: from the clinic to cellular biology and back again. Proc. Nutr. Soc. 58, 681–684. ( 10.1017/S0029665199000890) [DOI] [PubMed] [Google Scholar]
- 12.Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Caceres CE. 2009. Quality matters: resource quality for hosts and the timing of epidemics. Ecol. Lett. 12, 118–128. ( 10.1111/j.1461-0248.2008.01264.x) [DOI] [PubMed] [Google Scholar]
- 13.Brown MJF, Loosli R, Schmid-Hempel P. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91, 421–427. ( 10.1034/j.1600-0706.2000.910302.x) [DOI] [Google Scholar]
- 14.Bradley CA, Gibbs SEJ, Altizer S. 2008. Urban land use predicts West Nile virus exposure in songbirds. Ecol. Appl. 18, 1083–1092. ( 10.1890/07-0822.1) [DOI] [PubMed] [Google Scholar]
- 15.Smith VH, Holt RD. 1996. Resource competition and within-host disease dynamics. Trends Ecol. Evol. 11, 386–389. ( 10.1016/0169-5347(96)20067-9) [DOI] [PubMed] [Google Scholar]
- 16.Cressler CE, Nelson WA, Day T, McCauley E. 2014. Disentangling the interaction among host resources, the immune system and pathogens. Ecol. Lett. 17, 284–293. ( 10.1111/ele.12229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojko JL, Olsen RG. 1984. The immunobiology of the feline leukemia virus. Vet. Immunol. Immunopathol. 6, 107–165. ( 10.1016/0165-2427(84)90050-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eley RM, Strum SC, Muchemi G, Reid GDF. 1989. Nutrition, body condition, activity patterns, and parasitism of free-ranging troops of olive baboons (Papio anubis) in Kenya. Am. J. Primatol. 18, 209–219. ( 10.1002/ajp.1350180304) [DOI] [PubMed] [Google Scholar]
- 19.Cornet S, Bichet C, Larcombe S, Faivre B, Sorci G. 2014. Impact of host nutritional status on infection dynamics and parasite virulence in a bird–malaria system. J. Anim. Ecol. 83, 256–265. ( 10.1111/1365-2656.12113) [DOI] [PubMed] [Google Scholar]
- 20.Seppälä O, Liljeroos K, Karvonen A, Jokela J. 2008. Host condition as a constraint for parasite reproduction. Oikos 117, 749–753. ( 10.1111/j.0030-1299.2008.16396.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
R code simulating effects of provisioning on disease dynamics is provided online.