Abstract
Maternal effects have wide-ranging effects on life-history traits. Here, using the crustacean Daphnia magna, we document a new effect: maternal food quantity affects offspring feeding rate, with low quantities of food triggering mothers to produce slow-feeding offspring. Such a change in the rate of resource acquisition has broad implications for population growth or dynamics and for interactions with, for instance, predators and parasites. This maternal effect can also explain the previously puzzling situation that the offspring of well-fed mothers, despite being smaller, grow and reproduce better than the offspring of food-starved mothers. As an additional source of variation in resource acquisition, this maternal effect may also influence relationships between life-history traits, i.e. trade-offs, and thus constraints on adaptation. Maternal nutrition has long-lasting effects on health and particularly diet-related traits in humans; finding an effect of maternal nutrition on offspring feeding rate in Daphnia highlights the utility of this organism as a powerful experimental model for exploring the relationship between maternal diet and offspring fitness.
Keywords: maternal effects, transgenerational effects, resource acquisition, population dynamics, trade-offs
1. Introduction
A key challenge of life is to adapt to changing conditions, and an important mechanism of adaptation is phenotypic plasticity, which allows individuals to rapidly modify their phenotype to suit new conditions. Maternal effects, where the mother's phenotype or the conditions she experiences influence the phenotype of her offspring, are a type of phenotypic plasticity that potentially allow parents to modify their offspring's phenotype to suit expected conditions [1]. Alternatively, maternal effects can arise as simple by-products of changes in the maternal environment, for instance, when costs of poor maternal conditions are transferred from mothers to offspring [2].
The crustacean Daphnia has been the subject of much work on phenotypic plasticity and maternal effects, partly because it offers extensive and eloquent examples of these phenomena [3], but also because of its clonal reproduction, which facilitates powerful experiments that separate effects driven by environmental cues from those attributable to genotype. An established case of phenotypic plasticity in Daphnia concerns traits tied to feeding biology; when food is scarce, through modifications of both filter screen size and the rate at which they beat their filtering apparatus, Daphnia increase the efficiency with which they feed, resulting in improved growth and reproduction at lower food concentrations [4–10]. However, because Daphnia generations overlap (and thus parental conditions are likely to match offspring conditions) we might expect that adult Daphnia also respond to food scarcity by altering traits that affect the feeding rate of their offspring. This, however, has never been tested.
Yet, resource acquisition is a crucial component of life-history theory, especially concerning life-history trade-offs. Specifically, the degree of variation in resource acquisition critically modulates the relationships between traits, a factor that has likely had a large impact on our ability to detect trade-offs, and estimate constraints on evolution [11,12]. Thus, motivated by the fact that maternal effects on offspring feeding rate could have wide-ranging consequences for adaptation, and our ability to detect and understand it, here we test the effect of food quantity on offspring filter screen size and feeding rate in several Daphnia magna genotypes.
2. Material and methods
(a). Acclimation
To enable individual Daphnia to be treated as independent replicates, and to avoid the accumulation of uncontrolled transgenerational effects in the experimental animals, 20–24 replicate clonal lineages of each genotype were maintained under standard conditions for three generations prior to their use in experiments as previously described [13]. During this period, Daphnia received 5 × 106 Chorella algae/day, which was estimated by measuring the absorbance of the algae at 664 nm white light, and which was the same as the ‘high-food’ treatment below.
(b). Maternal effects on feeding rate
The essential feature of this experiment was to manipulate maternal food quantity and measure the feeding rate of offspring. To set up the maternal generation, we assigned two neonates from the second clutch of the third acclimatizing generation to high-food (5 × 106 Chorella algae/day) or low-food (1.5 × 106 Chorella algae/day) treatments. One offspring was taken from the second clutch of these maternal Daphnia; offspring were photographed on the day of birth to measure body size (as previously described [13]) and their feeding rate measured by determining how quickly they filter algae from the water. For this, individuals were placed in wells of a 24-well plate (Costar Corning, NY, USA) with 2 ml media containing 5 × 106 Chorella algae. The plates were incubated for 24 h, following which the contents of each well were mixed and three aliquots of 200 µl then removed to the wells of a new 96-well plate (Costar Corning). The optical absorbance of 650 nm white light by each well was determined using a plate-reading spectrophotomer (BioTek) and the mean calculated for the three replicate wells. Six control wells per plate did not contain any Daphnia, and feeding rate for each Daphnia was calculated by subtracting the mean absorbance of the three replicate wells from the mean absorbance of the six control wells.
We initially conducted this experiment with eight European genotypes (one Finnish, one German, one Israeli and five Scottish) to capture among-population variation and then repeated the experiment (except that we did not measure body size) with 10 Scottish genotypes from the same pond to study within-population variation.
(c). Maternal effects on filter screen size
Mothers were raised under conditions of high or low food (as above) and the filter screens of offspring dissected from their third and fourth appendage limbs. Filter screens were spread on a microscopic slide, photographed (as above) and measured using the Polygon Area Selection Tool in ImageJ v. 1.46r (http://rsbweb.nih.gov/ij/). Body length was also measured pre-dissection as above. Ten Scottish genotypes were used for this experiment (and there were 24 replicates in total).
(d). Analysis
We tested the effect of maternal food on offspring feeding rate in the eight European genotypes by fitting linear mixed effects models (package lme4 in R) with maternal food, body size at birth and genotype as fixed effects. The significance of each term was assessed by comparing models with and without that term using a likelihood-ratio test. We analysed the Scottish genotypes from the within-population experiment in the same way (omitting body size, which we did not measure). Filter screen size was calculated by adding together the areas of filter screens from the third and fourth appendage limbs and analysed in a linear model with maternal food treatment and body size (squared) as explanatory variables in JMP v. 10.00 (SAS Institute Inc., Cary, NC, USA). The body length by maternal food interaction term was not significant and so was dropped from the model.
3. Results
For the eight European D. magna genotypes, feeding rate increased with body length at birth (table 1). After accounting for this effect of body length, the offspring of low-food-treated mothers fed at a significantly lower rate than the offspring of high-food-treated mothers (figure 1a and table 1). For the 10 Scottish D. magna clones, the offspring of low-food-treated mothers fed at a significantly lower rate than the offspring of high-food-treated mothers (figure 1b and table 1). In both experiments, the effect of maternal food did not significantly vary across genotypes. Filter screen size increased with body length (figure 2 and table 1). After taking into account the effect of body length, filter screens from offspring born of food-restricted mothers were significantly larger than those from the offspring of well-fed mothers (figure 2 and table 1).
Table 1.
Feeding rate of the (a) eight European genotypes and (b) 10 Scottish genotypes was analysed using mixed models. Significance of each effect was assessed using likelihood-ratio tests (the likelihood-ratio test statistic, which is twice the difference in the log likelihoods of the models, follows the χ2 distribution). (c) Filter screen area was analysed with a linear model.
| response | effect | d.f. | F | χ2 | p |
|---|---|---|---|---|---|
| (a) feeding rate | maternal food | 6.26 | 0.01 | ||
| body length | 17.50 | <0.001 | |||
| maternal food × body length | 0.00 | 0.50 | |||
| genotype × body length | 1.10 | 0.15 | |||
| genotype × maternal food | 0.69 | 0.20 | |||
| (b) feeding rate | maternal food | 4.04 | 0.02 | ||
| (c) filter screen area | maternal food | 1, 41 | 5.62 | 0.023 | |
| square (body length) | 1, 41 | 8.67 | 0.005 |
Figure 1.
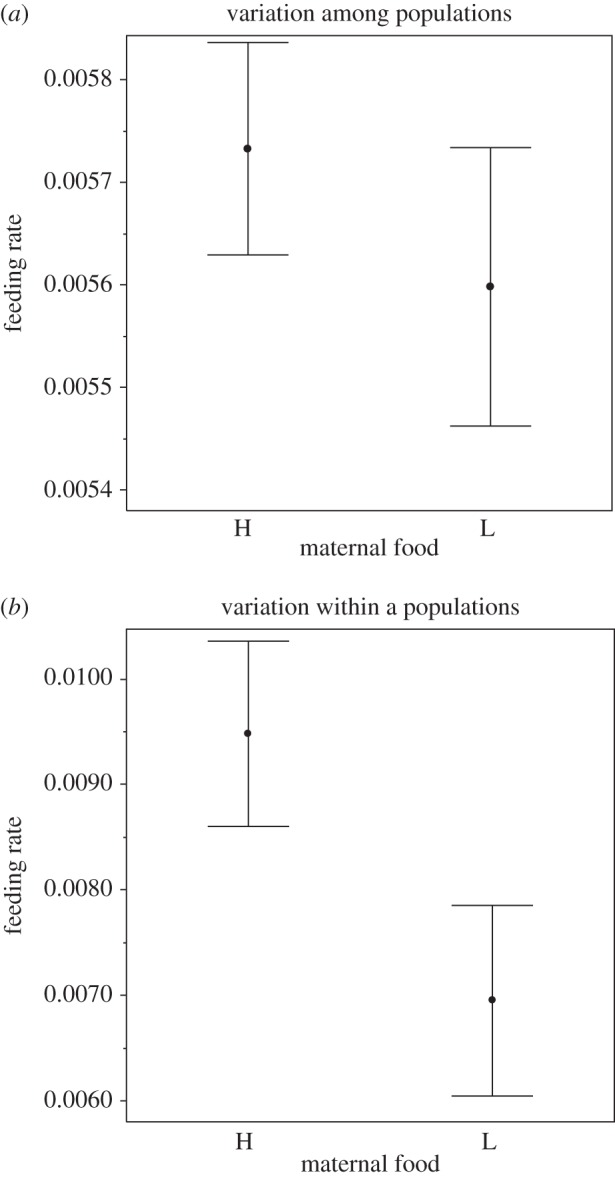
Feeding rate of offspring whose mothers received high (H) or low (L) food in two experiments with (a) eight European genotypes (to estimate among-population variation) and (b) 10 Scottish genotypes (to estimate within-population variation). Data are fitted means.
Figure 2.
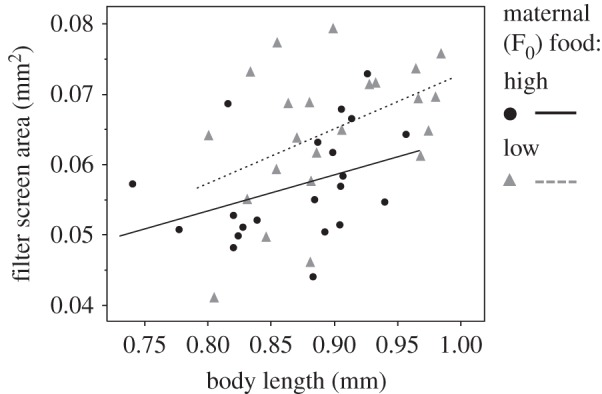
Additive third and fourth appendage limb filter screen area in offspring whose mothers received high or low food plotted against body length.
4. Discussion
We have shown that food-restricted D. magna mothers produce offspring with a low rate of feeding (figure 1), i.e. they clear a fixed amount of algae from the water more slowly than offspring from well-fed mothers do. This is the first report showing that the prevalence of a food resource (or prey) affects the rate at which it is consumed by the next generation of consumers (or predators). This maternal effect on feeding rate may act in the opposite direction to food-induced changes in feeding rate within a generation. The implications of this result are broad. Consumer–resource population dynamics are likely to be affected. We might expect, for example, that the maternal effect dampens large amplitude cycles, because as the algae decline, the rate at which Daphnia feed on them recedes. Changes in feeding rate may also affect aging [14], as well as interactions with predators and pathogens. Indeed, this maternal effect could explain why low maternal food reduces the risk of infection with the orally ingested pathogen Pasteuria ramosa [15–17], if we suppose that a lower rate of feeding results in ingestion of fewer infectious spores. Predation risk could also be affected because feeding rate is linked to body size, and because many Daphnia predators are size-selective [18].
Our results even offer intriguing potential parallels for the role of maternal nutrition on offspring feeding physiology in humans. For example, as revealed by the Dutch famine study, adults exposed to famine in utero have different rates of obesity, possibly linked to decreased glucose tolerance [19]. Just as for the human study, the adaptive nature of the maternal effect in Daphnia is difficult to pin down, partly because feeding rate will affect a great many life-history traits that themselves have context-dependent effects on fitness, and thus a substantial set of further experiments are required to fully address adaptation. Understanding the mechanism of feeding rate reduction would contribute to elucidating the adaptive nature of this plasticity. Towards this, we measured filter screen size, but found that the offspring of low-food mothers had larger filter screens (figure 2). In principle, larger screens would filter more algae, and thus we can only speculate that the reduction in feeding rate under low maternal food is caused by these offspring beating their filtering apparatus at a lower rate. Elsewhere, larger screens and reduced beat rates have been shown to improve feeding efficiency [4], and so it is conceivable that Daphnia born to poorly fed mothers could be maximizing efficiency. Another possibility is that the change in feeding rate simply represents a transgenerational cost of being malnourished, although this is unlikely given that the offspring of poorly fed mothers are relatively large and well provisioned at birth [20–23].
Our results can help us to understand several puzzling maternal effects in Daphnia. Previously, researchers have been confused by the observation that the offspring of high-food mothers, despite being smaller, lighter and containing less carbon at birth [20–23], perform better in terms of growth [17,24] and reproduction [20,21,23] than the offspring of low-food mothers. These results were puzzling because life-history theory generally predicts that larger, better provisioned, individuals should perform better than smaller individuals [25]. Lynch & Ennis [24] even remarked that high-food-derived offspring, ‘exhibit an enhanced incorporation of energy into early growth and reproduction far in excess of what can be attributed to maternal stores’. We speculate that the smaller offspring of well-fed mothers perform better because they are acquiring algae from the environment more rapidly than are the offspring of poorly fed mothers [24]. By revealing an additional source of variation in resource acquisition, our results also have important implications for our ability to detect trade-offs and understand constraints on evolution [11,12]. That cases where we expect, but do not find, trade-offs could be mediated by maternal effects, and experimentally tested, seems an exciting new possibility for the field of trade-offs. Daphnia are well suited to these experiments and so could become an important biological model for studying how maternal effects influence trade-offs, as well as for understanding how a mother's diet affects the fitness and health of her offspring.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank two anonymous referees for their helpful comments on the manuscript.
Data accessibility
The data supporting this article has been uploaded as part of the electronic supplementary material.
Funding statement
This work was supported by a NERC grant no. NE/I026405/1.
References
- 1.Mousseau TA, Fox CW. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407. ( 10.1016/S0169-5347(98)01472-4) [DOI] [PubMed] [Google Scholar]
- 2.Marshall DJ, Uller T. 2007. When is a maternal effect adaptive? Oikos 116, 1957–1963. ( 10.1111/j.2007.0030-1299.16203.x) [DOI] [Google Scholar]
- 3.Colbourne JK, et al. 2011. The ecoresponsive genome of Daphnia pulex. Science 331, 555–561. ( 10.1126/science.1197761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lampert W, Brendelberger H. 1996. Strategies of phenotypic low-food adaptation in Daphnia: filter screens, mesh sizes, and appendage beat rates. Limnol. Oceanogr. 41, 216–223. ( 10.4319/lo.1996.41.2.0216) [DOI] [Google Scholar]
- 5.Pop M. 1991. Mechanisms of the filtering area adaptation in Daphnia. Hydrobiologia 225, 169–176. ( 10.1007/BF00028394) [DOI] [Google Scholar]
- 6.Stuchlík E. 1991. Feeding behaviour and morphology of filtering combs of Daphnia galeata. Hydrobiologia 225, 155–167. ( 10.1007/BF00028393) [DOI] [Google Scholar]
- 7.Lampert W. 1994. Phenotypic plasticity of the filter screens in Daphnia: adaptation to a low-food environment. Limnol. Oceanogr. 39, 997–1006. ( 10.4319/lo.1994.39.5.0997) [DOI] [Google Scholar]
- 8.Hanazato T. 1996. Combined effects of food shortage and oxygen deficiency on life history characteristics and filter screens of Daphnia. J. Plankton Res. 18, 757–765. ( 10.1093/plankt/18.5.757) [DOI] [Google Scholar]
- 9.Repka S, Veen A, Vijverberg J. 1999. Morphological adaptations in filtering screens of Daphnia galeata to food quantity and food quality. J. Plankton Res. 21, 971–989. ( 10.1093/plankt/21.5.971) [DOI] [Google Scholar]
- 10.Repka S, Veselá S, Weber A, Schwenk K. 1999. Plasticity in filtering screens of Daphnia cucullata×galeata hybrids and parental species at two food concentrations. Oecologia 120, 485–491. ( 10.1007/s004420050881) [DOI] [PubMed] [Google Scholar]
- 11.Van Noordwijk AJ, de Jong G. 1986. Acquisition and allocation of resources: their influence on variation in life history tactics. Am. Nat. 128, 137–142. ( 10.1086/284547) [DOI] [Google Scholar]
- 12.De Jong GR, van Noordwijk AJ. 1992. Acquisition and allocation of resources: genetic (CO) variances, selection, and life histories. Am. Nat. 139, 749–770. ( 10.1086/285356) [DOI] [Google Scholar]
- 13.Garbutt JS, Scholefield JA, Vale PF, Little TJ. 2013. Elevated maternal temperature enhances offspring disease resistance in Daphnia magna. Funct. Ecol. 28, 424–431. ( 10.1111/1365-2435.12197) [DOI] [Google Scholar]
- 14.Masoro EJ. 2005. Overview of caloric restriction and ageing. Mech. Ageing Dev. 126, 913–922. ( 10.1016/j.mad.2005.03.012) [DOI] [PubMed] [Google Scholar]
- 15.Mitchell SE, Read AF. 2005. Poor maternal environment enhances offspring disease resistance in an invertebrate. Proc. R. Soc. B 272, 2601–2607. ( 10.1098/rspb.2005.3253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Ami F, Ebert D, Regoes RR. 2010. Pathogen dose infectivity curves as a method to analyze the distribution of host susceptibility: a quantitative assessment of maternal effects after food stress and pathogen exposure. Am. Nat. 175, 106–115. ( 10.1086/648672) [DOI] [PubMed] [Google Scholar]
- 17.Stjernman M, Little TJ. 2011. Genetic variation for maternal effects on parasite susceptibility. J. Evol. Biol. 24, 2357–2363. ( 10.1111/j.1420-9101.2011.02363.x) [DOI] [PubMed] [Google Scholar]
- 18.Riessen HP, Young JD. 2005. Daphnia defense strategies in fishless lakes and ponds: one size does not fit all. J. Plankton Res. 27, 531–544. ( 10.1093/plankt/fbi029) [DOI] [Google Scholar]
- 19.Ravelli A, van der Meulen J, Michels R, Osmond C, Barker D, Hales C, Bleker O. 1998. Glucose tolerance in adults after prenatal exposure to famine. Lancet 351, 173–177. ( 10.1016/S0140-6736(97)07244-9) [DOI] [PubMed] [Google Scholar]
- 20.Boersma M. 1997. Offspring size and parental fitness in Daphnia magna. Evol. Ecol. 11, 439–450. ( 10.1023/A:1018484824003) [DOI] [Google Scholar]
- 21.Boersma M. 1997. Offspring size in Daphnia: does it pay to be overweight? Hydrobiologia 360, 79–88. ( 10.1023/A:1003184214186) [DOI] [Google Scholar]
- 22.Ebert D. 1993. The trade-off between offspring size and number in Daphnia magna: the influence of genetic, environmental and maternal effects. Arch. Hydrobiol. Suppl 904, 453–473. [Google Scholar]
- 23.Guinnee MA, Gardner A, Howard AE, West SA, Little TJ. 2007. The causes and consequences of variation in offspring size: a case study using Daphnia. J. Evol. Biol. 20, 577–587. ( 10.1111/j.1420-9101.2006.01253.x) [DOI] [PubMed] [Google Scholar]
- 24.Lynch M, Ennis R. 1983. Resource availability, maternal effects, and longevity. Exp. Gerontol. 18, 147–165. ( 10.1016/0531-5565(83)90008-6) [DOI] [PubMed] [Google Scholar]
- 25.Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring. Am. Nat. 108, 499–506. ( 10.1086/282929) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article has been uploaded as part of the electronic supplementary material.


