Abstract
Uric acid stored in the fat body of cockroaches is a nitrogen reservoir mobilized in times of scarcity. The discovery of urease in Blattabacterium cuenoti, the primary endosymbiont of cockroaches, suggests that the endosymbiont may participate in cockroach nitrogen economy. However, bacterial urease may only be one piece in the entire nitrogen recycling process from insect uric acid. Thus, in addition to the uricolytic pathway to urea, there must be glutamine synthetase assimilating the released ammonia by the urease reaction to enable the stored nitrogen to be metabolically usable. None of the Blattabacterium genomes sequenced to date possess genes encoding for those enzymes. To test the host's contribution to the process, we have sequenced and analysed Blattella germanica transcriptomes from the fat body. We identified transcripts corresponding to all genes necessary for the synthesis of uric acid and its catabolism to urea, as well as for the synthesis of glutamine, asparagine, proline and glycine, i.e. the amino acids required by the endosymbiont. We also explored the changes in gene expression with different dietary protein levels. It appears that the ability to use uric acid as a nitrogen reservoir emerged in cockroaches after its age-old symbiotic association with bacteria.
Keywords: nitrogen metabolism, Blattabacterium, glutamine, asparagine, proline, glycine
1. Introduction
Insect endosymbionts supply their hosts with nutrients needed for their particular lifestyles, mainly essential amino acids or vitamins. Besides, many animals also rely on microbial endosymbionts to recycle their nitrogenous waste products. For instance, in the aphid Acyrthosiphon pisum, the ammonia generated in the bacteriocytes (cells containing bacterial endosymbionts) is incorporated into the carbon skeletons of essential amino acids that are generated by Buchnera aphidicola [1]. In other insects, like the shield bug, Parastrachia japonensis, or the brown planthopper, Nilaparvata lugens, endosymbionts enable the host to use uric acid as a nitrogen source during starvation periods [2,3].
It is well known that cockroaches are able to accumulate uric acid when they are fed on a protein-rich diet, and conversely the amount of uric acid stored decreases when they are deprived of proteins [4,5]. Classic observations have suggested that the endosymbiont Blattabacterium lies behind these fluctuations. For example, observations show that bacteriocytes are closely associated with uricocytes in the host's fat body, a cell type storing urates [5]. We also know that aposymbiotic individuals of Blattella germanica accumulate high amounts of uric acid [6]. The identification of genes encoding for all enzymes of the urea cycle and for urease in the Blattabacterium genome [7,8], as well as the results of flux balance analysis (FBA) carried out on the reconstructed metabolic networks of Blattabacterium strains from the cockroaches B. germanica and Periplaneta americana support the key role of the endosymbiont in cockroach nitrogen metabolism [9]. The analysis of six further strains reinforced this hypothesis as the genes for urease and most of the genes of the urea cycle are part of the core of the Blattabacterium pangenome [10,11]. Genome-scale metabolic modelling is consistent with these ideas and also shows that Blattabacterium is auxotrophic for several non-essential amino acids, including glutamine [9].
Based on these studies, a model was proposed where the uric acid accumulated in the cockroach fat body was used as a nitrogen reservoir, to be mobilized in periods of scarcity [7,9]. This model requires a host uricolytic pathway (i.e. urate oxidase, allantoinase and allantoicase) and also the supply of non-essential amino acids to the endosymbiont. Despite the presence of many of these enzymes among Bacteroidetes [8], none of the Blattabacterium genomes sequenced so far contains the necessary genes [10,11]. However, urate oxidase activity was detected in some tissues of the cockroaches Leucophaea maderae [12] and P. americana [13]. In the context of this metabolic model, we have also proposed the action of membrane facilitators for urea and glutamine coded in the Blattabacterium genome, i.e. glpF and gltP genes, respectively [7].
This work investigates the presence of transcripts for enzymes involved in nitrogen metabolism in the transcriptome of three B. germanica tissues. Two tissue types harbour Blattabacterium: the fat body where the bacterium is massively present, and the ovary, where only a small population of bacteria is present. The third tissue type (the epidermis, including cuticle layers) is a Blattabacterium-free tissue. We have also explored how genes involved in uric acid metabolism respond to dietary nitrogen levels. Additionally, we have been able to find the transcripts for the synthesis of the non-essential amino acids required by Blattabacterium metabolism.
2. Material and methods
Blattabacterium germanica specimens were obtained from a population reared at the facilities of the Institut de Biologia Evolutiva (CSIC-UPF) in Barcelona, Spain. RNA extraction and cDNA synthesis were performed using standard procedures. Each transcriptome library was sequenced on the 454-Flx platform, assembled and annotated (see the electronic supplementary material).
The relative expression of genes involved in uric acid metabolism was measured in animals fed on different experimental diets with different protein content (0, 5 and 50%), using animals fed on dog food (25% of protein content) as a control (see the electronic supplementary material). Results are represented as copies of target mRNA against the corresponding reference gene (actin 5c and elongation factor EF-Tu in the case of host and endosymbiont transcripts, respectively). Statistical analyses were run with REST [14] (see the electronic supplementary material for further details).
3. Results and discussion
(a). Uric acid metabolism is shared between host and endosymbiont
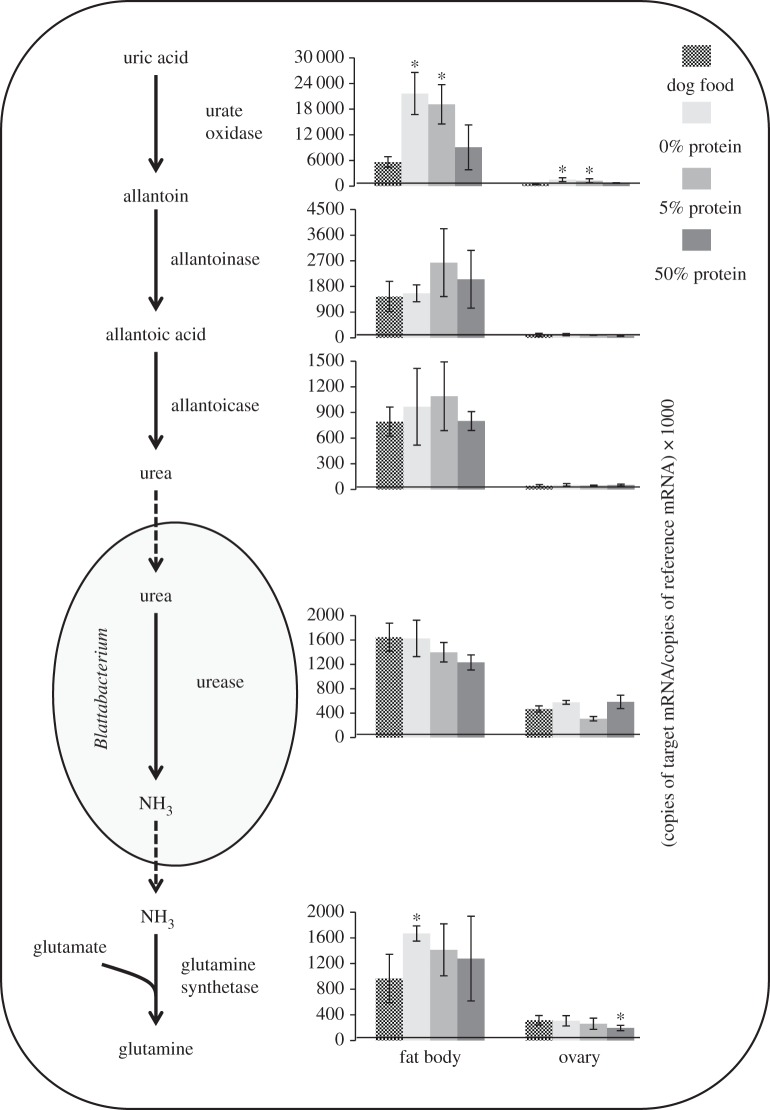
The nitrogen recycling process in cockroaches involves the degradation of uric acid to urea, and the later degradation of this metabolite by a Blattabacterium urease, generating ammonia and CO2. It has been postulated that endosymbiont-released ammonia would be used by a host-encoded glutamine synthetase to produce glutamine, thus incorporating nitrogen from uric acid to metabolism [7,9]. The expression of the genes for uricolytic enzymes was detected in the library obtained from the fat body (figure 1). Conversely, only urate oxidase and allantoicase transcripts were detected in the ovary library, whereas none of these genes were expressed in the epidermis library. With the expression of genes for all uricolytic enzymes and glutamine synthetase, the pathway postulated for uric acid recycling would be possible in the fat body (figure 1). On the strength of these results, we can propose that B. germanica possesses a nitrogen recycling system similar to the one observed in P. japonensis [3] or in N. lugens [2], albeit differing greatly with these systems where the uricolytic activities are supplied by the symbionts: in B. germanica, the pathway is chimeric with participation of enzymes from the host and the symbiont.
Figure 1.
Proposed model for uric acid mobilization. The expression pattern in response to dietary protein levels is expressed beside each gene as copies of mRNA from the target gene per 1000 copies of reference gene (actin 5c and EF-Tu for Blattella and Blattabacterium transcripts, respectively). The asterisk represent statistically significant differences with respect to control (p < 0.05, n = 3).
(b). Host metabolism complements non-essential amino acid auxotrophies of Blattabacterium
Glutamine is not the only non-essential amino acid required by the endosymbiont metabolism. The FBA of the genome-scale metabolic network of B. germanica–Blattabacterium would suggest that Blattabacterium is also auxotrophic for L-Asn, Gly and L-Pro [9]. Transcripts from all necessary genes for the synthesis of these amino acids were identified in the fat body library, but not in the ovary or the epidermis libraries (table 1). Interestingly, some of these non-essential amino acids are among the most abundant free amino acids in cockroach haemolymph, as measured in Blaberus discoidalis [15] and in P. americana [16], L-Pro and Gly being the most abundant in both species. The loss of the ability to synthesize non-essential amino acids seems to be a common feature in other insect endosymbionts such as Buchnera [17] or Blochmannia [18], which are endosymbionts of aphids and Camponotus ants, respectively. In aphids, like cockroaches, these non-essential amino acids are also among the most abundant in the haemolymph [1], and their availability in host tissues renders maintenance of biosynthetic pathways for them unnecessary in the endosymbiont. Blattabacterium germanica might use the amino acid supply to control the metabolic behaviour or growth rate of Blattabacterium, like the control that the aphid A. pisum exerts on the essential amino acid metabolism of Buchnera by modulating the supply of metabolic precursors [19,20]. This sort of control over the symbiotic population through amino acid supply has also been observed in plant hosts when controlling their nitrogen-fixing bacteria [21].
Table 1.
Presence (+) or absence (–) of transcripts related to non-essential amino acid biosynthesis in the three tissue libraries (fat body, ovary and epidermis) of B. germanica. (All transcripts, even those represented by a single read, were considered. See the electronic supplementary material, table S3, for accession numbers and best BLAST hits. EC, enzyme commission number.)
| gene | EC | fat body | ovary | epidermis |
|---|---|---|---|---|
| asparagine biosynthesis | ||||
| aspartate aminotransferase (mitochondrial-like and cytoplasmic) | 2.6.1.1 | + | + | + |
| asparagine synthetase | 6.3.5.4 | + | + | − |
| glutamine biosynthesis | ||||
| glutamate dehydrogenase | 1.4.1.3 | + | + | + |
| glutamine synthetase | 6.3.1.2 | + | + | + |
| proline biosynthesis | ||||
| glutamate-semialdehyde dehydrogenase | 2.7.2.11 | + | − | − |
| ornithine-δ-transaminase | 2.6.1.3 | + | + | − |
| pyrroline-5-carboxylate reductase (isozymes P5CR and P5CR2) | 1.5.1.2 | + | + | − |
| glycine biosynthesis | ||||
| phophoglycerate dehydrogenase | 1.1.1.95 | + | + | + |
| phosphoserine transaminase | 2.6.1.52 | + | + | − |
| phosphoserine phosphatase | 3.1.3.3 | + | − | − |
| serine hydroxymethyltransferase | 2.1.2.1 | + | + | + |
(c). Dietary nitrogen levels affect gene expression
Once we had confirmed that the fat body of B. germanica expresses genes involved in uric acid production and degradation, we measured the expression of these genes in the fat body and ovary in response to dietary nitrogen levels. Urate oxidase gene expression increased significantly in both tissues of animals fed on a low-protein diet (figure 1). The other gene showing a significant variation in expression is the one for glutamine synthetase, which is over-expressed in the fat body of animals fed on a non-protein diet, and downregulated in the ovary of those animals fed on a high-protein diet (figure 1). None of the other genes showed significant increases in expression, suggesting that the uricolytic pathway is expressed in a constitutive manner and other levels of flux regulation must exist.
Cockroaches accumulate uric acid in the fat body, especially specimens fed on protein-rich diets [5]. The amount of uric acid accumulated in these animals decreases dramatically when they are shifted to a low-protein diet [22]. Both observations suggest that uric acid is actually a reservoir of nitrogen, ready to be mobilized in periods of scarcity. Our observations on the increased gene expression for urate oxidase and glutamine synthetase in animals deprived of a dietary nitrogen source are consistent with this proposal.
We can conclude, thus, that after the symbiotic association between the ancestors of cockroaches and Blattabacterium, their metabolic networks merged and transformed a nitrogen waste product in insects, such as uric acid, into a metabolically useful source of nitrogen.
Data accessibility
Available in the electronic supplementary material, table S3.
Funding statement
This work was supported by the Ministerio de Ciencia e Innovación, Spain (BFU2012-39816-C02-01, co-financed by FEDER funds, to A.L., BFU2011-22404 to M.D.P. and CGL2008-03517/BOS and CGL2012-36251 to X.B.) and the Generalitat Valenciana, Spain (Prometeo/2009/092 to A.M.). R.P. was recipient of a fellowship from the Ministerio de Ciencia e Innovación, Spain.
Supplementary Material
References
- 1.Macdonald SJ, Lin GG, Russell CW, Thomas GH, Douglas AE. 2012. The central role of the host cell in symbiotic nitrogen metabolism. Proc. R. Soc. B 279, 2965–2973. ( 10.1098/rspb.2012.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hongoh Y, Ishikawa H. 1997. Uric acid as a nitrogen resource for the brown planthopper, Nilaparvata lugens: studies with synthetic diets and aposymbiotic insects. Zool. Sci. 14, 581–586. ( 10.2108/zsj.14.581) [DOI] [Google Scholar]
- 3.Kashima T, Nakamura T, Tojo S. 2006. Uric acid recycling in the shield bug, Parastrachia japonensis (Hemiptera: Parastrachiidae), during diapause. J. Insect Physiol. 52, 816–825. ( 10.1016/j.jinsphys.2006.05.003) [DOI] [PubMed] [Google Scholar]
- 4.Cochran D. 1985. Nitrogen excretion in cockroaches. Annu. Rev. Entomol. 30, 29–49. ( 10.1146/annurev.en.30.010185.000333) [DOI] [Google Scholar]
- 5.Cochran DG, Mullins DE, Mullins KJ. 1979. Cytological changes in the fat body of the American cockroach, Periplaneta americana, in relation to dietary nitrogen levels. Ann. Entomol. Soc. Am. 72, 197–205. [Google Scholar]
- 6.Valovage WD, Brooks MA. 1979. Uric acid quantities in the fat body of normal and aposymbiotic German cockroaches, Blattella germanica. Ann. Entomol. Soc. Am. 72, 687–689. [Google Scholar]
- 7.López-Sánchez M, Neef A, Peretó J, Patiño-Navarrete R, Pignatelli M, Latorre A, Moya A. 2009. Evolutionary convergence and nitrogen metabolism in Blattabacterium strain Bge, primary endosymbiont of the cockroach Blattella germanica. PLoS Genet. 5, e1000721 ( 10.1371/journal.pgen.1000721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabree ZL, Kambhampati S, Moran NA. 2009. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl Acad. Sci. USA 106, 19 521–19 526. ( 10.1073/pnas.0907504106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.González-Domenech CM, Belda E, Patiño-Navarrete R, Peretó J, Moya A, Latorre A. 2012. Metabolic stasis in an ancient symbiosis: genome-scale metabolic networks from two Blattabacterium cuenoti strains, primary endosymbionts of cockroaches. BMC Microbiol. 12, S5 ( 10.1186/1471-2180-12-S1-S5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patiño-Navarrete R, Moya A, Latorre A, Peretó J. 2013. Comparative genomics of Blattabacterium cuenoti: the frozen legacy of an ancient endosymbiont genome. Genome Biol. Evol. 5, 351–361. ( 10.1093/gbe/evt011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokuda G, et al. 2013. Maintenance of essential amino acid synthesis pathways in the Blattabacterium cuenoti symbiont of a wood-feeding cockroach. Biol. Lett. 9, 20121153 ( 10.1098/rsbl.2012.1153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lisa JD, Ludwig D. 1958. Uricase, guanase, and xanthine oxidase from the fat body of the cockroach, Leucophaea maderae. Ann. Entomol. Soc. Am. 52, 548–551. [Google Scholar]
- 13.Cordero S, Ludwig D. 1962. Purification and activities of purine enzymes from various tissues of the American cockroach Periplaneta americana Linnaeus (Orthoptera: Blattidae). New York Entomol. Soc. 71, 66–73. [Google Scholar]
- 14.Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30, e36 ( 10.1093/nar/30.9.e36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowa SM, Keeley LL. 1996. Free amino acids in the hemolymph of the cockroach, Blaberus discoidalis. Comp. Biochem. Physiol. A Physiol. 113, 131–134. ( 10.1016/0300-9629(95)02043-8) [DOI] [PubMed] [Google Scholar]
- 16.Stevens TM. 1961. Free amino acids in the hemolymph of the American cockroach, Periplaneta americana L. Comp. Biochem Physiol. 3, 304–309. ( 10.1016/0010-406X(61)90017-2) [DOI] [PubMed] [Google Scholar]
- 17.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407, 81–86. ( 10.1038/35024074) [DOI] [PubMed] [Google Scholar]
- 18.Gil R, et al. 2003. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc. Natl Acad. Sci. USA 100, 9388–9393. ( 10.1073/pnas.1533499100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacDonald SJ, Thomas GH, Douglas AE. 2011. Genetic and metabolic determinants of nutritional phenotype in an insect–bacterial symbiosis. Mol. Ecol. 20, 2073–2084. ( 10.1111/j.1365-294X.2011.05031.x.) [DOI] [PubMed] [Google Scholar]
- 20.Price DR, Feng H, Baker JD, Bavan S, Luetje CW, Wilson AC. 2014. Aphid amino acid transporter regulates glutamine supply to intracellular bacterial symbionts. Proc. Natl Acad. Sci. USA 111, 320–325. ( 10.1073/pnas.1306068111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prell J, Bourdès A, Kumar S, Lodwig E, Hosie A, Kinghorn S, White J, Poole P. 2010. Role of symbiotic auxotrophy in the Rhizobium–legume symbioses. PLoS ONE 5, e13933 ( 10.1371/journal.pone.0013933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullins DE, Cochran DG. 1974. Nitrogen metabolism in the American cockroach: an examination of whole body and fat body regulation of cations in response to nitrogen balance. J. Exp. Biol. 61, 557–570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available in the electronic supplementary material, table S3.