Abstract
How do animals determine when others are able and disposed to receive their communicative signals? In particular, it is futile to make a silent gesture when the intended audience cannot see it. Some non-human primates use the head and body orientation of their audience to infer visual attentiveness when signalling, but whether species relying less on visual information use such cues when producing visual signals is unknown. Here, we test whether African elephants (Loxodonta africana) are sensitive to the visual perspective of a human experimenter. We examined whether the frequency of gestures of head and trunk, produced to request food, was influenced by indications of an experimenter's visual attention. Elephants signalled significantly more towards the experimenter when her face was oriented towards them, except when her body faced away from them. These results suggest that elephants understand the importance of visual attention for effective communication.
Keywords: audience effect, theory of mind, perspective taking, communication
1. Introduction
For effective communication, it is essential that when a signal is produced, the intended recipient is able to perceive it; and by tracking conspecifics' gaze, animals can monitor the focus of others' visual attention and their interest in external events [1]. The understanding of visual attention has therefore been extensively studied, primarily in non-human primates [1], and for this reason primates will be used as the main comparison for our own results. Chimpanzees (Pan troglodytes) recognize the importance of the attentional focus of an audience [2] and all the great apes match the modality of their signals to their audience's attentional status [3–7]. Other species have also been found to successfully respond towards face cues that may show visual attentiveness (dogs [8], pigs [9] and scrub-jays [10]). For most species, it is unknown which cues are important for inferring whether a potential audience is able to see a signal and attending in the appropriate direction.
The African elephant (Loxodonta africana) lives in a complex multi-level fission–fusion society and regularly interacts with a large network of related and unrelated individuals [11]: effective communication is critical for everyday elephant interactions. Because elephants primarily rely on non-visual modes of communication, cognitive processes underlying their use of visual signals have been accorded little attention. Yet, elephants respond to subtle visual signals [12], and the form and contexts of wild elephant gestures have been described in detail [13]. It remains unknown whether the visual signals of African elephants are dependent on the presence or attentional status of an audience. In this study, we test whether African elephants modify the frequency of experimenter-directed signals in a food-requesting task, according to whether the experimenter (A.F.S., hereafter E) can see them, which we manipulate by varying E's body and face orientation. We do not test whether elephants use eye-gaze direction on its own, because we consider elephants' visual acuity unlikely to support the use of this cue in such a study [14].
2. Material and methods
(a). Subjects
Our subjects were 10 captive African elephants aged between 4 and 34 years old (six males, four females; electronic supplementary material, methods). They were only ever confined at night in stables, or while being saddled or unsaddled: we used this opportunity and tested elephants that were saddled early, or not going on the ride. Subjects never spent more than approximately 30 min restrained.
(b). Design
The order of presentation of conditions was pseudorandomized and counterbalanced. Each subject was presented with four trials of each of the seven conditions (electronic supplementary material, methods).
(c). Procedure
We tested elephants individually within the stables while secured. Experimental sessions began with ‘no delay’ trials: E stood behind a wooden tray (50 × 50 cm with a twine handle) positioned out of reach of that elephant, and facing the subject, E called its name, and dropped a piece of fruit (melon or orange piece approx. 15 cm long) onto the tray. E then immediately picked up the tray and set it down within reach of the subject's trunk, returning to her original position. E used the tray's handle to pull it back out of reach to its original position once the subject took the fruit. After three ‘no-delay’ trials, the testing phase began with the first ‘delay’ trial (electronic supplementary material, methods).
In ‘delay’ trials, after dropping the food and lifting the tray, E appeared to forget to move the tray, instead putting it back down out of reach. E waited 20 s before picking the tray up again and putting it in reach of the subject, using an earpiece which played a 20 s countdown. During the delay, E stood still and adopted one of six different postures which varied the orientation of her body and head. E oriented her body directly towards, away from, or with her side towards the subject. E also oriented her head so that her face was looking either towards or away from the subject (figure 1). To establish a baseline of actions, in a seventh condition, E walked away from the subject during the delay. As the experiment was conducted in the open stables, E could not easily leave completely, so instead she walked towards the exit without looking back for 20 s, and then returned to put the tray within the subject's reach. In the test phase, each delay trial alternated with a ‘no-delay’ trial and sessions always ended with a ‘no-delay’ trial.
Figure 1.
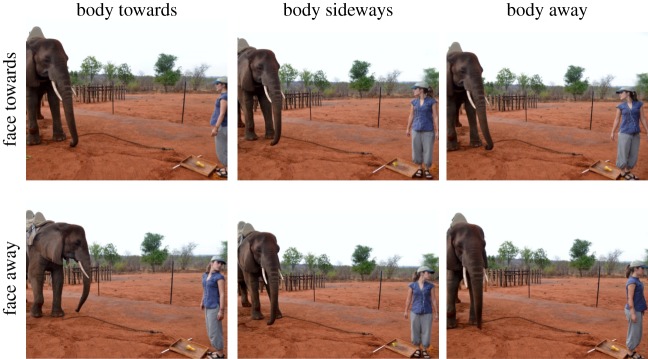
Experimental conditions. Experimenter body and face orientations. (Online version in colour.)
After a session, elephants left the stables. Experimentation necessarily ended when all elephants were saddled, so sometimes sessions had to be terminated before completing the planned trials (three to four per session). Then the remainder of the aborted session was done before the next session started. Trials were recorded using a video camera (Panasonic HDC-SD 90) on a tripod.
(d). Coding and analysis
A.F.S. coded ‘delay’ trials from the videos, beginning when E had assumed the prescribed orientation and ending after 20 s. For baseline trials, A.F.S. began coding 2 s after E had put the tray down out of reach, which was approximately the same time it took E to get into position for other conditions. All the subjects' actions directed towards the experimenter and the location of the wooden tray (baseline trials) were coded (electronic supplementary material, table S1). Briefly, the actions that were coded were: (i) forward-trunk-swing: lunging forward and tossing the trunk; (ii) head-nod: head bobbing up and down; (iii) mouth-open-beg: mouth opened, with trunk curled back; (iv) sniff-towards: extending some part of the trunk; (v) periscope-sniff: trunk upwards in an s-shape and (vi) horizontal-sniff: horizontal extension of the trunk.
We used the total frequency of these six experimenter-directed actions per subject in each condition for analyses. A second coder, blind to the experimental hypothesis, coded 35 randomly selected trials according to the descriptors. Inter-rater reliability was excellent for these data (rs = 0.854, p < 0.001). Tests are two-tailed and compared to an α-level of 5%. Data were analysed using SPSS. All confidence intervals are 95%.
3. Results
Elephants might have decreased signalling over trials, because they always got the fruit after each trial; in practice, however, we found that elephants' signalling was as frequent in the second half of trials of each condition compared with the first half (electronic supplementary material, figure S1).
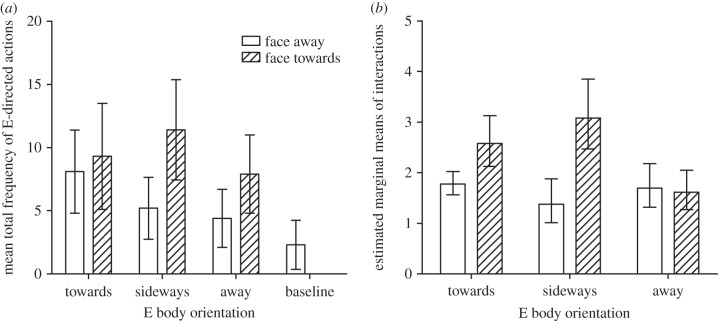
Responsiveness varied between subjects, with overall frequency of signalling tending to decline with increasing age (rs = −0.45, p = 0.191). As a group, elephants produced more visual signals when E was present compared with when she was not (baseline; figure 2a). We tested whether E's body and face orientation influenced the frequency with which elephants signalled towards E. Using generalized estimating equations, we created a model with 24 scores per subject including body and face orientation as categorical predictors, specifying an unstructured correlation matrix (electronic supplementary material, methods). We included the main effects of these predictors and their interaction in the model. We found significant main effects of body (Wald 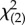 = 7.61, p = 0.022) and face orientation (Wald
= 7.61, p = 0.022) and face orientation (Wald 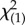 = 35.79, p < 0.001) as well as a significant effect of the interaction between body and face orientation (Wald
= 35.79, p < 0.001) as well as a significant effect of the interaction between body and face orientation (Wald 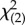 = 34.97, p < 0.001). Using pairwise comparisons, we found that elephants signalled significantly more often when E's face was turned towards them, but only when her body was oriented sideways or towards them, and not when her body was directed away (figure 2b).
= 34.97, p < 0.001). Using pairwise comparisons, we found that elephants signalled significantly more often when E's face was turned towards them, but only when her body was oriented sideways or towards them, and not when her body was directed away (figure 2b).
Figure 2.
(a) Mean total frequencies of experimenter-directed signals. Condition significantly affected the number of visual signals produced by the subjects (Friedman's ANOVA: 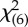 = 35.56, p < 0.001). Elephants produced more signals when E was present compared with when she was not (baseline: M = 2.30, CI 0.36, 4.24; body away–-face away: M = 4.40, CI 2.11, 6.69, T = 8, p = 0.053; body side–face away: M = 5.20, CI 2.75, 7.65, T = 7.50, p = 0.039) and for four conditions this difference was significant (Bonferroni correction: body away–face towards: M = 7.90, CI 4.80, 11.0, T = 0.00, p = 0.001; body towards–face away: M = 8.10, CI 4.81, 11.4, T = 0.00, p = 0.004; body towards–face towards: M = 9.30, CI 5.10, 13.5, T = 0.00, p = 0.004; body side–face towards: M = 11.4, CI 7.44, 15.4, T = 0.00, p = 0.002). Bars represent 95% CI. (b) Estimated marginal means of the interactions in the fitted hierarchical model. Elephants signalled significantly more often when E's face was turned towards them compared with when it was turned away, only when her body was oriented sideways (M difference = 1.70, Wald CI difference = 1.27, 2.14, p < 0.001) or towards them (M difference = 0.80, Wald CI difference = 0.24, 1.36, p = 0.005), but not when her body was directed away (M difference = −0.08, Wald CI difference = −0.49, 0.33, p = 0.698). Bars represent 95% Wald CI of the difference.
= 35.56, p < 0.001). Elephants produced more signals when E was present compared with when she was not (baseline: M = 2.30, CI 0.36, 4.24; body away–-face away: M = 4.40, CI 2.11, 6.69, T = 8, p = 0.053; body side–face away: M = 5.20, CI 2.75, 7.65, T = 7.50, p = 0.039) and for four conditions this difference was significant (Bonferroni correction: body away–face towards: M = 7.90, CI 4.80, 11.0, T = 0.00, p = 0.001; body towards–face away: M = 8.10, CI 4.81, 11.4, T = 0.00, p = 0.004; body towards–face towards: M = 9.30, CI 5.10, 13.5, T = 0.00, p = 0.004; body side–face towards: M = 11.4, CI 7.44, 15.4, T = 0.00, p = 0.002). Bars represent 95% CI. (b) Estimated marginal means of the interactions in the fitted hierarchical model. Elephants signalled significantly more often when E's face was turned towards them compared with when it was turned away, only when her body was oriented sideways (M difference = 1.70, Wald CI difference = 1.27, 2.14, p < 0.001) or towards them (M difference = 0.80, Wald CI difference = 0.24, 1.36, p = 0.005), but not when her body was directed away (M difference = −0.08, Wald CI difference = −0.49, 0.33, p = 0.698). Bars represent 95% Wald CI of the difference.
4. Discussion
African elephants produced more experimenter-directed signals when the experimenter was present compared with when she was not, showing that elephants' visual signals depend on the presence of an audience. When requesting food, elephants signalled more frequently when the experimenter's face was oriented towards them, compared with when it was facing away. While extensive research has been conducted on whether great apes in captivity can use facial orientation to flexibly adapt their own signalling to the perspective of another, here we show that another wild mammal—the African elephant—shares this ability. The data concern only the interpretation of human visual attention, but we predict that when studies look in greater depth at natural elephant communication, visual attention will be found to be a determinant in the African elephant's production of visual signals.
Elephants' sensitivity to experimenter face orientation was clear when the human's body was facing or directed sideways from the elephant, but not when her body faced directly away from the elephant. Great apes, when gesturing, and domestic horses, when choosing whom to approach, have also been found to discriminate between body and face orientations of a human experimenter, with a similar pattern of results [15,16]. In the case of great apes, the failure of the subjects to take account of face orientation when the experimenter's body was facing away from them was explained on the hypothesis that body orientation encodes the human's disposition to transfer food, while face orientation encodes their perceptual access to the animal itself [15]; when restrictions on the experimenter's physical ability to provide the food reward when turned away were removed, apes responded to face even when the experimenter's body was turned away [17]. That hypothesis can also explain the results of the elephants in our study, and the congruence between the pattern of results in elephant and great ape behaviour suggests an underlying similarity of cognitive mechanism.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to the Wild Horizons directors for permission to work with the elephants; Z. Sibanda and staff; R. Parry and J. Dawson of Victoria Falls Wildlife Trust; E. Bowman and K. Cross for help with data analysis; and E. Orr for secondary coding.
This experiment was approved by the School of Psychology and Neuroscience ethics committee, University of St Andrews.
Funding statement
This work was funded by the School of Psychology and Neuroscience, University of St Andrews.
References
- 1.Emery NJ. 2000. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci. Biobehav. Rev. 24, 581–604. ( 10.1016/S0149-7634(00)00025-7) [DOI] [PubMed] [Google Scholar]
- 2.Liebal K, Call J, Tomasello M. 2004. Use of gesture sequences in chimpanzees. Am. J. Primatol. 64, 377–396. ( 10.1002/ajp.20087) [DOI] [PubMed] [Google Scholar]
- 3.Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. 1994. The learning and use of gestural signals by young chimpanzees: a trans-generational study. Primates 35, 137–154. ( 10.1007/BF02382050) [DOI] [Google Scholar]
- 4.Hobaiter C, Byrne RW. 2011. The gestural repertoire of the wild chimpanzee. Anim. Cogn. 14, 745–767. ( 10.1007/s10071-011-0409-2) [DOI] [PubMed] [Google Scholar]
- 5.Pika S, Liebal K, Tomasello M. 2005. Gestural communication in subadult bonobos (Pan paniscus): repertoire and use. Am. J. Primatol. 65, 39–61. ( 10.1002/ajp.20096) [DOI] [PubMed] [Google Scholar]
- 6.Genty E, Breuer T, Hobaiter C, Byrne RW. 2009. Gestural communication of the gorilla (Gorilla gorilla): repertoire, intentionality and possible origins. Anim. Cogn. 12, 527–546. ( 10.1007/s10071-009-0213-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liebal K, Pika S, Tomasello M. 2006. Gestural communication of orangutans (Pongo pygmaeus). Gesture 6, 1–38. ( 10.1075/gest.6.1.02lie) [DOI] [Google Scholar]
- 8.Gácsi M, Miklósi Á, Varga O, Topál J, Csányi V. 2004. Are readers of our face readers of our minds? Dogs (Canis familiaris) show situation-dependent recognition of human's attention. Anim. Cogn. 7, 144–153. ( 10.1007/s10071-003-0205-8) [DOI] [PubMed] [Google Scholar]
- 9.Nawroth C, Ebersbach M, von Borell E. 2013. Are juvenile domestic pigs (Sus scrofa domestica) sensitive to the attentive states of humans? The impact of impulsivity on choice behaviour. Behav. Process. 96, 53–58. ( 10.1016/j.beproc.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 10.Clayton NS, Dally JM, Emery NJ. 2007. Social cognition by food-caching corvids. The western scrub-jay as a natural psychologist. Phil. Trans. R. Soc. B 362, 507–522. ( 10.1098/rstb.2006.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archie EA, Moss CJ, Alberts SC. 2011. Friends and relations: kinship and the nature of female elephant social relationships. In The Amboseli elephants. A long-term perspective on a long-lived mammal (eds Moss CJ, Croze H, Lee PC.), pp. 238–245. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 12.Smet AF, Byrne RW. 2013. African elephants can use human pointing cues to find hidden food. Curr. Biol. 23, 2033–2037. ( 10.1016/j.cub.2013.08.037) [DOI] [PubMed] [Google Scholar]
- 13.Poole JH, Granli PK. 2009. ElephantVoices Gestures Database See http://www.elephantvoices.org/multimedia-resources/elephant-gestures-database.html.
- 14.Shyan-Norwalt MR, Peterson J, Milankow King B, Staggs TE, Dale RHI. 2010. Initial findings on visual acuity thresholds in an African elephant (Loxodonta africana). Zoo Biol. 29, 30–35. ( 10.1002/zoo.20259) [DOI] [PubMed] [Google Scholar]
- 15.Kaminski J, Call J, Tomasello M. 2004. Body orientation and face orientation: two factors controlling apes’ behavior from humans. Anim. Cogn. 7, 216–223. ( 10.1007/s10071-004-0214-2) [DOI] [PubMed] [Google Scholar]
- 16.Proops L, McComb K. 2010. Attributing attention: the use of human-given cues by domestic horses (Equus caballus). Anim. Cogn. 13, 197–205. ( 10.1007/s10071-009-0257-5) [DOI] [PubMed] [Google Scholar]
- 17.Tempelmann S, Kaminski J, Liebal K. 2011. Focus on the essential: all great apes know when others are being attentive. Anim. Cogn. 14, 433–439. ( 10.1007/s10071-011-0378-5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.