Abstract
Recent work has demonstrated the feasibility of using decellularized lung extracellular matrix scaffolds to support the engineering of functional lung tissue in vitro. Rendered acellular through the use of detergents and other reagents, the scaffolds are mounted in organ-specific bioreactors where cells in the scaffold are provided with nutrients and appropriate mechanical stimuli such as ventilation and perfusion. Though initial studies are encouraging, a great deal remains to be done to advance the field and transition from rodent lungs to whole human tissue engineered lungs. To do so, a variety of hurdles must be overcome. In particular, a reliable source of human-sized scaffolds, as well as a method of terminal sterilization of scaffolds, must be identified. Continued research in lung cell and developmental biology will hopefully help identify the number and types of cells that will be required to regenerate functional lung tissue. Finally, bioreactor designs must be improved in order to provide more precise ventilation stimuli and vascular perfusion in order to avoid injury to or death of the cells cultivated within the scaffold. Ultimately, the success of efforts to engineer a functional lung in vitro will critically depend on the ability to create a fully endothelialized vascular network that provides sufficient barrier function and alveolar-capillary surface area to exchange gas at rates compatible with healthy lung function.
Index Terms: Decellularization, decellularized lung, lung engineering, organ engineering, tissue engineering
I. Introduction
LUNG disease is currently the third most common cause of death in the United States and is the only one of the top three causes of death that is increasing in prevalence [1]. Lung transplantation is the primary intervention for end stage lung disease, but this option is limited by a paucity of donor organs and the poor longevity of transplanted lungs. Of those that are implanted, over 75% of grafts fail in the first ten years, resulting in a patient survival rate of only 30% at the ten year time point [2]. Tissue engineering may offer an alternative solution to this unmet clinical need for functional, replacement lungs.
First and foremost, the engineered lung tissue must be able to exchange gas. The tissue, then, must possess a network of vascular conduits, with an adequate surface area and membranes thin enough to allow rapid diffusion of gases into and out of the blood. The vascular network must resist thrombosis and possess sufficient barrier function to prevent flooding of the alveoli with blood or blood components. By these criteria, two groups have met with modest success in regenerating functional lung tissues. Petersen et al., as well as Ott et al., have successfully implanted engineered lungs that exchanged gas for several hours [3], [4].
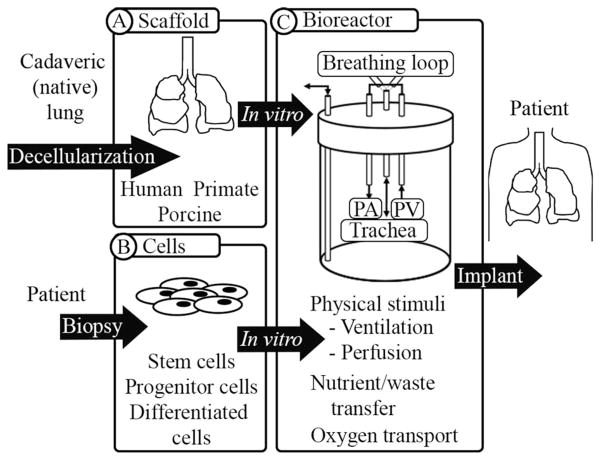
While these examples provide an encouraging foundation for the field of whole organ lung engineering, significant work remains to be done in three key areas: the scaffold used to construct the organ, the cells used to populate the organ, and the bioreactor in which to cultivate the organ (see Fig. 1).
Fig. 1.
Lung engineering includes three main components, including (A) an organ-specific scaffold, (B) appropriate numbers and types of cells, possibly obtained from the patient that requires the organ, and (C) a bioreactor in which to cultivate the tissue or organ (C). The leading source of scaffolds is currently a decellularized extracellular matrix scaffold, while the optimal cell source has yet to be determined. iPS cells and fetal-associated cells may be more tractable for the clinic due to fewer ethical hurdles that has led to greater access to these cell types. iPS cells have the additional benefit of fewer potential immunological complications. An effective bioreactor must provide physiological stimuli and support active cell growth.
The scaffold provides the architecture of the organ and supports the function of the organ by maintaining multiple, independent compartments and by providing a hierarchical, branching structure. The cells impart additional biological function, and the bioreactor facilitates growth and development via nutrient and waste transfer as well as oxygen transport. These three main components are undoubtedly intertwined. Seeding of endothelial and epithelial cells, which enhance the barrier function, also provides a renewable population that confers long-term stability of the engineered tissue and allows the transplanted organ to function for more than a few hours.
With this complex union of constituents in mind, we review the successes and challenges encountered thus far in the areas of scaffold generation, strategic recellularization, and bioreactor design. We aim to provide insight into a rapidly evolving field that ultimately aims to reconstitute functional lungs in vitro.
II. Lung Scaffolds
A. Design Criteria and Scaffold Properties
The ability to exchange gas falls primarily on three physical properties of the lung: 1) extensive surface area, 2) very thin alveolar capillary membranes, and 3) viscoelastic behavior.
For gas exchange to occur, the air and blood must be closely apposed (the walls of the alveoli are only 20–60 nm in thickness [5], [6]). Indeed, a set of adult human lungs has a surface area of 70–100 m2, approximately the surface area of a tennis court. An increase of the barrier thickness by twofold or loss of half of the surface area for gas exchange due to ruptured or absent alveolar walls would halve the diffusion capacity of oxygen. Although human lungs do possess some “reserve capacity,” a reduction of more than one-third of the diffusion capacity is incompatible with healthy lung function [7]. To date, gently decellularized whole organ scaffolds achieve this requirement more effectively than any other scaffold alternative. Other methods, such as the use of hydrogels, electrospun scaffolds, or other “bulk” synthetic methods fail to recapitulate the structural hierarchy of a lung due to insufficient control over form [8]. Techniques such as 3-D printing, while attractive due to reproducibility and impressive structural control, are so far unable to achieve adequate spatial resolution to recapitulate the gas-exchanging alveolar-capillary network.
The necessity for the lung to be both compliant and elastic also favors the use of decellularized tissue as a scaffold. Compliance or distensibility allows the lungs to inflate as a direct result of small pressure differences between the alveoli and the surrounding environment. Elastic recoil allows for passive exhalation, which helps to minimize the work of ventilation [7]. Various groups have decellularized rodent lungs using CHAPS [3] sodium dodecyl sulfate (SDS) and TritonX-100 [4], or TritonX-100 and sodium deoxycholate (SDC) [9]. All of these methods produce acellular scaffolds that exhibit hysteresis, but decreased compliance, compared to the native lung [3], [4], [9]. In addition to the absence of surfactant in decellularized lungs, this is likely due to lack of elastin, compared to native lungs [3], [9]. Additional work by Petersen et al. demonstrated that the ultimate tensile stress value of decellularized lung is similar to that of native lung [10].
In addition to mechanical considerations, a decellularized scaffold for lung regeneration must be hospitable to cell adhesion, survival, and proliferation. Decellularized scaffolds from the variety of methods in the literature retain many of the major basement membrane and extracellular matrix proteins present in the native organ, [3], [4], [9], [11]–[14], [15]–[19]. Among these are fibrillar collagens I and III, which provide structural integrity, and collagen IV, which forms the basement membrane. Other basement membrane proteins such as laminins and fibronectin are present in varying amounts. Compared to nonspecific, commercial substrates such as Matrigel or collagen I, organ specific extracellular matrices promote cellular phenotypes that are more appropriate to the organ [20]–[23].
In addition to the objectives of removing the cellular material and retaining ECM components, the scaffolds must be devoid of residual detergents, which are cytotoxic [19], [24]. Studies with pulmonary valves suggest that concentrations of < 50 mg/L in the final wash, as detected by high-performance liquid chromatography, indicate a fully rinsed, nontoxic scaffold [24]. Lungs decellularized with SDS that support epithelial cell growth contain undetectable levels of SDS per mg tissue after sufficient rinsing [16].
In addition to the residual quantity, the type of detergent(s) used impacts the results of cellular repopulation, though there are conflicting reports of the “best” substrate by this measure. Repopulation of mouse lung scaffolds decellularized with each of the aforementioned detergents with either mesenchymal stem cells or the C10 epithelial cell line demonstrated little difference between scaffolds [25]. Human microvascular endothelial cells seeded onto urinary bladder basement membrane decellularized with 3% TritonX-100, 4% SDC, 8 mM CHAPS, or 1% SDS have a more normal phenotype and form a more confluent monolayer when cultured on TritonX-treated matrix, compared to the other detergents [26]. Finally, human alveolar epithelial cells seeded onto human lung matrix decellularized with regimens similar to those above, showed fewer apoptotic cells, less T-cell activation, and induction of fewer cytokines on lungs decellularized with 1% SDS, compared to cells cultured on matrix treated with other detergents [17]. Although these data may reflect differences in the tissue response to the detergents applied or cell type-specific interactions with acellular matrix, there is clearly more work to be done. As efforts proceed, optimized decellularization regimens should be evaluated by 1) the effect that they have on whole lung mechanics, 2) the degree to which ECM components are retained, the extent to which 3) cellular components are removed, and 4) the viability, phenotype, and function of cells seeded onto the acellular matrix.
In sum, work on rodents [3], [4], [9], [12], [13], [15], macaques [11], and more recently with the human and pig tissue [14], [16]–[18], has established the feasibility of the decellularization approach. Acellular matrices are useful platforms to study cell behavior [3], [4], [11]–[15], [22], [27]–[29]. One major hurdle in transitioning from rodent to large animal lungs is establishing consistent and reliable scaffold production across species and across laboratories. The long-term structural integrity and the ability of the scaffold to support long-term cell survival will also need to be evaluated.
B. Use of Decellularized Pulmonary Scaffolds in the Clinic
In 2008, the first example of using a decellularized cadaveric trachea that was seeded with bone marrow cells and nasal epithelium to replace an airway segment in a patient was reported [30]. In 2008, nearly 11,000 lungs were deemed unsuitable for transplant due to the poor organ function and were therefore never procured, despite prior consent for lung procurement [31]. Whether these donated, but unused organs could be salvaged for scaffold generation in the future is unclear. If the extracellular matrix is significantly compromised, cadaveric human lungs may not be an option. Therefore, alternative sources such as nonhuman primate or porcine lungs may be critical to the advancement of the field.
Porcine organs in particular are an attractive option in the near-term. Much of the infrastructure for pig cultivation for other tissue-based products, such as heart valves, pericardium, and intestinal submucosa, already exists [32], [33]. Recent success in establishing a pig model of cystic fibrosis suggests that pigs may be good models for human lung disease as well [34], [35]. Additionally, fully cellular, porcine lungs that were transplanted into immune-depleted baboons were able to provide adequate gas exchange (“full respiratory support”) for up to 11 h, with little histological evidence of microvascular or alveolar damage upon explant [36]. At a minimum, this demonstrates sufficient surface area to support human gas exchange requirements if decellularized porcine lungs were to serve as a scaffold for generation of lung tissue that could be implanted in a human. The ability of a human immune system to accommodate a porcine extracellular matrix requires additional evaluation.
One additional consideration is the sterilization of scaffolds. Unfortunately, no single method of sterilizing matrix-based allografts or xenografts has been established [37]. Chemical and high-dose antibiotic treatments present the risk of toxicity or adverse reaction to residual compounds, while extreme conditions such as high heat (autoclaving) will denature collagens. Sterilization of the bone and anterior cruciate ligament allografts with gamma irradiation or electron beams, respectively, can have adverse effects on the mechanical properties of these grafts [38], [39]. Low doses of gamma irradiation (2M Rad) can be used to terminally sterilize decellularized tracheas, [40], but whether the more delicate structure of the lung can withstand similar treatment remains to be seen. Ethylene oxide would require extensive out-gassing, and toxic ethylene glycol byproducts are formed when gas comes into contact with water [37]. Ultimately, supercritical carbon dioxide may be the most promising option for terminal sterilization of complex 3-D biological products such as a decellularized lung scaffolds, though this technology is still in its infancy. First described as an efficient method of destroying microorganisms in 1995 [41], supercritical CO2 does not require temperatures above 37 °C, can penetrate tissues, and is nontoxic [42]. Terminal sterilization of acellular dermal matrix with supercritical CO2 has proven effective at reducing bioburden to levels below FDA guidelines, with minimal disruption of mechanical properties [43], [44]. For all of these reasons, supercritical CO2 is an attractive option for acellular scaffold sterilization, provided it does not negatively impact the structure, composition, or mechanics of the lung extracellular matrix.
In summary, a scaffold suitable for use in whole lung tissue engineering must first and foremost be devoid of cells and cell components prior to reseeding. Recommendations by the Badylak group, for example, stipulate a lack of visible nuclear material by DAPI or H&E staining, DNA fragment length of less than 200 bp, and less than 50 ng dsDNA/mg ECM by dry weight [45]. The scaffold must also provide a hospitable environment for cell adhesion and survival, which largely relies on retention of constituent basement membrane proteins. The scaffold must also meet structural criteria specific for lung function, such as sufficient surface area for gas exchange and an intact basement membrane between air and blood. At a minimum, this membrane should provide a barrier to cell-sized particles. At least two-thirds of the vascular compartment must be comprised of patent, perfusable conduits so that blood can be oxygenated. The overall mechanics of the scaffold must be compatible with continuous ventilation long term; that is, possessing of both a viscous and elastic region when examined on a stress–strain curve. Finally, though the scaffold must be sterile, there can be no toxic elements retained within the scaffold.
III. Strategies for Cellular Repopulation: Cell Types and Use With a Decellularized Whole Organ Scaffold
A. Essential Cellular Constituents of the Lung
The lung is largely comprised of three categories of cell type: epithelial, endothelial, and interstitial, or mesenchymal. The epithelia line the airways from trachea to alveoli; they provide the barrier function between air and blood and perform regionally specific functions such as mucociliary clearance and gas exchange (see Fig. 2). Unlike the gut epithelium, which is completely renewed in less than a week, the epithelial layer of the lung is renewed only once every four months during normal homeostasis [46], [47].
Fig. 2.
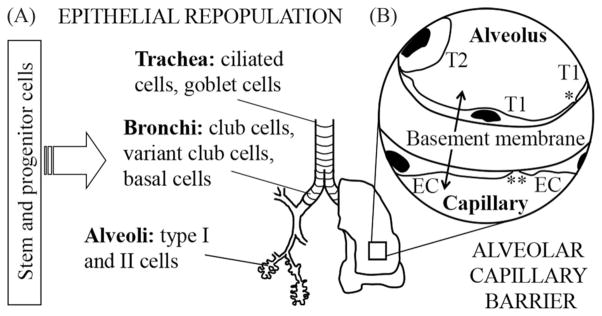
The lung includes (A) 23 generations of airways, terminating in the (B) alveolar compartment, which is the site of gas exchange. Two key challenges to successful lung engineering are 1) determining the source and type of epithelial cells with which to populate the airways with a sufficient number of cells and 2) engineering an effective alveolar-capillary barrier, to enable gas exchange. Key cell types are indicated along the airway branches of the lung (A). Components of the alveolar-capillary barrier are indicated in (B). This barrier includes type I (T1) and type II (T2) epithelial cells and microvascular endothelial cells (EC). Tight junctions between T1 cells (*) provide a barrier to components > 1 nm in diameter. Endothelial cell junctions (**) provide a barrier to material > 5 nm in diameter. The basement membrane separating the air and blood is < 100 μm thick and provides approximately half of the barrier function of an intact alveolar-capillary barrier.
Endothelial cells, which account for approximately 50% of the cells in the lung, also provide barrier function and accomplish the critical task of preventing thrombosis, both physically and by production of hemoactive substances. They additionally provide biochemical and molecular support for the epithelium.
Finally, interstitial cells, which are comprised of fibroblasts and other mesenchymal cells (MSCs), provide structural stability through extracellular matrix production and turnover. They are also critical for the survival and physiologically appropriate organization of the epithelium.
Taken together, there are nearly 250 billion cells in the human lung, of which 26% are found in the nonalveolar regions (i.e., trachea and upper airways) and 74% are found in the alveolar regions [48]. The nonalveolar regions of the lung total 65 billion cells, including 30–40% interstitial cells, approximately 40–50% endothelial cells, and a smaller fraction of epithelium—approximately 10 billion total [49]. The 184 billion cells found in the alveoli are approximately 50% endothelial, followed by 25% interstitial, and 25% epithelial, with the remaining small fraction accounted for by alveolar macrophages [50]. This translates to 80 billion endothelial cells (largely microvascular), 60 billion interstitial cells, and 35 billion epithelial cells in the alveolar region alone. For successful lung tissue engineering, each category of cells will have to be provided for.
B. Epithelial Cells
The two central questions in epithelial cell repopulation of the scaffold are 1) which cell types are both necessary and sufficient to generate functional lung tissue and 2) how can these cells be obtained?
1) Primary Lung Cell Types
In the trachea and upper airways, the primary function of the epithelium is to humidify the air taken in by the lungs and to provide immunological protection via mucociliary clearance. The production of secretoglobins and immunoglobulins confer additional immunity. In the adult trachea and primary bronchi (cartilaginous airways), the luminal epithelium performs these roles; this population includes ciliated columnar epithelial cells, mucus-producing goblet cells, neuroendocrine cells, basal cells, and both Clara (club) and club-like cells.
Neuroendocrine cells are located within the monolayer of epithelium and serve as a “specialized” epithelial phenotype. They are able to sense oxygen, produce bioactive compounds, and promote the secretory functions of other epithelial cell types [51]–[54]. Though they make up less than 10% of the airway epithelial cell population [51], these are likely an important component of the airway epithelium for lung engineering efforts.
Basal cells account for approximately 30% of the pseudos-tratified mucociliary population in the human lung (though only 2% of the rat airway population). They are located beneath the columnar epithelial cells in the proximal airways and constitute 90% of the cell surfaces in constant contact with the basement membrane [49]. More heavily represented in the trachea and proximal airways, the frequency of basal cells diminishes toward the distal lung. In the upper airways, they assist in resolving club cell injury in the mouse and give rise to ciliated cells, which otherwise do not replicate [55], [56]. Basal cells that are isolated from the human trachea are classified as stem cells for the region [57].
Club cells are also multifunctional. Characterized by the production of the secretoglobin club cell secretory protein (CCSP, also known as CC10), they are distributed asymmetrically along the airway tree. Although largely absent from the proximal airways of the human lung, club cells represent up to 22% of the total population in the respiratory bronchioles and account for 44% of the replicating cells at this level [58]. Indeed, they are the principle cells required for maintaining homeostasis of the bronchial airway epithelium and the bronchioles of normal adult mice after injury [59], [60].
The function of the distal lung is primarily gas exchange and the production of systemic exocrine and hemoactive factors. This region of the lung is organized into a complex system of alveoli that is comprised of two primary epithelial cells types: squamous type I epithelial cells and cuboidal type II cells. Type I cells line the majority of the alveoli (covering up to 95% of the alveolar surface area) and are the primary site of gas-exchange, while type II cells secrete alveolar surfactants and give rise to type I cells. Recent evidence suggests that type II cells are actually a heterogeneous population made up of subpopulations with differing regenerative capacities [61]. In addition, though type II epithelial cells have historically been considered the primary progenitor cell of the alveolus, careful lineage-tracing experiments by the Hogan group have recently shown that murine type II cells are capable of clonal expansion as well. This capacity for self-renewal promotes the status of murine type II cells to that of an alveolar stem cell [62].
2) Lung-Resident Stem and Progenitor Cells
The ability of certain epithelial cell populations to recapitulate healthy lung tissue in the context of various insults and injuries is an area of intense research interest and is extremely relevant to lung regeneration. Populations that have been identified in rodents and also have candidate analogs in human lung include a subpopulation of bronchial epithelium that is integrin α6 β4 positive [63] and a p-63 expressing “basal-like” cell that proliferates in response to influenza infection [64]. Putative OCT4+ progenitor cells, isolated from pig lungs [65], and cells from human lungs that express the stem cell marker c-kit, may have the capacity to regenerate damaged lung tissue [66].
The population of bronchial epithelial cells that are characterized by the α6 β4 integrin and a lack of surfactant protein C (SP-C) proliferate after severe injury—they are otherwise “dormant” after more mild insults [63]. When supplied with the appropriate mixed cell population from embryonic mouse lungs (including both epithelial and MSCs) in a kidney capsule “organoid” assay, these β4+ cells are able to recapitulate entire lung structures in a clonal manner. β4+ progenitor cells generate both CCSP+ airway structures and SP-C+ alveolar-like structures, but require both MSCs and a mixed population of embryonic epithelial cells in order to give rise to lung-specific progeny. Contributions of other elements of the kidney capsule environment are likely important as well, though these are as yet undefined.
Studies by Kumar et al. have used array-based techniques to distinguish regional epithelial progenitors [64]. Despite shared expression of 99% of the hybridized genes, epithelial progenitors from different regions of the airway tree can be separated by whole genome expression profiles. These cells give rise to different progeny in air–liquid interface experiments. Although the nasal and tracheal epithelium yield robust populations of mucus-producing and ciliated epithelial cells, the distal airway stem cells form hollow, alveoli-like spheres, and show expression of type I and type II cell markers.
One potentially important marker of lung progenitors may be OCT4. In the pig lung, there appears to be an OCT4+ epithelial progenitor that is also characterized by an ability to self-renew, form colonies, and give rise to type I and II alveolar epithelial cells [65]. Another study of type I epithelial cells which express OCT4 showed they had phenotypic plasticity in vitro [67]. These data indicate that OCT4 may characterize the capacity for phenotypic plasticity and self-renewal in the lung epithelium.
Finally, a somewhat controversial study published in 2011 asserts the existence of rare c-kit-expressing cells within the human lung that can be isolated and expanded in culture. These proposed “c-kit+ lung stem cells” are reported to form bronchioles, alveoli, and pulmonary vessels [66]. The ability of these cells to generate cell types of all three germ layers—a feat typically achieved by only embryonic or induced pluripotent stem (iPS) cells—would indicate a cell of great potential value. However, no reports replicating these data have been published to date.
3) Exogenous Stem Cells: Embryonic, Induced Pluripotent, and Fetal-Associated Cells
Human embryonic stem cells: Several groups have utilized murine, and then human, embryonic stem cells to derive distal epithelium in vitro [68]–[72]. These protocols produce cells that phenotypically appear to be “type II-like;” they possess lamellar bodies and show expression of surfactant proteins, including surfactant protein C [28], [70]–[73]. Derivation of airway epithelial cells from ESCs has also been demonstrated following culture of ESCs under an air–liquid interface [72]–[74]. Although these studies are important feasibility studies and can be used to inform strategies for directing the differentiation of other stem and progenitor cells, their clinical utility may ultimately be limited by the significant ethical hurdles associated with human embryonic stem cells. Therefore, other sources of stem and progenitor cells from which to generate lung cells are indispensable.
iPS cells: iPS cells are the product of adult somatic cell reprogramming to an embryonic-like state by inducing a “forced” expression of specific pluripotent genes [75], [76]. Several research groups have reported the successful differentiation of iPS cells toward a range of pulmonary epithelial types, including both type II-like cells and airway epithelium, using a variety of protocols [73], [77], [78]. For the purposes of cell transplantation and tissue engineering, many ways to generate integration-free iPS cells have been reported. Several techniques rely on nonintegrating DNA such as plasmids [75], [79], adenoviruses [80], [81], or episomes [82]. RNA-based methods such as Sendai virus [83] or messenger RNA (mRNA, [84], [85]) have also emerged. Additional trials have been done with proteins [86] or chemically defined reagents [87]. Though rapid progress has been made, it is still premature to predict which method of stem or iPS cell generation will be most germane for lung regeneration.
Fetal-associated stem cells: Recent literature has also described the use of “fetal associated” stem cells as a multi-or pluripotent cell source. These cells are derived from tissues and substances associated with a fetus such as the placenta, amnion and amniotic fluid, umbilical cord blood, Wharton’s jelly, and other components and may be used to generate lung cells [88]–[90]. Though less is known about these cells types, this population possesses growth kinetics and plasticity intermediate between adult and embryonic stem cells. In addition, these cells can be obtained with little difficulty; human amniotic fluid stem cells, for example, can be derived from discarded amniocentesis specimens [88], [89]. Similarly, placental- and umbilical cord-derived stem cells can be collected at birth and stored for potential future use, without the ethical hurdles that are associated with embryonic stem cells. Both placental- and umbilical cord-derived stem cells can be differentiated to cells similar to alveolar epithelium in vitro and express lung epithelial markers in vivo in a mouse model [88], [90].
4) Strategy for Lung Epithelial Regeneration In Vitro
To recapitulate the composition of the lung, initial functions include the ability of the cells to adhere to the decellularized scaffold when delivered to the appropriate compartment, to survive, proliferate, and differentiate. The repopulated lung must also contain diverse groups of cell types. Each type must exist in an anatomically appropriate region of the lung. Several groups have used a variety of cell types to repopulate acellular lung scaffolds. Despite differences in the decellularization method, cell type, culture medium, and bioreactor conditions, these studies highlight several key strategies for successful repopulation. These include 1) the use of a mature cell phenotype or support to become such during culture, 2) the application of appropriate physiological stimuli such as ventilation and perfusion during culture, and 3) exposure of the airway compartment to air when possible. The culture time required to achieve mature phenotypes distributed throughout the lung will depend in part on the maturity of the cells used, and may range from 7–10 days to several weeks or months [3], [13], [28], [91].
When supplied with a minimal medium and cultured on the acellular lung in vitro, mature cell types outperform less differentiated populations. Mixed, fully differentiated lung cells from neonatal rats expressed a greater variety and abundance of mature lung cell types under these conditions, compared to a mixed population of fetal rat lung cells [3], [4]. Neonatal rat lung cells also exhibit regional specificity of epithelial marker expression when seeded into the airway compartment of decellularized lungs. In addition to the alveolar epithelial cell markers AQP-5 and pro-SPC, markers of types I and II cells, respectively, CCSP was also abundant in cells lining proximal airways. In some cases, cytokeratin-14 positive cells were located beneath the club cell populations, suggestive of a basal cell-like phenotype [3]. Though fetal lung cell cultures showed evidence of distal lung markers SP-A, SP-C, TTF-1 and T1α, the variety of cell types and regional localization of neonatal cultures was largely not observed in fetal lung cell populations [4], [92].
Immature, progenitor populations can be used to produce differentiated epithelium within an acellular matrix when additional support is provided, however. Subcutaneous implantation of a decellularized mouse lung repopulated with “predifferentiated” murine embryonic stem cells (mESC) is one way to provide this support. Much like the kidney capsule assay, subcutaneous implantation provides a physiological microenvironment that favors cell survival, proliferation, and differentiation. After two weeks of cultivation, the repopulated, implanted lung contained cells positive for the distal lung epithelial markers TTF-1 and pro-SPC. There were also scattered FOXJ1+ cells indicating the presence of proximal airway cells [13]. Additional studies with lung progenitors derived from mESC or human iPS cells resulted in mature lung cell markers after treatment with differentiation and maturation medium during culture [13], [28], [91]. After ten days of culture, the mESC-derived progenitors showed both regionally appropriate and robust expression of alveolar epithelial markers associated with maturation [28]. In contrast, the undifferentiated mESC cultured in a minimal medium were relatively unorganized and somewhat lacking in definitive lung epithelial markers both at 14 and 21 days [15]. Most impressively, the iPS-derived human lung progenitor cultures (cultured on human lung ECM for up to 55 days) contained both the messenger RNA and the protein for a diverse array of proximal and distal lung epithelial markers. These included the basal cell marker p63 and the club cell marker CCSP. In addition, these cells expressed TTF-1 (NKX2.1). These markers are associated with distal lung progenitors, club cells, and type II epithelial cells, respectively. Multiple surfactant proteins characteristic of type II cells were detected. The type I epithelial marker T1α (podoplanin, PDN) was also present. Additionally, the SP-B+ cells demonstrated the ability to take up labeled surfactant protein B, indicating the functional capacity to recycle surfactant [91]. Clearly, biochemical signals are an important part of encouraging regionally and functionally specific lung epithelial cell types and are a fruitful avenue for additional pursuit.
In addition to biochemical signals, mechanical signals such as ventilation are also important stimuli for maturation of lung cells during development [93]. Ventilation of lung constructs has been used to promote lung phenotypes in repopulated decellularized constructs [3], [4], [9], [92]. Indeed, lack of ventilation leads to decreased cell coverage of the extracellular matrix scaffold and accumulation of the protein in the airways of lungs cultured for eight days [3]. When whole, native rat lungs are cultured in vitro, ventilation leads to maintenance of CCSP expression in the airways and robust expression of pro-SPC in punctate, cytoplasmic locations, similar to the native lung. Lungs that were perfused only displayed more diffuse and somewhat less abundant pro-SPC staining. A count of apoptotic cells in lungs that were simultaneously ventilated and perfused showed cell viability equal to that of freshly explanted rat lungs; either ventilation or perfusion alone were vastly better than static lung culture [94].
Finally, the creation of an air–liquid interface by ventilation with air during culture has a positive effect on the lung cell phenotype. When exposed to the air rather than submerged in the liquid, type II cells produce more surfactant [95], and proximal cells maintain a ciliated phenotype [96]. Cilia were also observed in neonatal-seeded scaffolds when ventilated with air [3]. The appearance of T1-α, a type I marker, and cilia in the experiments by Longmire et al. [28] is also likely derived from the tendency of unanchored lung slices to float in the liquid media (unpublished observations), thereby creating an air–liquid interface.
Ultimately, for effective epithelial repopulation, the key stem and progenitor cells that must be recapitulated are basal cells, club cells, and alveolar type II cells. In fact, these cells are likely indispensable to any serious lung tissue engineering effort, as they provide antecedents for the epithelium of each of the three major regions of the lung, including the trachea, airways (bronchi, bronchioles, etc.), and alveoli. Administration of basal cells, club cells, or other lung-resident progenitors to a decellularized scaffold has not been evaluated. The appearance of some of the markers of each of these cells in the Huang studies is encouraging. However, the degree of organization is not clear. Studies designed to explore the regional specificity of a progenitor cell population’s survival in the whole lung and the ability of the cells to constitute an organized epithelium in an acellular extracellular matrix scaffold long term would provide additional necessary information for lung engineering efforts.
If these cells can be induced to differentiate in a regionally specific way within a whole acellular lung scaffold, perhaps based on microenvironmental cues, such a “one cell type” approach would be appealing. This approach may also have the potential to capitulate the heterogeneity that exists within the cell type. Basal cells, club cells, and alveolar type II cells are all heterogeneous populations [59], [61], [97], [98]. However, if this type of organization cannot be achieved, more mature phenotypes, such as bona fide basal, club, or type II cells may be required. In that case, site-specific cell delivery will become an important element of repopulation efforts.
In the case of any repopulation efforts, time points at which repopulated cells are evaluated are critical. A phenomenon of “dedifferentiation,” followed by “redifferentiation,” has been described under a variety of conditions. Early experiments using basal cells and secretory cells to repopulate denuded trachea, for example, followed this sequence of events [98], as did repair following basal cell ablation [97]. In the case of human iPS cell-derived lung field progenitor cells, the appearance of mRNA for distal lung markers was apparent at d15 in the absence of protein. This was followed by disappearance of the RNA signal, then recurrence, accompanied by the robust protein expression of distal markers [91]. Though the time required to reach this “maturation” stage was sometime between 15 and 48 days, regeneration of an epithelial layer may be possible in 7–14 days [97], [98]. This will require both a sufficient density of cells seeded into the scaffold, and culture conditions that will foster the proliferation and differentiation of regional epithelial populations. Because an acellular substrate may mimic an “injured” substrate in some respects, cells cultured in this environment may activate “repair” programs, compared to cells in a confluent monolayer. This state, described for basal cells in both mice [99] and humans [100], is characterized by a high proliferative index and extensive expansion of the basal cell pool. The use of cells in this state would facilitate rapid repopulation of the scaffold and provide a pool of cells to recapitulate multiple differentiated phenotypes, as basal cell hyperplasia gives way to differentiated phenotypes of the tracheal and bronchial epithelium [99], [100].
More primitive stem and progenitor cells also possess a greater capacity for proliferation than differentiated cell types. The 10 billion proximal airway cells and 35 billion alveolar epithelial cells required to recellularize a set of human lungs will demand extensive expansion. Whether this should occur prior to seeding on the lung or during culture on the lung matrix must be further evaluated.
C. Endothelial Cells
1) Endothelial Cell Functions
Like the epithelium, the endothelium exhibits significant heterogeneity that needs to be understood in order to modulate the contribution of each type to the engineered lung tissue. An intact endothelial monolayer prevents the formation of fibrin clots largely by preventing contact of blood with the underlying collagen of the basement membrane and interstitial extracellular matrix [101]. Heparan sulfate also binds antithrombin III and chondroitin sulfate, and dermatan sulfate facilitates binding of the thrombomodulin in the EC membrane with the circulating thrombin [102]. Additionally, the endothelium produces hemoactive compounds such as nitric oxide and prostacylins, which actively inhibit platelet aggregation.
In response to mechanical stimuli, pulmonary endothelial cells of arterial origin produce more prostacyclin than venous endothelium [103]. Pulmonary artery endothelial cells also express more tissue plasminogen activator than microvascular endothelial cells or HUVECs, though microvascular endothelial cells express more urokinase-type plasminogen activator [102], [104]. Both of these enzymes convert plasminogen to plasmin, which lyses fibrin clots. Therefore, repopulation of an otherwise thrombogenic substrate, such as decellularized lung with cells that produce fibrinolytic substances would be advantageous. By actively mitigating the formation or stability of fibrin clots, the presence of plasminogen activators could potentially prolong the life of an implanted graft.
An effective endothelial layer, particularly in the lung, also depends critically on successful barrier function. Compared to the endothelium of the large vessels, microvascular endothelial cells express more cell–cell junction molecules such as N-cadherin, VE-cadherin, and activated leukocyte cell adhesion molecule, a component of adherins junctions [105]. Consequently, microvascular endothelial cells form tighter cell–cell junctions [105], [106]. Human lung microvascular endothelial cells may also enhance the barrier function of epithelial cells [107], which actually provide a barrier with a smaller transport threshold than that of the endothelium. Compared to the 5-nm cutoff for endothelial cell–cell barriers, the epithelium provides a barrier to molecules greater than 1 nm.
Finally, the endothelium, especially the microvascular endothelium, plays a critical role in the support of regional epithelial stem and progenitor cell proliferation [108]. VEGF receptor inhibitor [109] or knockout of endothelial nitric oxide synthase [110] leads to fewer airspaces and a disrupted alveolar structure. These results highlight the importance of the endothelium, not only for its role in blood interactions and trans epi-and endothelial transport, but also for supporting normal epithelial behavior and lung structure. A repopulated scaffold that lacks the endothelium may not, in fact, be able to recapitulate appropriate function without the paracrine factors endowed by the endothelium.
2) Endothelial Cell Sources
Several groups have reported the differentiation of endothelium from human iPSs and human embryonic stem cells [71], [111]–[118]. Human iPS cell-derived ECs differentiated in the static culture express general endothelial markers CD31 and VE-Cadherin, but are functionally and phenotypically heterogeneous, with arterial, venous, or lymphatic subtypes present [115]. Whereas some groups report that ECs derived from human ES or iPS cells resemble a primarily arterial phenotype [117], others report that a larger percentage of cells are of a venous phenotype [119]. This is likely accounted for by the use of different protocols and the variety of soluble factors used to drive differentiation. Nevertheless, these cells appear to be efficacious in vivo—transplantation of iPS-ECs improved neovascularization and blood flow recovery in a hind limb ischemic model [120]. Furthermore, iPS-ECs displayed good attachment, stabilization, patency, and characteristic vascular structure when seeded on decellularized vessel scaffolds [121], [122]. Therefore, in vitro differentiation of the pluripotent cells could indicate a viable population to scale up for the vascularization of the engineered lung [121], [122]
3) Strategy for Lung Endothelial Regeneration In Vitro
For successful vascular engineering of the lung, derivation of the endothelium from iPS cells for this purpose will likely include a combination of arterial and microvascular endothelial cells. Approximately half of the cells used to reseed a lung must be vascular endothelium. For a human lung, this tallies over 100 billion cells. Few definitive data have been reported thus far regarding comprehensive endothelialization of acellular organ scaffolds. The only reports of endothelial cell seeding of decellularized lung extracellular matrix scaffold are those of implanted engineered rat lungs. Each of these constructs included a population of endothelial cells within the vascular compartment of the engineered tissue. In addition to an uneven distribution of endothelial cells within the compartment, the constructs lacked complete coverage of the basement membrane by a continuous endothelial monolayer. This led to thrombosis and failure of the organ within hours [3], [4], to days [124]. The only other report of endothelial cells invested in a decellularized matrix was the ingrowth of vessels in a subcutaneously implanted construct. Whether these vessels essentially “tunneled” through the matrix or faithfully traversed the existing vascular conduits is unclear [13].
For advances to be made in this area, considerations for repopulating an acellular scaffold include the cell size in comparison to the capillary diameter, cell density, and cell type. Ott et al. used human umbilical vein endothelial cells and Petersen et al. used rat lung microvascular endothelial cells. While the venous endothelium is widely available, arterial and microvascular endothelium possess more appropriate characteristics for the lung endothelium. They possess greater capacity for anticoagulation and proliferate more quickly. Rapid proliferation will facilitate coverage of the extensive vascular surface area of a lung scaffold on a short time scale.
Regardless of the type of the endothelium used, the single most important task is to create a monolayer of cells within as many capillaries as possible. The capillary beds possess the highest portion of the vascular surface area and are also at greatest risk for thrombosis, due to the low flow in these regions. Capillaries are also the sites of gas exchange. For all of these reasons, successful endothelialization is the single most important (and most challenging) component of the lung or any organ tissue-engineering endeavor.
D. Interstitial and MSCs
Interstitial cells comprise approximately 30% of the cells in the lung—approximately 75 billion cells in a full set of human lungs. They primarily serve to provide structural stability, as well as biochemical and physical support for the surrounding endothelial and epithelial cell populations. There are many different types of interstitial cells, each with distinct functions. Fibroblasts are differentiated cells that produce and degrade the extracellular matrix. Though the extracellular matrix turnover declines with age, approximately 2–10% of the collagen in an adult lung may be renewed every day [125].
Additional in vitro experiments have demonstrated the ability of various stromal cells to accelerate epithelial wound repair via migration and proliferation [126]. The lung mesenchyme promotes branching and distal lung morphogenesis, and specifies regional epithelial patterning during development [127]. Interstitial cells promote polarization of the bronchial epithelium and ciliogenesis via direct contact with the epithelium [128]. Finally, PDGFRα+ MSCs foster the survival of alveolar epithelial cells and the formation of “alveolospheres” in vitro [62]. Other specialized interstitial cells of the lung include lipofibroblasts, telocytes, and pericytes. These interact with epithelial and endothelial cells, respectively, to process surfactant and promote vascular formation and stabilization [129]–[131].
Overall, the objective of using interstitial cells in lung tissue engineering is to confer the physical and biochemical support enjoyed by the normal lung on the engineered tissue. The central question is which interstitial cells to use for lung tissue engineering. The prolific production of ECM by fibroblasts, while important and useful when well controlled, may induce fibrosis in an engineered lung context. Lung MSCs are located in the perivascular area in the bronchioalveolar region of the lung. While MSCs are generally mesodermal in origin, studies have also found human lung-specific MSCs to express nestin (a neural stem cell marker) [132]. In vitro differentiation of the MSC into lung phenotypes has received great attention, but after almost a decade of research in this area, incontrovertible evidence of such differentiation remains limited and controversial.
1) Role of MSCs During Injury In Vivo
Bone marrow-derived MSCs have been shown to play a role in mitigating lung injury in animal models of disease. For example, Tropea et al. demonstrated that systemic delivery of MSCs in a mouse model of bronchopulmonary dysplasia led to significant increase of bronchioalveolar stem cells as compared with controls [133]. Studies have highlighted the importance of the soluble factors secreted by MSCs that seem to play a role in the therapeutic efficacy of MSCs [134]. Some of the critical factors may include keratinocyte growth factor (fibroblast growth factor 7), interleukin-10, angiopoietin-1, interleukin-1 receptor antagonist, and prostaglandin E2.
2) In Vivo and Clinical Uses of MSCs
Both systemic as well as intratracheal administration of MSCs in a spectrum of animal models demonstrate the efficacy of MSCs for resolving lung disease and injury. For a more detailed review, please refer [135]. MSCs from the bone marrow, placenta, and amniotic fluid have been utilized in human models of lung injury [136], [137]. A more recent milestone involves a Phase II clinical trial involving administration of bone marrow MSCs in patients with COPD. Although not efficacious in mitigating the disease, no adverse effects were reported over the two-year follow-up period [138]. MSCs have also been used in ex vivo tissue regeneration models. Macchiarini et al. recently achieved clinical success using autologous bone marrow MSCs and the nasal epithelium for chondrocytes and luminal epithelium regeneration, respectively, to create bioartificial airways from decellularized cadaveric tracheas [30], [139], [140].
3) Strategies for the Use of MSCs in Lung Tissue Engineering
To date, several kinds of stromal cells, such as bone marrow and adipose-derived mesenchymal stem cells, have been introduced to decellularized lung scaffolds. Decellularized lungs that are inoculated with MSCs result in cellular deposition mainly in the parenchyma, and to a lesser extent in the small and larger airways. Where the MSCs attach and bind is largely determined by the ECM components of the decellularized scaffold, which in turn is highly defined by the method utilized to de-cellularize the scaffold. Bonvillain et al. found that the murine MSC initially adhere to areas rich in collagen I and laminin as well as fibronectin-rich areas, while in a macaque model they showed substantial preference for laminin above all other matrix molecules [11]. Studies by Daly et al. revealed substantial “homing” of inoculated MSCs to collagen I, collagen IV, and laminin-rich areas, but very little binding of MSCs to fibronectin [12]. There is mounting evidence that the ECM contributes to the functional behavior of MSCs [141], [142]. Cells with different known integrin expression have different binding characteristics on the decellularized matrix [12]. Overall, determination of cell-matrix interactions will be key in understanding the regional specification of MSCs on a decellularized lung scaffold.
Although MSCs bind to and proliferate on decellularized scaffolds, it is unclear what phenotypes they assume under different culture conditions. Over a 28-day culture of MSCs on decellularized mouse lungs in small airway growth medium and basal medium, only transient expression of TTF1 was detected, and no other epithelial markers [12]. Other groups reported that rhesus marrow-derived MSCs as well as adipose MSCs in the decellularized matrix did not have epithelial marker expression after seven days of culture [11].
Overall, successful employment of interstitial cells for lung tissue engineering may require a combination of fibroblasts and MSCs. Based on the ratio of cell types in an intact lung, a 1:1 or 2:1 ratio of interstitial cells to epithelium would be most appropriate. A combination of MSCs with epithelium, followed by supplementation with differentiated fibroblasts, would provide epithelial support while minimizing the likelihood of the epithelium being overrun by MSCs. MSCs are less proliferative, less migratory, and produce less ECM than fibroblasts. As a source of MSCs, bone marrow and adipose-derived MSCs are most accessible. Each of these cell types has been used clinically for other applications; these populations are generally regarded as safe. Bone marrow MSCs have even demonstrated the ability to support the lung epithelium during acute injury by mitochondrial transfer [143]. However, the ability of these cells to perform appropriately in the context of lung tissue engineering has not been clearly established. Efforts in the near term will center on exploring bone marrow and adipose-derived MSCs for tissue engineering.
Long-term, lung-resident MSCs will be the most appropriate MSC source. Human lung-MSCs instilled into mouse lungs localize to the alveolar compartment, where they secrete keratinocyte growth factor, a key growth factor for lung epithelium. They also show higher initial retention and greater longevity in the lung, as compared to fibroblasts [144]. One caveat is that there may be a variety of lung-MSC subpopulations. One recent report of MSCs derived from the mature lung, for example, indicates that CD166- lung-MSCs support the formation of lung epithelium stem cell colonies in vitro, while CD166+ lung-MSCs do not [145]. Like epithelial cells used for lung tissue engineering, the MSC population that is used may ultimately have to be a heterogeneous one. Regardless, interstitial cells, including both MSCs and fibroblasts, will be an indispensable component of a successfully engineered lung.
IV. Bioreactors
A. Bioreactor Design Criteria
To facilitate seeding of multiple cell types, the bioreactor-mounted decellularized scaffold should provide independent access to the vascular and airway compartments. Additionally, the bioreactor must maintain sterility, adequate nutrient transfer and waste removal, and sufficient oxygen transport to prevent the growing cells from becoming hypoxic. For a lung, the bioreactor should be capable of providing tissue-specific environmental or mechanical stimuli such as an air–liquid interface and cyclic stretch (i.e., ventilation), generated by negative pressure [94], [146]. Like any 3-D tissue, perfusion should also be provided and it should be pulsatile to mimic the blood supplied to the body by the heart [147]. Bioreactors such as those used by Price et al. [9] and Petersen et al. [94] are relatively easy to modify to accommodate different connections or additional ports or probes. Specifically, the ability to monitor biologically relevant parameters such as pH, lactate, glucose, and CO2 and O2 levels in real time could provide information about the state of the cells populating the matrix and, ultimately, the function of the organ. Lactate concentration, for example, directly reflects cellular hypoxia and metabolic state [148].
Obtaining information about cell growth, proliferation, and differentiation over the course of culture without terminating the experiment, as typical analytical techniques such as RNA or protein analysis require, would be a significant advancement in whole organ engineering and culture. In addition to assays for soluble factors, noninvasive evaluation of engineered lungs may be possible through imaging techniques such as multiphoton microscopy [149], [150] or magnetoacoustic-tomography with magnetic induction (MAT-MI) [151]. Multiphoton microscopy allows imaging of multiple tissue components based on inherent differences in nonlinear excitation and with minimal photo damage to living cells, while MAT-MI can be used to construct image of a composite “tissue” (i.e., a phantom with regions of different salinity) by exploiting differences in electrical impedance.
Additional design considerations particularly important to human lung-sized bioreactors include cost, overall “handle-ability,” and the availability of pumps and ventilators or other mechanisms to drive physiological stimuli. The organ size may also make orientation of the organ within the bioreactor important to successful culture, as gravitational influences on cell seeding and matrix distension may be important for organs larger than rodent-sized. Culture medium requirements will also pose a unique challenge to whole lung engineering, as the volume of the medium required to support a set of adult human lungs, extrapolating from the medium requirements of a confluent T75 flask of microvascular endothelial cells, could translate to >300 L of medium per week. If growth factors or small molecules are added to this volume for the purpose of stem cell differentiation or the support of highly specialized epithelial phenotypes, the cost could become unsustainable very quickly. Though the use of multiple cell types simultaneously may provide some “endogenous” growth factor and paracrine factor support, advances in the recombinant protein production hold greater potential for reducing the cost of medium additives.
B. Challenges for the Bioreactor Use
With the advent of large-scale cell culture for bioprocess applications (e.g., antibody production in the pharmaceutical industry), steady advances have increased the bioprocess protein yield 100-fold since the mid-1980s [152], [153]. Hence, it is not unreasonable to think that, while current projected costs for whole lung culture are very high, these costs may come down with increased efficiency of culture medium utilization and bioreactor improvements.
Finally, biologically relevant mechanical stimuli, while provided by the first generation of bioreactors reported [9], [94], may not be finely tuned enough to impart the greatest advantage possible for the developing organ. Ventilation in particular may be the most important mechanical cue to “get right.” Plentiful evidence exists that the frequency and the volume of breaths administered to both patients, and to tissues in vitro, have significant impacts on the health of both the endothelial and epithelial layers [154]–[157]. While mechanical distension is important for the differentiation of type I and II cells and the balance between the number of each population in utero [93], [158] and in vitro [159], overdistension can disrupt barrier integrity [155] and even induce apoptosis of type II cells [160], [161]. In addition to the tidal volume, ventilation frequency is important—a program of variable stretch has been shown to impart less injury to and greater surfactant production by type II epithelium than regular stretch cycles [162]. Ventilation strategy can also significantly influence pulmonary vascular resistance, thereby affecting perfusion and vascular integrity [163].
Therefore, defining an “appropriate ventilation protocol” will likely be critical to cultivating successful lung tissue. When ex vivo lung perfusion (EVLP) is applied to whole human or pig lungs, a tidal volume of 6–8 mL/kg, at 7 breaths/min provides adequate ventilation without adversely affecting cell viability or vascular resistance [163] In addition, Cypel et al. perform “lung recruitment maneuvers” every hour during the course of EVLP to recruit regions of collapsed alveoli. A similar strategy may be useful for long-term lung culture.
An appropriate ventilation program for the engineered lung tissue may also necessitate reconciling the scaffold with the repopulating cell type, as a full-sized adult human lung containing differentiated cells would typically require 500 ml of air, at a ventilation rate of 12 breaths/min [7]. In contrast, liquid ventilation at approximately 40 breaths/min for several minutes per hour would be a more logical choice for fetal or primitive cell types, as this more closely mimics fetal ventilation movements in the human [93]. Ultimately, exposure to air is beneficial for maintaining and promoting epithelial differentiation [95], [96], so a transition may have to be made from liquid to air ventilation in vitro. Because of the “leaky” nature of acellular and newly seeded extracellular matrix scaffolds, quantitative models or visual information about the “actual stretch” experienced by the cells within the scaffold will be of use as well.
V. Conclusion
Overall, significant progress has been made in recent years toward engineering functional lung tissue in vitro. Although the first report of whole heart engineering [123] preceded that of whole lung by two years [3], [4], work in lung tissue engineering has outpaced that of the heart, liver (also 2010, [164]), and kidney (2012, [92], [165], [166]), as judged by the abundance, frequency, and scope of manuscripts produced on this topic. In addition to more than one group implanting the recellularized tissue and a large number of groups seeding various cells onto the decellularized tissue, at least three independent groups have decellularized human and pig lungs [16], [17], [19], scaled up from rodent lungs. Collectively, groups working on whole lung engineering have been early adopters of new technology (six papers using proteomics to query the composition of lung scaffolds [11], [12], [14], [19], [22], [167] exist, for example, compared to only one paper each that uses this technique to analyze kidney [167] or liver [22] matrix). Though the use of such techniques will require refinement and greater depth of understanding in order to truly inform and direct future efforts; the dissemination and use of such tools is a positive sign of the interest and commitment of multiple groups to advancing lung engineering efforts. There is also active comparison of decellularization regimens, which will hopefully accelerate the specification of an “optimized” protocol.
Despite these auspicious first few years, much work remains. Each of the three central elements of tissue engineering—the scaffold, the cells, and recellularization strategies designed to deliver the cells to the scaffold, and the bioreactor in which the tissue is cultivated—require more detailed study and additional improvements.
While decellularized extracellular matrix scaffolds remain the state of the art in whole organ engineering presently, identifying a reliable scaffold source that will provide consistent quality and minimal variability between scaffolds will be critical. In addition, a defined method of terminal sterilization for a biologically active scaffold of the size and structure required for whole organ engineering is a tractable but challenging element of moving this technology to the clinic.
One additional consideration, which perhaps should be examined in tandem with select cell populations, is the composition of the extracellular matrix after decellularization. Although many different detergents and other reagents are available to effect cell removal, an optimal decellularization method for lung tissue engineering has yet to be identified. Clearly, defining appropriate evaluation criteria for decellularized matrices will be a critical first step. The ability to articulate specific standards may hinge on the use of tools such as extracellular matrix proteomics, only recently applied to decellularized lung tissue [11], [12], [14], [16], [22], [168], that may provide insight heretofore unavailable in the field.
For the purpose of repopulation of the scaffold and generation of the lung tissue, cell selection will require careful consideration of regionally specific lung stem and progenitor cells identified to date, as well as continued investigation of lung cell and developmental biology. In addition, the field will certainly benefit as stem cell biology advances. Unlike the ability of investigators to generate differentiated cardiomyocytes and hepatocytes from iPS cells, no one has yet produced a method for directed differentiation of definitive lung cells. The principle objective of continued work with primary cells is to determine the numbers and types of cells required to generate an adequate amount of the functional lung tissue to support an organism.
Advancements in the areas of the bioreactor design will center on improved monitoring of the developing tissue, as well as more precise control of physical stimuli such as ventilation and perfusion. In particular, tuning the volume and frequency of ventilation will be important to both epithelial and endothelial survival [157], [161], and proliferation. Refining ventilation and perfusion will require bringing the considerable molecular and cellular tools available for 2-D stretch experiments into three dimensions. Drawing comparisons between models of compromised alveolar barrier integrity and a partially repopulated acellular scaffold may also be productive, since the engineered lung tissue may initially resemble an injured lung more closely than it approximates an intact whole lung.
Ultimately, many if not all of the challenges posed by tissue sterility, scaffold procurement, bioreactor improvement, and even epithelial cell differentiation and scale up are surmountable, given sufficient resources, time, and effort. In our opinion, the ultimate success of lung regeneration in vitro will depend most heavily on the ability of researchers to produce a sufficiently endothelialized organ. The provision of a nonthrom-bogenic surface for blood flow, the creation of tight cell–cell junctions [103], [105], and the production of various dilatory vasoactive substances [103] are needed to ensure patency of the most extensive vascular bed in the body [169]. Without a profusion of perfused conduits in close proximity to air, the lung will not function for gas exchange.
The principle consideration to note is that the challenges are many and varied but are not insurmountable. The combination of talented physicians, scientists, engineers, biologists, and others will continue to move the field forward. Importantly, there are many opportunities for technological innovation and for bridges to be built across disciplines. Therefore, concerted, collaborative efforts among biologists, engineers, clinicians, chemists, and emerging areas must be leveraged to overcome the physical and biological challenges of repopulating a whole organ scaffold with the aim of developing a transplantable organ in vitro.
Acknowledgments
The work of Laura E. Niklason was supported by the National Institutes of Health under Grants U01 HL111016 and R01 HL098220. The work of Sumati Sundaram was supported by Connecticut Innovations # 13SC36.
Footnotes
Disclosures: L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Authors’ photographs and biographies not available at the time of publication.
Contributor Information
Elizabeth A. Calle, Email: elizabeth.calle@yale.edu, Department of Biomedical Engineering, Yale University, New Haven, CT 06519 USA
Mahboobe Ghaedi, Email: mahboobe.ghaedi@yale.edu, Department of Anesthesia, Yale University, New Haven, CT 06519 USA.
Sumati Sundaram, Email: sumati.sundaram@yale.edu, Department of Anesthesia, Yale University, New Haven, CT 06519 USA.
Amogh Sivarapatna, Email: amogh.sivarapatna@yale.edu, Department of Biomedical Engineering, Yale University, New Haven, CT 06519 USA.
Michelle K. Tseng, Email: michelle.tseng@yale.edu, Department of Biomedical Engineering, Yale University, New Haven, CT 06519 USA
Laura E. Niklason, Email: laura.niklason@yale.edu, Department of Anesthesia and Department of Biomedical Engineering, Yale University, New Haven, CT 06519 USA
References
- 1.Hoyert DL, Xu J. [accessed: Dec. 28, 2012];LDD_2008.pdf. [Online]. Available: http://sacredvancouver.com/nchs/data/nvsr/nvsr61/nvsr61_06.pdf.
- 2.H. R. A. S. A. U S Department of Health and Human Services. United states organ transplant OPTN and SRTR annual data report 2011. 2011 [Online]. Available: srtr.transplant.hrsa.gov.
- 3.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 5.Hachenberg T, Rettig R. Stress failure of the blood-gas barrier. Curr Opin Anaesthesiol. 1998;11:37–44. doi: 10.1097/00001503-199802000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Weibel ER. Morphometry of the Human Lung. New York, NY, USA: Academic; 1963. [Google Scholar]
- 7.Boron WF, Boulpaep EL. Medical Physiology. Amsterdam, The Netherlands: Elsevier; 2008. [Google Scholar]
- 8.Li M, Mondrinos MJ, Chen X, Gandhi MR, Ko FK, Lelkes PI. Co-electrospun poly(lactide-co-glycolide), gelatin, and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79:963–973. doi: 10.1002/jbm.a.30833. [DOI] [PubMed] [Google Scholar]
- 9.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung biore-actor system for bioengineering the lung: The matrix reloaded. Tissue Eng A. 2010;16:2581–2591. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Org. 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, Mayeux JP, Gregory AN, Wang G, Townley IK, Borg ZD, Weiss DJ, Bunnell BA. A nonhuman primate model of lung regeneration: Detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng. 2012 Dec;18:2437–2452. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng. 2012 Jan;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, Finck C. A rapid lung decellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng C Methods. 2012;18:632–646. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Amer J Respir Crit Care Med. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. 2010;16:2565–2580. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 16.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, Vacanti JP, Ott HC. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J Heart Lung Transplantation. 2013;32:S69–S70. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Nichols JEE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, Edward K, Lafrancesca S, Sakamoto J, Vega S, Ogedegbe M, Mlcak R, Deyo D, Woodson L, McQuitty C, Lick S, Beckles D, Melo E, Cortiella J. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng A. 2013;19:2045–2062. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, Singh G, Freytes DO, Bacchetta MD, Sonett JR, Vunjak-Novakovic G. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–1055. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner DE, Bonenfant NR, Sokocevic D, Desarno MJ, Borg ZD, Parsons CS, Brooks EM, Platz JJ, Khalpey ZI, Hoganson DM, Deng B, Lam YW, Oldinski RA, Ashikaga T, Weiss DJ. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2014 Jan;35:2664–2679. doi: 10.1016/j.biomaterials.2013.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French KM, Boopathy AV, Dequach JA, Chingozha L, Lu H, Christman KL, Davis ME. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012 Dec;8:4357–4364. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neill JD, Freytes DO, Anandappa AJ, Oliver JA, Vunjak-Novakovic GV. The regulation of growth and metabolism of kidney stem cells with regional specificity using extracellular matrix derived from kidney. Biomaterials. 2013;34:9830–9841. doi: 10.1016/j.biomaterials.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamis Y, Hasson E, Soroker A, Bassat E, Shimoni Y, Ziv T, et al. Organ-specific scaffolds for in vitro expansion, differentiation, and organization of primary lung cells. Tissue Eng C Methods. 2011;17:861–870. doi: 10.1089/ten.tec.2010.0717. [DOI] [PubMed] [Google Scholar]
- 23.DeQuach JA, Yuan SH, Goldstein LS, Christman KL. De-cellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng A. 2011 Nov;17:2583–2592. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cebotari S, Tudorache I, Jaekel T, Hilfiker A, Dorfman S, Ternes W, Haverich A, Lichtenberg A. Detergent decellularization of heart valves for tissue engineering: Toxicological effects of residual detergents on human endothelial cells. Artif Organs. 2010;34:206–210. doi: 10.1111/j.1525-1594.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 25.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng C Methods. 2012;18:420–432. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faulk DM, Carruthers CA, Warner HJ, Kramer CR, Reing JE, Zhang L, D’Amore A, Badylak SF. The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 2013;10:183–193. doi: 10.1016/j.actbio.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lwebuga-Mukasa JS, Ingbar DH, Madri JA, et al. Repopulation of a human alveolar matrix by adult rat type II pneumocytes in vitro. A novel system for type II pneumocyte culture. Exp Cell Res. 1986;162:423–435. doi: 10.1016/0014-4827(86)90347-2. [DOI] [PubMed] [Google Scholar]
- 28.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra DK, Thrall MJ, Baird BN, Ott HC, Blackmon SH, Kurie JM, Kim MP. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macchiarini P, Jungebluth P, Go T, Asnaghi M, Rees L, Cogan T, Dodson A, Martorell J, Bellini S, Parnigotto P, Dickinson S, Hollander A, Mantero S, Conconi M, Birchall M. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 31.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the united states, 1999–2008. Amer J Transplantation. 2010;10:973–986. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 32.Schoen FJ. Cardiac valves and valvular pathology: Update on function, disease, repair, and replacement. Cardiovasc Pathol. 2005;14:189–194. doi: 10.1016/j.carpath.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Keane TJ, Londono R, Turner NJ, Badylak SF. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 2011;36:1771–1781. doi: 10.1016/j.biomaterials.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 34.Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, Mccray PB, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29–31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daggett CW, Yeatman M, Lodge AJ, Chen EP, Linn SS, Gullotto C, Frank MM, Platt JL, Davis RD. Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg. 1998;115:19–27. doi: 10.1016/s0022-5223(98)70438-6. [DOI] [PubMed] [Google Scholar]
- 37.Vangsness CT, Wagner PP, Moore TM, Roberts MR. Overview of safety issues concerning the preparation and processing of soft-tissue allografts. Arthroscopy. 2006;22:1351–1358. doi: 10.1016/j.arthro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt T, Hoburg A, Broziat C, Smith MD, Gohs U, Pruss A, Scheffler S. Sterilization with electron beam irradiation influences the biomechanical properties and the early remodeling of tendon allografts for reconstruction of the anterior cruciate ligament (ACL) Cell Tissue Bank. 2012;13:387–400. doi: 10.1007/s10561-011-9289-6. [DOI] [PubMed] [Google Scholar]
- 39.Kaminski A, Jastrzebska A, Grazka E, Marowska J, Gut G, Wojciechowski A, Uhrynowska-Tyszkiewicz I. Effect of gamma irradiation on mechanical properties of human cortical bone: Influence of different processing methods. Cell Tissue Bank. 2012;13:363–374. doi: 10.1007/s10561-012-9308-2. [DOI] [PubMed] [Google Scholar]
- 40.Remlinger NT, Czajka CA, Vorp DA, Stolz DB, Badylak SF, Gilbert S, Gilbert TW. Hydrated xenogeneic decellularized tracheal matrix as a scaffold for tracheal reconstruction. Biomaterials. 2010 May;31:3520–3526. doi: 10.1016/j.biomaterials.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa H, Shimoda M, Shiratsuchi H, Osajima Y. Sterilization of microorganisms by the supercritical carbon dioxide micro-bubble method. Biosci Biotechnol Biochem. 1995;59:1949–1950. doi: 10.1271/bbb.59.1949. [DOI] [PubMed] [Google Scholar]
- 42.Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R. Bacterial inactivation by using near- and supercritical carbon dioxide. Proc Nat Acad Sci USA. 1999;96:10344–10348. doi: 10.1073/pnas.96.18.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu QQ, Leamy P, Brittingham J, Pomerleau J, Kabaria N, Connor J. Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater. 2009;91:572–578. doi: 10.1002/jbm.b.31431. [DOI] [PubMed] [Google Scholar]
- 44.Webb S. ‘Supercritical’ CO2 emerging as transplant tissue sterilization option. Sci Amer. 2009 [Google Scholar]
- 45.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Amer J Physiol Lung Cell Mol Physiol. 2010;298:L715–L731. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rawlins EL, Hogan BLM. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 48.Stone KC, Mercer RR, Freeman BA, Chang LY, Crapo JD. Distribution of lung cell numbers and volumes between alveolar and nonalveolar tissue. Amer Rev Respir Dis. 1992;146:454–456. doi: 10.1164/ajrccm/146.2.454. [DOI] [PubMed] [Google Scholar]
- 49.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Amer J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 50.Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD. Allometric relationships of cell numbers and size in the mammalian lung. Amer J Respir Cell Mol Biol. 1992 Feb;6:235–243. doi: 10.1165/ajrcmb/6.2.235. [DOI] [PubMed] [Google Scholar]
- 51.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, Deutsch GH. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139:1060–1071. doi: 10.1378/chest.10-1304. [DOI] [PubMed] [Google Scholar]
- 52.Peake JL, Reynolds SD, Stripp BR, Stephens KE, Pinkerton KE. Alteration of pulmonary neuroendocrine cells during epithelial repair of naphthalene-induced airway injury. Amer J Pathol. 2000;156:279–286. doi: 10.1016/S0002-9440(10)64728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Amer J Physiol Lung Cell Mol Physiol. 2000 Jun;278:L1256–L1263. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Amer J Pathol. 2000 Jan;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rawlins EL, Ostrowski LE, Randell SH, Hogan BLM. Lung development and repair: Contribution of the ciliated lineage. Proc Nat Acad Sci USA. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Amer J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BLM. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Nat Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of Clara cells in normal human airway epithelium. Amer J Respir Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- 59.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BLM. The role of scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Amer J Pathol. 1986;123:126–133. [PMC free article] [PubMed] [Google Scholar]
- 61.Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, Woo AJ, Chen H, Conner DA, Fujiwara Y, Stripp BR, Kim CF. Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C-expressing lung cells. Amer J Respir Cell Mol Biol. 2013;48:288–298. doi: 10.1165/rcmb.2011-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BLM. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang DY, Lim B, Chow VT, Crum CP, Xian W, Mckeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khatri M, Goyal SM, Saif YM. Oct4+ stem/progenitor swine lung epithelial cells are targets for influenza virus replication. J Virol. 2012;86:6427–6433. doi: 10.1128/JVI.00341-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kajstura J, Rota M, Hall SR, Hosoda T, D’Amario D, Sanada F, Zheng H, Ogrek B, Rondon-Clavo C, Ferreira-Martins J, Matsuda A, Arranto C, Goichberg P, Giordano G, Haley KJ, Bardelli S, Rayatzadeh H, Liu X, Quaini F, Liao R, Leri A, Perrella MA, Loscalzo J, Anversa P. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Amer J Physiol Lung Cell Mol Physiol. 2009;297:L1045–L1055. doi: 10.1152/ajplung.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, Bishop AE. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- 69.Winkler ME, Mauritz C, Groos S, Kispert A, Menke S, Hoffmann A, Gruh I, Schwanke K, Haverich A, Martin U. Serum-free differentiation of murine embryonic stem cells into alveolar type II epithelial cells. Cloning Stem Cells. 2008;10:49–64. doi: 10.1089/clo.2007.0075. [DOI] [PubMed] [Google Scholar]
- 70.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Nat Acad Sci USA. 2007;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banerjee ER, Laflamme MA, Papayannopoulou T, Kahn M, Murry CE, Henderson WR. Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One. 2012;7:e33165, 1–15. doi: 10.1371/journal.pone.0033165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10:385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 76.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007 Jul 19;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 77.Green MD, Chen A, Nostro M-C, d’Souza SL, Schaniel C, Lemischka IR, Gouon-Evans V, Keller G, Snoeck H-W. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29:267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okita K, Hong H, Takahashi K, Yamanaka S. Generation of mouse-induced pluripotent stem cells with plasmid vectors. Nat Protoc. 2010;5:418–428. doi: 10.1038/nprot.2009.231. [DOI] [PubMed] [Google Scholar]
- 80.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem Cells. 2009;27:2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 82.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Phys Biol Sci. 2009;85:348–362. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warren L, Ni Y, Wang J, Guo X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci Rep. 2012;2:1–7. doi: 10.1038/srep00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warren L, Ni Y, Wang J, Guo X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1038/srep00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009 Jun 5;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011 May;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carraro G, Perin L, Sedrakyan S, Giuliani S, Tiozzo C, Lee J, Turcatel G, De Langhe SP, Driscoll B, Bellusci S, Minoo P, Atala A, De Filippo RE, Warburton D. Human amniotic fluid stem cells can integrate and differentiate into epithelial lung lineages. Stem Cells. 2008;26:2902–2911. doi: 10.1634/stemcells.2008-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 90.De Paepe ME, Mao Q, Ghanta S, Hovanesian V, Padbury JF. Alveolar epithelial cell therapy with human cord blood-derived hematopoietic progenitor cells. Amer J Pathol. 2011;178:1329–1339. doi: 10.1016/j.ajpath.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang SXL, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91. doi: 10.1038/nbt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med. 2013;19:646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Inanlou MR, Baguma-Nibasheka M, Kablar B. The role of fetal breathing-like movements in lung organogenesis. Histol Histopathol. 2005;20:1261–1266. doi: 10.14670/HH-20.1261. [DOI] [PubMed] [Google Scholar]
- 94.Petersen TH, Calle EA, Colehour MB, Niklason LE. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20:1117–1126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. Amer J Resp Crit Care Med. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 96.Ostrowski LE, Nettesheim P. Inhibition of ciliated cell differentiation by fluid submersion. Expt Lung Res. 1995;21:957–970. doi: 10.3109/01902149509031773. [DOI] [PubMed] [Google Scholar]
- 97.Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, Medoff BD, Rajagopal J. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu JY, Nettesheim P, Randell SH. Growth and differentiation of tracheal epithelial progenitor cells. Amer J Physiol. 1994;266:L296–L307. doi: 10.1152/ajplung.1994.266.3.L296. [DOI] [PubMed] [Google Scholar]
- 99.Musah S, Chen J, Hoyle GW. Repair of tracheal epithelium by basal cells after chlorine-induced injury. Respir Res. 2012;13:107–119. doi: 10.1186/1465-9921-13-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh M, Ahmad S, Jian A, Li B, Smith RW, Helm KM, Seibold MA, Groshong SD, White CW, Reynolds SD. Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro. Amer J Respir Cell Mol Biol. 2013 Dec;49:1127–1134. doi: 10.1165/rcmb.2013-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vlodavsky I, Eldor A, HyAm E, Atzom R, Fuks Z. Platelet interaction with the extracellular matrix produced by cultured endothelial cells: A model to study the thrombogenicity of isolated subendothelial basal lamina. Thrombosis Res. 1982;28:179–191. doi: 10.1016/0049-3848(82)90260-2. [DOI] [PubMed] [Google Scholar]
- 102.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- 103.Johnson AR. Human pulmonary endothelial cells in culture: Activities of cells from arteries and cells from veins. J Clin Invest. 1980;65:841–850. doi: 10.1172/JCI109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Muth H, Maus U, Wygrecka M, Lohmeyer J, Grimminger F, Seeger W, Günther A. Pro- and antifibrinolytic properties of human pulmonary microvascular versus artery endothelial cells: Impact of endotoxin and tumor necrosis factor-alpha. Crit Care Med. 2004;32:217–226. doi: 10.1097/01.CCM.0000104941.89570.5F. [DOI] [PubMed] [Google Scholar]
- 105.Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res. 2008;75:391–402. doi: 10.1016/j.mvr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stevens T. Functional and molecular heterogeneity of pulmonary endothelial cells. Proc Amer Thorac Soc. 2011;8:453–457. doi: 10.1513/pats.201101-004MW. [DOI] [PubMed] [Google Scholar]
- 107.Neuhaus W, Samwer F, Kunzmann S, Muellenbach RM, Wirth M, Speer CP, Roewer N, Förster CY. Lung endothelial cells strengthen, but brain endothelial cells weaken barrier properties of a human alveolar epithelium cell culture model. Differentiation. 2012;84:294–304. doi: 10.1016/j.diff.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 108.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, Shido K, Rabbany SY, Rafii S. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Amer J Physiol Lung Cell Mol Physiol. 2002;283:L555–L562. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 110.Han RNN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase-deficient mice: A model of alveolar capillary dysplasia? Circulation Res. doi: 10.1161/01.RES.0000125624.85852.1E. [DOI] [PubMed] [Google Scholar]
- 111.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, Zaninovic N, Rosenwaks Z, Rabbany SY, Rafii S. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levenberg S, Ferreira LS, Chen-Konak L, Kraehenbuehl TP, Langer R. Isolation, differentiation and characterization of vascular cells derived from human embryonic stem cells. Nat Prot. 2010;5:1115–1126. doi: 10.1038/nprot.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Nat Acad Sci USA. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Z, Hu S, Ghosh Z, Han Z, Wu JC. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem Cells Development. 2011;20:1701–1710. doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, Lee JC, Zambidis ET, Reijo-Pera R, Cooke JP. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Amer J Transl Res. 2013;5:21–35. [PMC free article] [PubMed] [Google Scholar]
- 116.Tatsumi R, Suzuki Y, Sumi T, Sone M, Suemori H, Nakatsuji N. Simple and highly efficient method for production of endothelial cells from human embryonic stem cells. Cell Transplant. 2011;20:1423–1430. doi: 10.3727/096368910X547444. [DOI] [PubMed] [Google Scholar]
- 117.White MP, Rufaihah AJ, Liu L, Ghebremariam YT, Ivey KN, Cooke JP, Srivastava D. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2012;31:92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Nat Biotechnol. 2007 Mar;25:317–318. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glaser DE, Gower RM, Lauer NE, Tam K, Blancas AA, Shih AJ, Simon SI, McCloskey KE. Functional characterization of embryonic stem cell-derived endothelial cells. J Vasc Res. 2011;48:415–428. doi: 10.1159/000324752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lai WH, Ho JCY, Chan YC, Ng JHL, Au KW, Wong LY, Siu CW, Tse HF. Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS ONE. 2013;8:e57876, 1–10. doi: 10.1371/journal.pone.0057876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JDJ, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc Nat Acad Sci USA. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–567. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 124.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 125.Townsley MI. Structure and composition of pulmonary arteries, capillaries, and veins. Compr Physiol. 2012 Jan;2:675–709. doi: 10.1002/cphy.c100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Akram KM, Samad S, Spiteri MA, Forsyth NR. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res. 2013;14:1–16. doi: 10.1186/1465-9921-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 128.Myerburg MM, Latoche JD, McKenna EE, Stabile LP, Siegfried JS, Feghali-Bostwick CA, Pilewski JM. Hepatocyte growth factor and other fibroblast secretions modulate the phenotype of human bronchial epithelial cells. Amer J Physiol Lung Cell Mol Physiol. 2007;292:L1352–L1360. doi: 10.1152/ajplung.00328.2006. [DOI] [PubMed] [Google Scholar]
- 129.Rehan VK, Sugano S, Wang Y, Santos J, Romero S, Dasgupta C, Keane MP, Stahlman MT, Torday JS. Evidence for the presence of lipofibroblasts in human lung. Expt Lung Res. 2006;32:379–393. doi: 10.1080/01902140600880257. [DOI] [PubMed] [Google Scholar]
- 130.Zheng Y, Zhang M, Qian M, Wang L, Cismasiu VB, Bai C, Popescu LM, Wang X. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013 Apr;17:567–577. doi: 10.1111/jcmm.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chow K, Fessel JP, Kaoriihida-Stansbury, Schmidt EP, Gaskill C, Alvarez D, Graham B, Harrison DG, Wagner DH, Jr, Nozik-Grayck E, West JD, Klemm DJ, Majka SM. Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulmonary Circulation. 2013;3:31–49. doi: 10.4103/2045-8932.109912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ricciardi M, Malpeli G, Bifari F, Bassi G, Pacelli L, Kamdje AHN, Chilosi M, Krampera M. Comparison of epithelial differentiation and immune regulatory properties of mesenchymal stromal cells derived from human lung and bone marrow. PLoS ONE. 2012;7:e35639, 1–10. doi: 10.1371/journal.pone.0035639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee J-H, Balasubramaniam V, Fredenburgh LE, Alex Mitsialis S, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Amer J Physiol Lung Cell Mol Physiol. 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matthay MA, Thompson BT, Read EJ, McKenna DH, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138:965–972. doi: 10.1378/chest.10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hodges RJ, Lim R, Jenkin G, Wallace EM. Amnion epithelial cells as a candidate therapy for acute and chronic lung injury. Stem Cells Int. 2012;2012(709763):1–7. doi: 10.1155/2012/709763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009 Oct;9:1259–1270. doi: 10.1517/14712590903213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013 Jun;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baiguera S, Jungebluth P, Burns A, Mavilia C, Haag J, De Coppi P, Macchiarini P. Tissue engineered human tracheas for in vivo implantation. Biomaterials. 2010;31:8931–8938. doi: 10.1016/j.biomaterials.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 140.Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, Mantero S, Birchall M, Bader A, Macchiarini P. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010;139:437–443. doi: 10.1016/j.jtcvs.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 141.Lindner U, Kramer J, Behrends J, Driller B. Improved proliferation and differentiation capacity of human mesenchymal stromal cells cultured with basement-membrane extracellular matrix proteins. Cytotherapy. 2010;12:992–1005. doi: 10.3109/14653249.2010.510503. [DOI] [PubMed] [Google Scholar]
- 142.Ode A, Duda GN, Glaeser JD. Toward biomimetic materials in bone regeneration: Functional behavior of mesenchymal stem cells on a broad spectrum of extracellular matrix components. J Biomed Mater Res A. 2010 doi: 10.1002/jbm.a.32909. [DOI] [PubMed] [Google Scholar]
- 143.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN. Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Amer J Respir Cell Mol Biol. 2011;45:809–816. doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McQualter JL, McCarty RC, Van der Velden J, O’Donoghue RJJ, Asselin-Labat ML, Bozinovski S, Bertoncello I. TGF-β signal ing in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res. 2013;11:1222–1233. doi: 10.1016/j.scr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 146.Soni N, Williams P. Positive pressure ventilation: What is the real cost? Br J Anaes. 2008;101:446–457. doi: 10.1093/bja/aen240. [DOI] [PubMed] [Google Scholar]
- 147.Solan A, Dahl S, Niklason LE. Effects of mechanical stretch on collagen and cross-linking in engineered blood vessels. Cell Transplant. 2009 doi: 10.3727/096368909X471161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Phypers B. Lactate physiology in health and disease. Continuing Edu Anaesthesia, Critical Care Amp Pain. 2006;6:128–132. [Google Scholar]
- 149.Merna N, Robertson C, La A, George SC. Optical imaging pre dicts mechanical properties during decellularization of cardiac tissue. Tissue Eng Part C Methods. 2013;19:802–809. doi: 10.1089/ten.tec.2012.0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Niklason LE, Yeh AT, Calle EA, Bai Y, Valentín A, Humphrey JD. Enabling tools for engineering collagenous tissues in tegrating bioreactors, intravital imaging, and biomechanical modeling. Proc Nat Acad Sci USA. 2010;107:3335–3339. doi: 10.1073/pnas.0907813106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li X, Xu Y, He B. Imaging electrical impedance from acoustic measurements by means of magnetoacoustic tomography with magnetic induction (MAT-MI) IEEE Trans Biomed Eng. 2007 Feb;54(2):323–330. doi: 10.1109/TBME.2006.883827. [DOI] [PubMed] [Google Scholar]
- 152.Wang P, Dong S, Brailsford JA, Iyer K, Townsend SD, Zhang Q, Hendrickson RC, Shieh J, Moore MAS, Danishefsky SJ. At last: Erythropoietin as a single glycoform. Angew Chem Int Ed Engl. 2012;51:11576–11584. doi: 10.1002/anie.201206090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wurm FM. Production of recombinant protein therapeutics in cul tivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 154.Birukova AA, Rios A, Birukov KG. Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Exp Cell Res. 2008;314:3466–3477. doi: 10.1016/j.yexcr.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oeckler RA, Hubmayr RD. Cell wounding and repair in ventila tor injured lungs. Respir Physiol Amp Neurobiol. 2008;163:44–53. doi: 10.1016/j.resp.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119:1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 157.Raaz U, Kuhn H, Wirtz H, Hammerschmidt S. Rapamycin reduces high-amplitude, mechanical stretch-induced apoptosis in pul monary microvascular endothelial cells. Microvascular Res. 2009;77:297–303. doi: 10.1016/j.mvr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 158.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human type II pneumono cytes: Maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Amer J Respir Cell Mol Biol. 1997;17:672–682. doi: 10.1165/ajrcmb.17.6.2858. [DOI] [PubMed] [Google Scholar]
- 159.Gutierrez JA, Gonzalez RF, Dobbs LG. Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. Amer J Physiol. 1998;274:L196–L202. doi: 10.1152/ajplung.1998.274.2.L196. [DOI] [PubMed] [Google Scholar]
- 160.Edwards YS, Sutherland LM, Power JH, Nicholas TE, Murray AW. Cyclic stretch induces both apoptosis and secretion in rat alveolar type II cells. FEBS Lett. 1999;448:127–130. doi: 10.1016/s0014-5793(99)00357-9. [DOI] [PubMed] [Google Scholar]
- 161.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Amer J Respir Cell Mol Biol. 2004;30:396–402. doi: 10.1165/rcmb.2003-0136OC. [DOI] [PubMed] [Google Scholar]
- 162.Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Amer J Physiol Lung Cell Mol Physiol. 2009;296:L574–L581. doi: 10.1152/ajplung.90454.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, Sato M, Harwood S, Pierre A, Waddell TK, de Perrot M, Liu M, Keshavjee S. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 164.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010 Jul;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sullivan C, Leon JB, Sayre SS, Marbury M, Ivers M, Pencak JA, Bodziak KA, Hricik DE, Morrison EJ, Albert JM, Navaneethan SD, Reyes CMD, Sehgal AR. Impact of navigators on completion of steps in the kidney transplant process: A randomized, controlled trial. Clin J Amer Soc Nephrol. 2012 Oct;7:1639–1645. doi: 10.2215/CJN.11731111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, Aboushwareb T, De Coppi P, Wood KJ, Stratta RJ, Atala A, Yoo JJ, Soker S. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg. 2012 Aug;256:363–370. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 167.Nakayama KH, Lee CC, Batchelder CA, Tarantal AF. Tissue specificity of decellularized rhesus monkey kidney and lung scaffolds. PLoS One. 2013;8:e64134, 1–10. doi: 10.1371/journal.pone.0064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, Hoffman AM, Weiss DJ. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18:895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ochoa CD, Wu S, Stevens T. New developments in lung endothelial heterogeneity: Von willebrand factor, P-selectin, and the Weibel-palade body. Semin Thromb Hemost. 2010;36:301–308. doi: 10.1055/s-0030-1253452. [DOI] [PMC free article] [PubMed] [Google Scholar]