Abstract
Excessive ethanol drinking in rodent models may involve activation of the innate immune system, especially toll-like receptor 4 (TLR4) signaling pathways. We used intracellular recording of evoked GABAergic inhibitory postsynaptic potentials (eIPSPs) in central amygdala (CeA) neurons to examine the role of TLR4 activation by lipopolysaccharide (LPS) and deletion of its adapter protein CD14 in acute ethanol effects on the GABAergic system. Ethanol (44, 66 or 100 mM) and LPS (25 and 50 µg/ml) both augmented eIPSPs in CeA of wild type (WT) mice. Ethanol (44 mM) decreased paired-pulse facilitation (PPF), suggesting a presynaptic mechanism of action. Acute LPS (25 µg/ml) had no effect on PPF and significantly increased the mean miniature IPSC amplitude, indicating a postsynaptic mechanism of action. Acute LPS pre-treatment potentiated ethanol (44 mM) effects on eIPSPs in WT mice and restored ethanol’s augmenting effects on the eIPSP amplitude in CD14 knockout (CD14 KO) mice. Both the LPS and ethanol (44–66 mM) augmentation of eIPSPs was diminished significantly in most CeA neurons of CD14 KO mice; however, ethanol at the highest concentration tested (100 mM) still increased eIPSP amplitudes. By contrast, ethanol pre-treatment occluded LPS augmentation of eIPSPs in WT mice and had no significant effect in CD14 KO mice. Furthermore, (+)-naloxone, a TLR4-MD-2 complex inhibitor, blocked LPS effects on eIPSPs in WT mice and delayed the ethanol-induced potentiation of GABAergic transmission. In CeA neurons of CD14 KO mice, (+)-naloxone alone diminished eIPSPs, and subsequent co-application of 100 mM ethanol restored the eIPSPs to baseline levels. In summary, our results indicate that TLR4 and CD14 signaling play an important role in the acute ethanol effects on GABAergic transmission in the CeA and support the idea that CD14 and TLR4 may be therapeutic targets for treatment of alcohol abuse.
Keywords: alcohol, LPS, neuroimmune, inflammation, drug abuse, extended amygdala, toll-like receptor
INTRODUCTION
Recent evidence points to a role for neuroimmune mechanisms, and particularly the innate immunity mediated by the toll-like receptors (TLRs), in ethanol effects and drinking (for review see (Crews et al, 2011; Harris and Blednov, 2012). TLR4 plays an especially critical role in innate immunity and this pathway is activated by both exogenous [e.g. LPS, exhibiting a microbe-/pathogen-associated molecular pattern (MAMP/PAMP)] and endogenous signals or ligands [e.g., high mobility group box 1, heat shock proteins, nucleic acids and fibronectin, exhibiting damage-associated molecular patterns (DAMPs)] (Piccinini and Midwood, 2010). Activation of TLR4 triggers transcriptional activation of pro-interleukin-1β and TNFα and initiation of the innate immune response (Hanamsagar et al., 2012; Kielian, 2009).
Evidence supporting a key role of TLR4 in alcohol effects and drinking includes the following: 1) the alcohol-induced activation of glia, induction of inflammatory mediators, apoptosis, and behavioral and anxiety impairments seen in WT mice are not found in TLR4 deficient mice (Alfonso-Loeches and Guerri, 2011; Alfonso-Loeches et al, 2010; Alfonso-Loeches et al, 2012; Blanco and Guerri, 2007; Blanco et al, 2005; Fernandez-Lizarbe et al, 2013; Fernandez-Lizarbe et al, 2009; Pascual et al, 2011; Valles et al, 2004; Wu et al, 2012); 2) TLR4 is involved in excessive alcohol drinking in preclinical models (Blednov et al, 2011a; Blednov et al, 2012; Liu et al, 2011; Mulligan et al, 2006); 3) alcohol releases endogenous ligands for TLR4 in brain (Crews et al, 2012; He and Crews, 2008; Vetreno and Crews, 2012); and 4) LPS leaks from the gut in human alcoholics to activate pro-inflammatory signaling that may contribute to neuroinflammation and neurodegeneration (Qin et al, 2007). Examination of the TLR4 inflammatory pathways suggests possible approaches to study ethanol effects and drinking. Notably, knockout of the accessory protein CD14, that plays a critical role in LPS activation of TLR4, reduces ethanol preference and blocks the LPS-induced increase in ethanol drinking seen in wild type (WT) mice (Blednov et al, 2011a; Blednov et al, 2012). The reduction of TLR4 expression in the central nucleus of the amygdala (CeA) modulates ethanol binge drinking in a mouse model via a GABAA receptor effect (Liu et al, 2011). Moreover, it has been shown that TLR4-MyD88 signaling is involved in the acute behavioral actions of alcohol, as both pharmacological inhibition of TLR4 signaling with (+)-naloxone, a TLR4-MD-2 complex inhibitor and genetic deficiency of TLR4 or MyD88 significantly reduced the sedation and motor impairment induced by a single dose of alcohol in mice (Wu et al, 2012). Ethyl glucuronide, an ethanol metabolite, causes TLR4-dependent pain allodynia that can be blocked by (+)-naloxone (Lewis et al, 2013). Naloxone has two stereotactic isoforms, (+)-naloxone and (−)-naloxone, that are both potent TLR4 signaling inhibitors (Hutchinson et al, 2008). Whereas (+)-naloxone is selective for TLR4, (−)-naloxone also acts on opioid receptors (Hutchinson et al, 2008). Because another opioid antagonist, naltrexone, is now used in the treatment of alcohol addiction (Jarosz et al, 2013; Thorsell, 2013), there has been an effort to evaluate the therapeutic potential of TLR4-MD-2 complex specific (+)-naloxone and (+)-naltrexone.
The CeA, a major component of the extended amygdala (Heimer and Alheid, 1991), is a brain region known to be critically involved in anxiety and fear-conditioning, as well as in alcohol and drug dependence (Davis and Shi, 1999; Koob and Volkow, 2010; Rosen, 2004). In alcohol dependence, the CeA participates in the learning of stimulus-reward responses and mediation of alcohol’s motivational effects, self-administration, and stress-induced reinstatement of drinking (Koob, 1998; Koob and Volkow, 2010). The great majority of CeA neurons are GABAergic, and the GABAergic system is a key player in ethanol effects in the CeA (Nie et al, 2004; Nie et al, 2009; Roberto et al, 2008; Roberto et al, 2003; Roberto et al, 2004a; Siggins et al, 2005).
Knocking out TLR4 or CD14 reduced ethanol drinking and ethanol-related behavior in rodents (Alfonso-Loeches et al, 2010; Blednov et al, 2011a; Blednov et al, 2012; Pascual et al, 2011). Importantly, decreased TLR4 expression in CeA but not in basolateral amygdala (BLA) reduced ethanol binge drinking, indicating a critical role of the TLR4 system in the CeA for ethanol drinking (Liu et al, 2011). Although TLR4 receptors are expressed primarily by microglia in the CNS (Lehnardt et al, 2002; Chakravarty and Herkenham, 2005; Pascual et al, 2012), several studies also have shown neuronal expression of TLR4 (Acosta and Davies, 2008; Okun et al, 2011; Rolls et al, 2007; Tu et al, 2011). In the CeA, TLR4 receptors appear to be expressed in neurons, and their expression is regulated by the α2 GABAA subunit (Liu et al, 2011). Overall, these findings indicate that both TLR4 and the GABAergic system, and their cellular interactions in the CeA, may play an important role in ethanol drinking. However, little is known about the cellular aspects of TLR4 activation on neurophysiology and GABAergic transmission or on ethanol-induced potentiation of GABAergic transmission in the CeA (Bajo et al, 2008; Cruz et al, 2011; Roberto et al, 2012; Roberto et al, 2003; Roberto et al, 2004b). Therefore, in the present study we explored these issues using electrophysiological methods in CeA slices from CD14 KO mice, with exogenous administration of LPS and the TLR4 antagonist (+)-naloxone to activate and inhibit TLR4, respectively. We report that acute ethanol effects on GABAergic transmission in the CeA involve, or are mimicked by, components of the innate immune system such as TLR4 and CD14.
METHODS
Slice Preparation
We prepared in vitro brain slices (300 and 400 µm thick for whole-cell and sharp electrode recordings, respectively) containing CeA as previously described (Bajo et al, 2008; Bajo et al, 2011) from male (20–30 weeks old; 25–31 g) C57Bl/6J mice (Jackson Laboratory and the rodent breeding colony of The Scripps Research Institute) and from male CD 14 KO mice (provided by Drs. Blednov and Harris of The University of Texas at Austin; see (Blednov et al, 2011a)). For more detailed information on the mice and slice preparation, see the Supplemental Information (SI). We conducted all mouse breeding and care procedures in accordance with the Institutional Animal Care and Use Committee (IACUC) policies of The University of Texas at Austin and The Scripps Research Institute.
Electrophysiology
Intracellular recording of evoked responses
We recorded from CeA neurons with sharp micropipettes containing 3 M KCl (65–80 mΩ resistance) using current-clamp mode. The CeA is divided into medial and lateral subdivisions, but they cannot be easily identified in acute slices maintained in vitro (Sah et al, 2003). Therefore, we recorded from both subdivisions and did not distinguish between neurons from the two subdivisions. We held most neurons near their resting membrane potential (RMP), acquired data with an Axoclamp-2A preamplifier (Axon Instruments, now Molecular Devices, Sunnyvale, CA) and analyzed the recordings using pClamp software (Molecular Devices). We evoked pharmacologically-isolated GABAA receptor-mediated inhibitory postsynaptic potentials (eIPSPs) by stimulating locally within the CeA through a bipolar stimulating electrode, positioned medially close to the lateral globus pallidus or internal capsule, while superfusing the slices with the glutamate receptor blockers, 6-cyano-7-nitroquinoxaline-2,3-dione (DNQX, 20 µM) and DL-2-amino-5-phosphonovalerate (DL-AP5, 30 µM), and a GABAB receptor antagonist (CGP 55845A; 1 µM).
To determine half-maximal eIPSP amplitudes, we generated input/output (I/O) curves by measuring eIPSP amplitudes at 5 incrementally-increasing stimulus strengths, threshold to maximum stimulation. We measured the eIPSP amplitude I/O curves before (control), during and after (washout) drug application. We also used the paired-pulse facilitation (PPF) protocol to determine if the ethanol and LPS effects on eIPSPs were mediated by pre- or postsynaptic mechanisms. We examined PPF using 100 ms inter-stimulus intervals with the stimulus strength adjusted to give a 50% maximal amplitude of the first eIPSP, as determined from the I/O relationship. We calculated PPF as the ratio of the second eIPSP amplitude over that of the first eIPSP*100 (see SI). It has been shown that changes in the PPF ratio vary inversely with the presynaptic release of transmitter (Bonci and Williams, 1997; Mennerick and Zorumski, 1995; Salin et al, 1996).
Whole-cell patch-clamp recording of miniature IPSCs
We also recorded spontaneous action potential-independent GABAAergic mIPSCs (miniature inhibitory postsynaptic currents) to verify pre- versus postsynaptic mechanisms of action of LPS. Generally, a change in the frequency of mIPSCs suggests an altered probability of transmitter release, whereas a change in the amplitude of mIPSCs reflects alterations in the efficacy of postsynaptic GABAA receptors (De Koninck and Mody, 1994; Otis et al, 1994). The mIPSCs were pharmacologically isolated by superfusion of slices with 20 µM DNQX, 30 µM DL-AP5, 1 µM CGP 55845A and 1 µM tetrodotoxin (TTX). We used pipettes (input resistance 3–4 MΩ) filled with an internal solution containing (in mM): 135 KCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 5 ATP, and 1 GTP, pH 7.3–7.4, osmolarity 275–290 mOsm). We determined the frequency, amplitude, rise-time, decay and area of mIPSCs using Mini Analysis (Synaptosoft) software, and we analyzed the effects of LPS on these parameters in individual neurons by the Kolmogorov-Smirnov test (Mini Analysis; Synaptosoft).
Statistical Analysis
The recordings were taken continuously throughout the entire application of the tested drug or combination of drugs. We used 4-min time bins (1–4, 5–8, 9–12, 13–16, 17–20, etc. min after application of a tested drug), and thus, the values used f or statistical analyses corresponded to average responses within each 4-min bin. We recorded for 20–25 min following each drug application. For statistical analyses, we used the maximum effects of the drugs determined by one of the time bins in the span of 8–20 min after the start of drug application. Statistical analyses were performed with GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA) and Systat (Systat, Chicago, IL). We analyzed the data using t-tests and one-way or two-way ANOVAs, with or without repeated measures, as indicated, followed by Newman-Keuls and Bonferroni post hoc tests. With regard to t-tests, we used a paired t-test, as well as parametric or Mann-Whitney (non-parametric) t-tests, depending upon the results from normality tests of the corresponding data sets. Because concentration-response testing utilized a between-subjects design, we used analyses of covariance (ANCOVA) to assess genotype differences in concentration responsiveness, covarying for pre-treatment baseline, to determine treatment effects regardless of baseline differences. In all cases, the threshold for statistical significance was set to p < 0.05. We express all values as mean ± S.E.M.
Drugs
We purchased CGP 52432, DL-AP5, TTX and DNQX from Tocris Cookson (Holloway Road, MO), and bicuculline and LPS from Sigma (St Louis, MO); (+)-naloxone was synthesized by Dr. Kenner Rice at NIH and Dr. Edward Roberts at The Scripps Research Institute. We obtained ethanol from Remet (La Mirada, CA).
RESULTS
We recorded from a total of 181 mouse CeA neurons from control (WT; n = 99) and null mutant (CD14 KO; n= 82) mice and found no significant differences in passive membrane properties or basal GABAergic transmission (Student’s t-test; see Table S1 in SI). For example, neither the mean amplitudes of GABAA-IPSPs evoked by a half-maximal stimulation intensity, nor paired-pulse facilitation, were significantly different between WT and CD 14 KO mice (Table S1 in SI). Thus, expression of CD14 does not appear to play a significant role in the regulation of membrane properties or basal evoked GABAergic transmission in mouse CeA neurons.
CD14 involvement in ethanol modulation of GABAA-IPSPs in CeA neurons
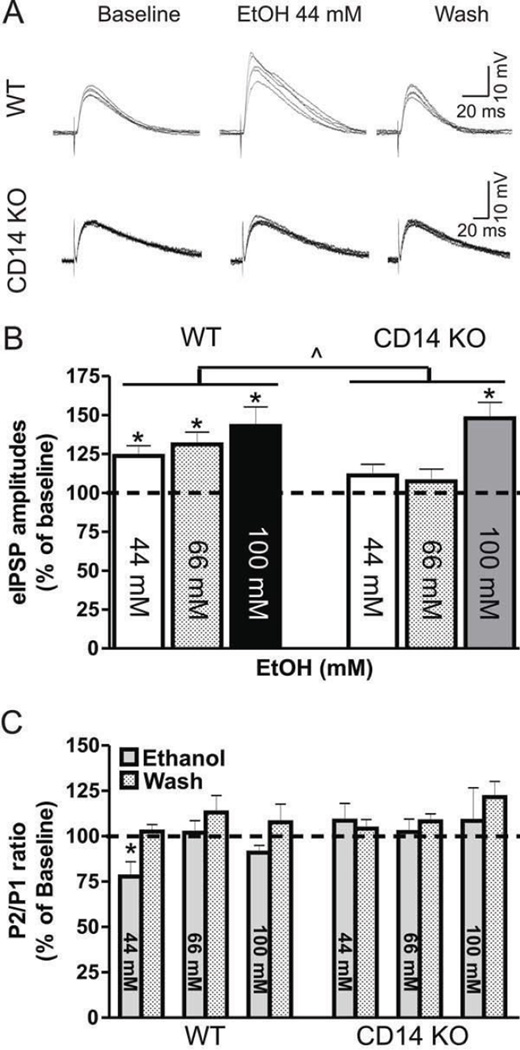
We compared the effects of acute ethanol on the eIPSPs in WT and CD 14 KO mice (Fig. 1) and found altered ethanol responses that were dose-specific (significant dose × strain interaction by ANCOVA analysis (F(2,56) = 3.2, p < 0.05), with no main effects of strain or dose. This interaction was further explored by two-way repeated measures ANOVA to test dose responsiveness within each genotype. In WT CeA, ethanol increased mean eIPSP amplitudes (Fig. 1A and B) compared to baseline, showing a main effect of ethanol application (F(1,22) = 31.5, p < 0.001) without significant effect of dose or ethanol application × dose interaction. However, in CeA neurons of CD 14 KO mice, there was an interaction between ethanol application and dose (two-way repeated measures ANOVA, F(2,28) = 7.1, p < 0.01), with mean eIPSP amplitudes increasing only after 100 mM (47.9 ± 10.3% of baseline; Bonferroni posthoc test, p < 0.05) but not after 44 mM (111 ± 7% of baseline) or 66 mM (109 ± 7% of baseline) ethanol (Fig. 1A and B ). In addition, the number of individual cells that were ethanol-responsive (defined as a ≥15% increase in a single eIPSP amplitude) increased from 4 of 12 to 8 of 12, and to 8 of 8 cells in the presence of 44, 66, and 100 mM ethanol, respectively.
Figure 1. Ethanol concentration-response analysis in CeA of WT control and CD14 KO mice.
A) Representative traces from intracellular recordings of evoked GABAA-IPSPs (eIPSPs) in CeA neurons from control WT (top panel) and CD14 KO mice (bottom panel). B) Ethanol at concentrations of 44 (n = 12), 66 (n = 8) and 100 mM (n = 4) significantly increased mean eIPSP amplitudes in WT mice. In CD14 KO mice, with superfusion of 44 mM (n = 12), 66 mM (n = 12) and 100 mM (n = 8), only the supramaximal (in WT mice) ethanol concentration of 100 mM increased mean eIPSP amplitudes. C) Ethanol effects on paired-pulse facilitation (PPF) in CeA of WT and CD 14 KO mice. Values represent mean ± SEM, and statistical significance (* and ^) was set at p < 0.05. (^) was calculated by ANCOVA and (*) by Bonferroni post-hoc test. Note that only the 44 mM ethanol concentration in WT CeA significantly affected PPF, suggesting a presynaptic increase in GABA release in this case.
Analysis of acute ethanol effects on PPF by ANCOVA showed no significant dose × strain interactions or main effects of dose or strain. However, because we have previously shown that application of 44 mM ethanol always augments PPF in outbred rodents (Bajo et al, 2008; Cruz et al, 2011; Roberto et al, 2003; Roberto et al, 2004a; Roberto et al, 2004b), we tested whether the lack of ethanol effect on PPF in CD 14 KO CeA might result from the inclusion of higher doses, which do not generate PPF. When the effects of 44 mM ethanol were analyzed separately by two-way repeated measures ANOVA, we found a significant ethanol application × mouse strain interaction (F(1,17) = 5.6; p < 0.05) with no main effects of mouse strain or ethanol application. Bonferroni posthoc test showed that 44 mM ethanol significantly reduced the paired-pulse ratio by 22.2 ± 8.2% (p < 0.05; Fig. 1C) in WT but not CD14 KO CeA (108.5 ± 9.5% of baseline), suggesting increased presynaptic GABA release in control WT neurons only. Notably, we did not see a significant ethanol application × mouse strain interaction or main effect of mouse strain or acute ethanol application alone on PPF at ethanol concentrations of 66 or 100 mM in WT (66 mM: 101.9 ± 6.7% of baseline; 100 mM: 91.0 ± 4.0% of baseline) or CD14 KO mice (66 mM: 100.0 ± 6.8% of baseline; and 100 mM: 108.4 ± 18.3% of baseline; Fig. 1C), suggesting that most changes in eIPSPs are likely to be postsynaptic at these higher concentrations.
TLR4 and CD14 may play different roles in acute ethanol effects on CeA GABAergic transmission
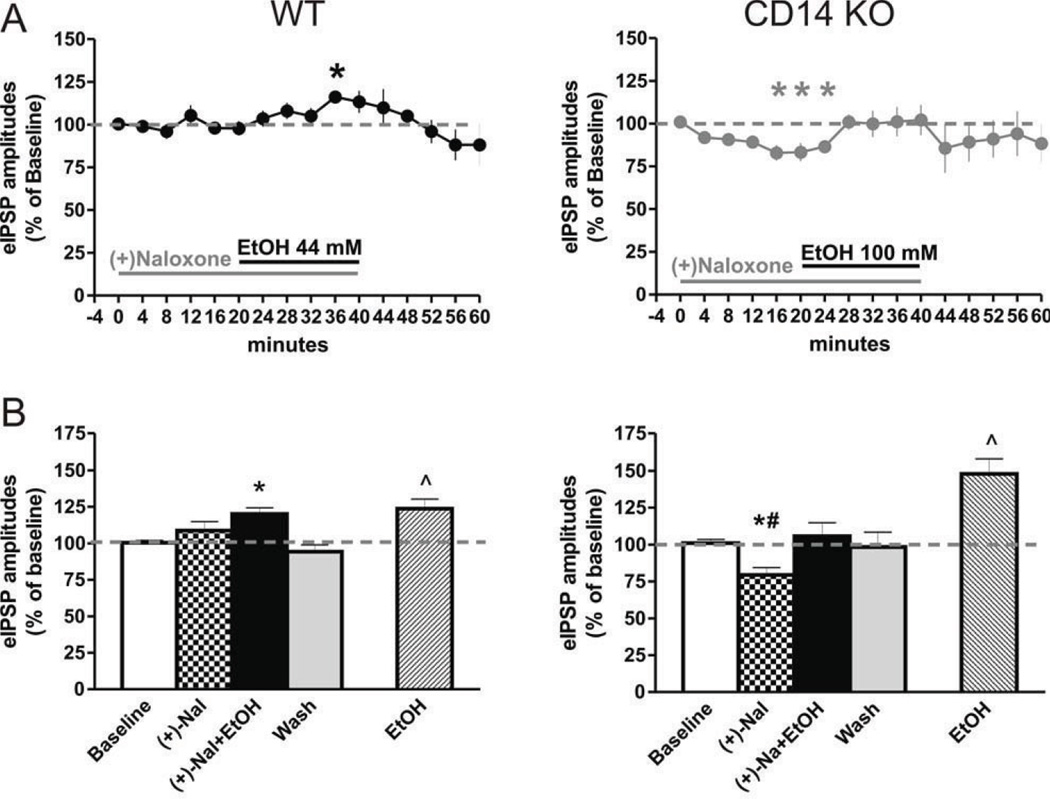
To assess the role of TLR4 in ethanol action, we used (+)-naloxone, a non-opioid TLR4 inhibitor that binds specifically to the TLR4-MD-2 complex, with no affinity for mu or other opioid receptors (Hutchinson et al, 2008; Iijima et al, 1978). We superfused 10 µM (+)-naloxone, a concentration shown to be effective in blocking TLR4-MD-2 complex activation (Hutchinson et al, 2008; Hutchinson et al, 2010), with the minimally effective ethanol concentrations in the CeA of WT (44 mM ethanol) or CD14 KO (100 mM ethanol) mice. Co-application of ethanol during (+)-naloxone superfusion did not significantly alter the membrane properties or I/V relationships in neurons from either mouse strain (data not shown). In WT CeA, one-way ANOVA (F(2,10) = 9.8; p < 0.01) followed by Newman-Keuls posthoc test showed that (+)-naloxone alone had no significant effect on the mean eIPSP amplitudes (108.7 ± 6.1% of baseline; Fig. 2A and B). Pretreatment with (+)-naloxone delayed and blunted 44 mM ethanol-induced potentiation of GABAergic transmission in the CeA neurons; ethanol co-applied with (+)-naloxone eventually increased the mean eIPSP amplitude by 20.1 ± 4.2%, but only after 20 minutes of ethanol application (Fig. 2A and B). Neither (+)-naloxone alone (106.5 ± 12.6% of baseline) nor (+)-naloxone+ethanol co-application (93.6 ± 2.7% of baseline) changed PPF significantly.
Figure 2. A possible role for TLR4 in ethanol effects on GABAergic transmission.
A) Time-course of effects of (+)-naloxone (10 µM) pre-treatment on ethanol-induced increases in eIPSP amplitudes in WT and CD 14 KO mice. Ethanol 44 mM, a maximally effective concentration in controls, was added to the (+)-naloxone superfusing CeA slices from WT mice (see bars, left panel), and 100 mM ethanol (maximal in CD14 CeA) added to the CeA slices of CD14 KO mice (right panel). Time points represent 4 min bins of mean evoked IPSP amplitudes following (+)-naloxone, (+)-naloxone+ethanol, and washout. B) There was no effect of (+)-naloxone alone on the mean amplitudes of eIPSPs in CeA of WT mice (n = 6), but (+)-naloxone delayed and weakened the ethanol potentiation of the mean amplitude of eIPSPs (left panel) compared to untreated slices (see Figure 1B). (+)-naloxone alone significantly decreased the mean eIPSP amplitudes in CeA slices of CD14 KO (n = 7) mice, but did not prevent the 100 mM ethanol augmentation of eIPSP amplitudes back to baseline levels (right panel). Statistics: *compares effects of (+)-naloxone and (+)-naloxone+ethanol to baseline; p < 0.05. # indicates significant differences between effects of (+)-naloxone and (+)-naloxone+ethanol on eIPSPs with p < 0.05.
Notably, in CD 14 KO mice, there was a significant difference between drug treatments (one-way ANOVA, F(2,12) = 5.6, p < 0.05). (+)-Naloxone alone significantly decreased mean eIPSP amplitudes by 20.6 ± 4.9% (Newman-Keuls test, p < 0.05; Fig. 2A and B). (+)-Naloxone pre-treatment did not inhibit 100 mM ethanol-induced increase in mean eIPSP amplitudes (see above), as 100 mM ethanol increased amplitudes by 25 ± 9.3% compared to the (+)-naloxone response (Newman-Keuls test, p < 0.05) and returned the mean eIPSP amplitudes to baseline levels (105 ± 9.3% of baseline). PPF was not significantly changed by (+)-naloxone (104.1 ± 15.1% of baseline) or by (+)-naloxone+ethanol (96.0 ± 11.3% of baseline) as shown by one-way ANOVA (F(2,10) = 0.4; p > 0.05), suggesting a postsynaptic mechanism of action. These results suggest that, unlike CD 14, the TLR4-MD-2 complex may not be critically involved in the slowly-developing acute effects of 44 mM ethanol on GABAergic transmission in CeA neurons or of the high (100 mM) ethanol concentration in CD14 KO mice. Moreover, our results suggest that concurrent inhibition of the TLR4-MD-2 complex and a deficiency of CD 14 may act via independent and opposing mechanisms in the action of 100 mM ethanol, perhaps due to the opposing effects of (+)-naloxone and ethanol on the eIPSPs in CD 14 KO mice.
LPS increases the amplitude of eIPSPs in the CeA
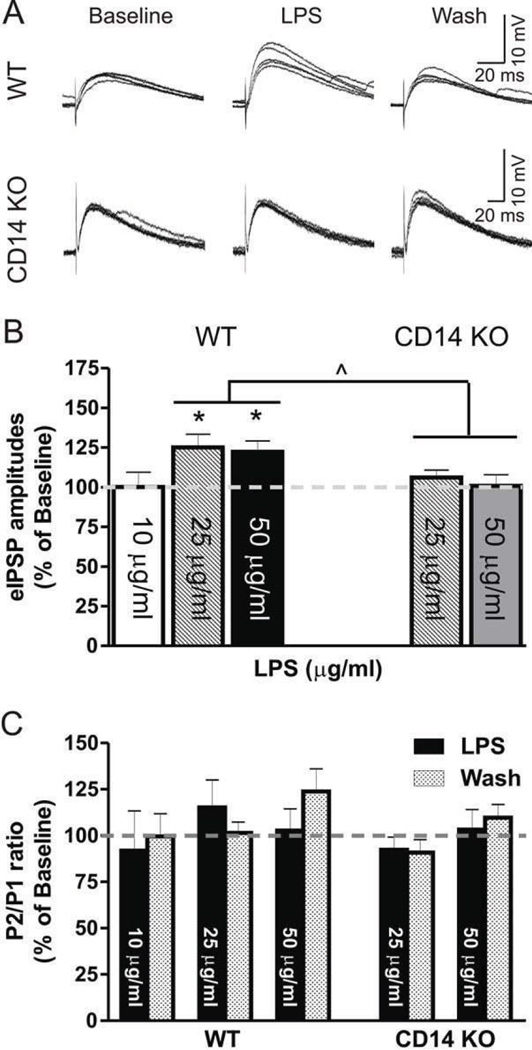
We constructed a LPS concentration-response curve in the CeA of control mice by superfusing 10, 25 or 50 µg/ml of LPS; we found no significant changes in the membrane properties or I/V relationships at any of these concentrations (Student’s t-test, data not shown). Two-way repeated measures ANOVA demonstrated a significant main effect of LPS (F(1,20) = 13.8, p < 0.01) but no main effect of dose or LPS × dose interaction (F(2,20) = 3.4, p = 0.053) on the mean IPSP amplitude (Fig. 3A and B). In the case of PPF, two-way ANOVA showed a significant main effect of dose (F(2,20) = 4.0, p < 0.05) but no significant main effect of LPS or LPS × dose interaction, suggesting that LPS application did not alter PPF (Fig. 3C). Given the lack of effect at 10 µg/ml (99.9 ± 0.9% of baseline) and the near statistical significance of LPS × dose interaction, we analyzed effects of each LPS dose separately. LPS significantly increased the mean amplitude of eIPSPs at 25 (133.2 ± 9.4% of baseline; one-way ANOVA, F(2,10) = 11.40; p < 0.01) and 50 (122.3 ± 6.9% of baseline; F(2,10) = 6.4; p < 0.05), whereas it had no effect at 10 µg/ml (99.9 ± 0.9% of baseline; Fig. 3A and B). Therefore, we chose higher doses of LPS (25 and 50 µg/ml) for further testing. As with WT mice, we found no changes in membrane properties or I/V relationships during LPS superfusion (data not shown) in CeA neurons of CD14 KO mice. Using analyses of covariance to determine the relationship between mouse strain and LPS dose, we found a significant main effect of strain (F(1,39) = 8.1, p < 0.01) with no significant concentration × strain interaction, suggesting that deletion of CD 14 alters the response to LPS in a concentration-independent manner. Separate analyses of LPS responses by strain demonstrated a significant main effect of LPS application in the CeA of WT mice (two-way repeated measures ANOVA, F(1,15) = 26.2, p < 0.01), without LPS dose or dose × LPS application interaction, whereas we observed no significant effects in CD 14 KO mice (Fig. 3A and B ). Analyses of covariance showed no significant dose × mouse strain interaction, main effects of dose, or main effects of mouse strain. These results suggest an involvement of CD 14 in mediating LPS effects on GABAergic transmission in CeA neurons.
Figure 3. LPS augments eIPSPs in WT CeA: concentration-response analysis.
A) Representative recordings from CeA neurons of WT and CD 14 KO mice following 25 µg/ml LPS superfusion. B) LPS at 25 (n = 8) and 50 (n = 6), but not 10 µg/ml (n = 6) significantly increased mean amplitudes of eIPSPs in WT mice, whereas the tested concentrations were ineffective in CeA of CD14 KO mice (25 µg/ml: n = 13; 50 µg/ml: n = 8). D) LPS had no significant effect on the PPF ratios in CeA of WT or CD 14 KO mice. Statistical significance was set at ^ p < 0.05, and values correspond to mean ± SEM.
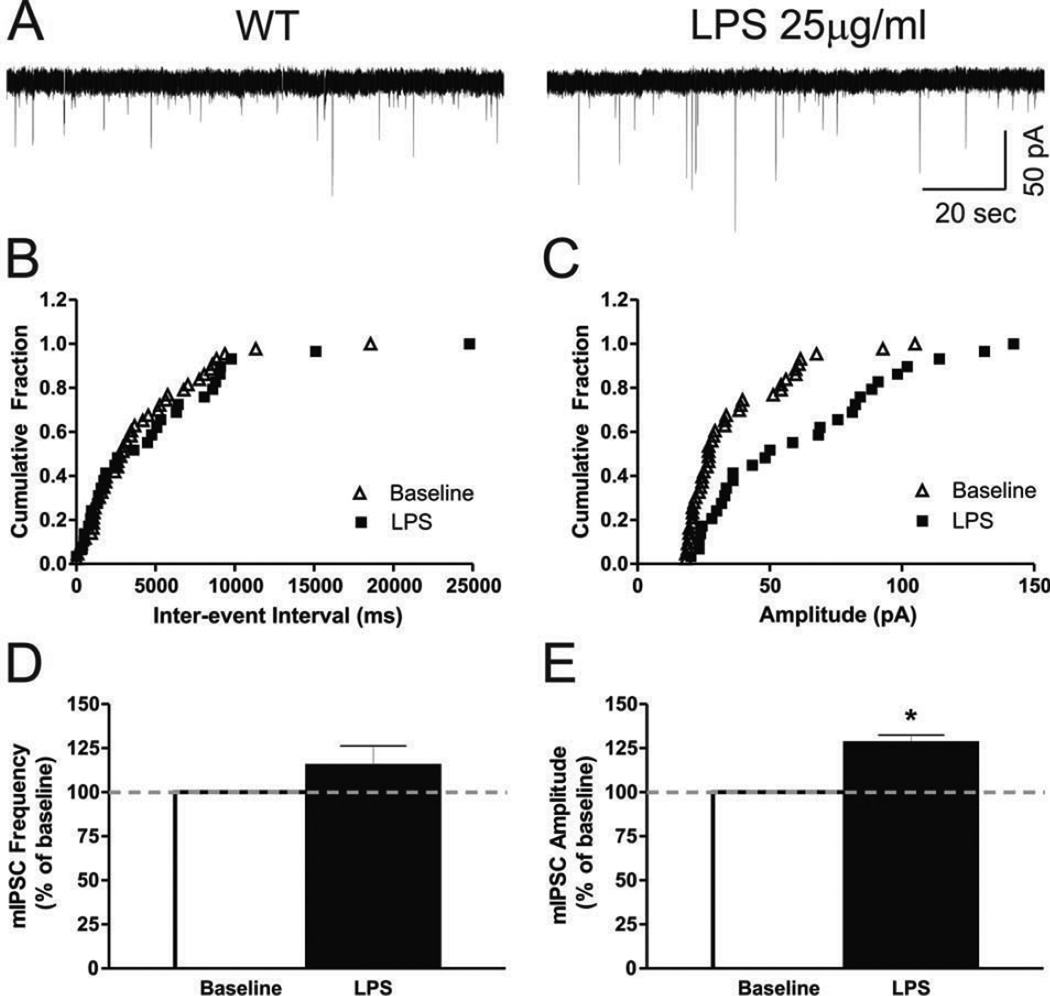
Our PPF data indicate that acute LPS effects on GABAergic transmission in WT mice may be mediated by postsynaptic mechanisms rather than presynaptic GABA release. This suggestion was supported by the LPS-induced modulation of mIPSCs: 25 µg/ml LPS increased mIPSC amplitudes by 27.8 ± 4.6% (t(8) = 7.52, p < 0.01; Fig. 4A, C and E) but had no significant effect on mIPSC frequencies (114.9 ± 11.5% of baseline; Fig. 4A, B and D). Moreover, LPS significantly increased the mean decay time by 18.5 ± 6.7% (t(8) = 2.92, p < 0.05) and the area of mIPSCs by 55.1 ± 10.4% (t(8) = 6.78, p < 0.01), whereas it had no effect on the mean rise time of mIPSCs (108.8 ± 7.9% of baseline; Table S2). Overall, these results suggest that LPS modulates kinetic properties of GABAA receptors and supports postsynaptic sites of LPS action on GABAergic transmission in the CeA.
Figure 4. LPS effects on mIPSCs suggest postsynaptic mechanisms of action.
A) Representative whole-cell current recording of mini IPSCs in a mouse CeA neuron (Vh = −60 mV) superfused with LPS (25 µg/ml). B) Cumulative fractions calculated by Kolmogorov-Smirnov sample test show that LPS did not change mIPSC frequency fractions. C) However LPS significantly shifted the amplitude fractions to the right, representing an increase in amplitude (p < 0.05). D) Summed data: LPS had no significant effect on mean GABAergic mIPSC frequency, but E) increased the mean amplitude of mIPSCs . Statistical significance * was set at p < 0.05 and calculated by Student’s t-test.
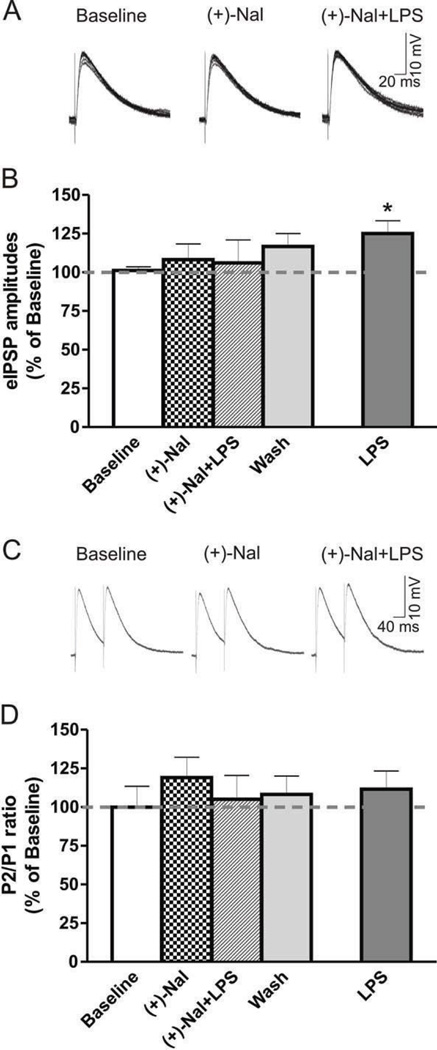
To assess the role of the TLR4-MD2 complex in the LPS effects, we used 10 µM (+)-naloxone and 25 µg/ml LPS, the minimum concentration eliciting a potent effect on GABAergic transmission in the CeA (Fig. 2). Notably, (+)-naloxone superfused alone had no significant effect on membrane properties or I/V relationships of eIPSPs. We found no significant difference between the treatment groups (one-way ANOVA, F(3,10) = 0.4; p >0.05), indicating that (+)-naloxone alone (108.2 ± 10.1% of baseline) had no effect on IPSP amplitudes and that (+)-naloxone pre-treatment for 20 min prevented the LPS-induced increase in mean IPSP amplitudes (106.0 ± 14.9% of baseline; Fig. 5A and B). Neither (+)-naloxone (119.1 ± 13.4% of baseline) alone nor (+)-naloxone+LPS (105.0 ± 15.3% of baseline) had a significant effect on PPF (F(3,5) = 0.5; p >0.05; Fig. 5C and D). These results indicate a critical role for the TLR4-MD2 complex in LPS-induced effects on GABAergic transmission in CeA neurons. Thus, LPS modulation of GABAergic transmission in the CeA may require both CD 14 and the TLR4-MD2 complex.
Figure 5. LPS effects on eIPSPs are likely mediated by the TLR4-MD-2 complex.
A) Representative multiple overlapping recordings from an individual CeA neuron from WT mouse following superfusion of ACSF only (baseline), (+)-naloxone (10 µM) and co-application of (+)-naloxone and LPS (25 µg/ml). B) Summed data: (+)-naloxone (10 µM), a selective TLR4 inhibitor, had no effect on mean eIPSP amplitudes but blocked the usual (compare to Figure 3A and B) 25 µg/ml LPS-induced increase in mean amplitudes of eIPSPs in WT mice (n = 6). C) Representative traces of PPF from an individual neuron, showing no change after LPS. D) (+)-naloxone and subsequent co-application of LPS did not alter PPF ratio significantly. Dashed horizontal lines represent an average of 4 minutes of recorded baseline
Co-application of LPS and ethanol modulates GABAAergic transmission
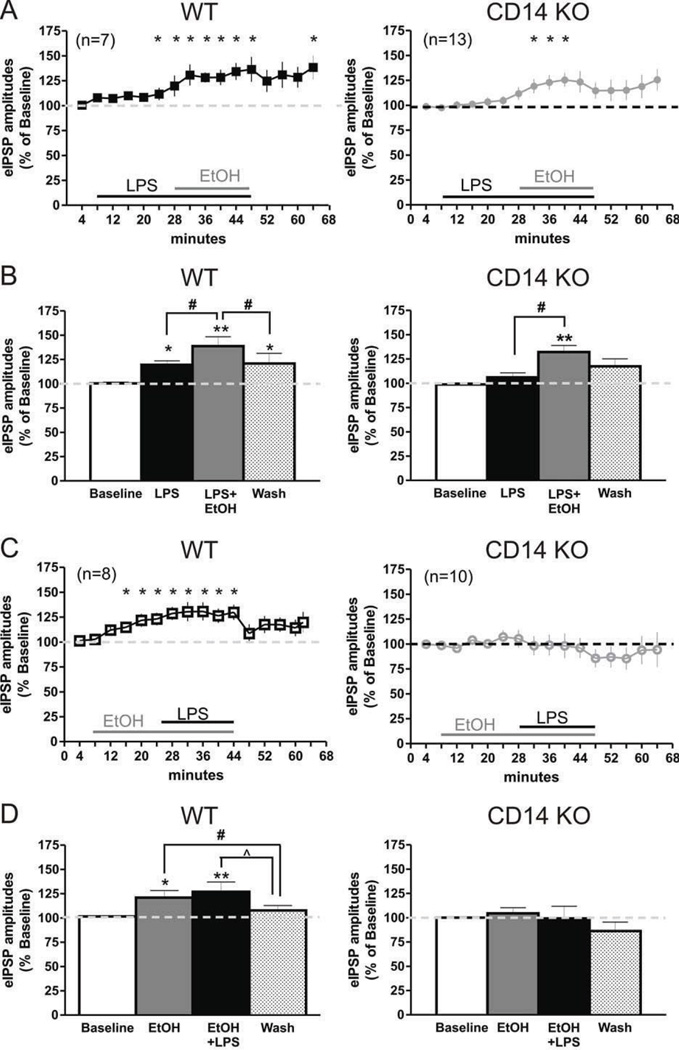
Because ethanol and LPS both augmented eIPSP amplitudes in control mice but appeared to act through different mechanisms (pre- versus postsynaptic sites), we characterized the effects of co-application of LPS and ethanol. We used an effective concentration of LPS (25 µg/ml) (Fig. 1 and 2) and ethanol (44 mM) that both augment GABAergic transmission (Fig. 1), as shown here and reported in our previous publications (Roberto et al, 2003; Roberto et al, 2004b). We first applied 25 µg/ml LPS for 20 min followed by subsequent co-application of 44 mM ethanol. In separate CeA neurons, the reverse application was tested, using superfusion of 44 mM ethanol for 20 min followed by co-application of LPS (25 µg/ml) for an additional 20 min. A three-way ANOVA showed significant differences between genotypes (F(1,34) = 7.0, p < 0.05) and treatments between the mouse strains (F(2,68) = 6.1, p < 0.01). Using three-way ANOVA for effects on PPF, we found a significant difference only in treatment order (F(1,32) = 5.4, p < 0.05). When we analyzed the data separately by genotype, one-way ANOVA (F(2,12) = 12.9; p < 0.01) followed by Newman-Keuls posthoc test (p < 0.05) showed that LPS alone significantly increased mean IPSP amplitudes in CeA of WT mice by 19.5 ± 4.1% compared to baseline, and subsequent co-application of ethanol further potentiated the mean amplitude to 138.8 ± 9.6% of baseline (Fig. 6A and B). In addition, there was a significant difference between effects of ethanol pretreatment and ethanol+LPS co-application on the IPSP amplitude (Newman-Keuls, p < 0.05). There was no significant change in PPF (data not shown) elicited by either LPS alone (90.2 ± 5.1% of baseline) or LPS-ethanol co-application (112.8 ± 11.7% of baseline).
Figure 6. LPS and ethanol interactions in CeA of WT and CD14 KO mice.
A) Time-course of the influence of LPS (25 µg/ml) pre-treatment on ethanol (44 mM) effects on evoked GABAA-IPSPs in CeA neurons from WT (n = 7) and CD14 KO (n = 13) mice. Statistical significance: *represents p < 0.05 and was calculated by one-sample t-test. B) Summed data showing that LPS alone increased mean eIPSPs and potentiated the 44 mM ethanol-induced increase in mean eIPSP amplitudes in WT mice and restored ethanol effects in the CeA of CD 14 KO mice, without showing a direct of LPS. Statistical significance: *LPS pre-treatment and LPS+ethanol co-application effects compared to baseline; #LPS+ethanol effects compared to LPS pre-treatment and washout. *represents p < 0.05, and **is p < 0.01. C) Time-course of the effects of ethanol pre-treatment and ethanol+LPS co-application on eIPSPs, showing a robust increase in eIPSP amplitudes by ethanol followed by an apparent occlusive effect on LPS action in WT mice; both effects are abolished in the CD 14 KO. Statistical significance *represents p < 0.05. D) Summed data: ethanol 44 mM pre-treatment occluded LPS-induced increases in evoked IPSP amplitudes in WT (n = 8) mice but neither ethanol nor ethanol plus LPS had any effect in CD14 KO (n = 10) mice. Statistical significance: * and ** are p < 0.05 and p < 0.01, respectively; effects compared to baseline; # and ^ are p < 0.05 and represent differences in the effects of ethanol and ethanol+LPS application compared to washout.
There also was a significant effect of ethanol and ethanol-LPS treatment (one way ANOVA, F(2,14) = 10.5; p < 0.01; Fig. 6C and D) when we reversed the order of LPS and ethanol co-applications. Ethanol pretreatment increased the mean IPSP amplitudes by 21 ± 7.3% (Newman-Keuls, p < 0.05). Surprisingly, subsequent ethanol+LPS co-application did not significantly potentiate the ethanol effect: the mean IPSP amplitude increased by only 26.9 ± 10.1% compared to baseline (p < 0.05). Although initial ethanol alone slightly decreased PPF (to 90.4 ± 7.6% of baseline), there was no significant difference in effects on PPF between the groups (F(2,10) = 0.6; p > 0.05).
We found a significant difference between the treatment groups in CeA neurons of CD 14 KO mice (one-way ANOVA, F(2,24) = 12.5; p < 0.01). LPS alone did not significantly alter the mean IPSP amplitudes (107.5 ± 4.3% of baseline; Newman-Keuls), but subsequent co-application of 44 mM ethanol with LPS significantly increased mean amplitude by 32.3 ± 6.7% compared to baseline (Newman-Keuls, p < 0.05) and compared to LPS alone (Newman-Keuls, p < 0.05; Fig. 6A and B). This increase was not associated with changes in PPF (94.3 ± 8.1% of baseline; data not shown), suggesting a predominantly postsynaptic mechanism of action. Analysis of the inverse relationship (ethanol treatment followed by LPS co-application) showed no significant difference in effects on IPSP amplitudes (F(2,18) = 0.22, p > 0.05) and PPF (F(2,18) = 0.28, p > 0.05) between treatment groups of CD14 KO mice (Fig. 6C and D).
Overall, these data indicate that LPS pretreatment may be additive with ethanol effects on eIPSP amplitudes in CeA neurons of WT mice and pre-emptive for ethanol effects in CeA of CD 14 KO mice. By contrast, ethanol pretreatment seems to occlude the usual LPS effects on eIPSPs in the CeA of control mice. Thus, ethanol and LPS likely act on GABAergic transmission in CeA via distinct mechanisms, such as pre- versus postsynaptic actions.
DISCUSSION
To our knowledge this is the first report of the effects of LPS on GABAergic transmission in the CeA and identifies a role for CD 14 and TLR4 in acute ethanol effects on this transmissionresponses. Our data indicate that moderate (44 mM) but not high ethanol concentrations (100 mM) require CD14 for augmentation of GABAergic neurotransmission. In addition, our findings with (+)-naloxone suggest that the TLR4-MD-2 complex may be involved in the early acute effects of 44 mM ethanol, but may not be required for the late potentiation of GABAergic transmission by ethanol or by high ethanol concentrations involving a postsynaptic mechanism. LPS potentiates GABAergic transmission in a majority of CeA neurons, probably by a postsynaptic mechanism that is both CD 14- and TLR4-MD-2 complex-dependent. Studies suggest that LPS pre-treatment may elicit additive effects, whereas ethanol pre-treatment may occlude LPS effects. Overall, our data suggest different roles for CD 14 and TLR4, particularly those likely mediated by the TLR4-MD-2 complex, in the acute effects of ethanol on CeA GABAergic transmission.
GABAergic responses in CeA are known to play a critical role in ethanol drinking and dependence (Foster et al, 2004; Roberts et al, 1996). Acute application of ethanol increases GABAergic transmission in rodent CeA neurons predominantly via presynaptic increase in GABA release (Bajo et al, 2008; Herman et al, 2013; Roberto et al, 2003; Roberto et al, 2004b). In the present study, CD14 played a critical role in ethanol effects on GABAergic transmission, given that 44 mM ethanol had no significant effect on eIPSPs in the CeA from CD14 KO mice. Ethanol potentiation of GABAergic transmission in CeA neurons from CD14 KO mice at high (100 mM) but not moderate (< 66 mM) ethanol concentrations suggests that ethanol-induced presynaptic GABA release is decreased or absent in these null mutant mice. We reported previously that ethanol had maximum effects at 44 mM in the rodent CeA (Nie et al, 2009; Roberto et al, 2003) in agreement with our current findings. Although ethanol acts predominantly via presynaptic mechanisms in this region, it also has postsynaptic actions (Herman et al, 2013; Roberto et al, 2003). Our previous and current findings also support both pre-and postsynaptic mechanisms for ethanol action. The lack of significance for higher ethanol concentrations on PPF or ethanol and LPS interactions may result from concomitant pre- and post-synaptic effects that could mask individual responses, in agreement with previous findings (Kallupi et al, 2013). We speculate that in CD 14 KO mice, the pharmacodynamic profile for ethanol in the CeA is different than that in WT mice, with significant ethanol effects only at a high concentration (100 mM) but not concentrations (44 and 66 mM) that are maximally effective in WT CeA (Roberto et al, 2003; Roberto et al, 2004b). Differences in postsynaptic GABAA receptor sensitivity to 80 mM ethanol have been reported in high versus low alcohol preferring rats (Poelchen et al, 2000). In addition, several reports have shown dose-dependent differences in ethanol effects on GABAergic transmission at postsynaptic sites in various experimental conditions (Blednov et al, 2011b; Chung and Moore, 2009; Jia et al, 2005; McCool et al, 2003; Sebe et al, 2003). In general, major factors determining ethanol action and sensitivity of GABAA receptors include the synaptic and cellular localization, subunit composition and intracellular modulation (e.g., phosphorylation) of GABAA receptors (Criswell et al, 2008; Farrant and Nusser, 2005; Weiner and Valenzuela, 2006). Thus, we hypothesize that the deletion of CD 14 may not only block ethanol-induced presynaptic GABA release but also alter postsynaptic GABAA receptors (e.g., subunit composition and/or intracellular signaling modulation). Moreover, aspecific or off-target sites, including extrasynaptic GABA receptor activation, may be involved in mediating effects of high doses of ethanol (Herman et al, 2013; Jia et al, 2005; McCool et al, 2003; Sebe et al, 2003).
In addition to the requirement for CD 14, the LPS-induced augmentation of eIPSPs may require the TLR4-MD-2 complex. MD-2 is an accessory protein that binds to the extracellular domain of TLR4 (Nagai et al, 2002). MD-2 and CD14 play different roles in activation of TLR4: CD 14 appears to be a universal adaptor for both damage-associated molecular patterns (DAMPs) and microbial-associated molecular patterns (MAMPs), whereas MD-2 only recognizes exogenous MAMPs such as LPS (Chun and Seong, 2010). Our results with (+)-naloxone support a TLR4-dependent mechanism for acute, moderate concentrations of ethanol and a TLR4-MD-2-independent role for prolonged or high-dose ethanol in WT mice. We speculate that prolonged ethanol application or a high concentration (100 mM) may release DAMPs (endogenous ligands for TLR4 such as HMGB-1 and heat-shock proteins) that activate TLR4 pathways via CD 14. Although effects of acute ethanol on release of DAMPS in brain slices are unknown, both prolonged, acute ethanol (> 1 hour) and chronic ethanol may release DAMPs (Bowers et al, 2006; Crews et al, 2012). These findings may support DAMPs as potential mediators in ethanol activation of neuroimmune responses, especially following prolonged and chronic ethanol exposure (Crews et al, 2011).
The direct (+)-naloxone-induced reduction of GABAergic transmission in the CeA of CD 14 KO mice suggests that TLR4-MD2 signaling may be involved in the ongoing regulation of Ce A GABAergic responses, perhaps as a compensation for the CD 14 deletion. The inability of LPS to potentiate GABAergic transmission in CD 14 KO mice supports a critical role for CD 14 in LPS-mediated activation of TLR4. However, it is possible that (+)-naloxone also acts via TLR4-MD-2- and CD14-independent mechanisms, such as the TLR4-independent activation of PI3K (phosphoinositide-3 kinase) signaling detected in macrophages from TLR4−/− mice (Miller Yi Fau - Viriyakosol et al; Miller et al, 2005), the increased localization of PI3K to lipid rafts, hyperphosphorylation of Akt (also called Protein kinase B), and/or reduced activation of p38 shown in macrophages from CD14 deficient mice (Miller et al, 2005; Sahay et al, 2009). Moreover, (+)-naloxone binding to the TLR4-MD2 complex, also localized in lipid-rafts (Blanco and Guerri, 2007; Szabo et al, 2007), could indirectly induce intracellular signaling, resulting in decreased GABAergic responses in CeA neurons of CD14 KO mice.
Although the TLR4-MD-2 complex may not be required for prolonged or high-dose ethanol effects on GABAergic neurotransmission, there is evidence for complex interactions between TLR4 and ethanol. In our LPS+ethanol co-application studies, when LPS presumably activated TLR4 and augmented eIPSPs prior to ethanol application, the ethanol-LPS co-application resulted in further enhancement of GABAergic transmission, probably via distinct additive mechanisms. By contrast, pre-treatment with ethanol occluded LPS elicited TLR4-dependent enhancement of GABAergic transmission. It has been reported that low to moderate, physiologically-relevant concentrations of ethanol (10–50 mM) can induce/recruit the TLR4/IL-1RI complex into lipid rafts, triggering downstream signaling events (Blanco et al, 2008; Fernandez-Lizarbe et al, 2013; Fernandez-Lizarbe et al, 2008; Lewis et al, 2013; Wu et al, 2012), similar to LPS activation of TLR4 signaling.
Ethanol promotes the association of TLR4 with TLR2 in lipid rafts, suggesting a mechanism for ethanol enhancement of TLR function (Fernandez-Lizarbe et al, 2013). For our studies, a critical question is the possible neuronal localization of TLR4. In CeA, TLR4 is expressed in neurons as well as in microglia (Liu et al, 2011). Thus, LPS- and ethanol-induced modulation of CeA GABAergic transmission could result from direct neuronal effects in addition to effects mediated via neuron-microglia interactions. Notably, it has been shown in brain slices that microglia can play a role in an LPS-induced, rapid-transient increase in the frequency of excitatory postsynaptic currents in hippocampal slices, supporting microglial involvement in the modulation of synaptic transmission (Pascual et al, 2012). Both LPS and ethanol activate TLR4 in microglia, although differences in the intracellular signaling pathways activated by LPS and ethanol have been reported (Kacimi et al, 2011; Wu et al, 2012). Overall effects of LPS and ethanol pre-treatment followed by LPS-ethanol and ethanol-LPS co-application, respectively, on GABAergic transmission in the CeA seem likely to be determined by both direct neuronal effects and interactions of inflammatory mediators (cytokines and chemokines) (Ikonomidou et al, 2000; Mukherjee et al, 2008) and neurotransmitters (e.g., GAB A, ATP) released from microglia and neurons (Fontainhas et al, 2011; Lee, 2013; Mead et al, 2012; Wong et al, 2011). Thus, we speculate that the increase in GABAergic transmission induced by LPS-ethanol co-application following LPS pre-treatment may involve increases in pro-inflammatory cytokines and in extracellular ATP levels, all reported to facilitate GABAergic transmission (Bowser and Khakh, 2004; Ikonomidou et al, 2000; Mukherjee et al, 2008). By contrast, although ethanol stimulates the TLR response, this stimulation is inhibited by TLR4 ligands (Fernandez-Lizarbe et al, 2008), possibly explaining our finding of occlusion of LPS effects by ethanol pre-treatment. Another possible mechanism for this occlusive effect is the inactivation of microglia by GABA, a negative regulator of LPS activation in microglia and astrocytes (Lee et al, 2011). We speculate that ethanol-induced increases in GABA release may inhibit activation of microglia and thus prevent further TLR4 signaling, blocking LPS potentiation of GABAergic transmission.
In CD 14 KO mice, we found no overall changes in CeA GABAergic transmission elicited by ethanol pretreatment followed by ethanol-LPS co-application. However, it is puzzling that ethanol effects on GABAergic transmission were restored in CD14 KO mice following LPS-pre-treatment. As with (+)-naloxone effects, a possible explanation may be altered signal transduction, particularly via PI3K, Akt and p38, found in CD14 deficient mice (Sahay et al, 2009). Thus, acute LPS may “prime” microglia and/or neurons by modulating intracellular signaling through CD 14-independent mechanisms, allowing ethanol to enhance CeA GABAergic transmission despite CD 14 deficiency.
Genomic and behavioral studies have shown that CD 14 and TLR4 also play a role in ethanol intake and preference. Expression of CD 14 is down-regulated in brain of alcohol preferring mice (Mulligan et al, 2006). In contrast, CD14 deficiency reduces ethanol preference in male mice, and ethanol preference and intake in females, in a 24-hr two-bottle choice test but not under limited access to ethanol (Blednov et al, 2012). CD14 deficiency also blocks the long-lasting increases in ethanol intake induced by systemic administration of LPS (Blednov et al, 2011a). Because GABAergic transmission in the CeA is involved in ethanol drinking behavior (Foster et al, 2004; Hyytia and Koob, 1995; Roberts et al, 1996), our results showing CD14-dependent enhancement of GABAergic transmission by ethanol may represent a cellular mechanism underlying the ethanol drinking behavior previously reported for CD 14 KO mice. We speculate that down-regulated CD 14 may decrease the reinforcing ethanol effects that are overcome by the increased ethanol drinking of the alcohol preferring mice (Mulligan et al, 2006). Binge drinking is reported to depend on GABAA receptor a2-subunit-dependent regulation of TLR4 expression in the CeA (Liu et al, 2011), suggesting that CeA TLR4 signaling plays a critical role in alcohol self-administration. In addition, TLR4 signaling is important for the acute sedative and motor impairing effects of ethanol (Wu et al, 2012), consistent with an interaction between TLR4 and GABAergic signaling (Blednov et al, 2013). Thus, these data combined with our present findings suggest that CD 14 and TLR4 in the CeA both play important roles in alcohol drinking behavior that is mediated, at least partially, by CD 14- and TLR4-dependent modulation of GABAergic transmission.
In summary, our study highlights a crucial role for CD 14 in the potentiation of GABAergic transmission by ethanol (< 66 mM) and suggests that LPS activation of TLR4 signaling potentiates GABAergic transmission in the CeA via a postsynaptic mechanism. Ethanol and LPS-TLR4 signaling may act via distinct mechanisms resulting in additive or occlusive interactions. Our findings also indicate that both CD 14 and the TLR4-MD-2 complex are required for LPS-induced potentiation of CeA GABAergic transmission. These complex interactions of ethanol with neuroimmune mechanisms and GABAergic transmission could ultimately form the basis for a new therapeutic approach to alcohol dependence.
Supplementary Material
Table 1.
The results are summarized in Table 1 and Table S2
| Treatment | eIPSPs | PPF | |||
|---|---|---|---|---|---|
| WT | CD14KO | WT | CD14KO | ||
| Ethanol (44 mM) | ↑ | = | =/↓ | = | |
| Ethanol (66 mM) | ↑ | = | = | = | |
| Ethanol (100 mM) | ↑ | ↑ | = | = | |
| LPS (10 µg/ml) | =/↑ | NT | = | NT | |
| LPS (25 µg/ml) | ↑ | = | = | = | |
| LPS (50 µg/ml) | ↑ | = | = | = | |
| Pre-treatment | Co-application | ||||
| (+)-naloxone (10 µM) | (+)-naloxone + LPS (25 µg/ml) |
= | NT | = | NT |
| LPS (25 µg/ml) | LPS + Ethanol (44mM) | ↑ & ↑ | = &↑ | = & = | = & = |
| Ethanol (44mM) | Ethanol + LPS (25 µg/ml) |
↑ & ↑ | = & = | = & = | = & = |
| (+)-naloxone (10 µM) | (+)-naloxone + Ethanol (44mM) |
= &↑* | NT | = & = | NT |
| (+)-naloxone (10 µM) | (+)-naloxone + Ethanol (100 mM) |
NT | ↓& = | NT | = &= |
Summary of changes in the evoked inhibitory postsynaptic potentials (eIPSPs) and paired-pulse facilitation (PPF) in central amygdala neurons of wild-type (WT) and CD 14 knockout (CD14KO) mice after various treatments. Concentrations for pre-application: (+)-naloxone (10 µM); LPS (25 µg/ml); ethanol (44 mM). ↑, significant increase; ↓, significant decrease; =, no changes; =/↑ or =/↓ significant difference determined by one-way ANOVA; all changes considered against baseline; NT, not tested. * delay in the effect of the co-treatment.
ACKNOWLEDGEMENTS
We thank Drs. George Koob, Floyd Bloom and Cecilia Borghese for their critical reviews and comments on the manuscript, Dr. Logrip for her help with statistical analyses, and Dr. George Koob for the gift of (+)-naloxone. The Scripps Research Institute’s manuscript number for this paper is 24053.
FUNDING AND DISCLOSURE
Support for this study was provided by NIH/NIAAA grants U01-AA013498, U01-AA013520, and U01-AA013517 (as part of the INIA West consortium). A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism. (+)-naloxone was provided by Dr. George Koob and synthesized by Drs. Kenner Rice from the NIH/NIAA and Edward Roberts from The Scripps Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
REFERENCES
- Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J Neurosci Res. 2008;86:1077–1086. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia. 2012;60:948–964. doi: 10.1002/glia.22327. [DOI] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A. 2008;105:8410–8415. doi: 10.1073/pnas.0802302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Roberto M, Madamba SG, Siggins GR. Neuroadaptation of GABAergic transmission in the central amygdala during chronic morphine treatment. Addict Biol. 2011;16:551–564. doi: 10.1111/j.1369-1600.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Perez-Arago A, Fernandez-Lizarbe S, Guerri C. Ethanol mimics ligand- mediated activation and endocytosis of IL-1RI/TLR4 receptors via lipid rafts caveolae in astroglial cells. J Neurochem. 2008;106:625–639. doi: 10.1111/j.1471-4159.2008.05425.x. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Valles SL, Pascual M, Guerri C. Involvement of TLR4/type I IL-1 receptor signaling in the induction of inflammatory mediators and cell death induced by ethanol in cultured astrocytes. J Immunol. 2005;175:6893–6899. doi: 10.4049/jimmunol.175.10.6893. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Black M, Chandra D, Homanics GE, Rudolph U, Harris RA. Linking GABA(A) receptor subunits to alcohol-induced conditioned taste aversion and recovery from acute alcohol intoxication. Neuropharmacology. 2013;67:46–56. doi: 10.1016/j.neuropharm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun. 2011a;25 Suppl 1:S92–S105. doi: 10.1016/j.bbi.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, Benavidez JM, Geil CR, Osterndorff- Kahanek E, Werner DF, Iyer S, Swihart A, Harrison NL, Homanics GE, Harris RA. Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive alpha2-containing GABA(A) receptors. J Pharmacol Exp Ther. 2011b;336:145–154. doi: 10.1124/jpet.110.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict Biol. 2012;17:108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers BJ, Radcliffe RA, Smith AM, Miyamoto-Ditmon J, Wehner JM. Microarray analysis identifies cerebellar genes sensitive to chronic ethanol treatment in PKCgamma mice. Alcohol. 2006;40:19–33. doi: 10.1016/j.alcohol.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KH, Seong SY. CD14 but not MD2 transmit signals from DAMP. Int Immunopharmacol. 2010;10:98–106. doi: 10.1016/j.intimp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Chung L, Moore SD. Neuropeptides modulate compound postsynaptic potentials in basolateral amygdala. Neuroscience. 2009;164:1389–1397. doi: 10.1016/j.neuroscience.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High Mobility Group Box 1/Toll- like Receptor Danger Signaling Increases Brain Neuroimmune Activation in Alcohol Dependence. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Zou J, Qin L. Induction of innate immune genes in brain create the neurobiology of addiction. Brain Behav Immun. 2011;(25 Suppl 1):S4–S12. doi: 10.1016/j.bbi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Bajo M, Maragnoli ME, Tabakoff B, Siggins GR, Roberto M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front Neurosci. 2011;4:207. doi: 10.3389/fnins.2010.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 1999;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. The Journal of Neurophysiology. 1994;71:1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces tlr4/tlr2 association, triggering an inflammatory response in microglial cells. J Neurochem. 2013 doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Gascon MS, Blanco A, Guerri C. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol Immunol. 2008;45:2007–2016. doi: 10.1016/j.molimm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C. Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol. 2009;183:4733–4744. doi: 10.4049/jimmunol.0803590. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6:e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, Cook JM, June HL. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–284. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Blednov YA. From concept to drugs: Neuroimmune gene expression, alcohol consumption, and potential intervention targets. Alcohol Clin Exp Res. 2012;78A(36 Suppl) [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Alheid GF. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel Subunit-Specific Tonic GABA Currents and Differential Effects of Ethanol in the Central Amygdala of CRF Receptor-1 Reporter Mice. J Neurosci. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) Eur J Neurosci. 2008;28:20–29. doi: 10.1111/j.1460-9568.2008.06321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2010;24:83–95. doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF. GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol. 1995;283:151–159. doi: 10.1016/0014-2999(95)00314-b. [DOI] [PubMed] [Google Scholar]
- Iijima I, Minamikawa J, Jacobson AE, Brossi A, Rice KC. Studies in the (+)- morphinan series. 5. Synthesis and biological properties of (+)-naloxone. J Med Chem. 1978;21:398–400. doi: 10.1021/jm00202a018. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jarosz J, Miernik K, Wachal M, Walczak J, Krumpl G. Naltrexone (50 mg) plus psychotherapy in alcohol-dependent patients: a meta-analysis of randomized controlled trials. Am J Drug Alcohol Abuse. 2013;39:144–160. doi: 10.3109/00952990.2013.796961. [DOI] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Kacimi R, Giffard RG, Yenari MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-kappaB, JAK-STAT and JNK stress kinase pathways. J Inflamm (Lond) 2011;8:7. doi: 10.1186/1476-9255-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallupi M, Varodayan FP, Oleata CS, Correia D, Luu G, Roberto M. Nociceptin/Orphanin FQ Decreases Glutamate Transmission and Blocks Ethanol-Induced Effects in the Central Amygdala of Naive and Ethanol-Dependent Rats. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian T. Overview of toll-like receptors in the CNS. Curr Top Microbiol Immunol. 2009;336:1–14. doi: 10.1007/978-3-642-00549-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Circuits, drugs, and drug addiction. Adv Pharmacol. 1998;42:978–982. doi: 10.1016/s1054-3589(08)60910-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. Neurotransmitters and microglial-mediated neuroinflammation. Curr Protein Pept Sci. 2013;14:21–32. doi: 10.2174/1389203711314010005. [DOI] [PubMed] [Google Scholar]
- Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- Lewis SS, Hutchinson MR, Zhang Y, Hund DK, Maier SF, Rice KC, Watkins LR. Glucuronic acid and the ethanol metabolite ethyl-glucuronide cause toll-like receptor 4 activation and enhanced pain. Brain Behav Immun. 2013;30:24–32. doi: 10.1016/j.bbi.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, Jr, Elnabawi A, Merchenthaler I, Sieghart W, June HL, Aurelian L. Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 2011;108:4465–4470. doi: 10.1073/pnas.1019020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Frye GD, Pulido MD, Botting SK. Effects of chronic ethanol consumption on rat GABA(A) and strychnine-sensitive glycine receptors expressed by lateral/basolateral amygdala neurons. Brain Res. 2003;963:165–177. doi: 10.1016/s0006-8993(02)03966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EL, Mosley A, Eaton S, Dobson L, Heales SJ, Pocock JM. Microglial neurotransmitter receptors trigger superoxide production in microglia; consequences for microglial-neuronal interactions. J Neurochem. 2012;121:287–301. doi: 10.1111/j.1471-4159.2012.07659.x. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol. 1995;488(Pt 1):85–101. doi: 10.1113/jphysiol.1995.sp020948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Yi Fau - Viriyakosol S, Viriyakosol S Fau - Worrall DS, Worrall Ds Fau - Boullier A, Boullier A Fau - Butler S, Butler S Fau - Witztum JL, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Das SK, Vaidyanathan K, Vasudevan DM. Consequences of alcohol consumption on neurotransmitters -an overview. Curr Neurovasc Res. 2008;5:266–272. doi: 10.2174/156720208786413415. [DOI] [PubMed] [Google Scholar]
- Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, Finn DA, Hoffman PL, Iyer VR, Koob GF, Bergeson SE. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc Natl Acad Sci U S A. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004;303:1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proceedings of the National Academy of Sciences USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 25 Suppl. 2011;1:S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm 2010. 2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchen W, Proctor WR, Dunwiddie TV. The in vitro ethanol sensitivity of hippocampal synaptic gamma-aminobutyric acid(A) responses differs in lines of mice and rats genetically selected for behavioral sensitivity or insensitivity to ethanol. J Pharmacol Exp Ther. 2000;295:741–746. [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, ODell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, Siggins GR. The Central Amygdala and Alcohol: Role of gamma-Aminobutyric Acid, Glutamate, and Neuropeptides. Cold Spring Harb Perspect Med. 2012;2 doi: 10.1101/cshperspect.a012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons L, Siggins GR. Increased GABA release in the central amygdala of ethanol dependent rats. Journal of Neuroscience. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Ethanol increases GABA release in rat central amygdala. Alcoholism, Clinical and Experimental Research. 2004b;28:92A. [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rosen JB. The neurobiology of conditioned and unconditioned fear: a neurobehavior al system analysis of the amygdala. Behav Cogn Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sahay B, Patsey RL, Eggers CH, Salazar JC, Radolf JD, Sellati TJ. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 2009;5:e1000687. doi: 10.1371/journal.ppat.1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABA(A) and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Thorsell A. The mu-opioid receptor and treatment response to naltrexone. Alcohol Alcohol. 2013;48:402–408. doi: 10.1093/alcalc/agt030. [DOI] [PubMed] [Google Scholar]
- Tu Z, Portillo JA, Howell S, Bu H, Subauste CS, Al-Ubaidi MR, Pearlman E, Lin F. Photoreceptor cells constitutively express functional TLR4. J Neuroimmunol. 2011;230:183–187. doi: 10.1016/j.jneuroim.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles SL, Blanco AM, Pascual M, Guerri C. Chronic ethanol treatment enhances inflammatory mediators and cell death in the brain and in astrocytes. Brain Pathol. 2004;14:365–371. doi: 10.1111/j.1750-3639.2004.tb00079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–488. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wang M, Li W. Regulation of microglia by ionotropic glutamatergic and GABAergic neurotransmission. Neuron Glia Biol. 2011;7:41–46. doi: 10.1017/S1740925X11000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Lousberg EL, Moldenhauer LM, Hayball JD, Coller JK, Rice KC, Watkins LR, Somogyi AA, Hutchinson MR. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br J Pharmacol. 2012;165:1319–1329. doi: 10.1111/j.1476-5381.2011.01572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.