Abstract
K63-linked polyubiquitination of proteins regulates their trafficking into specific cellular pathways such as endocytosis and autophagy. CYLD, a deubiquitinase specific for K63-linked polyubiquitins, is present in high quantities at the postsynaptic density (PSD). It was previously shown that, under excitatory conditions, CaMKII activates CYLD in a Ca2+-dependent manner. The observation that CYLD can also be phosphorylated in the absence of Ca2+ in isolated PSDs led us to further explore the regulation of CYLD under basal conditions. A possible involvement of the autonomous form CaMKII and IKK, both kinases known to be localized at the PSD, was examined. A CaMKII inhibitor CN21 had no effect on CYLD phosphorylation in the absence of Ca 2+, but two different IKK inhibitors, IKK16 and tatNEMO, inhibited its phosphorylation. Immuno electronmicroscopy on hippocampal cultures, using an antibody for CYLD phosphorylated at S-418, revealed that the phosphorylated form of CYLD is present at the PSD under basal conditions. Phosphorylation of CYLD under basal conditions was inhibited by IKK16. NMDA treatment further promoted phosphorylation of CYLD at the PSD, but IKK16 failed to block the NMDA-induced effect. In vitro experiments using purified proteins demonstrated direct phosphorylation and activation of CYLD by the beta catalytic subunit of IKK. Activation of IKK in isolated PSDs also promoted phosphorylation of CYLD and an increase in endogenous deubiquitinase activity specific for K63-linked polyubiquitins. Altogether, the results suggest that in the absence of excitatory conditions, constitutive IKK activity at the PSD regulates CYLD and maintains basal levels of K63-linkage specific deubiquitination at the synapse.
Keywords: Ubiquitin, CYLD, Deubiquitinase, IKK, PSD, Postsynaptic density
1. Introduction
Postsynaptic density (PSD) is a specialized protein complex that lies at the cytoplasmic side of postsynaptic membranes of glutamatergic synapses. It contains high concentrations of receptors, scaffolding proteins and regulatory enzymes (review: [1]). Attachment of ubiquitin chains to PSD proteins regulates their trafficking and turnover (reviews: [2], [3]).
Different types of ubiquitination regulate different cellular events. Generally, K48-linked polyubiquitination promotes proteasomal degradation [4] while K63-linked polyubiquitination signals for endocytosis [5] and autophagic degradation [6]. Ubiquitination by ubiquitin ligases is counteracted by the action of deubiquitinases (DUB). While much research on ubiquitination at the PSD has focused on ubiquitin ligases (review: [2]), relatively little is known about how DUBs are regulated.
CYLD, a DUB specific for K63-linked polyubiquitins (review: [7]), is an abundant protein in affinity-purified PSDs [8]. Immuno electronmicroscopy (EM) on hippocampal cultures revealed that even though more CYLD accumulates at the PSD under excitatory conditions, the protein is also present at the synapse in the absence of stimulation, although at lower quantity [9]. We previously reported that CaMKII mediates NMDA-induced recruitment and activation of CYLD at the PSD [10], but the regulation of CYLD under basal conditions is still elusive.
CYLD has been shown to be a substrate of IKK (inhibitor of κB kinase) in non-neuronal cell types [11], [12]. IKK, a complex of two catalytic subunits typically α, β, and a regulatory subunit γ, is increasingly recognized as an important protein in synaptic function. The kinase is an activator of nuclear factor κB (NF-κB ) signaling (review: [13]), a mechanism proposed to relay signal from synapse to the nucleus (review: [14]). IKK plays a role in proper synapse formation [15], synaptic [16] and behavioral plasticity [16], [17]. Loss of IKKβ activity confers abnormal PSD morphology as well as impairment in synaptic transmission machinery [15]. While IKK is present in PSD fractions [15], its function at the PSD requires further elucidation.
Here we use a combination of in situ EM and in vitro biochemical experiments to explore the role of IKK in the regulation of CYLD. Our results identify IKK as an activator of CYLD at the PSD and reveal a mechanism for the regulation of K63-linked polyubiquitination in unstimulated synapses.
2. Materials and Methods
2.1 Materials
Antibody to CYLD is a rabbit polyclonal (1:200 for Western, 1:100 for EM) from Sigma (SAB42000060). Antibody to CYLD phosphorylated at S-418 is a rabbit polyclonal (1:100 for Western, 1:50 for EM) from Cell Signaling (Antibody#4500).
Tetrameric K63-linked polyubiquitins are from LifeSensors (Malvern, PA). Purified recombinant human CYLD transcript variant 2 is from OriGene Technologies (Rockville, MD), and purified IKKβ is from Life technologies (Carlsbad, CA). N-methyl-D-aspartic acid (NMDA) is from Tocris (Ellisville, CO). TatNEMO (DRQIKIWFQNRRMKWKKTALDWSWLQTE) and tatcontrol (DRQIKIWFQNRRMKWKK) are from Imgenex (San Diego, CA). IKK16, N-(4-Pyrrolidin-1-yl-piperidin-1-yl)-[4-(4-benzo[b]thiophen-2-yl-pyrimidin-2-ylamino)phenyl]carboxamide hydrochloride, is from Tocris (Ellisville, CO). CN21 is a 21-amino acid sequence (42–62) derived from the natural CaMKII inhibitor CaM-KIIN [18].
2.2 Preparation and treatment of dissociated hippocampal cultures, pre-embedding immunogold electron microscopy
The protocols for obtaining brains for hippocampal cultures were approved by the NIH Animal Use and Care Committee and conformed to NIH guidelines. Hippocampal cells from 21-day embryonic Sprague-Dawley rats were dissociated and grown on a glial cell layer for 3–4 weeks, as previously described [19].
Control medium (124 mM NaCl, 2 mM KCl, 1.24 mM KH2PO4 1.3 mM MgCl2, 2.5 mM CaCl2, 30 mM glucose in 25 mM HEPES at pH 7.4) was prepared, and where indicated, was supplemented to include 50 μM NMDA, and/or 20 μM IKK16. Experiments were performed with dishes on a floating platform in a water bath maintained at 37°C. Cell cultures were washed once with control medium, and incubated for 20 min with or without IKK16, followed by incubation with NMDA or control medium in the presence or absence of IKK16 for 2 minutes. After treatment, neuronal cultures were processed for pre-embedding immunogold-labeling as described previously [20].
2.3 Morphometry and statistical analysis
Excitatory synapses were identified by clustered synaptic vesicles in the presynaptic compartment, the uniform 20 nm separation of the pre- and postsynaptic membranes, and the presence of a PSD, a layer of dense material underneath the postsynaptic membrane. The PSD complex was defined as the postsynaptic specialization that comprises the electron dense PSD core and a network contiguous to it. The area of the PSD complex to be measured was outlined as described previously [9]. CYLD and p-CYLD labels appeared as individual black grains at the PSD complex, and immunolabeling intensity was expressed as the number of labels per μm length of the PSD. Kruskal-Wallis–rank sum test was performed to assess statistical significance of the differences between experimental groups with significance level set at P<0.05.
2.4 Preparation of PSD fraction
PSD fraction from cerebral cortex was prepared as described previously [21] using brains from adult Sprague Dawley rats collected and frozen in liquid nitrogen within 2 min of decapitation by either Pel-Freeze Biologicals (Rogers, AR) or Rockland (Gilbertsville, PA).
2.5 Endogenous phosphorylation and DUB assay with isolated PSDs
To assess the level of CYLD phosphorylation by endogenous kinase activity, PSD fractions were pre-incubated in 0.1 M DTT on ice for two hours. The PSD fractions (18–23 μg protein) were then incubated in phosphorylation medium in a final concentration of 1 mM EGTA, 5 mM MgCl2, 50 μg/mL leupeptin, 20 mM DTT, 0.4 μM Microcystin-LR, with or without 100 μM ATP, in 20 mM HEPES, pH 7.4 in a final volume of 100 μL for 15 minutes at 37°C. When indicated, IKK16 was included at a final concentration of 1 μM or 20 μM, and tatNEMO at a final concentration of 5 μM or 20 μM. Control peptide, the tat-portion of tatNEMO, was included in a final concentration of 20 μM. An equal volume of SDS-PAGE treatment buffer was added to stop the reaction.
For endogenous DUB assays, PSD fractions (26–45 μg protein) were incubated in the phosphorylation medium in a final volume of 44 μL as described above, in the presence or absence of 20 μM IKK16 or 2.5 μM CN21. Immediately after incubation, an equal volume of solution containing 10 mM EGTA, 10 mM EDTA, 0.1 M DTT in 100 mM HEPES, pH 7.5 was added to the reaction mixture. Aliquots of 20 μl (6–10 μg of PSD protein) were incubated with 5 μL of tetrameric K63-linked ubiquitin chains (total 0.5 μg in 0.5 mg/mL BSA) at 37 °C for indicated time intervals. The heat-inactivated control consisted of boiling the sample for two minutes prior to incubation with tetrameric K63-linked ubiquitin chains for an hour. Reactions were terminated by adding SDS-PAGE electrophoresis sample buffer. The proteins were resolved via electrophoresis, and the resulting gel was cut directly below 100 kDa molecular weight. The bottom part of the gel was stained (Product #24630F from Thermo Scientific) to visualize ubiquitin chains, while the top portion was used for Western immunoblotting with p-CYLD antibody, then stripped (Product# 46430 from Thermo Scientific), and probed with CYLD antibody.
2.6 In vitro phosphorylation and DUB assay using purified proteins
To examine if IKK directly phosphorylates CYLD, 0.25 μg of purified CYLD was incubated with purified IKKβ, at a final concentration of 15 nM, in medium containing 1mM EGTA, 5 mM MgCl2, 50 μg/uL leupeptin, 2.5 mM DTT, 1 mg/mL BSA, 6.5% glycerol, in 20 mM HEPES, pH 7.4, with or without 100 μM ATP in a final volume of 20 μL at 37°C for one hour. An equal volume of SDS-PAGE treatment buffer was added to stop the reaction.
For DUB assays, 0.25 μg of purified CYLD was incubated with or without purified IKKβ at a final concentration of 257 nM in the phosphorylation medium in a final volume of 70 μL for 15 min. An equal volume of the solution containing 10 mM EGTA, 10 mM EDTA, 0.1 M DTT in 100 mM HEPES pH 7.5 was added to the reaction. Subsequently, 34 μL aliquots containing ~0.05 μg of CYLD were incubated with 3 μL of tetrameric K63-linked ubiquitin (0.3 μg total in 0.5 mg/ml BSA) at 37°C for indicated time intervals. The heat-inactivated control consisted of boiling the sample for two minutes prior to incubation with K63-linked ubiquitin chains for one hour. Reactions were terminated by adding SDS-PAGE treatment buffer. The proteins were resolved via electrophoresis, and changes in CYLD phosphorylation and DUB activity were assessed as described above in ‘Endogenous phosphorylation and DUB assay in isolated PSDs’.
2.7 Electrophoresis and immunoblotting
Samples were resolved by SDS-PAGE on 4–15% Mini-PROTEAN TGX gels from BioRAD, and transferred to PVDF membranes using Trans-Blot Turbo transfer system from BioRad, blocked, incubated with specified primary antibodies and then with horseradish peroxidase-conjugated secondary antibodies (1:50,000 dilution), and the signal was finally visualized by chemiluminescence (SuperSignal West Pico, Thermo Scientific).
3. Results and Discussion
We have previously reported CaMKII-mediated phosphorylation of CYLD in isolated PSDs in the presence of Ca2+, with concomitant increase of DUB activity [10]. In the same study it was observed that phosphorylation of CYLD in the absence of Ca2+ also correlated with enhanced DUB activity, albeit at a more modest level [10]. In the present study, we set out to identify the protein kinase responsible for the phosphorylation of CYLD in the absence of Ca2+. The first possibility tested was phosphorylation by an autonomous (i.e., Ca2+-independent) form of CaMKII.
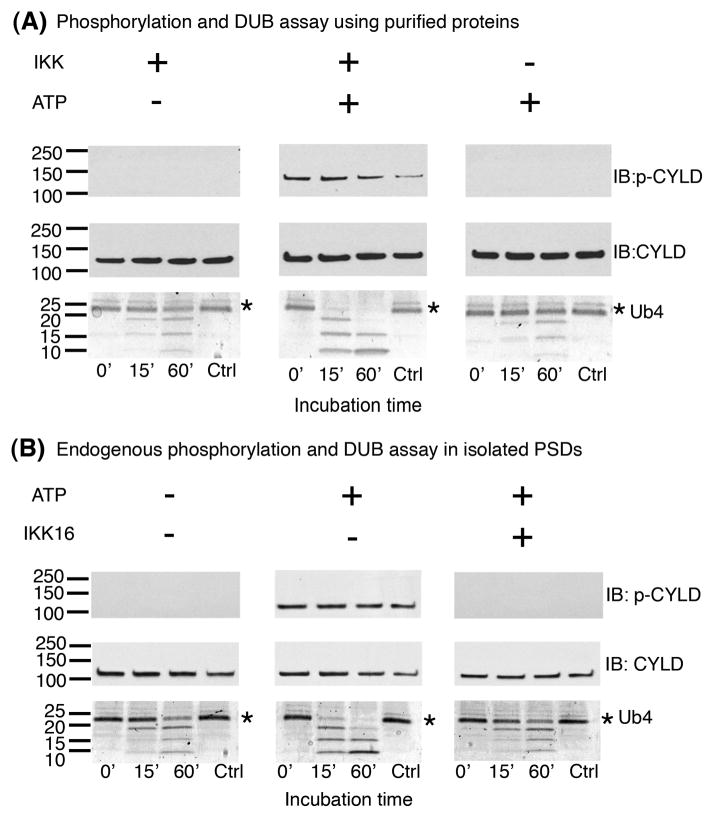
Experiments with an antibody specific to CYLD phosphorylated at S-418 (p-CYLD) show that incubation of PSD fractions in the presence of ATP in EGTA-containing medium induces phosphorylation of CYLD (Fig. 1, top panels), confirming previous results by mass spectrometry [10]. Increase in CYLD phosphorylation is accompanied by an increase in PSD associated DUB activity, as shown by an increase in the degradation rate of added K63-linked tetra-ubiquitin chains (Ub4) (Fig. 1, bottom panels). Preincubation of PSD fractions with CN21, a peptide inhibitor for both the Ca2+-dependent and autonomous forms of CaMKII, had no effect on either the phosphorylation of CYLD or increase in DUB activity (Fig. 1), indicating that neither of these events are mediated by CaMKII.
Figure 1. CaMKII does not mediate phosphorylation or activation of CYLD in isolated PSDs under Ca2+-free conditions.
PSD fractions were pre-incubated for 15 min with or without ATP and CN21, an inhibitor of both Ca2+-dependent and autonomous forms CaMKII, as indicated. Samples were subsequently incubated for indicated times with K63-linked tetra ubiquitin (Ub4) to test DUB activity. Western immunoblots show CYLD phosphorylation at S-418 using a phospho-specific antibody (p-CYLD) (top panels) in comparison to total CYLD levels (middle panels). DUB activity was monitored as the rate of degradation of Ub4, as shown in the coomassie gel stain (bottom panels). Addition of ATP promoted CYLD phosphorylation and increased Ub4 breakdown and inclusion of the CaMKII inhibitor CN21 had no effect on either of these reactions. Two independent experiments yielded similar results.
The second possibility tested was phosphorylation of CYLD by IKK. IKK, a Ca2+-independent kinase, known to phosphorylate CYLD at S-418 in non-neuronal cells [11], [12], is present in the PSD fractions [15]. Two different IKK inhibitors, IKK16 and tatNEMO, were used to assess possible involvement of IKK in mediating CYLD phosphorylation at the PSD. Application of either inhibitor reduced CYLD phosphorylation at S-418 in a dose-dependent manner (Fig. 2A). These observations indicate that endogenous IKK activity in isolated PSDs mediates CYLD phosphorylation in the absence of Ca2+. To ensure that IKK could directly phosphorylate CYLD rather than via sequential phosphorylation through other PSD-associated kinases, purified CYLD and purified IKKβ (catalytic subunit) were co-incubated in the presence or the absence of ATP. Addition of ATP induced phosphorylation of CYLD at S-418 (Fig. 2B), indicating that IKK can directly phosphorylate CYLD.
Figure 2. IKK phosphorylates CYLD at S-418 in vitro.
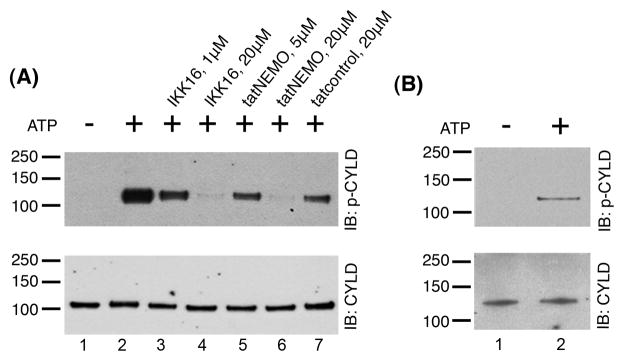
(A) PSD fractions were incubated under different conditions designed to manipulate IKK activity, followed by Western immunobloting with an antibody specific to CYLD phosphorylated at S-418 (p-CYLD) (top panels) or an antibody to CYLD (bottom panels). Addition of ATP induced phosphorylation of CYLD at S-418, but inclusion of the IKK inhibitors, IKK16 or tatNEMO reduced CYLD phosphorylation in a dose-dependent manner. (B) Purified CYLD and purified IKKβ were incubated in the presence or the absence of ATP, followed by Western immunoblotting as described above. Addition of ATP induced phosphorylation of CYLD at S-418.Two experiments yielded similar results.
The two IKK inhibitors utilized possess distinct inhibitory actions on the IKK complex, providing clues on the mechanism of IKK-mediated phosphorylation of CYLD at the PSD. IKK16 is an ATP-competitive inhibitor for the catalytic subunits of IKK, whereas tatNEMO blocks the binding of the regulatory subunit IKKγ to the catalytic subunits. The fact that phosphorylation of CYLD can be attenuated by tatNEMO suggests that phosphorylation of CYLD at the PSD requires the regulatory subunit IKKγ, in addition to the catalytic subunits of IKK. Interestingly, in contrast to the situation in PSD fractions, addition of purified IKKβ was sufficient to phosphorylate purified CYLD. Thus, at the PSD, co-localization and proper orientation of CYLD and the catalytic subunits through IKKγ may be necessary for optimal phosphorylation. Indeed, IKKγ binds CYLD as well as the catalytic subunits of IKK [11]. Interestingly, a similar scenario has been described by Reiley et al., who showed that IKK-mediated phosphorylation of CYLD was blocked in IKKγ-deficient Jurkat T-cell line [11].
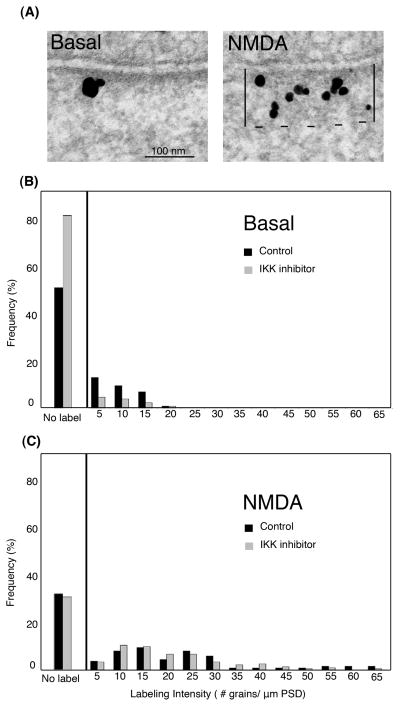
To examine regulation of CYLD phosphorylation in intact cells, immunoEM studies were carried out in hippocampal cultures using an antibody specific for p-CYLD. Immunogold label for p-CYLD was observed at the PSD under basal conditions, but increased dramatically upon NMDA treatment (Fig. 3A). It had previously been shown that NMDA also causes a net increase in CYLD labeling at the PSD [10]. The role of IKK in promoting CYLD phosphorylation at the PSD under both basal and excitatory conditions was examined using a cell permeable IKK inhibitor, IKK16.
Figure 3. The phosphorylation state of CYLD at the PSD of hippocampal neurons is controlled by IKK under basal conditions.
Hippocampal cultures were pre-incubated with or without the IKK inhibitor IKK16 for 20 min, followed by treatment with either NMDA or control medium for 2 min before fixation and immunogold labeling with an antibody specific for CYLD phosphorylated on S-418 (p-CYLD). (A) Electron micrographs of the synaptic region showing immunogold labeling for p-CYLD, seen as dark grains of heterogeneous sizes. On the right panel is shown the zone for measuring amount of labeling at the PSD complex, delineated by two perpendicular lines between the postsynaptic membrane and a parallel dashed line at 120 nm distance from the postsynaptic membrane. NMDA treatment promoted an increase in p-CYLD labels at the PSD. (B & C) The histograms depict the frequency of PSDs either with no label (left) or with labels of varying intensities (right). Immunolabeling intensities were binned into groups from 5 to 65. Under basal conditions (B), the IKK inhibitor IKK16 (gray bars) reduced the labeling for phosphorylated CYLD at the PSD. However, preincubation with the inhibitor had no significant effect on labeling for phosphorylated CYLD following NMDA treatment (C). The histograms represent one experiment out of a total of three experiments with similar results.
The cultures were incubated in control medium with or without IKK16 and the labeling intensity for p-CYLD was quantified (Fig. 3B). In the absence of IKK16, ~50% of synapses did not show any p-CYLD label. The percentage of synapses devoid of p-CYLD label rose to ~80% upon inclusion of IKK16 (Fig. 3B, left panel) together with a general decrease in the labeling intensity at the PSDs (Fig 3B, right panel). Under the same conditions, labeling intensity of CYLD at the PSD did not differ between IKK16-treated and control groups, indicating that IKK16 does not affect the levels of CYLD at the PSD (Supplementary Figure 1). These results suggest that IKK phosphorylates CYLD at the PSD under basal condition.
Interestingly, pre-incubation of hippocampal cultures with IKK16 neither reduced NMDA-induced CYLD phosphorylation (Fig. 3C) nor prevented recruitment of more CYLD to the PSD (Supplementary Figure 1). These results indicate that while IKK regulates CYLD under basal conditions, it plays no or a minimal role in mediating further enhanced CYLD phosphorylation that is induced by stimulation of NMDA receptors. As reported earlier, under the latter conditions regulation of CYLD appears to be mediated by CaMKII [10].
In a next series of experiments the effect of IKK-mediated phosphorylation on CYLD activity was examined. First, experiments with purified CYLD and purified IKKβ were carried out. When the two proteins were incubated in the presence of ATP, CYLD became phosphorylated at S-418 and the rate of degradation of added K63-linked Ub4 increased (Fig. 4A). These results establish activation of CYLD through IKK-mediated phosphorylation. However, while western immunoblotting indicates phosphorylation on S-418, the effect of phosphorylation on additional residues cannot be excluded. In fact, mass-spec analysis on purified CYLD incubated with purified IKKβ revealed additional phosphorylated residues (data not shown). In agreement with observations using purified proteins, inclusion of IKK inhibitor attenuated phosphorylation of CYLD on S-418 as well as the degradation of K63-linked polyubiquitins in the PSD fractions (Fig. 4B). These results indicate that IKK at the PSD promotes degradation of K63-linked polyubiquitins through phosphorylation of CYLD.
Figure 4. IKK regulates DUB activity targeting K63-linked polyubiquitins.
(A) Purified CYLD was pre-incubated with purified IKKβ in the presence or absence of ATP, followed by incubation with tetrameric K63-linked polyubiquitin chains (Ub4). IKKβ was omitted in additional controls (right column). The upper two panels are Western immunoblots with antibodies specific for CYLD phosphorylated at S-418 (p-CYLD) and for CYLD respectively, while the bottom panels shows the lower portions of coomassie blue stained gels containing Ub4 and degradation products. Addition of both IKKβ and ATP promotes CYLD phosphorylation (upper panels), with a concomitant increase in the rate of cleavage of Ub4 (bottom panels). Two experiments yielded similar results. (B) PSD fractions were incubated under different conditions designed to manipulate IKK activity, followed by incubation with tetrameric K63-linked polyubiquitin chains (Ub4). CYLD phosphorylation status (top panels) in comparison to total CYLD levels (middle panels) and DUB activity (bottom panels) were monitored as described above. Inclusion of ATP promoted CYLD phosphorylation, and an increase in the rate of Ub4 breakdown. Addition of IKK16 prevented both CYLD phosphorylation and ATP-dependent upregulation of Ub4 breakdown (bottom panels). Three independent experiments yielded similar results.
Our finding appears to be in contradiction with previous reports, indicating that IKK-mediated phosphorylation of CYLD promotes ubiquitination of TRAF2 in HeLa and HEK cells [11], [12] and of IKKγ in HEK cells [12]. However, it should be noted that, while our work establishes a direct link between phosphorylation of CYLD and its catalytic activity, the earlier studies followed ubiquitination of specific proteins in intact cells. Therefore, one possible explanation for the discrepancy may be that the effect of CYLD on the ubiquitination of certain proteins in intact cells is indirect, mediated by factors downstream from CYLD phosphorylation. In future studies, it would be beneficial to identify CYLD’s substrates and compare factors involved in orchestrating downstream effects of CYLD phosphorylation between neuronal and non-neuronal cells.
IKK-mediated CYLD activation is expected to remove K63-linked polyubiquitins from PSD proteins and thus regulate their trafficking. IKK at the PSD is active under basal conditions. It is probable that the level of constitutive IKK activity at a particular synapse determines the activity of CYLD under basal conditions and thus helps maintain the pattern of protein trafficking in the absence of excitatory conditions. A role of IKK has been implied in several synaptic events, including the regulation of levels of specific PSD proteins [15]. The demonstration that CYLD activity in the PSD is regulated by IKK implies a potential downstream role of CYLD in IKK-mediated pathways in neurons. Thus, future work should examine how the interaction of CYLD and IKK fits into overall landscape of IKK-mediated neuronal function and pathology.
Supplementary Material
CYLD is phosphorylated by IKK in isolated PSDs in the absence of Ca2+.
CYLD is phosphorylated by IKK at the PSDs of intact neurons in basal conditions.
Phosphorylation of CYLD by IKK increases its deubiquitinase activity.
The process is likely to influence protein trafficking at the PSD in basal conditions.
Acknowledgments
We thank Christine A. Winters for hippocampal neuronal cultures, Virginia Crocker and Rita Azzam for EM technical support, Dr. Yan Li for performing and analyzing mass-spectrometry and Dr. Thomas Reese for helpful discussions and a critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, NINDS, and by R01NS052644 (to K.U.B.).
Abbreviations
- PSD
Postsynaptic density
- DUB
deubiquitinase
- EM
electronmicroscopy
- IKK
inhibitor of κB kinase
- NF-κB
Nuclear factor κB
- CaMKII
Ca2+/calmodulin-dependent kinase
- p-CYLD
CYLD phosphorylated at S-418
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 2.Lin AW, Man HY. Ubiquitination of neurotransmitter receptors and postsynaptic scaffolding proteins. Neural Plast. 2013;2013:432057. doi: 10.1155/2013/432057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi JJ, Ehlers MD. Ubiquitin and protein turnover in synapse function. Neuron. 2005;47:629–632. doi: 10.1016/j.neuron.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. Embo J. 2006;25:1635–1645. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan JM, Wong ES, Kirkpatrick DS, Pletnikova O, Ko HS, Tay SP, Ho MW, Troncoso J, Gygi SP, Lee MK, Dawson VL, Dawson TM, Lim KL. Lysine 63-linked ubiquitination promotes the formation and autophagic clearance of protein inclusions associated with neurodegenerative diseases. Hum Mol Genet. 2008;17:431–439. doi: 10.1093/hmg/ddm320. [DOI] [PubMed] [Google Scholar]
- 7.Massoumi R. Ubiquitin chain cleavage: CYLD at work. Trends Biochem Sci. 2010;35:392–399. doi: 10.1016/j.tibs.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Dosemeci A, Makusky AJ, Jankowska-Stephens E, Yang X, Slotta DJ, Markey SP. Composition of the synaptic PSD-95 complex. Mol Cell Proteomics. 2007;6:1749–1760. doi: 10.1074/mcp.M700040-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dosemeci A, Thein S, Yang Y, Reese TS, Tao-Cheng JH. CYLD, a deubiquitinase specific for lysine63-linked polyubiquitins, accumulates at the postsynaptic density in an activity-dependent manner. Biochem Biophys Res Commun. 2013;430:245–249. doi: 10.1016/j.bbrc.2012.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thein S, Tao-Cheng JH, Li Y, Bayer KU, Reese TS, Dosemeci A. CaMKII mediates recruitment and activation of the deubiquitinase CYLD at the postsynaptic density. PLoS One. 2014;9:e91312. doi: 10.1371/journal.pone.0091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiley W, Zhang M, Wu X, Granger E, Sun SC. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–3895. doi: 10.1128/MCB.25.10.3886-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, Hahn WC, Cantley LC. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–472. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1:a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmeisser MJ, Baumann B, Johannsen S, Vindedal GF, Jensen V, Hvalby OC, Sprengel R, Seither J, Maqbool A, Magnutzki A, Lattke M, Oswald F, Boeckers TM, Wirth T. IkappaB kinase/nuclear factor kappaB-dependent insulin-like growth factor 2 (Igf2) expression regulates synapse formation and spine maturation via Igf2 receptor signaling. J Neurosci. 2012;32:5688–5703. doi: 10.1523/JNEUROSCI.0111-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vest RS, Davies KD, O’Leary H, Port JD, Bayer KU. Dual mechanism of a natural CaMKII inhibitor. Mol Biol Cell. 2007;18:5024–5033. doi: 10.1091/mbc.E07-02-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z, McLaren RS, Winters CA, Ralston E. Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol Cell Neurosci. 1998;12:363–375. doi: 10.1006/mcne.1998.0723. [DOI] [PubMed] [Google Scholar]
- 20.Tao-Cheng JH, Yang Y, Bayer KU, Reese TS, Dosemeci A. Effects of CaMKII inhibitor tatCN21 on activity-dependent redistribution of CaMKII in hippocampal neurons. Neuroscience. 2013;244:188–196. doi: 10.1016/j.neuroscience.2013.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca(2+)/calmodulin-dependent [correction of Ca(2+)/CaMKII-dependent] protein kinase II in neurons. J Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.