Abstract
The mechanism of chloroquine (CQ) resistance in Plasmodium falciparum is not clearly understood. However, CQ resistance has been shown to be associated with point mutations in Pfcrt and Pfmdr1. These genes encode for digestive vacuole transmembrane proteins Pfcrt and Pgh1, respectively. The present study was carried out to analyze the association of Pfcrt-K76T and Pfmdr1-N86Y mutations with CQ resistance in Northeast Indian P. falciparum isolates. 115 P. falciparum isolates were subjected to in vitro CQ sensitivity testing and PCR-RFLP analysis for the Pfmdr1-N86Y and Pfcrt-K76T mutations. 100 isolates of P. falciparum were found to be resistant to CQ by the in vitro susceptibility test (geometric mean EC50 2.21 µM/L blood) while 15 were found to be CQ sensitive (geometric mean EC50 0.32 µM/L blood). All the CQ resistant isolates showed the presence of Pfmdr1 and Pfcrt mutations. CQ sensitive isolates were negative for these mutations. Strong linkage disequilibrium was observed between the alleles at these two loci (Pfmdr1-N86Y and Pfcrt-K76T). The results indicate that Pfmdr1-N86Y and Pfcrt-K76T mutations can be used as molecular markers to identify CQ resistance in P. falciparum. The result necessitates the evaluation of CQ in vivo therapeutic efficacy in endemic areas for more effective malaria control strategies.
Introduction
Malaria is one of the major public health problems of the malaria affected countries, including India. In India, around 1.5 million laboratory confirmed cases of malaria are reported annually, out of which 50% cases are due to Plasmodium falciparum alone. Chloroquine (CQ) has been the most effective drug in the treatment of non-complicated malaria. A sudden rise in P. falciparum cases has been caused by resistance towards CQ, which was used for a long time as the first line of treatment of malaria cases [1]. CQ resistance may lead to high morbidity and mortality in P. falciparum cases, if not treated timely. CQ acts by interfering with heme metabolism in the digestive vacuole of P. falciparum and CQ resistance results from reduced accumulation of the drug by the parasites [2]–[4].
Various genetic alterations have been shown to be associated with CQ resistance. Mainly, two genes known as P. falciparum multidrug resistance gene Pfmdr1, which codes for Pgh1, a P-glycoprotein homologue, and the CQ resistance transporter gene Pfcrt, which codes for CQ resistance transporter protein have been identified as potential candidates of CQ resistance. Several point mutations in Pfmdr1 gene at positions 754, 1049, 3598, 3622 and 4234 result in amino acid changes at codons 86, 184, 1034, 1042 & 1246, respectively. These amino acid changes have been shown to be associated with CQ resistance [5]–[12]. Out of the several mutations described, the mutation in codon 86 (from asparagine to tyrosine, N86Y), involved in the substrate specificity of the gene product (P- glycoprotein), appears to be the most important as this may alter the transport activity of the protein [4]. However, a few studies have reported contrasting observations with regard to the role of Pfmdr1 gene mutations in CQ resistance [13]. Southeast Asian CQ resistant isolates (K1 genotype) have shown N86Y mutation while CQ resistant South American isolates (7G8 genotype) were negative for N86Y, and showed mutations at positions 184, 1034, 1042 and 1246 [5]. Mutation in codon 86 has also been correlated to CQ resistance in parasites selected in vitro for CQ resistance [13]. Similarly, mutations in the Pfcrt (codon 74, 75, 76, 220, 271, 326, 371) have also been shown to play a role in in vitro CQ resistance in laboratory lines of P. falciparum from all over the world [14]–[16]. Pfcrt K76T mutation has not been observed in CQ responders, and therefore, has been accepted as a good molecular marker for CQ resistance in P. falciparum [10], [17]–[19].
Malaria is a serious health problem in India, especially in the Northeast. However, to date, the role of mutations in genes Pfmdr1 and Pfcrt has not been studied in the emergence of P. falciparum CQ resistance. Although studies from other parts of India have reported poor association of CQ resistance with these gene mutations, but no extensive study has been carried out, yet [20]. In P. falciparum endemic areas, CQ was the recommended first line treatment for uncomplicated malaria. However, now a days this has been changed to artesunate-based combination therapies. Despite this, in many malaria-affected areas CQ is still used for non-complicated malaria [11], [21]. Therefore, constant observation of the existing parasite population genetic makeup and determination of the presence of CQ resistance is important [22]. Keeping this in mind, the present study was planned to explore the correlation between in vitro CQ sensitivity and Pfcrt-K76T and Pfmdr1-N86Y mutations in a large number of clinical isolates of P. falciparum from the Northeast India. Since fresh isolates often have a mixture of clones of both CQ sensitive and resistant clones, the culture-adapted line derived from an isolate often responds differently. Therefore, in the present study, fresh clinical isolates were used. The linkage disequilibrium between the alleles in codon 86 of Pfmdr1 (N86 & 86Y) and in codon 76 of Pfcrt (K76 & 76T) gene were also analyzed.
Materials and Methods
Ethics Statement
Ethical Committee of Post Graduate Institute of Medical Education & Research, Chandigarh approved the study protocol. The institutional ethics committee adhered to guidelines of national regulatory agency i.e. ICMR for conductance of experiments on humans and animals.
Study area and sample collection
This study was carried out in the remote villages of (Tinsukhia, Assam and Lohit, Arunachal Pradesh) of Northeast India. These area's have big tea estates and heavy rainfall throughout the year, and hence malaria too. The study participants were poor tea estate workers, lacking ready access to medical services. Informed written consent was obtained from the patients. For drug sensitivity and molecular analysis, approximately 5 ml of blood was collected from the asymptomatic patients (25–45 years old) who were tested positive for P. falciparum using Giemsa staining. The blood was stored in vials containing citrate and stored in at 2o–8°C. A total of 115 blood samples were included in the study, with 65 from Assam and 50 from Arunachal Pradesh. Samples with multiple infections were excluded from the study. The present study was carried out as a part of PhD work of Mr. Sandeep K. Shrivastava and data generated during this study was submitted to the Institute Academics Committee, and can also be accessed from the institute central library after due permission from authorities.
In vitro sensitivity testing
An in vitro micro test (Mark III) protocol recommended by WHO [23] was followed for the sensitivity testing. The CQ sensitivity test was performed immediately after the collection of blood. The test was considered valid and interpretable if ≥10 percent of the parasites in the control well (drug free well) had developed into the schizonts after 24–36 hours incubation. Isolates were considered resistant if they showed schizont maturation at CQ concentrations ≥ 8 pmol/well (1.6 µmol/L blood). To evaluate the drug-parasite response, the EC50 value (50% inhibition) was calculated by Probit analysis.
DNA isolation
DNA was isolated according to method of Foley et al. [24] with slight modifications. Briefly, the cells from 100 µl whole blood were lysed with 1 ml of ice-cold 5 mM Na2HPO4 (pH 8.0) and the pellet was collected by centrifugation at 10000 X g for 10 minutes. This step was repeated two times more. Finally, the pellet obtained was re-suspended in 50 µl of sterile, double distilled water. The re-suspended pellet was heated in a boiling water bath for 10 minutes, followed by cooling to room temperature and centrifugation as above. For the PCR analysis, 3 µl of the supernatant was used as DNA template.
Detection of N86Y mutation in Pfmdr1 gene
Nested PCR, as reported previously was performed to amplify codon 86 of Pfmdr1 [21]. During nest1 reaction, primers P1- 5′ATGGGTAAAGAGCAGAAAGA3′ and P2-5′AACGCAAGTAATACATAAAGTCA3′ were used to amplify the region flanking codon 86. Nested primers P3 5′TGGTAACCTCAGTATCAAAGAA3′ and P4 5′ATAAACCTAAAAAGGAACTGG3′ were used to amplify the PCR product in nest2 reaction. In nest 1, PCR parameters were, initial denaturation at 94°C for 3 minutes, followed by 45 cycles, each of 30 sec at 92°C, 45 sec at 48°C, 1 min at 65°C followed by the final extension at 65°C for 5 min. In nest 2, only 20 cycles of PCR were run (Mastercycler, Eppendorf, USA).
Restriction Digestion with ApoI and Afl III
The finally amplified product was subjected to restriction digestion with Afl III (mutational allele) and Apo I (wild type allele) (New England Biolabs, UK) by incubating at 37°C for one hour with the one unit of each enzyme. The digests were resolved on 3% agarose gel, stained with ethidium bromide, and results were recorded on the gel documentation system (UVITEC, UK).
Detection of the K76T mutation in Pfcrt gene
For the K76T mutation, during nest1, primers CRTP1 5′ CCGTTAATAATAAATACACGCAG3′ and CRTP2 5′GCATGTTACAAAACTATA GTTACC3′ were used, and for nest2 CRTD1: 5′TGTGCTCATGTGTTTAAACTT3′ and CRTD2: 5′CAAAACTATAGTTACCAATTTT3′ were used [25]. The nest1 PCR parameters were, initial denaturation at 95°C for 5 minutes followed by 45 cycles, each of 30 sec at 92°C, 56 sec at 30°C, 1 min at 60°C followed by the final extension at 60°C for 3 min. In nest2 PCR, initial denaturation at 95°C for 5 minutes followed by 25 cycles, each of 30 sec at 92°C, 30 sec at 48°C, 30 sec at 65°C followed by the final extension at 65°C for 3 min were done. The nested PCR product was digested with ApoI as described above.
Statistical analysis
All the experiments were repeated thrice to validate the reproducibility. The results were expressed as geometric means and were reported with 95% confidence intervals. For statistical analysis (Chi squared test), SPSS 10 and Epi-Info 6.04 (CDC, Atlanta, GA, USA) were used. Probit analysis was used to calculate the CQ EC50 for each isolate [22], [26]. Linkage disequilibrium constants (D' and r2) were also calculated [27]–[28].
Results
A total of 115 P. falciparum isolates were used in the study. The in vitro CQ susceptibility of these isolates was carried out according to WHO guidelines and isolates were categorized as sensitive and resistant strains. Out of 65 isolates from Assam, 50 were found to be CQ resistant in the in vitro sensitivity test with the geometric mean EC50 value of 1.06 µmol/L blood (Chi2 value 68.44, P<10−5 with 95% confidence intervals), and 15 showed the sensitivity towards CQ with geometric mean EC50 of 0.32 µmol/L blood (Chi2 value 161.07, P<10−5 with 95% confidence intervals). On the other hand, all 50 isolates collected from Arunachal Pradesh also showed a high level of CQ resistance with geometric mean EC50 of 2.94 µmol/L blood (the chi2 or p value could not be computed due to uniformity of one parameter). In total, 100 P. falciparum isolates were found to be the CQ resistant (geometric mean EC50 2.21 µmol/L blood) and 15 isolates were found to be the CQ sensitive (geometric mean EC50 0.32 µmol/L blood) (Chi2 value 161.07, P<10−5 with 95% confidence intervals).
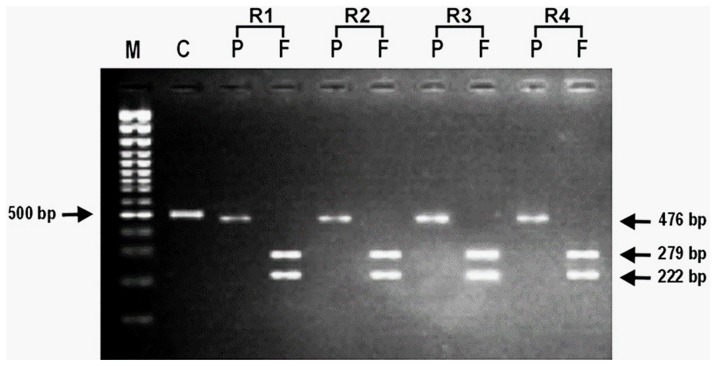
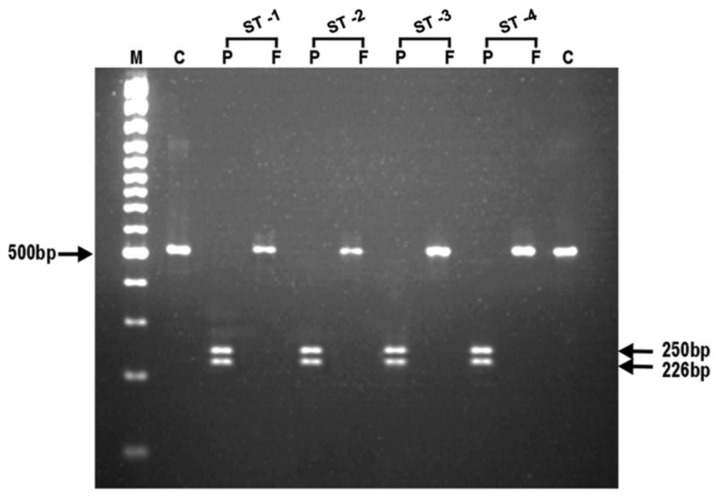
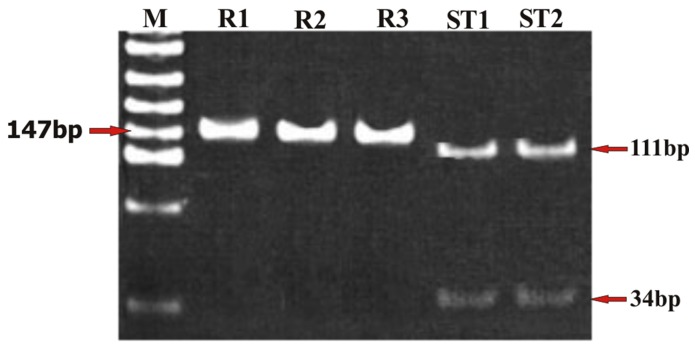
To ascertain the genetic polymorphism leading to the CQ resistance, nested PCR for Pfmdr1 and Pfcrt genes was performed. To confirm the status of the amplicon, the PCR product was digested with Apo1 and Afl III. On nested PCR for Pfmdr1, all the isolates showed the Pfmdr1-codon 86 region amplicon with the product size of 501 bp. On digestion with the ApoI in the case of CQ sensitive isolates, ApoI digested the amplicon at codon 86, and generated three fragments of 250 bp, 226 bp and 25 bp, respectively. Afl III was unable to digest the amplicon of the sensitive isolates (Table 1; Figure 1). However, Afl III generated two fragments of 279 bp and 222 bp, respectively from the amplicon of all the CQ resistant isolates indicating a mutant allele at codon 86 (86T). ApoI did not digest the amplicon of the CQ resistant strains at this site, and generated fragments of 476 bp and 25 bp (Table 1; Figure 2). The nested PCR of Pfcrt gene showed an amplicon of 145 bp. In the case of CQ sensitive isolates, the ApoI digestion of amplicon resulted in two fragments of 111 bp and 34 bp respectively, suggesting the presence of K76 allele at codon 76. On the other hand, mutant allele 76T was observed in all the CQ resistant isolates with an undigested fragment of 145 bp (Table 1; Figure 3).
Table 1. Chloroquine sensitivity status and presence of pfmdr1-N86Y and pfcrt-K76T mutation in P. falciparum isolates from Northeast India.
| Area of isolation | In vitro CQ sensitivity status | Pfmdr1- codon 86 | Pfcrt-codon76 | ||
| Allele-N86 | Allele-86Y | Allele-K76 | Allele-76T | ||
| Assam (n = 65) | CQR* (n = 50) | 0 | 50 | 0 | 50 |
| CQS** (n = 15) | 15 | 0 | 15 | 0 | |
| Arunachal Pradesh (n = 50) | CQR (n = 50) | 0 | 50 | 0 | 50 |
* chloroquine resistant; ** chloroquine sensitive.
Figure 1. Representative photomicrograph showing the results of RFLP after digestion of PCR amplified product with endonucleases, Apo1 and AfI III for Pfmdr1 gene (86Y) in chloroquine resistant Plasmodium falciparum isolates.
In photograph, (M) is 100 bp DNA ladder; (C) is undigested product as control; (P) is Apo1; (F) is Afl III and (R1- R4) are chloroquine resistant P. falciparum isolates.
Figure 2. Representative photomicrograph showing the results of RFLP after digestion of PCR amplified product with endonucleases, Apo1 and AfI III for Pfmdr1 gene (N86) in chloroquine sensitive Plasmodium falciparum isolates.
In photograph, (M) is 100 bp DNA ladder; (C) is undigested product as control; (P) is Apo1; (F) is Afl III and (ST1-ST4) are chloroquine sensitive P. falciparum isolates.
Figure 3. Representative photomicrograph showing the results of RFLP after digestion of PCR amplified product with endonuclease, Apo1 for Pcfrt gene (K76T) in chloroquine resistant and sensitive Plasmodium falciparum isolates.
In photograph, (M) is molecular weight marker pUC mix8; (R1-R3) are chloroquine resistant P. falciparum isolates and (ST1-ST2) are chloroquine sensitive P. falciparum isolates.
In total, 100 CQ resistant isolates (from in vitro) showed the mutation at codon 86Y, while in CQ sensitive isolates, wild type allele (N86) was observed. In these isolates, N86Y mutation was found to be associated with the in vitro CQ sensitivity status (Chi2 test, P<10−5). The K76T mutation was also found to be associated with the in vitro CQ susceptibility in all the isolates (Chi2 test, P<10−5). The linkage disequilibrium (LD) between the alleles of these two loci (Pfmdr1-N86Y and Pfcrt-K76T) was analyzed by calculating the LD constants D' [23] and r2 [24]. The LD constants show strong LD between these loci (D' = 1.00; r2 = 1.00) (Table 2).
Table 2. Linkage disequilibrium between alleles of Pfmdr1 codon 86 (on chromosome) five and Pfcrt codon 76 (on chromosome seven).
| Pfcrt-76T | Pfcrt-K76 | D' | r2 | P | |
| Pfmdr1-86Y | 100 | 0 | 1.00 | 1.00 | <10−5 |
| Pfmdr1-N86 | 0 | 15 |
Discussion
Since its first report in 1950's, CQ resistance had spread worldwide [29]. In India, the first case of CQ resistance was reported in 1973 from Karbi-Anglong district in the Assam [30]. Since then, it has gradually spread West and South [11]. The fast rate of emergence of CQ resistance has become a major hurdle in the control of malaria.
The development of molecular techniques for the rapid identification of drug resistant parasites is of immense importance for the epidemiology, and information on the choice of antimalarial treatment regimens. In the present study, the in vitro CQ susceptibility pattern of 115 isolates was compared with that of point mutations in the genes Pfmdr 1 and Pfcrt. The development of the CQ resistance phenotype is a complex and probably cumulative phenomenon where more than one gene with one or more mutation(s)/polymorphism(s) might contribute to the development of CQ resistance [4].
In the present study, the CQ sensitivity assay and DNA isolation were performed with the fresh clinical isolates of P. falciparum, immediately after the collection of blood. Continuous in vitro culture induces the selection of sub population of parasites, and hence does not truly represent parasites of the in vivo infection [33]. In previous studies, culture-adapted isolates or clones of mutations in Pfmdr1were used [20]. The CQ resistant strains become CQ sensitive on withdrawal of the drug or on continuous passages during culture [34]. Therefore, genotyping of such culture-adapted isolates would be misleading. The results of in vitro CQ susceptibility tests showed that out of a total 115 clinical isolates, 100 isolates were resistant towards the CQ while only 15 isolates showed sensitivity to CQ. Interestingly, 100% of the isolates from the Arunachal Pradesh and 77% isolates of the Assam showed the in vitro CQ resistance indicating the alarming situation of CQ resistance in P. falciparum.
On molecular detection of point mutations in Pfmdr1, a strong association was observed between codon 86Y mutation and in vitro CQ resistance in these isolates. These findings corroborate well with the previous findings [12], [17], [19], [32]. In the present study, all the CQ resistant isolates showed mutational change at position 86Y confirming the role of this mutation in the CQ resistance. It is likely that the 86Y allele of Pfmdr1 is of functional relevance. A point mutation in the Pfmdr1 gene results in a change of an amino acid at codon 86, 184, 1034, 1042 and 1246. However, contrasting observations are available in this regard. It has been observed that the Southeast Asian CQ resistant isolates have a change in the amino acid at codon 86 from asparagine to tyrosine (N86Y) [31]. While CQ resistant South American isolates have shown mutational changes at codon 184, 1034, 1042 and 1246. Out of these, the mutation at codon 86 appears to be important since it is involved in the substrate specificity of the gene product (P- glycoprotein), and hence may alter the transport activity of the protein [31], [38]. Similarly, due to mutations in Pfcrt gene, mutations involving the substitution from lysine (K) to threonine (T) at position 76 (K76T) has also been observed consistently in the CQ resistant strains [4], [11].
The analysis of K76T mutation in Pfcrt gene revealed its 100% association with the in vitro CQ resistance. In an earlier study, significant association (linkage disequilibrium, LD) between the alleles Pfmdr1 86Y and Pfcrt 76T was observed [12], [35]–[36]. Significant association between the Pfmdr1 86Y and the EC50 of CQ among clones with the Pfcrt 76T allele suggests the role of both the mutations in CQ resistance. The results of the present study corroborate with the earlier studies, which showed significant association between Pfmdr1 N86Y and Pfcrt K76T [19], [23], [37].
The LD test measures the linkage between genes/alleles on different chromosomes under a restrictive set of conditions or external influence (selection under the drug pressure) [21]–[22]. To analyze the LD between alleles at codon 86 of Pfmdr1 gene on chromosome 5 and alleles at codon 76 of Pfcrt gene on chromosome 7, LD constants (D' and r2) were calculated. A high LD between these two loci (D' = 1.00 and r2 = 1.00) was observed. These findings are in agreement with the findings of Adagu and Warhurst, [38], where high LD values were observed between these two alleles. The CQ has been the first line drug for treatment of uncomplicated malaria in the Assam and Arunachal Pradesh. Thus, our findings in combination with previous evidence suggested that the LD between Pfcrt-K76T and Pfmdr1-N86Y alleles might be due to the strong directional selection of the alleles by CQ drug pressure in the population of the studied area. These results further suggest that Pfmdr1 (N86Y) and Pfcrt (K76T) are potentially useful markers of the assessment of in vitro CQ resistance in the Northeast India. These can be used for the rapid diagnosis and surveillance of CQ resistance and may have potential use in the monitoring of in vivo therapeutic efficacy of CQ in malaria endemic areas.
Funding Statement
The authors have no support or funding to report.
References
- 1.National Drug Policy on Malaria (2013) Directorate General of National Vector Borne Disease Control Programme, Ministry of Health & Family Welfare, Government of India. New Delhi: 1–15.
- 2. Fitch CD (1970) Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science 169(942): 289–90. [DOI] [PubMed] [Google Scholar]
- 3. Douki JBL, Boutamba SDD, Zatra R, Edou SEZ, Ekomy H, et al. (2011) Increased prevalence of the Plasmodium falciparum Pfmdr1 86N genotype among field isolates from France ville, Gabon after replacement of chloroquine by artemether–lumefantrine and artesunate–mefloquine. Infect Gen Evo 11: 512–517. [DOI] [PubMed] [Google Scholar]
- 4. Sanchez CP, Dave A, Stein WD, Lanzer M (2010) Transporters as mediators of drug resistance in Plasmodium falciparum . Int J Parasitol 40: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 5. Foote SJ, Kyle DE, Martin RK, Oduola AM, Forsyth K, et al. (1990) Several alleles of the multidrug-resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum . Nature 17 345(6272): 255–58. [DOI] [PubMed] [Google Scholar]
- 6. Basco LK, Le Bras J, Rhoades Z, Wilson CM (1995) Analysis of pfmdr1 and drug susceptibility in fresh isolates of Plasmodium falciparum from sub-Saharan Africa.Mol Biochem Parasitol. 74(2): 157–66. [DOI] [PubMed] [Google Scholar]
- 7. Cox-Singh J, Singh B, Alias A, Abdullah MS (1995) Assessment of the association between three pfmdr1 point mutations and chloroquine resistance in vitro of Malaysian Plasmodium falciparum isolates. Trans R Soc Trop Med Hyg 89(4): 436–7. [DOI] [PubMed] [Google Scholar]
- 8. Adagu IS, Dias F, Pinheiro L, Rombo L, do Rosario V, Warhurst DC (1996) Guinea Bissau: association of chloroquine resistance of Plasmodium falciparum with the Tyr86 allele of the multiple drug-resistance gene Pfmdr1. Trans R Soc Trop Med Hyg 90(1): 90–91. [DOI] [PubMed] [Google Scholar]
- 9. Duraisingh MT, Drakeley CJ, Muller O, Bailey R, Snounou G, et al. (1997) Evidence for selection for the tyrosine-86 allele of the pfmdr 1 gene of Plasmodium falciparum by chloroquine and amodiaquine. Parasitol 114: 205–11. [DOI] [PubMed] [Google Scholar]
- 10. Chaijaroenkul W, Ward SA, Mungthin M (2011) Sequence and gene expression of chloroquine resistance transporter (pfcrt) in the association of in vitro drugs resistance of Plasmodium falciparum . Malaria J 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murambiwa P, Masola B, Govender T (2011) Anti-malarial drug formulations and novel delivery systems: A review. Acta Tropica1 18: 71–79. [DOI] [PubMed] [Google Scholar]
- 12. Atroosh WM, Mekhlafi HM, Mahdy MAK, Surin J (2012) The detection of pfcrt and pfmdr1point mutations as molecular markers of chloroquine drug resistance, Pahang, Malaysia. Malaria J 11: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pickard AL, Wongsrichanalai C, Purfield A, Kamwendo D, Emery K, et al. (2003) Resistance to antimalarials in Southeast Asia and genetic polymorphisms in pfmdr1 . Antimicrob Agents Chemother 47 2418–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fidock DA, Nomura T, Talley AK (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein Pfcrt and evidence for their role in chloroquine resistance. Mol Cell 6(4): 861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sidhu AB, Verdier-Pinard D, Fidock DA (2002) Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298(5591): 210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vathsala PG, Pramanik A, Dhanasekaran S (2004) Widespread occurrence of the Plasmodium falciparum chloroquine resistance transporter (Pfcrt) gene haplotype SVMNT in P. falciparum malaria in India. Am J Trop Med Hyg 70(3): 256–9. [PubMed] [Google Scholar]
- 17. Ojurongbe O, Ogungbamigbe TO, Beyioku AFF (2007) Rapid detection of Pfcrt and Pfmdr1 mutations in Plasmodium falciparum isolates by FRET and in vivo response to chloroquine among children from Osogbo, Nigeria. Malaria J 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mekhlafi AMA, Mahdy MAK, Mekhlafi HM (2011) High frequency of Plasmodium falciparum chloroquine resistance marker (pfcrt T76 mutation) in Yemen: An urgent need to re-examine malaria drug policy. Parasit Vect 4: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veiga MI, Ferreira PE, Jornhagen L (2011) Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS ONE 6(5): e20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhattacharya PR, Pillai CR (1999) Strong association, but incomplete correlation, between chloroquine resistance and allelic variation in the pfmdr-1 gene of Plasmodium falciparum isolates from India. Ann Trop Med Parasitol 93(7): 679–84. [DOI] [PubMed] [Google Scholar]
- 21. Ranjitkar S, Schousboe ML, Thomsen TT (2011) Prevalence of molecular markers of anti-malarial drug resistance in Plasmodium vivax and Plasmodium falciparum in two districts of Nepal. Malaria J 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alam MT, Souza DK, Vinayak S (2011) Selective sweeps and genetic lineages of Plasmodium falciparum drug -resistant alleles in Ghana. J Infect Dis 203: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization (2001) In vitro micro-test (mark III) for the assessment of the response of P. falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin.
- 24. Foley M, Ranford-Cartwright LC, Babiker HA (1992) Rapid and simple method for isolating malaria DNA from finger prick samples of blood. Mol Biochem Parasitol 53(2): 241–4. [DOI] [PubMed] [Google Scholar]
- 25. Djimdé A, Doumbo OK, Cortese JF (2001) A molecular marker for chloroquine resistant falciparum malaria. New Engl J Med 344(4): 257–63. [DOI] [PubMed] [Google Scholar]
- 26. Wernsdorfer & Wernsdorfer (1995) The evaluation of in vitro tests for the assessment of drug response in Plasmodium falciparum . Mitt Österr Ges Trop Med Paras 17: 221–228. [Google Scholar]
- 27.Smith JM (1968) Evolutionary Genetics. Oxford University Press, Oxford.
- 28. Hill WG, Robertson A (1968) The effects of inbreeding at loci with heterozygote advantage. Genetics 60(3): 615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore DV, Lanier JE (1961) Observations on two Plasmodium falciparum infections with an abnormal response to chloroquine. Am J Trop Med Hyg 10: 5–9. [DOI] [PubMed] [Google Scholar]
- 30. Sehgal PN, Sharma MID, Sharma SL (1973) Resistance to chloroquine in falciparum malaria in Assam State, India. J Com Dis 5(4): 175–80. [Google Scholar]
- 31. Choi KH, Chen CJ, Kriegler M, Roninson IB (1988) An altered pattern of cross-resistance in multidrug-resistant human cells results from spontaneous mutations in the mdr1 (P-glycoprotein) gene. Cell 53(4): 519–29. [DOI] [PubMed] [Google Scholar]
- 32. Figueiredo P, Benchimol C, Lopes D (2008) Prevalence of pfmdr1, pfcrt, pfdhfr and pfdhps mutations associated with drug resistance, in Luanda, Angola. Malaria J 7: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Le Bras J, Deloron P, Ricour A (1983) Plasmodium falciparum: drug sensitivity in vitro of isolates before and after adaptation to continuous culture. Exp Parasitol 56(1): 9–14. [DOI] [PubMed] [Google Scholar]
- 34. Walliker D, Hunt P, Babiker H (2005) Fitness of drug-resistant malaria parasites. Acta Trop 94(3): 251–9. [DOI] [PubMed] [Google Scholar]
- 35. Costanzo MS, Kyle M, Brown D, Hart L (2011) Fitness Trade-Offs in the Evolution of Dihydrofolate Reductase and Drug Resistance in Plasmodium falciparum . PLoS ONE 6(5): e19636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghanchi NK, Ursing J, Beg M (2011) Prevalence of resistance associated polymorphisms in Plasmodium falciparum field isolates from southern Pakistan. Malaria J 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dajem SM, Ahmed A (2010) Analysis of gene mutations involved in chloroquine resistance in Plasmodium falciparum parasites isolated from patients in the southwest of Saudi Arabia. Ann Saudi Med 30(3): 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adagu IS, Warhurst DC (2001) Plasmodium falciparum: linkage disequilibrium between loci in chromosomes 7 and 5 and chloroquine selective pressure in Northern Nigeria. Parasitol 123 (3): 219–24. [DOI] [PubMed] [Google Scholar]