Abstract
Purpose of review
Racial/ethnic and socioeconomic disparities in chronic kidney disease (CKD) have been documented for decades, yet little progress has been made in mitigating them. Several recent studies offer new insights into the root causes of these disparities, point to areas where future research is warranted and identify opportunities for changes in policy and clinical practice.
Recent findings
Recently published evidence suggests that geographic disparities in CKD prevalence exist and vary by race. CKD progression is more rapid for racial/ethnic minority groups as compared to whites and may be largely, but not completely, explained by genetic factors. Stark socioeconomic disparities in outcomes for dialysis patients exist, and vary by race, place of residence and treatment facility. Disparities in access to living kidney donation may be driven primarily by the socioeconomic status of the donor as opposed to recipient factors.
Summary
Recent studies highlight opportunities to eliminate disparities in CKD, including efforts to direct resources to areas and populations where disparities are most prevalent, efforts to understand how to best use emerging information on the contribution of genetic factors to disparities, and continued work to identify modifiable environmental, social, and behavioral factors for targeted interventions among high-risk populations.
Keywords: socioeconomic status, race, ethnicity, renal, kidney transplantation
INTRODUCTION
Over the past two decades, disparities in chronic kidney disease (CKD) have been well-documented throughout much of the world. [1–4] Racial and ethnic minorities suffer disproportionally from advanced and progressive pre-end stage renal disease (ESRD) CKD, and more than 3 times the incidence of ESRD when compared to whites. [5] Socioeconomic gradients in ESRD risk have also been well-described, [6,7] with lower socioeconomic status (SES) individuals bearing the greatest burden. Additionally, interactions of race/ethnicity and SES in determining disparities in CKD have been suggested, with racial/ethnic minorities of low SES suffering the worst outcomes. [3,7–9] In this review, we highlight several studies on disparities in CKD published in 2013, and we offer insights regarding studies’ implications on efforts to address the root causes of disparities.
DISPARITIES IN THE BURDEN OF CKD
CKD prevalence varies substantially across different nations and geographic variability has also been reported within several nations. In the U.S., Tanner et al [10*] described regional variations in prevalence of albuminuria and decreased estimated glomerular filtration rate (eGFR) among black and white adults 45 years or older in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. However, differences correlated only modestly with U.S. Renal Data System (USRDS) network-specific ESRD incidence. Further, for whites in the study, the highest correlation was between network-specific mean income <$20,000 with ESRD incidence rates. Whereas, for blacks, network-specific obesity and history of cardiovascular disease were most correlated with ESRD incidence. These data suggest that CKD prevalence may not explain the well-documented geographic variation in ESRD incidence, and regions with high CKD prevalence may warrant greater resources to prevent the complications and health expenditures associated with CKD [11], independent of their ESRD burden. [10*] These data suggest a potential differential impact of ESRD risk factors across race, and highlight the need for further studies elucidating potential race differences in modifiable CKD risk factors.
In a related analysis of the REGARDS study, Plantinga et al found that blacks but not whites residing in the Southeastern U.S. their entire lives were at greater risk of ESRD, but there was no clear geographic pattern for earlier-stage CKD. This potential effect modification by race was strongest among individuals earning less than $35,000. [12] The Southeastern U.S. is well-known to have higher prevalence of diabetes [13] and hypertension [14] than other regions, and the Southeastern states overlay what is called the ‘stroke belt’ due to high incidence of cerebrovascular events. [15] While the causes of geographic disparities in CKD are likely multifaceted, and, especially in the case of the racial variation, may include psychosocial stressors such as discrimination [16]--they may be surmountable. In Japan, for example, marked regional variation in ESRD incidence was noted in 1984 to 1991 [17] and was inversely correlated with use of renin-angiotensin system (RAS) inhibitors [18]. This disparity disappeared by 2001 to 2008, presumably due, in part, to increased RAS inhibitor use in the previously disparate regions. [19]
Vart et al [20*] investigated the relation of ncome level and educational attainment to prevalent CKD in general population-based cohorts in the United States [1999–2002 National Health and Nutritional Examination Survey (NHANES)] and The Netherlands [Prevention of Renal and Vascular End-stage Disease (PREVEND 1997–1998)]. In NHANES, income was strongly and independently associated with CKD but education was not. In contrast, in PREVEND, low income was weakly associated with CKD whereas low education had a strong association. If validated in longitudinal studies, these findings imply that improved access to healthcare in the U.S. could pose an opportunity to mitigate socioeconomic disparities in CKD; while efforts to modify health behaviors through education might reflect a more salient modifiable risk factor in countries with long-standing universal access to health care.
CONTRIBUTION OF GENETIC FACTORS TO DISPARITIES IN CKD PROGRESSION
In a study by Parsa and Kao et al [21**], the effects of variants in the gene encoding apolipoprotein L1 (APOL1) on CKD progression was examined in the African American Study of Kidney Disease and Hypertension (AASK), a clinical trial of the effectiveness antihypertensive medications and blood pressure targets on outcomes among blacks with CKD attributed to hypertension, and the Chronic Renal Insufficiency Cohort (CRIC) study, an observational cohort study including both white and black participants with CKD (46% with diabetes). Participants were grouped according to whether they had 2 copies of high-risk APOL1 variants (high-risk group) or 0 or 1 copy (low-risk group). Fifty-eight percent of the high-risk variant participants in AASK experienced ESRD or doubling of serum creatinine during follow up, compared to 37% of the low-risk group, and APOL1 status did not modify the effects of proteinuria or the treatment regimens evaluated. In the CRIC study, the multivariable adjusted hazard ratio for the composite renal outcome (ESRD or a 50% reduction in eGFR during follow up) comparing blacks in the high-risk group to all whites was 1.95 (95% CI, 1.39 to 2.73) for persons with diabetes and was 2.68 for those without diabetes. (Table 1) Notably, among blacks in the low-risk group compared to all whites, there remained a 40% greater risk of the composite renal outcome.
Table 1.
Multivariable Analyses of Differences in the eGFR Slope and Risk of the ^Composite Renal Outcome in the CRIC Study
| Multivariate Model and Comparison Group |
Difference in eGFR Slope | Risk of Composite Renal Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| With Diabetes | Without Diabetes | With Diabetes | Without Diabetes | |||||
| ml/min/1.73 m2/yr (95% CI) |
P value | ml/min/1.73 m2/yr (95% CI) |
P value |
hazard ratio (95% CI) |
P value | hazard ratio (95% CI) |
P value | |
| Model 3 | ||||||||
| All black patients vs. all white patients | −0.48 (−0.88 to −0.09) | 0.02 | −0.17 (−0.48 to 0.13) | 0.27 | 1.49 (1.18 to 1.88) | <0.001 | 1.80 (1.31 to 2.49) | <0.001 |
| Black patients with APOLI high risk vs. all white patients | −1.32 (−2.02 to −0.63) | <0.001 | −1.05 (−1.54 to −0.56) | <0.001 | 1.95 (1.39 to 2.73) | <0.001 | 2.68 (1.78 to 4.05) | <0.001 |
| Black patients with APOLI low risk vs. all white patients | −0.35 (−0.75 to 0.06) | 0.09 | 0.08 (−0.25 to −0.40) | 0.65 | 1.40 (1.10 to 1.78) | 0.006 | 1.57 (1.11 to 2.21) | 0.01 |
| Model 4 | ||||||||
| All black patients vs. all white patients | −.0.21 (−0.55 to 0.14) | 0.25 | −0.21 (−0.50 to 0.08) | 0.16 | 1.34 (1.06to 1.70) | 0.02 | 1.98 (1.44 to 2.72) | <0.001 |
| Black patients with APOLI high risk vs. all white patients | −0.79 (−1.41 to −0.17) | 0.01 | −0.81 (−1.26 10−0.35) | <0.001 | 1.58 (1.12 to 2.24) | 0.009 | 2.84 (1.84 to 4.38) | <0.001 |
| Black patients with APOLI l ow risk vs. all white patients | −.0.11 (−0.47 to 0.25) | 0.55 | −0.03 (−0.34 to 0.27) | 0.84 | 1.29 (1.01 to 1.65) | 0.04 | 1.78 (1.28 to 2.49) | <0.001 |
The composite outcome was incident end-stage renal disease or a reduction of 50% in the eGFR from baseline. The APOL1 risk was based on the recessive genetic model. Model 3 is the multivariable-adjusted base model with adjustment for age, sex, clinical site, baseline eGFR, education level, treatment by a nephrologist, the use of either an angiotensin-converting–enzyme inhibitor or an angiotensin-receptor blocker, systolic blood pressure, body-mass index, glycated hemoglobin level, and smoking status. Model 4 includes all the variables in model 3 plus the total 24-hour urinary protein excretion. Adapted with permission PENDING from Ref [21**].
There are several important implications of the study by Parsa and Kao et al. First, it appears that APOL1 high-risk variants may increase the risk of progression of CKD among blacks, regardless of the cause of CKD (ie. diabetic or non-diabetic nephropathies), extending insight gained from previous studies addressing this question [22–24]. Second, in AASK, participants’ response to hypertension treatment regimen did not differ by APOL1 variant group, suggesting no current role for genotyping black patients for determination of best pharmacologic therapy [25] or blood pressure target. Rather, the same mainstays of treatment (e.g. angiotensin converting enzyme inhibitors and control of hypertension) appear to be warranted for black patients (and have similar effects on CKD progression) regardless of genotype. Third, as not all AASK participants in the APOL1 high risk group experienced CKD progression (40% did not), an additional genetic or environmental ‘hit’ (e.g. HIV infection) is likely necessary to potentiate APOL1-associated nephropathy. A recent study suggested that non-HIV infections such as urinary tract JC polyoma virus infection may have a protective role against APOL1-mediated risk of kidney disease. [26] Fourth, even blacks in the low-risk APOL1 variant group (77%) in the CRIC study had greater risk of CKD progression than whites (Table 1), suggesting racial disparities in CKD progression are not fully explained by genetic factors. As we await further elucidation of the role of high-risk variants in the onset and progression of CKD among blacks, studies performed among this ‘lower-risk’ population may present the most promising opportunities to identify modifiable root cause for disparities.
CONTRIBUTION OF NON-GENETIC FACTORS TO DISPARITIES IN CKD PROGRESSION
In a study by Derose et al [27*] of over 1 million adults in the Kaiser Permanente Southern California managed care system, more extreme rates of eGFR decline were observed in blacks. Projected kidney failure (based on predicted eGFR <15 ml/min/1.73m2 at specified times) during CKD stages 3 and 4 was high in blacks, Hispanics, and Asians relative to whites. Death prior to developing ESRD was most common among whites and least common among Asians. Notably, even among the group with projected ESRD, death was 7 times more common than ESRD in whites and about twice as common in Hispanics, Asians, and blacks. In analyses accounting for both, differences in eGFR decline and mortality contributed to racial disparities in the incidence of ESRD. These findings highlight CKD as a strong risk factor for mortality among each major racial/ethnic group, and the importance of considering CKD-related mortality in studies of racial differences in CKD progression. For example, in a U.S. Veteran’s Administration study of male patients, black patients with CKD had lower mortality compared with white patients. The survival advantage seen in blacks was accentuated in the setting of more advanced stages of CKD, which was postulated to be due to changes in case-mix and laboratory characteristics occurring during the course of kidney disease. [28]
In the Kidney Early Evaluation Program, a nationwide community-based health screening study of over 120,000 individuals at high risk of kidney disease, Chang et al [29*] found that, among persons with preserved eGFR at baseline, the risk of developing treated ESRD during follow up was increased among blacks (as compared to Non-Hispanic whites), irrespective of their presence of albuminuria at baseline. Notably, this study found no differences in incident ESRD among Hispanics compared to Non-Hispanic whites, and no differences according to education or health insurance status. These findings suggest screening for CKD and CKD risk modification may be warranted among blacks with or without albuminuria. A study by Gutiérrez et al demonstrated that that higher urinary albumin-to-creatinine ratio (ACR) was associated with greater risk of incident but not recurrent coronary heart disease in black individuals when compared with whites, suggesting that blacks have greater susceptibility to vascular disease and underscoring the potential benefits of screening among blacks. [30*]
Towards understanding risk factors for CKD progression among the urban poor, Hall et al [31*] conducted a study of this population being treated at the Community Health Network (CHN) in San Francisco, California. They found that younger age, male sex, non-White race/ ethnicity, public health insurance coverage (Medicare and Medicaid), diabetes, lower eGFR, higher proteinuria, lower hemoglobin level, and lower serum albumin concentration were significantly associated with a higher adjusted risk of progression to ESRD. In contrast, they observed no significant association between other factors including substance abuse, HIV/ AIDS, viral hepatitis (HBV or HCV), and non- English language with higher risk of ESRD after adjustment for traditional risk factors. Importantly, the authors note that the availability of interpreter services and access to ambulatory care in the CHN may have attenuated unfavorable outcomes typically associated with linguistic barriers to care. These findings emphasize the importance of continued focus on control of traditional risk factors to mitigate disparities in CKD progression among disadvantaged populations.
A study in Alberta, Canada found that progression to kidney failure was 3 times faster for First Nations as compared to Non-First Nations participants in an analysis of over 1.8 million adults. Similar to the findings by Chang et al among U.S. racial/ethnic groups [29*], this difference was not explained by the greater prevalence of albuminuria among the First Nations participants, and albuminuria conferred a similar risk of progression to kidney failure for First Nations and Non–First Nations people [32]. Potential explanations for this disparity offered by the authors, and opportunities for intervention, include First Nations people’s documented receipt of lower quality health care and limited access to specialist care. [33,34]
In the PREdialysis PAtient REcord study based in the The Netherlands, black and white adults were compared at the initiation of pre-dialysis nephrology care in a universal health care system. Blacks initiated pre-dialysis care at higher eGFRs than whites, and blacks experienced more rapid renal function decline. [35] These findings underscore the likely additional contributions of behavioral and genetic contributions to racial disparities beyond access to health care.
DISPARITIES IN DIALYSIS OUTCOMES
In a 2013 analysis of all USRDS patients (nearly 600,000) initiating hemodialysis between 2000 and 2008, patients’ residence in areas with higher median household income was associated with improved survival. Using measures of income distributional inequality and residential racial segregation, Kimmel et al [36**] reported that among whites, income inequality was associated with increased risk of mortality. Among blacks exclusively, residence in highly racially segregated areas was associated with increased mortality. (Table 2) These complex results both identify high-risk populations for mortality on dialysis and shed light on knowledge gaps in ESRD disparities. Studies are urgently needed that measure contextual experiences of CKD patients (i.e. discrimination [16]), neighborhood factors that vary by median income and residential segregation (i.e. availability of healthy foods [37]), and relate these to dialysis outcomes.
Table 2.
Adjusted Hazard Ratios (95% Confidence Interval, CI) for Death Associated with Income, Gini Index (measure of income inequality), and Residential Segregation, by Race
| Characteristics | White (n=370,861) | Black (n=209,768) | ||
|---|---|---|---|---|
| HR (95% Cl) | P Value | HR (95% Cl) | P Value | |
| Income | ||||
| $2,500 to <$20,000 | 1.02 (0.96–1.09) | 0.53 | 1.08 (1.05–1.11) | <0.001 |
| $20,000 to <$30,000 | 1.04 (1.02–1.05) | <0.001 | 1.04 (1.01–1.06) | 0.002 |
| $30,000 to <$40,000 | 1.00 | 1.00 | ||
| $40,000 to <$50,000 | 0.99 (0.98–1.01) | 0.30 | 0.96 (0.93–0.99) | 0.01 |
| $50,000 to <$60,000 | 0.97 (0.96–0.99) | <0.001 | 0.95 (0.91–1.00) | 0.03 |
| $60,000 to <$70,000 | 0.95 (0.93–0.98) | <0.001 | 0.91 (0.86–0.96) | 0.001 |
| $70,000 | 0.94 (0.92–0.97) | <0.001 | 0.95 (0.89–1.00) | 0.05 |
| Gini Index^ | ||||
| First quartile (lowest) | 1.00 | 1.00 | ||
| Second quartile | 1.00 (0.98–1.02) | 1.00 | 0.98 (0.93–1.04) | 0.51 |
| Third quartile | 1.01 (0.99–1.03) | 0.20 | 0.96 (0.91–1.01) | 0.10 |
| Fourth quartile (highest) | 1.06 (1.04–1.09) | <0.001 | 0.99 (0.94–1.04) | 0.63 |
| Residential segregation | ||||
| First quartile (lowest) | 1.00 | 1.00 | ||
| Second quartile | 1.00 (0.98–1.03) | 0.81 | 1.06 (1.02–1.11) | 0.006 |
| Third quartile | 1.01 (0.99–1.03) | 0.35 | 1.07 (1.03–1.12) | 0.002 |
| Fourth quartile (highest) | 1.01 (0.99–1.04) | 0.27 | 1.13 (1.09–1.18) | <0.001 |
Cox proportional hazards model adjusted for income, Gini Index, segregation, sex, age and year of dialysis initiation, body mass index, smoking, diabetes, polycystic kidney disease, AIDS nephropathy, insurance, prior employment status, and comorbid conditions (chronic obstructive pulmonary disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, atherosclerotic heart disease, and cancer).
Quartile definitions for Gini Index and residential segregation were as follows: for Gini Index, 0.33–0.41, 0.41–0.43, 0.43–0.46, and 0.46–0.60; for residential segregation, 0–39.50, 39.50–48.95, 48.95–58.60, and 58.60–94.00. Adapted with permission PENDING from Ref [36**].
Hall et al [38**] conducted an analysis of the USRDS data and categorized dialysis facilities according to the proportion of racial-ethnic minority patients initiating dialysis at each facility during 2005–2008. The predominantly ‘minority-serving’ facilities (i.e. those with the greatest proportion of minorities initiating dialysis) were markedly larger, and were less likely to offer home dialysis therapies than facilities serving predominantly white patients. A significantly greater proportion of minority-serving dialysis facilities reported worse than expected survival as compared with facilities serving predominantly white patients, despite similar clinical performance measures for anemia and dialysis adequacy across minority-serving status. Further, wait-listing for transplantation was lower among the predominantly minority-serving facilities. Authors suggested differences may have been, in part, attributable to differences in the presence and quality of pre-dialysis care.
DISPARITIES IN ACCESS TO KIDNEY TRANSPLANTATION
Several studies in 2013 attempted to unravel the complex relation of race, SES and access to kidney transplantation. In Australia, a study of non-Indigenous adults by Grace et al [39*] revealed that patients from socioeconomically advantaged areas were more likely to receive a kidney from a living donor, both before and after dialysis initiation. However, SES was not associated with the receipt of a kidney from a deceased donor. Authors suggest that financial barriers and comorbid disease of potential donors may limit access to living kidney donation (LKD) in low SES areas, whereas the provision of subsidized immunosuppressive drugs for the life of the allograft may contribute to equal access to deceased donor transplants.
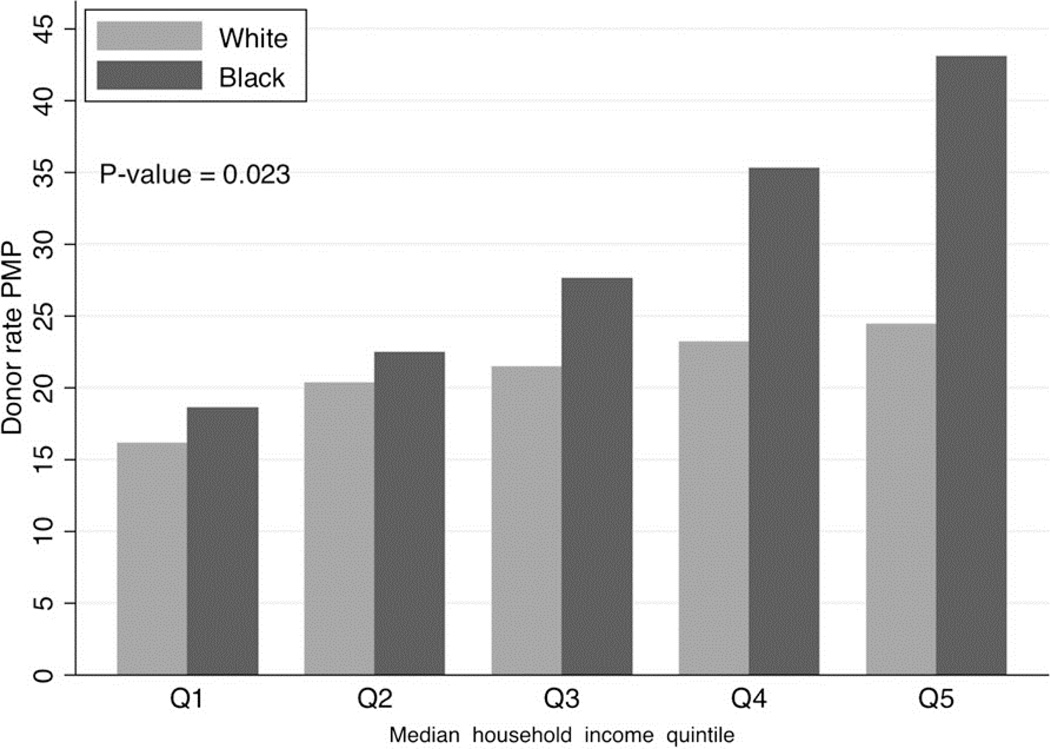
In a study of race-specific barriers to LKD in the U.S., Gill et al [40**] used data on all African-American and white living kidney donors to examine the associations between LKD and donor median household income and race. They found that LKD was greater in the higher-income quintiles for both racial groups. Of particular note, the total incidence of LKD was higher in the African-American population than in the white population, but ratios varied by income. The incidence of LKD was lower among African-Americans than whites in the lowest income quintile, but higher among African-Americans in the three highest income quintiles (Figure 1). These findings suggest racial disparities in access to LKD are strongly influenced by financial barriers, which could disproportionately influence racial minorities’ access to LKD.
Figure 1.
Age and sex standardized living donor rates per million population (PMP) by quintile of median household income in African-American and white populations. Reprinted with permission from Ref [40**]
In a Canadian study, socioeconomic disparities in access to transplantation varied by age as well as race. Promislow et al found that rates of both live and deceased donor transplants were lower among Aboriginals under the age of 60 years compared to Caucasians, but were similar among those older than 60 years. [41] A single-center study conducted in Miami, Florida found black and Hispanic patients had longer waiting times from initiating dialysis to transplant wait listing when compared to whites. This disparity was largely explained by differences in SES and lower rates of preemptive transplantation among racial/ethnic minority patients. [42]
CONCLUSION
Worldwide disparities persist in CKD prevalence, progression and treatment. Recent studies point to geographic disparities in CKD, racial/ethnic disparities in kidney function decline, and disparities in ESRD treatment-related outcomes. Studies highlight numerous opportunities to eliminate disparities, including efforts to direct resources to areas and populations where disparities are most prevalent, efforts to understand how to best use emerging information on the contribution of genetic factors to disparities, and continued work to identify modifiable environmental, social, and behavioral factors for targeted interventions to improve the care and outcomes of CKD for all patients.
KEY POINTS.
Geographic disparities in CKD prevalence exist and vary by race.
CKD progression is more rapid for racial/ethnic minority groups as compared to whites and may be largely, but not completely, explained by genetic factors.
Stark socioeconomic disparities in outcomes for dialysis patients exist, and vary by race, place of residence and treatment facility.
Disparities in access to living kidney donation may be driven primarily by the socioeconomic status of the donor as opposed to recipient factors.
ACKNOWLEDGEMENTS
Dr. Crews was supported by grant K23DK097184 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH), Bethesda, Maryland and the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton, New Jersey USA. Dr. Boulware was supported by grants P50 HL105187 and HHSN26820130047C from the National Heart, Lung and Blood Institute and grants R34 DK094116, R01 DK098759 and R01 DK102134 from the NIDDK, NIH.
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors have no relevant conflicts of interest.
References
- 1.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–174. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 2.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Seminars in nephrology. 2013;33:409–415. doi: 10.1016/j.semnephrol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews DC, Pfaff T, Powe NR. Socioeconomic factors and racial disparities in kidney disease outcomes. Seminars in nephrology. 2013;33:468–475. doi: 10.1016/j.semnephrol.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Amaral S, Patzer RE, Kutner N, McClellan W. Racial disparities in access to pediatric kidney transplantation since share 35. J Am Soc Nephrol. 2012;23:1069–1077. doi: 10.1681/ASN.2011121145. [DOI] [PubMed] [Google Scholar]
- 5.US Renal Data System. Bethesda, MD: 2012. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease. [Google Scholar]
- 6.Young EW, Mauger EA, Jiang KH, et al. Socioeconomic status and end-stage renal disease in the United States. Kidney Int. 1994;45:907–911. doi: 10.1038/ki.1994.120. [DOI] [PubMed] [Google Scholar]
- 7.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews DC, Charles RF, Evans MK, et al. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crews DC, McClellan WM, Shoham DA, et al. Low income and albuminuria among REGARDS (Reasons for Geographic and Racial Differences in Stroke) study participants. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;60:779–786. doi: 10.1053/j.ajkd.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner RM, Gutierrez OM, Judd S, et al. Geographic variation in CKD prevalence and ESRD incidence in the United States: results from the reasons for geographic and racial differences in stroke (REGARDS) study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:395–403. doi: 10.1053/j.ajkd.2012.10.018. Regional variations in prevalence of albuminuria and decreased estimated glomerular filtration rate correlated only modestly with U.S. Renal Data System network-specific ESRD incidence. For whites, the highest correlation was between network-specific mean income <$20,000 with ESRD incidence rates, whereas, for blacks, network-specific obesity and history of cardiovascular disease were most correlated with ESRD incidence.
- 11.Smith DH, Gullion CM, Nichols G, et al. Cost of medical care for chronic kidney disease and comorbidity among enrollees in a large HMO population. Journal of the American Society of Nephrology : JASN. 2004;15:1300–1306. doi: 10.1097/01.asn.0000125670.64996.bb. [DOI] [PubMed] [Google Scholar]
- 12.Plantinga L, Howard VJ, Judd S, et al. Association of duration of residence in the southeastern United States with chronic kidney disease may differ by race: the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Int J Health Geogr. 2013;12:17. doi: 10.1186/1476-072X-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker LE, Kirtland KA, Gregg EW, et al. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. American journal of preventive medicine. 2011;40:434–439. doi: 10.1016/j.amepre.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. Journal of the American Society of Hypertension : JASH. 2010;4:32–41. doi: 10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glymour MM, Kosheleva A, Boden-Albala B. Birth and adult residence in the Stroke Belt independently predict stroke mortality. Neurology. 2009;73:1858–1865. doi: 10.1212/WNL.0b013e3181c47cad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce MA, Beech BM, Sims M, et al. Social environmental stressors, psychological factors, and kidney disease. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2009;57:583–589. doi: 10.231/JIM.0b013e31819dbb91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usami T, Koyama K, Takeuchi O, et al. Regional variations in the incidence of end-stage renal failure in Japan. JAMA : the journal of the American Medical Association. 2000;284:2622–2624. doi: 10.1001/jama.284.20.2622. [DOI] [PubMed] [Google Scholar]
- 18.Usami T, Nakao N, Fukuda M, et al. Maps of end-stage renal disease and amounts of angiotensin-converting enzyme inhibitors prescribed in Japan. Kidney international. 2003;64:1445–1449. doi: 10.1046/j.1523-1755.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 19.Wakamatsu-Yamanaka T, Fukuda M, Sato R, et al. Geographic differences in the increasing ESRD rate have disappeared in Japan. Clinical and experimental nephrology. 2011;15:708–713. doi: 10.1007/s10157-011-0466-5. [DOI] [PubMed] [Google Scholar]
- 20. Vart P, Gansevoort RT, Coresh J, et al. Socioeconomic Measures and CKD in the United States and The Netherlands. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1685–1693. doi: 10.2215/CJN.12521212. In the U.S., income was strongly and independently associated with CKD but education was not. In contrast, in The Netherlands, low income was weakly associated with CKD whereas low education had a strong association.
- 21. Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. Renal risk variants in APOL1 were associated with higher rates of end-stage renal disease and progressive chronic kidney disease in a study of blacks, and in a study comparing black and white patients with CKD. Higher risk was observed regardless of diabetes status. Among blacks in the low-risk group compared to all whites, there remained a 40% greater risk of the composite renal outcomes. Findings suggest that genetic factors account for some, but not all, of black-white disparities in CKD progression.
- 22.Pollak MR, Genovese G, Friedman DJ. APOL1 and kidney disease. Current opinion in nephrology and hypertension. 2012;21:179–182. doi: 10.1097/MNH.0b013e32835012ab. [DOI] [PubMed] [Google Scholar]
- 23.Friedman DJ, Kozlitina J, Genovese G, et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–2105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24:1484–1491. doi: 10.1681/ASN.2013010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams WW, Pollak MR. Health disparities in kidney disease--emerging data from the human genome. The New England journal of medicine. 2013;369:2260–2261. doi: 10.1056/NEJMe1312797. [DOI] [PubMed] [Google Scholar]
- 26.Divers J, Nunez M, High KP, et al. JC polyoma virus interacts with APOL1 in African Americans with nondiabetic nephropathy. Kidney Int. 2013;8:1207–1213. doi: 10.1038/ki.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Derose SF, Rutkowski MP, Crooks PW, et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:236–244. doi: 10.1053/j.ajkd.2013.01.019. Projected kidney failure during CKD stages 3 and 4 was high in blacks, Hispanics, and Asians relative to whites. Even among the group with projected ESRD, death was 7 times more common than ESRD in whites and about twice as common in Hispanics, Asians, and blacks.
- 28.Kovesdy CP, Quarles LD, Lott EH, et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;62:228–235. doi: 10.1053/j.ajkd.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang TI, Li S, Chen SC, et al. Risk factors for ESRD in individuals with preserved estimated GFR with and without albuminuria: results from the Kidney Early Evaluation Program (KEEP) American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:S4–S11. doi: 10.1053/j.ajkd.2012.12.016. Risk of developing treated ESRD during follow up was increased among blacks (as compared to Non-Hispanic whites), irrespective of baseline albuminuria. There were no differences in incident ESRD among Hispanics compared to Non-Hispanic whites, and no differences according to education or health insurance status.
- 30. Gutierrez OM, Khodneva YA, Muntner P, et al. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706–714. doi: 10.1001/jama.2013.8777. Higher urinary albumin-to-creatinine ratio was associated with greater risk of incident but not recurrent coronary heart disease in blacks compared to whites, suggesting that blacks have greater susceptibility to vascular disease.
- 31. Hall YN, Choi AI, Xu P, et al. Predictors of end-stage renal disease in the urban poor. Journal of health care for the poor and underserved. 2013;24:1686–1700. doi: 10.1353/hpu.2013.0189. Traditional risk factors, such as younger age, male sex, non-White race/ ethnicity, public health insurance coverage, diabetes, and higher proteinuria were associated with higher risk of ESRD among the urban poor, however social or societally-determined factors such as substance abuse and HIV/AIDS were not. Results highlight the importance of addressing traditional risk factors in efforts to reduce disparities.
- 32.Samuel SM, Palacios-Derflingher L, Tonelli M, et al. Association between First Nations ethnicity and progression to kidney failure by presence and severity of albuminuria. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2013 doi: 10.1503/cmaj.130776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah BR, Gunraj N, Hux JE. Markers of access to and quality of primary care for aboriginal people in Ontario, Canada. American journal of public health. 2003;93:798–802. doi: 10.2105/ajph.93.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao S, Manns BJ, Culleton BF, et al. Access to health care among status Aboriginal people with chronic kidney disease. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2008;179:1007–1012. doi: 10.1503/cmaj.080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Beukel TO, de Goeij MC, Dekker FW, et al. Differences in Progression to ESRD between Black and White Patients Receiving Predialysis Care in a Universal Health Care System. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:1540–1547. doi: 10.2215/CJN.10761012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2013;24:293–301. doi: 10.1681/ASN.2012070659. Among whites, income inequality was associated with increased risk of mortality. Among blacks exclusively, residence in highly racially segregated areas was associated with increased mortality. Findings call for further study into the contextual factors responsible for these disparities.
- 37.Franco M, Diez Roux AV, Glass TA, et al. Neighborhood characteristics and availability of healthy foods in Baltimore. American journal of preventive medicine. 2008;35:561–567. doi: 10.1016/j.amepre.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall YN, Xu P, Chertow GM, Himmelfarb J. Characteristics and Performance of Minority-Serving Dialysis Facilities. Health services research. 2013 doi: 10.1111/1475-6773.12144. U.S. predominantly minority-serving facilities were markedly larger, and less likely to offer home dialysis therapies than those serving predominantly white patients. A greater proportion of minority-serving dialysis facilities reported worse than expected survival, despite similar clinical performance measures for anemia and dialysis adequacy across minority-serving status. Differences may have been, in part, attributable to differences in the presence and quality of pre-dialysis care.
- 39. Grace BS, Clayton PA, Cass A, McDonald SP. Transplantation rates for living- but not deceased-donor kidneys vary with socioeconomic status in Australia. Kidney international. 2013;83:138–145. doi: 10.1038/ki.2012.304. Patients from socioeconomically advantaged areas were more likely to receive a kidney from a living donor, both before and after dialysis initiation. However, SES was not associated with the receipt of a kidney from a deceased donor. Financial barriers of potential donors may limit access to living kidney donation in low SES areas, whereas the provision of subsidized immuno-suppressive drugs may contribute to equal access to deceased donor transplants.
- 40. Gill J, Dong J, Rose C, et al. The effect of race and income on living kidney donation in the United States. Journal of the American Society of Nephrology : JASN. 2013;24:1872–1879. doi: 10.1681/ASN.2013010049. Living kidney donation (LKD) was greater in the higher-income quintiles for both African Americans and whites. The total incidence of LKD was higher among African-Americans than whites, but ratios varied by income. The incidence of LKD was lower among African-Americans than whites in the lowest income quintile, but higher among African-Americans in the three highest income quintiles, suggesting that racial disparities in access to LKD are strongly influenced by financial barriers.
- 41.Promislow S, Hemmelgarn B, Rigatto C, et al. Young aboriginals are less likely to receive a renal transplant: a Canadian national study. BMC nephrology. 2013;14:11. doi: 10.1186/1471-2369-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95:309–318. doi: 10.1097/TP.0b013e31827191d4. [DOI] [PubMed] [Google Scholar]