Abstract
We evaluated the competition among stored-product psocid species by conducting two series of laboratory experiments. In the first series, three species of Liposcelididae were used: Liposcelis bostrychophila, Liposcelis decolor, and Liposcelis paeta. Five adult females of these species were placed in vials containing wheat, either alone or in all possible combinations of two species. The number of adults in the vials was counted after 35, 70, 105, 140, and 175 days. These tests were performed at 25 and 30°C. At 25°C, there were no differences in numbers of L. bostrychophila when this species was reared either alone or with each of the other two species. At 30°C, L. bostrychophila was the dominant species. The presence of L. bostrychophila had a negative effect on the growth of populations of L. decolor and L. paeta. The presence of L. paeta did not affect growth of populations of L. decolor, although the presence of L. decolor occasionally reduced growth of populations of L. paeta. In the second series of tests, L. bostrychophila adult females were placed in vials of wheat either alone or with adult females of Lepinotus reticulatus, at the ratios of (L. bostrychophila: L. reticulatus) 10∶0, 9∶1, 7∶3, 5∶5, 3∶7, 1∶9, and 0∶10. These tests were carried out only at 30°C, and the observation periods were the same as for the first series of tests. Liposcelis bostrychophila was the dominant species in this case as well, regardless of the ratio of the parental females. At the end of the experimental period, L. reticulatus was present only in vials that contained this species alone. Our results showed that L. bostrychophila outcompetes the other stored-product psocid species tested.
Introduction
The insect community in the stored-grain agroecosystem consists of several categories of species, such as primary and secondary colonizers, fungus feeders, scavengers, predators, and parasitoids [1]. These species coexist and infest the product at various population densities [2], [3]. Hence, in contrast with field pests where pest control measures often can be directed toward control of one major pest species, pest control measures for durable stored products need to be selected to control the multiple species that coexist in these commodities. Despite this, the factors that determine species coexistence in durable stored products are poorly understood. During the last two decades only a few studies have examined the coexistence of multiple species and its possible incorporation into management practices. Nansen et al. [4], by examining large sample data sets from silos in Kansas, reported that the numbers of the red flour beetle, Tribolium castaneum, increased with the increase of the presence of the lesser grain borer, Rhyzopertha dominica, in the same sampling unit, but the numbers of these two species were negatively correlated with the presence of the rusty grain beetle, Cryptolestes ferrugineus. Athanassiou and Saitanis [5], in a flat wheat storage, found different spatiotemporal distribution between the Mediterranean flour moth, Ephestia kuehniella, and its parasitoids Harbobracon hebetor and Venturia canescens.
There are numerous laboratory studies investigating the competition among different stored-product insect species [6]–[11]. These studies were conducted at constant abiotic conditions, and the outcome of the competition was found to be highly affected by food availability [7], [12], life table characteristics of each species [9], [11], temperature and humidity [13], and the type of diet [14]. Theoretically, in studies where food availability is limited, it is generally expected that some species would be the “superior competitors” and that the “inferior competitors” are likely to become extinct [10], [15]. However, there were cases where the competitors were able to coexist for a long period, as long as food was available [8]. Nicholson [16] proposed two forms of intraspecific competition: contest and scramble competition. Contest competition was defined as the competition in which the winner species “obtains as much of the governing requisite as it needs for survival and reproduction” and the loser “relinquishes the requisite to its successful competitors”. Scramble competition was defined as the competition in which all the individuals of the population have equal access to the limited resource. These same two types of competition can also apply to interspecific competition.
Most of the studies that examine competition among stored-product insect species under laboratory conditions tested beetles, and, to a lesser extent, moths. There are no published data on competition among psocid (Psocoptera) pest species. Psocids, particularly some species of the family Liposcelididae, are major pests that can easily build up high populations, especially in stored grains [17]. They cause serious weight losses by consuming the germ and endosperm of grains, they contaminate commodities with feces and exuviae, they are a potential threat to human health through transfer of microorganisms, and they can cause allergic reactions in sensitized group of humans [18]–[24]. The elevated moisture content (>13%) of commodities allows microorganisms (e.g., fungi) to grow and consequently to affect their properties [25]–[29]. Psocid infestations are favored when the moisture content of commodities is high, and psocids can feed on fungi [30]. The coexistence of several psocid species in the same habitat can impact pest management because psocid species vary remarkably in their susceptibility to the insecticides commonly used for control of stored-product insect pests [31], [32]. Apart from their variable susceptibility to insecticides, psocid populations also develop at different rates on different types of food. In laboratory experiments with single species of psocids, Athanassiou et al. [33] showed that the type of food was one of the most important factors in population growth of four psocid species. Furthermore, abiotic factors also may have a substantial influence on developmental parameters [34]. Paradoxically, and despite the fact that species coexistence is very common in the case of stored-product psocids [35], [36], there are studies in which only one psocid species was primarily or even solely detected throughout long monitoring periods. For example, Opit et al. [37] found only Liposcelis entomophila in two bins of wheat in 2005, while in the same bins, they found only L. decolor one year later (2006) after fumigating the grain between years.
Despite the fact that coexistence of some major stored-product insect species has been investigated, there are no data available describing competition among species of stored-grain psocid pests. Thus, our study aims to provide the first series of data on the interspecific competition among species of stored-product psocid pests examining the long-term population growth of several psocid species placed alone (one species) or in pairs of species in the same food source.
Materials and Methods
Psocid rearing
Liposcelis bostrychophila, L. decolor, L. paeta, and Lepinotus reticulatus were used in the tests. L. bostrychophila and L. reticulatus are predominantly parthenogenetic, although a few males of L. bostrychophila were recently found [38]. All species were reared on a mixture of 97% cracked wheat kernels, 2% rice krispies, and 1% brewer's yeast at 30°C and 70% relative humidity, as suggested by Opit and Throne [34]. Adult females, <14-days old, were used in the tests.
Experiment 1: Combinations of two species at constant densities
In these tests, we assessed each possible two-way combination of the three Liposcelis spp. (L. bostrychophila with L. decolor, L. bostrychophila with L. paeta, L. decolor with L. paeta), and also each species alone. For this purpose, 5 adult females of each species were placed in plastic vials (3 cm in diameter, 8 cm in height) with 5 g of untreated, clean, whole wheat (variety Fuller) adjusted to 13% moisture content. Five adult females were placed in vials where only one species was used. All vials then were placed in incubators set at 30°C and 75% relative humidity. The vials were opened after 35 days, and the adults of the two species were sieved from the wheat using a #30 sieve, identified, and counted. Nymphs were not identified nor counted, given that identification of nymphs of different species of the genus Liposcelis is difficult [39], and their inclusion in the counts might not have produced reliable results. This same procedure was repeated at 35-day intervals until 175 days from the start of the test (i.e., observation times were at 35, 70, 105, 140, and 175 days), using separate sets of vials for each observation time. The entire procedure was repeated at 25°C and 75% relative humidity. There were three sub-replicates, and the whole experiment was repeated 3 times by preparing new series of vials each time. Thus, 9 vials (3 replicates×3 sub-replicates) in total were prepared for each insect combination-temperature-observation for a total of 154 vials (9 vials×5 observations periods×2 temperatures×6 species combinations) altogether for the entire experiment.
Experiment 2: Combinations of two parthenogenetic species at various densities
In these tests, L. bostrychophila and L. reticulatus were used. Ten adult females were placed in each vial at the following combinations of L. bostrychophila: L. reticulatus adult ratios: 10∶0, 9∶1, 7∶3, 5∶5, 3∶7, 1∶9, and 0∶10. The experimental procedure, the conditions, and the observation periods were as described above, with the exception that this experiment was carried out only at 30°C. In these tests, both adults and nymphs were identified and counted, given that all stages of the genera Liposcelis and Lepinotus can be distinguished easily [40], [41]. There were three replicates with three sub-replicates each time (9 vials for each case) for a total of 315 vials (9 vials×5 observation periods×7 species combination ratios).
Data analysis
For Experiment 1, the data were analyzed, separately for each species, using a three-way ANOVA with temperature, observation period, and psocid species as main effects, with psocid counts as the response variable. Similarly, for Experiment 2, the data were analyzed using a two-way ANOVA, with observation period and species ratio as main effects. All analyses were conducted using the JMP 10 software (SAS Institute Inc., Cary, NC, U.S.A.). Means were separated by the use of the Tukey-Kramer HSD test at α = 0.05 [42].
The evolution of adult counts for the different insect species was modelled as a function of time using a quadratic regression model with temperature and initial counts of insects as covariates. The quadratic term for time was preferable to alternatives given the fact that it allows the possibility of capturing U-shaped patterns and steep rates of decline as time increases [43]. This strategy was also used for modelling the evolution of nymph counts for the different insect species. Suitability of models was determined by examination of residuals and R2. All analyses were performed using R version 3.0.2 (R Project for Statistical Computing, http://www.r-project.org)
Results
Experiment 1
For L. bostrychophila, all main effects were significant, but only the Period×Temperature interaction was significant (Table 1). At 25°C, there were no significant differences in the numbers of L. bostrychophila among the three species combinations at any observation time (Table 2). At 30°C, differences among species combinations were observed only after 70 and 105 days. After 70 days at 30°C, there were more L. bostrychophila when reared alone than when they were reared with L. decolor, while after 105 days where there were more L. bostrychophila when reared alone than when they were reared with L. paeta. The general pattern was that there were numerically more L. bostrychophila when reared alone at 30°C than when they were reared with another species. At 25°C, populations exceeded 300 psocids in all treatments at their peak, while, at 30°C, this population level was approached only when L. bostrychophila were reared alone and peak population levels were about half that in the other two treatments. Populations at both temperatures began to decrease during the course of the study, with this occurring at 175 days at 25°C and generally at 140 days at 30°C.
Table 1. ANOVA parameters for main effects and interactions for the three psocid species tested.
| Psocid species | |||||||
| L. bostrychophila | L. decolor | L. paeta | |||||
| Source | df | F | p | F | p | F | p |
| Period | 4 | 50.7 | <0.01 | 14.5 | <0.01 | 2.6 | 0.04 |
| Temperature | 1 | 75.8 | <0.01 | 33.3 | <0.01 | 182.3 | <0.01 |
| Species | 2 | 8.2 | <0.01 | 40.2 | <0.01 | 69.5 | <0.01 |
| Period×Temperature | 4 | 48.4 | <0.01 | 4.8 | <0.01 | 6.4 | <0.01 |
| Period×Species | 8 | 1.4 | 0.20 | 3.1 | <0.01 | 1.1 | 0.34 |
| Temperature×Species | 2 | 3.0 | 0.05 | 5.5 | <0.01 | 46.5 | <0.01 |
| Period×Temperature×Species | 8 | 1.2 | 0.32 | 1.8 | 0.08 | 2.6 | <0.01 |
In all cases, total df = 269.
Table 2. Mean number of L. bostrychophila adults ± SE per vial (for vials in which the initial population was 5 adult females of L. bostrychophila alone, or 5 adult females of L. bostrychophila with 5 adult females of L. decolor, or 5 adult females of L. bostrychophila with 5 adult females of L. paeta) at 35, 70, 105, 140, and 175 days after the introduction of the initial population at two temperatures.
| Period (days after introduction of the first females) | |||||
| Initial species in the vial | 35 | 70 | 105 | 140 | 175 |
| 25°C | |||||
| L. bostrychophila | 28.0±2.7 | 121.8±7.1 | 256.9±29.1 | 344.7±37.0 | 266.4±28.2 |
| L. bostrychophila with L. decolor | 21.7±3.0 | 106.2±10.3 | 231.3±35.5 | 341.6±41.2 | 211.5±35.8 |
| L. bostrychophila with L. paeta | 25.9±3.3 | 118.4±18.2 | 240.7±19.5 | 320.8±33.0 | 258.6±23.2 |
| F | 1.1 | 0.4 | 0.2 | 0.1 | 1.0 |
| p | 0.34 | 0.66 | 0.82 | 0.89 | 0.38 |
| 30°C | |||||
| L. bostrychophila | 65.1±14.2 | 253.2±30.0 a | 278.7±32.7 a | 157.7±46.8 | 18.6±8.9 |
| L. bostrychophila with L. decolor | 46.2±6.0 | 146.2±25.4 b | 166.2±39.5 ab | 92.9±32.8 | 14.6±6.9 |
| L. bostrychophila with L. paeta | 54.8±6.8 | 188.6±16.1 ab | 114.9±31.1 b | 64.7±10.6 | 30.5±9.0 |
| F | 1.0 | 4.8 | 5.8 | 2.0 | 0.99 |
| p | 0.40 | 0.02 | <0.01 | 0.15 | 0.39 |
Within each column and temperature, means followed by the same letter are not significantly different; where no letters exist, no significant differences were noted (Tukey-Kramer HSD test at p = 0.05; in all cases, df = 2, 24).
For L. decolor, all main effects and interactions were significant, with the exception of Period×Temperature×Species (Table 1). At 25°C and at all observation times, significantly more L. decolor adults were found in vials containing either L. decolor alone or L. decolor with L. paeta than in vials containing L. decolor with L. bostrychophila (Table 3). The same pattern was observed at 30°C after 35 days, but there were no differences among the species combination treatments at later observation times. The L. decolor population died out by 140 and 105 days when reared with L. bostrychophila at 25 and 30°C, respectively. L. decolor populations never reached 30 individuals at 25°C in any treatment and never reached 20 individuals at 30°C. Only a few L. decolor adults were found in any of the species combination treatments after 175 days at 30°C, and only in vials where this species was reared alone.
Table 3. Mean number of L. decolor adults ± SE per vial (for vials in which the initial population was 5 adult females of L. decolor alone, or 5 adult females of L. decolor with 5 adult females of L. bostrychophila, or 5 adult females of L. decolor with 5 adult females of L. paeta) at 35, 70, 105, 140, and 175 days after the introduction of the initial population at two temperatures.
| Period (days after introduction of the first females) | |||||
| Initial species in the vial | 35 | 70 | 105 | 140 | 175 |
| 25°C | |||||
| L. decolor | 13.1±3.4 a | 19.6±10.3 a | 29.9±7.3 a | 6.9±2.7 a | 7.6±2.9 a |
| L. decolor with L. bostrychophila | 5.0±1.2 b | 4.1±1.0 b | 0.6±0.4 b | 0.0±0.0 b | 0.0±0.0 b |
| L. decolor with L. paeta | 13.0±1.6 a | 20.4±3.0 a | 18.7±3.8 a | 9.2±1.8 a | 6.9±2.0 a |
| F | 4.4 | 6.4 | 9.6 | 6.6 | 4.2 |
| p | 0.03 | <0.01 | <0.01 | <0.01 | 0.03 |
| 30°C | |||||
| L. decolor | 18.6±4.6 a | 10.0±4.6 | 7.7±3.5 | 2.2±1.1 | 0.2±0.1 |
| L. decolor with L. bostrychophila | 3.8±0.9 b | 0.4±0.2 | 0.0±0.0 | 0.1±0.1 | 0.0±0.0 |
| L. decolor with L. paeta | 9.9±1.1 ab | 10.6±2.9 | 4.1±1.1 | 2.4±1.5 | 0.0±0.0 |
| F | 7.3 | 3.3 | 3.3 | 1.3 | 2.3 |
| p | <0.01 | 0.06 | 0.06 | 0.28 | 0.12 |
Within each column and temperature, means followed by the same letter are not significantly different; where no letters exist, no significant differences were noted (Tukey-Kramer HSD test at p = 0.05; in all cases, df = 2, 24).
For L. paeta, all main effects and interactions were significant, with the exception of Period×Species (Table 1). At 25°C at all observation times other than the first, there were generally fewer L. paeta when reared with L. bostrychophila than when L. paeta was reared alone (Table 4). L. paeta population levels did not differ significantly whether reared with L. decolor or L. bostrychophila. L. paeta population levels did not differ significantly when reared alone or with L. decolor, except after 175 days. At 30°C for the first 105 days, there were more L. paeta when they were reared alone than when they were reared with L. decolor, and the fewest L. paeta were found when they were reared with L. bostrychophila. After 105 days at 30°C, the numbers of L. paeta did not differ when they were reared alone or with L. decolor, but fewer were found when L. paeta was reared with L. bostrychophila. The number of L. paeta adults was considerably higher at 30 than at 25°C (Table 4). At 25°C, the number of adults found never exceeded 10 individuals, except after 175 days when L. paeta was reared alone. At 30°C, a maximum of 69 L. paeta were found after 70 days when they were reared alone, 37 after 70 days when reared with L. decolor, and only 9 at the first observation time when reared with L. bostrychophila.
Table 4. Mean number of L. paeta adults ± SE per vial (for vials in which the initial population was 5 adult females of L. paeta alone, 5 adult females of L. paeta with 5 adult females of L. bostrychophila, or 5 adult females of L. paeta with 5 adult females of L. decolor) at 35, 70, 105, 140, and 175 days after the introduction of the initial population at two temperatures.
| Period (days after introduction of the first females) | |||||
| Initial species in the vial | 35 | 70 | 105 | 140 | 175 |
| 25°C | |||||
| L. paeta | 4.0±0.6 | 7.3±1.5 a | 5.2±0.5 a | 4.1±1.4 | 19.2±3.7 a |
| L. paeta with L. bostrychophila | 4.4±1.2 | 2.9±0.9 b | 3.1±0.5 b | 2.0±0.7 | 3.7±0.8 b |
| L. paeta with L. decolor | 3.8±0.7 | 3.4±0.9 ab | 3.2±0.7 ab | 3.4±0.6 | 7.1±2.5 b |
| F | 0.1 | 4.4 | 3.7 | 0.2 | 9.1 |
| p | 0.87 | 0.02 | 0.04 | 0.79 | <0.01 |
| 30°C | |||||
| L. paeta | 48.3±2.5 a | 69.4±10.8 a | 48.4±7.6 a | 43.0±6.3 a | 24.9±7.6 a |
| L. paeta with L. bostrychophila | 8.7±2.7 c | 6.6±1.0 c | 2.6±1.1 c | 2.4±1.1 b | 2.7±1.1 b |
| L. paeta with L. decolor | 28.9±6.5 b | 37.1±7.4 b | 27.1±6.7 b | 31.3±10.1 a | 25.5±5.2 a |
| F | 20.9 | 17.1 | 15.2 | 9.1 | 6.0 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Within each column and temperature, means followed by the same letter are not significantly different; where no letters exist, no significant differences were noted (Tukey-Kramer HSD test at p = 0.05; in all cases, df = 2, 24).
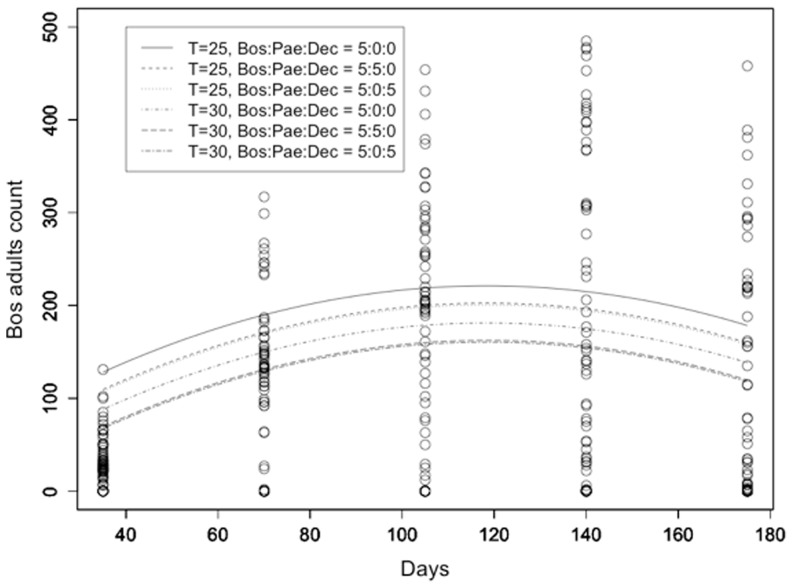
The population growth (population evolution) of L. bostrychophila adult counts as a function of time (in days) was modeled using a quadratic regression model taking into account the L. bostrychophila: L. paeta: L. decolor initial counts and temperature (R2 = 0.535; Fig. 1). All predictors had a significant effect (p<0.05). Specifically, there was a significant evolution of L. bostrychophila adults counts during time (t = 8.719, p<0.001 for the linear and t = −7.881, p<0.001 for the quadratic term). The initial number of L. paeta had a significant effect on L. bostrychophila adults counts (t = −2.193, p = 0.029) and similarly for L. decolor (t = −2.422, p = 0.016). The effect of temperature was highly significant with lower temperature resulting in higher adult L. bostrychophila counts (t = −5.763, p<0.001).
Figure 1. L. bostrychophila adult counts.
L. bostrychophila adult counts at 25 and 30°C as a function of time along with the fitted models under different competition scenarios (Bos = L. bostrychophila, Pae = L. paeta, Dec = L. decolor).
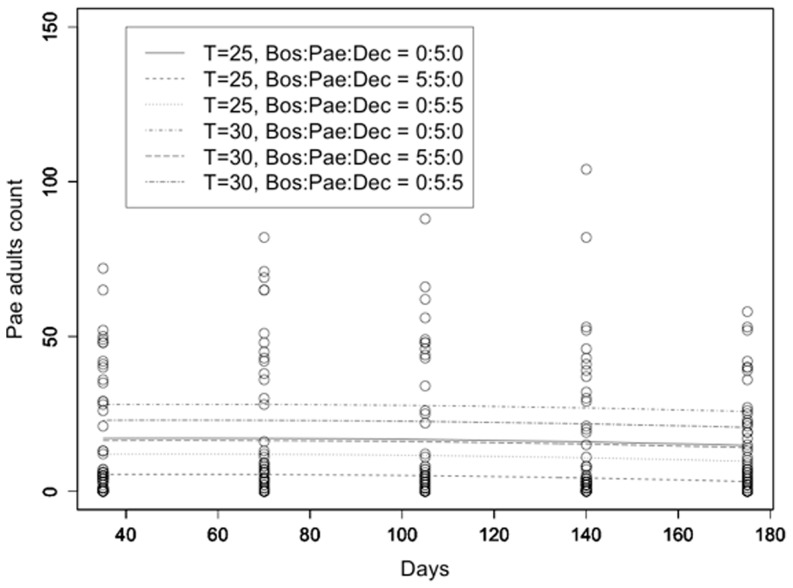
The population evolution of L. paeta adult counts as a function of time (in days) was modeled as for L. bostrychophila (R2 = 0.394; Fig. 2). L. paeta adult counts did not significantly change over time, while all other predictors had a significant effect (p<0.001). There was no significant evolution of L. paeta adult counts over time (t = 0.251, p = 0.802 for the linear and t = −0.530, p = 0.596 for the quadratic term). However, the initial number of L. bostrychophila had a significant effect on L. paeta adult counts (t = −8.132, p<0.001), while the same was found for L. decolor (t = −3.583, p<0.001). The effect of temperature was highly significant with lower temperature resulting in lower adult L. paeta counts (t = 9.338, p<0.001).
Figure 2. L. paeta adult counts.
L. paeta adult counts at 25 and 30°C as a function of time along with the fitted models under different competion scenarios (Bos = L. bostrychophila, Pae = L. paeta, Dec = L. decolor).
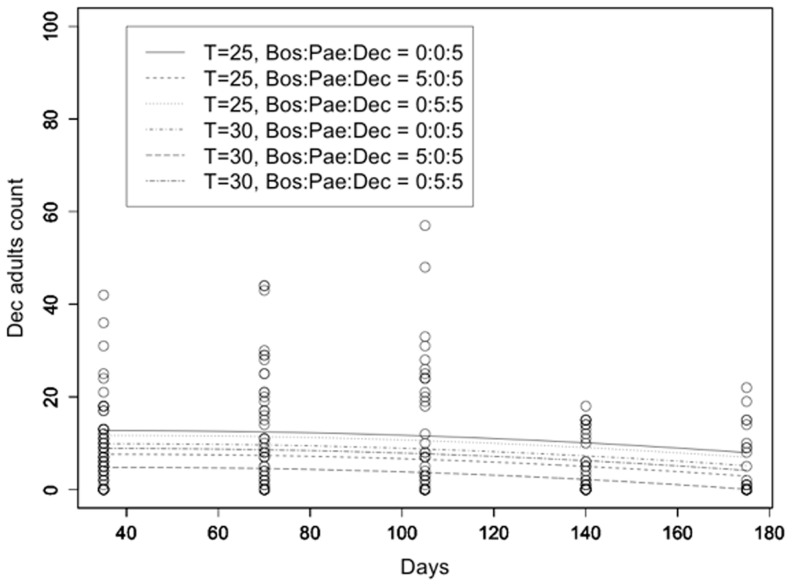
Finally, the population of L. decolor adult counts was similarly modeled (R2 = 0.333; Fig. 3). L. decolor adult counts did not significantly change over time and were independent of the initial number of L. paeta. Predictors such as temperature and initial number of L. bostrychophila had a significant effect (p<0.001). There was no significant evolution of L. decolor adults' counts over time (t = 0.603, p = 0.547 for the linear and t = −1.714, p = 0.087 for the quadratic term). The initial number of L. bostrychophila had a significant effect on L. decolor adult counts (t = −6.935, p<0.001). However, the initial number of L. paeta did not significantly affect L. decolor counts (t = −1.401, p = 0.162). The effect of temperature was highly significant with lower temperature resulting in higher adult L. decolor counts (t = −4.717, p<0.001).
Figure 3. L. decolor adult counts.
L. decolor adult counts at 25 and 30°C as a function of time along with the fitted models under different competition scenarios (Bos = L. bostrychophila, Pae = L. paeta, Dec = L. decolor).
Experiment 2
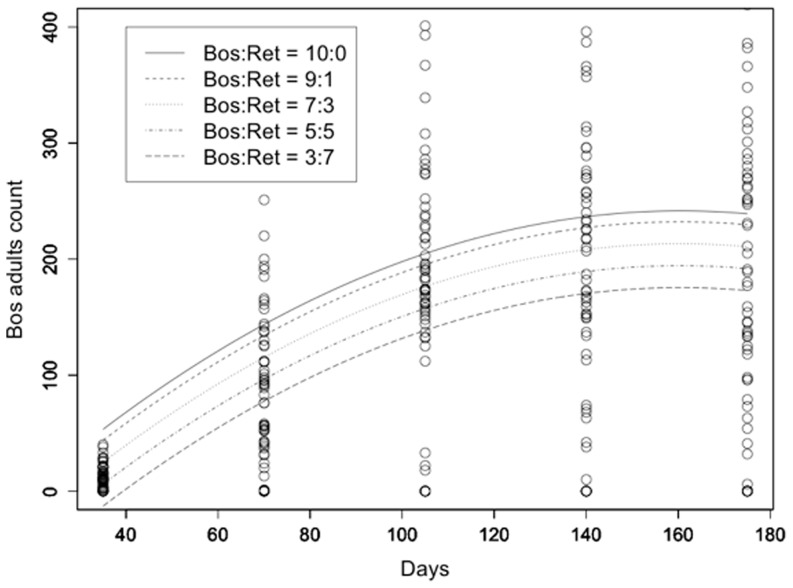
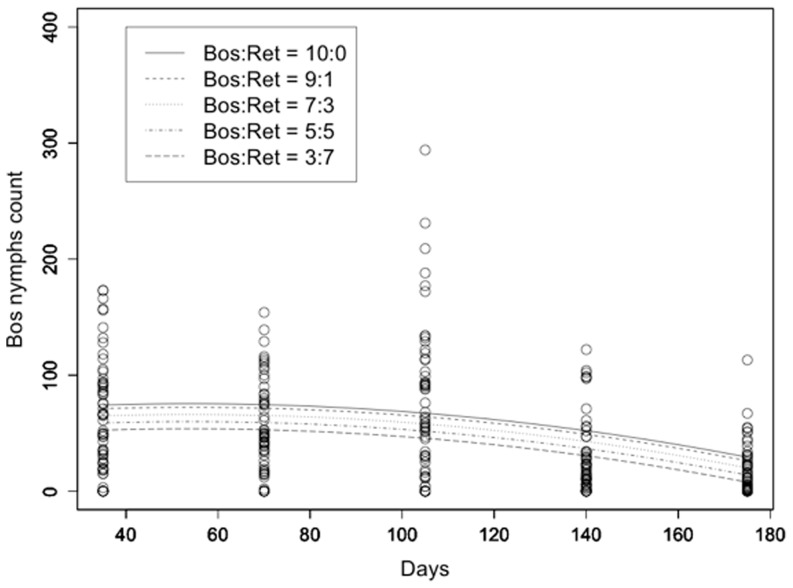
For L. bostrychophila adults and nymphs, Period and Species ratio×Period were significant, but Species ratio was not (Table 5). After 35 and 70 days, the number of L. bostrychophila adults was significantly greater as the initial number of L. bostrychophila adults in the vial increased (Table 6), with numbers of adults ranging from 3 to 24 and 119 to 168 after 35 and 70 days, respectively. Numbers of nymphs varied with initial density only after 35 days. After 105 and 140 days, there were no significant differences among treatments in the numbers of nymphs or adults. In contrast, after 175 days, the number of adults tended to decrease as the initial number of parental females increased. In fact, greater than 2 times more adults were found in vials that initially contained 1 parental L. bostrychophila female than in vials that initially contained 10 parental L. bostrychophila females.
Table 5. ANOVA parameters for main effects and their interaction for the two psocid species tested.
| Species/life stage | |||||||||||||
| L. bostrychophila | L. reticulatus | ||||||||||||
| adults | nymphs | total | adults | nymphs | Total | ||||||||
| Source | df | F | p | F | p | F | p | F | p | F | p | F | p |
| Species ratio | 5 | 66.7 | 0.65 | 0.3 | 0.92 | 0.4 | 0.85 | 50.2 | <0.01 | 26.0 | <0.01 | 57.0 | <0.01 |
| Period | 4 | 66.7 | <0.01 | 24.0 | <0.01 | 316.7 | <0.01 | 12.5 | <0.01 | 50.0 | <0.01 | 13.5 | <0.01 |
| Species ratio×Period | 20 | 2.5 | <0.01 | 4.3 | <0.01 | 2.3 | <0.01 | 3.4 | <0.01 | 2.0 | <0.01 | 1.7 | 0.03 |
In all cases, total df = 269.
Table 6. Mean number of adults, nymphs, or total number of adults and nymphs of L. bostrychophila ± SE per vial (for vials in which the initial population was adult females of L. bostrychophila with adult females of L. reticulatus at a ratio of 1∶9, 3∶7, 5∶5, 7∶3, 9∶1, and 10∶0 adults (L. bostrychophila: L. reticulatus)) at 35, 70, 105, 140, and 175 days after the introduction of the initial population.
| Period (days after introduction of the first females) | |||||
| Adult ratio | 35 | 70 | 105 | 140 | 175 |
| Adults | |||||
| 1∶9 | 3.4±1.3 c | 19.2±5.5 d | 165.1±29.5 | 209.3±44.1 | 313.6±49.9 a |
| 3∶7 | 9.2±1.6 bc | 58.4±7.7 c | 185.1±19.3 | 200.7±32.3 | 277.2±38.0 ab |
| 5∶5 | 11.7±1.6 bc | 86.2±9.7 c | 182.1±32.1 | 226.3±40.1 | 183.6±29.4 ab |
| 7∶3 | 14.3±1.3 b | 93.1±11.0 bc | 236.9±30.7 | 238.4±47.7 | 217.1±34.9 ab |
| 9∶1 | 18.5±2.8 ab | 139.2±14.6 ab | 219.0±25.7 | 233.0±40.6 | 208.6±37.3 ab |
| 10∶0 | 24.3±3.8 a | 168.0±18.1 a | 209.0±16.5 | 200.9±32.0 | 134.7±31.3 b |
| F | 10.4 | 20.5 | 1.0 | 0.2 | 3.0 |
| p | <0.01 | <0.01 | 0.41 | 0.97 | 0.02 |
| Nymphs | |||||
| 1∶9 | 14.7±2.7 d | 50.7±15.0 | 94.6±16.7 | 43.4±14.8 | 34.4±12.6 |
| 3∶7 | 34.0±3.0 c | 69.6±6.3 | 86.8±22.9 | 33.8±11.3 | 19.7±4.1 |
| 5∶5 | 34.0±8.6 bc | 76.2±15.0 | 90.9±22.3 | 33.3±11.0 | 19.6±5.5 |
| 7∶3 | 74.1±5.2 b | 44.4±10.2 | 91.1±29.7 | 22.2±6.4 | 20.2±7.1 |
| 9∶1 | 96.7±8.5 a | 59.9±13.6 | 61.0±17.7 | 26.1±12.2 | 15.1±5.4 |
| 10∶0 | 141.4±9.6 a | 50.8±11.9 | 42.4±14.3 | 17.1±3.3 | 8.1±3.2 |
| F | 43.4 | 0.9 | 0.9 | 0.8 | 1.6 |
| p | <0.01 | 0.43 | 0.43 | 0.50 | 0.17 |
| Total | |||||
| 1∶9 | 18.1±3.5d | 69.9±20.0c | 259.7±43.0 | 254.8±56.5 | 349.0±60.5 a |
| 3∶7 | 43.2±3.2c | 128.0±11.9bc | 271.9±33.4 | 234.4±38.3 | 296.9±41.1 ab |
| 5∶5 | 75.4±9.7bc | 162.4±20.9ab | 273.0±49.4 | 259.7±49.0 | 203.1±33.6 ab |
| 7∶3 | 88.4±6.2b | 137.6±14.2bc | 328.0 ± 52.8 | 260.7±52.4 | 237.3±33.6 ab |
| 9∶1 | 115.8±8.8a | 199.1±20.2ab | 280.0±40.4 | 259.1±50.6 | 223.7±41.6 ab |
| 10∶0 | 165.8±9.5a | 218.7±17.3a | 251.4±16.8 | 218.6±34.3 | 142.8±32.0 b |
| F | 51.2 | 9.1 | 0.4 | 0.1 | 3.0 |
| p | <0.01 | <0.01 | 0.82 | 0.98 | 0.02 |
Within each column and life stage, means followed by the same letter are not significantly different; where no letters exist, no significant differences were noted; HSD test at P = 0.05; in all cases, df = 5, 53).
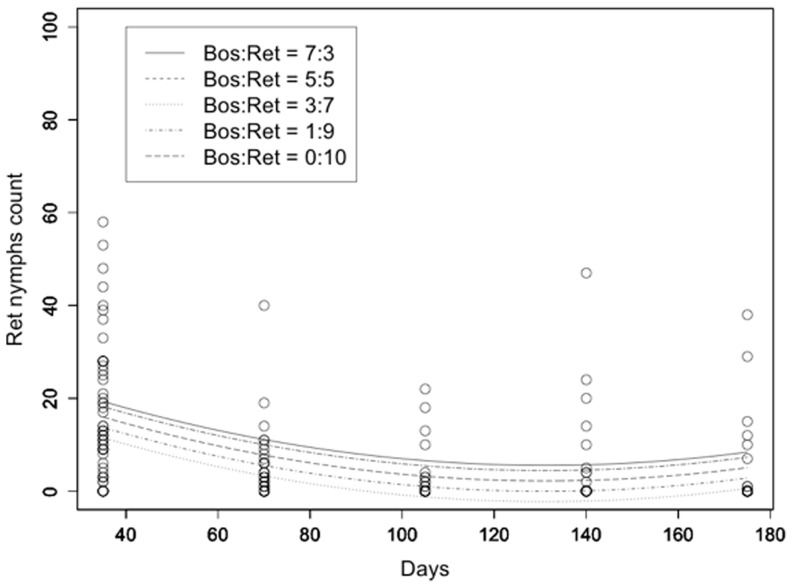
For L. reticulatus, all main effects and their interaction were significant (Table 5). Throughout the study, both the numbers of L. reticulatus nymphs and adults increased as the initial number of L. reticulatus parental females in the vials increased (Table 7). A maximum of only 35 L. reticulatus adults or 33 nymphs was produced during the study and only when this species was reared alone, and the initial number of adults was exceeded in only a few cases. After 175 days, L. reticulatus had gone extinct except in vials where they were reared alone.
Table 7. Mean number of adults, nymphs, or total number of adults and nymphs of L. reticulatus ± SE per vial (for vials in which the initial population was females of L. reticulatus with adult females of L. bostrychophila at a ratio of 1∶9, 3∶7, 5∶5, 7∶3, 9∶1, and 10∶0 adults (L. reticulatus: L. bostrychophila)) at 35, 70, 105, 140, and 175 days after the introduction of the initial population.
| Period (days after introduction of the first females) | |||||
| Adult ratio | 35 | 70 | 105 | 140 | 175 |
| Adults | |||||
| 1∶9 | 0.6±0.3 c | 2.7±1.7 c | 1.6±1.1 b | 0.0±0.0 b | 0.0±0.0 b |
| 3∶7 | 1.7±0.4 bc | 2.4±0.8 c | 4.6±2.7 b | 0.0±0.0 b | 0.0±0.0 b |
| 5∶5 | 2.2±0.8 bc | 9.2±2.7 bc | 4.4±2.6 b | 0.0±0.0 b | 0.0±0.0 b |
| 7∶ 3 | 2.9±0.8 bc | 11.4±2.9 bc | 3.1±2.0 b | 0.0±0.0 b | 0.0±0.0 b |
| 9∶1 | 5.7±1.3 ab | 20.8±4.3 ab | 5.2±3.6 b | 1.9±1.9 b | 0.0±0.0 b |
| 10∶0 | 8.2±2.2 a | 31.9±6.9 a | 35.3±6.3 a | 33.7±8.2 a | 17.4±4.1 a |
| F | 5.9 | 9.0 | 14.0 | 15.7 | 17.7 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Nymphs | |||||
| 1∶9 | 5.4±1.9 c | 0.6±0.4 b | 0.0±0.0 b | 0.0±0.0 b | 0.0±0.0 b |
| 3∶7 | 10.2±2.0 bc | 1.6±0.7 b | 0.0±0.0 b | 0.0±0.0 b | 0.0±0.0 b |
| 5∶5 | 14.3±2.7 bc | 2.4±0.8 ab | 0.0±0.0 b | 0.0±0.0 b | 0.0±0.0 b |
| 7∶3 | 16.8±3.6 bc | 2.2±1.2 ab | 0.2±0.2 b | 0.0±0.0 b | 0.0±0.0 b |
| 9∶1 | 24.1±4.2 ab | 5.2±2.0 ab | 0.2±0.1 b | 0.2±0.2 b | 0.0±0.0 b |
| 10∶0 | 33.1±6.6 a | 10.3±4.0 a | 8.3±2.6 a | 14.2±4.9 a | 12.6±4.4 a |
| F | 6.7 | 3.4 | 9.9 | 8.4 | 8.2 |
| p | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 |
| Total | |||||
| 1∶ 9 | 6.0±2.2 c | 3.2±2.2 c | 1.5±1.1 b | 0.0±0.0 b | 0.0±0.0 b |
| 3∶ 7 | 11.9±2.3 bc | 4.0±1.2 c | 4.5±2.7 b | 0.0±0.0 b | 0.0±0.0 b |
| 5∶ 5 | 16.6±3.1 bc | 11.7±3.1 bc | 4.4±2.6 b | 0.0±0.0 b | 0.0±0.0 b |
| 7∶ 3 | 19.7±4.2 bc | 13.6±4.0 bc | 3.3±2.2 b | 0.0±0.0 b | 0.0±0.0 b |
| 9∶ 1 | 27.7±5.3 ab | 26.0±5.3 ab | 5.4±3.7 b | 2.1±2.1 b | 0.0±0.0 b |
| 10∶ 0 | 41.3±8.2 a | 42.2±9.9 a | 43.7±7.0 a | 47.9±10.7 a | 30.0±11.9 a |
| F | 7.4 | 8.5 | 19.5 | 19.1 | 14.6 |
| p | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
Within each column and life stage, means followed by the same letter are not significantly different; where no letters exist, no significant differences were noted; HSD test at P = 0.05; in all cases, df = 5, 53).
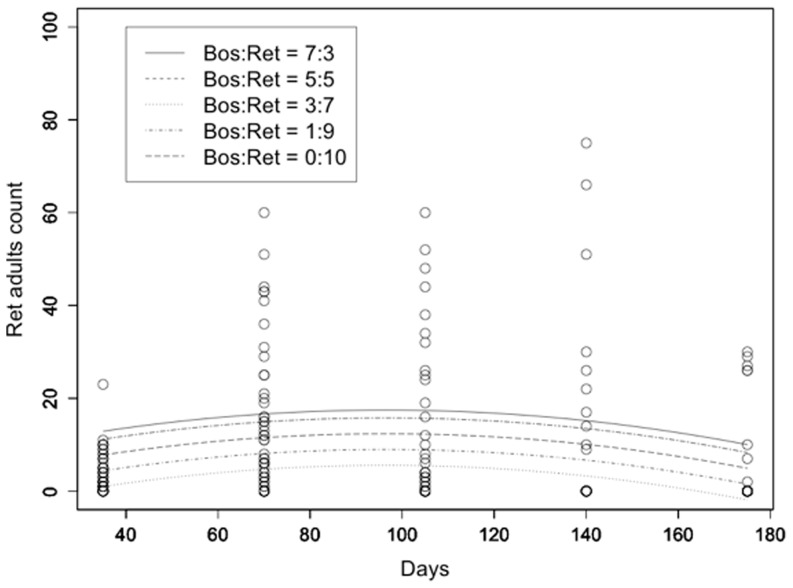
The model used for the description of the population evolution of L. reticulatus adult counts over time included only the linear and quadratic terms for time and the initial L. reticulatus: L. bostrychophila rate. All terms were highly statistically significant with t>3.5 and p<0.001. R2 was equal to 0.289 (Fig. 4). Similar results were obtained for the evolution of L. reticulatus nymph counts with R2 = 0.412 (Fig. 5). Respective results for L. bostrychophila are shown in Figs. 6, and 7. R2 for L. bostrychophila adult counts was 0.404 and for L. bostrychophila nymph counts 0.183.
Figure 4. L. reticulatus adult counts.
L. reticulatus adult counts as a function of time along with model fit under different competition scenarios (Bos = L. bostrychophila, Ret = L. reticulatus).
Figure 5. L. reticulatus nymph counts.
L. reticulatus nymph counts as a function of time along with model fit under different competition scenarios (Bos = L. bostrychophila, Ret = L. reticulatus).
Figure 6. L. bostrychophila adult counts.
L. bostrychophila adult counts as a function of time along with model fit under different competition scenarios (Bos = L. bostrychophila, Ret = L. reticulatus).
Figure 7. L. bostrychophila nymph counts.
L. bostrychophila nymph counts as a function of time along with model fit under different competition scenarios (Bos = L. bostrychophila, Ret = L. reticulatus).
Discussion
The results of the present study document that psocid species can coexist for a relatively long interval, but this coexistence is directly affected by the species that share the same food source. In the series of tests with the three Liposcelis species, our tests showed that L. decolor and L. paeta can coexist; hence, scramble competition seems to be the dominant competition type for these two species. Nevertheless, for both species, the simultaneous presence of L. bostrychophila negatively affected their population growth, regardless of the temperature level. In fact, coexistence with L. bostrychophila caused extinction of L. decolor by the end of the study, and the number of L. decolor adults was reduced, even after the first 35 days of observation. Despite the fact that L. paeta populations did not go extinct, the presence of L. bostrychophila drastically reduced its population growth in comparison with either L. paeta alone, or L. paeta with L. decolor. In contrast, L. bostrychophila population growth was not affected by the presence of either of the other species. Consequently, among the species tested, L. bostrychophila has the ability to be the “superior colonizer”, a species that can easily build up high populations in relatively short periods, and remain unaffected by the presence of other species, at least under the conditions tested here. Several studies documented that L. bostrychophila is one of the most common psocid species in stored grains and related commodities [23], [44]–[47]. Our results show that the rapid population growth of L. bostrychophila could be partially attributed to its ability to overcome competition with other major Liposcelis species.
Long-term laboratory studies on the competition of stored-product insects have been based on protocols in which the medium is replaced to ensure continuous food availability [8], [9], [11]. Conversely, the basic characteristic of the current study was that the entire experiment was carried out in a specific quantity of the commodity, which should be regarded as the decisive factor that defined the outcome of the competition at the end of the experimental period, but also regulated the interspecific competition trends. In cases of sufficient food availability, several species may coexist for a long interval by modifying their spatiotemporal distributions to local foci of infestation [3]. However, limited availability of food is directly related to individual (per capita) vital rates, which lead to environmentally-mediated density dependent characteristics [8],[48],[49]. During the last observation period of the experiment (175 days), we noticed that there was no food available in the vials or in some cases the food available in the vials was negligible due to the continuous infestation and the resulting degradation. Food exhaustion seriously moderates reproduction and survival. In our study, we observed that, even for L. bostrychophila which had the highest numbers in both tests, populations started to decline after a peak, which usually occurred during the 105 and/or 140 days observation period. Moreover, this trend also was observed even when only one species was present in the vials, which clearly indicates density-dependent regulating mechanisms [11], [49]. It is generally considered that population growth has a certain cost in fecundity rates. For example, Giga and Smith [8] reported that fecundity of the cowpea weevil, Callosobruchus maculatus, was negatively affected by an increase in adult density. Still, Lale and Vidal [11] reported that this species had the ability to develop easily under conditions of high population density. It should be noted that the adults of this species may use semiochemicals to mark the seeds infested [50]. According to Cameron et al. [15], the patterns of density-dependent resource competition are expressed more vigorously under resource limitations.
Kučerova [23] found that L. bostrychophila was able to cause approx. 10% weight loss in broken wheat kernels after 3 months, and that during this interval populations increased more than 100 times. Our results are in accordance with that observation. This clearly implies that some stored-product psocids are able to develop, probably at different food uptake rates, in waste products, which allow growth at very low levels of food availability. For both tests, populations decreased at the end of the observation period, so we assume that as food availability becomes limited, severe intraspecific resource competition bottlenecks regulate population growth [51].
In a recent study, Athanassiou et al. [33] found that L. bostrychophila was able to develop higher numbers than L. decolor and L. paeta in various types of grain commodities, especially when cracked kernels were present. L. decolor had the lowest rate of population growth regardless of the commodity. This stands in accordance with the results of the present study, where L. decolor had the lowest number of adults among the three Liposcelis species tested, even in vials where it was the only species. Hence, population growth trends of this species may be an indirect outcome of its slower developmental rates on this commodity or varieties of this commodity so far used for experiments, rather than an outcome of competition with other species. On an optimal commodity, L. decolor development rates are quicker than for L. bostrychophila or L. paeta at 25 and 30°C [17].
As expected, temperature played an important role in population growth and the concomitant competition. For L. bostrychophila, there was no effect of the presence of other species at 25°C, throughout the entire experimental period. In contrast, at 30°C, during the observation periods when psocid populations were high (70–140 days), L. bostrychophila population levels were lower when other species were present. The analysis performed here suggested that despite the fact that this species was always the winner of the competition, its population growth was highly mediated by the presence of other species, which means that competition is likely to slow population increase in a more realistic scenario of a larger spatial scale. This clearly indicates that competition, at least for L. bostrychophila, is highly mediated by temperature, and, at low temperatures, coexistence may occur. In contrast, the adult population of L. decolor was continuously higher when this species was reared alone at 25°C than when this species coexisted with L. bostrychophila. Moreover, there were no differences in L. decolor adult numbers at 30°C for the last 140 days of the observation period. L. paeta adult numbers were reduced at both temperatures when reared with other species. Rees and Walker [52] found that at 30°C, population growth of L. bostrychophila was three times higher than that of L. paeta, but the differences were minimal at lower temperatures. Finally, for both temperature levels tested here, egg-to-adult survivorship was higher for L. bostrychophila than for L. decolor or L. paeta [53]–[55]. These studies may partially explain our results for the effect of temperature on the competition of different species. Our results clearly indicate that reproduction was rapid for L. bostrychophila at 25°C during the first 35 days of the observation period, as indicated by the high number of adults that were recorded during this period. In contrast, the number of L. paeta adults only increased slightly, and practically remained unaffected for L. decolor.
Regarding the second series of experiments, L. bostrychophila was always the dominant competitor. Again, this species was superior in population growth and life table parameters than L. reticulatus. For example, L. reticulatus egg-to-adult development is slower than that of L. bostrychophila [34], [54], [56]. Population growth of L. bostrychophila is almost 40 times higher than that of L. reticulatus at 30°C [34], [52]. The dominance of L. bostrychophila over L. reticulatus was manifested regardless of the initial number of L. bostrychophila parental females. Hence, even in the vials that contained only one parental L. bostrychophila female, L. reticulatus became extinct after 140 days. However, the population growth of L. bostrychophila was proportional to the initial number of adults, at least during the first 70 days of the observation period. Interestingly, population growth was inversely related to the initial number of parental adults at the last observation period (175 days). We assume that, as noted above, density-dependent mechanisms regulated single-species population dynamics, given that higher numbers of parental females produced higher populations earlier in the observation period, which resulted in more vigorous intraspecific competition and faster food exhaustion. On the other hand, L. reticulatus population drastically declined very early in the experiment, when L. bostrychophila was present, and this reduction was proportional to the number of parental L. reticulatus females. Eventually, L. reticulatus was present only when it was alone, which suggests that this species has lower population growth rates and does not colonize as well as L. bostrychophila. This species has shorter adult longevity and lower fecundity than L. bostrychophila, which clearly stands in agreement with the data of the current study [34], [54]. Throne et al. [57] in a survey in stored wheat in Kansas found that L. reticulatus was <1% of the total number of psocid individuals recorded.
We used statistical models with linear and quadratic terms for time as predictors in order to capture U-shaped evolutions of counts. Models with more terms or with time added as a predictor with degree higher than 2 were also explored without adding much to the results. We concluded that coexistence plays a significant role in the population growth and probably on the developmental parameters of psocids, regardless of the final outcome of this competition. Hence, despite that, based on our tests, L. bostrychophila was the superior colonizer of the competition, the initial coexistence rate plays an important role in the time that this outcome will occur. In this context, our data documented that coexistence always delays population increase, regardless of the psocid species that coexist. Temperature also plays an important role in this outcome.
To our knowledge, this is the first study in which the inter- and intraspecific competition of stored-product psocid species, expressed as population growth, was assessed, under the basis of limited availability of food or food exhaustion. From the species spectrum tested here, L. bostrychophila was clearly the dominant competitor, but the outcome of this competition was based on the other competitor species, temperature, observation period, and initial population. Overall, our tests show that, under conditions of limited food availability, the simultaneous presence of L. bostrychophila will lead the populations of L. decolor and, especially, L. reticulatus to extinction. Conversely, L. paeta could coexist with L. bostrychophila for a longer period, but in low numbers, which eventually is likely to lead to extinction as well. Further experimentation is needed to assess, apart from population growth, additional factors that are responsible for the superiority of L. bostrychophila over the other species tested here, with emphasis on the possible interactions among individuals, such as possible predation activity among individuals or the existence of semiochemical-based population regulators. The results of the present study will contribute by providing the inferences necessary for the development of long-term prediction plans for psocid colonization and extinction patterns in stored grains and related commodities.
Acknowledgments
Disclaimer: Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA, the University of Thessaly, or the Benaki Phytopathological Institute. USDA is an equal opportunity provider and employer.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
These authors have no support or funding to report.
References
- 1.White NDG (1995) Insects, mites and insecticides in stored-grain ecosystems. In: Jayas DS, White NDG, Muir WE, editors. Stored-grain ecosystems. New York: Marcel Dekker. pp. 123–167. [Google Scholar]
- 2. Arbogast RT, Throne JE (1997) Insect infestation of farm-stored maize in South Carolina: towards characterization of a habitat. J Stored Prod Res 33: 187–198. [Google Scholar]
- 3. Athanassiou CG, Kavallieratos NG, Palyvos NE, Sciaretta A, Trematerra P (2005) Spatiotemporal distribution of insects and mites in horizontally stored wheat. J Econ Entomol 98: 1058–1069. [DOI] [PubMed] [Google Scholar]
- 4. Nansen C, Flinn P, Hagstrum D, Toews MD, Meikle WG (2009) Interspecific associations among stored-grain beetles. J Stored Prod Res 45: 254–260. [Google Scholar]
- 5. Athanassiou CG, Saitanis C (2006) Spatiotemporal clustering and association of Ephestia kuehniella (Lepidoptera: Pyralidae) and two of its parasitoids in bulk-stored wheat. J Econ Entomol 99: 2191–2201. [DOI] [PubMed] [Google Scholar]
- 6. Birch LC (1954) Experiments on the relative abundance of two sibling species of grain weevils. Austral J Zool 2: 66–74. [Google Scholar]
- 7. Lefkovitch LP (1968) Interaction between four species of beetles in wheat and wheat feed. J Stored Prod Res 4: 1–8. [Google Scholar]
- 8. Giga DP, Smith RH (1991) Intraspecific competition in the bean weevils Callosobruchus maculatus and Callosobruchus rhodesianus (Coleoptera: Bruchidae). J Appl Ecol 28: 918–929. [Google Scholar]
- 9. Giga DP, Canhao SJ (1993) Competition between Prostephanus truncatus (Horn) and Sitophilus zeamais (Motsch.) in maize at two temperatures. J Stored Prod Res 29: 63–70. [Google Scholar]
- 10. White NDG, Demianyk CJ, Kawamoto H, Sinha RN (1995) Population growth of Cryptolestes ferrugineus and C. pusillus (Coleoptera: Cucujidae) alone, or in competition in stored wheat or maize at different temperatures. Bull Entomol Res 85: 425–429. [Google Scholar]
- 11. Lale NES, Vidal S (2001) Intraspecific and interspecific competition of Callosobruchus maculatus (F.) and Callosobruchus subinnotatus (Pic) on stored bambara groundnut, Vigna subterranean (L.) Verdcourt. J Stored Prod Res 37: 329–338. [DOI] [PubMed] [Google Scholar]
- 12. Shazali MEH (1987) Weight loss caused by development of Sitophilus oryzae (L.) and Sitotroga cerealella (Oliv.) in sorghum grains of two size classes. J Stored Prod Res 23: 233–238. [Google Scholar]
- 13. Lale NES, Vidal S (2003) Effect of constant temperature and humidity on oviposition and development of Callosobruchus maculatus (F.) and Callosobruchus subinnotatus (Pic) on stored bambara groundnut, Vigna subterranean (L.) Verdcourt. J Stored Prod Res 39: 459–470. [DOI] [PubMed] [Google Scholar]
- 14. Sokoloff A, Franklin IR, Lakhanpal RK (1966) Comparative studies with Tribolium (Coleoptera: Tenebrionidae) - II: Productivity of T. castaneum (Herbst) and T. confusum Duv. on natural, semi-synthetic and synthetic diets. J Stored Prod Res 1: 313–324. [Google Scholar]
- 15. Cameron TC, Wearing HJ, Rohani P, Sait SM (2007) Two-species asymmetric competition: effect of age structure on intra- and interspecific interactions. J Anim Ecol 76: 83–93. [DOI] [PubMed] [Google Scholar]
- 16. Nicholson AJ (1954) An outline of the dynamics of animal populations. Austr J Zool 2: 9–65. [Google Scholar]
- 17. Nayak MK, Collins PJ, Throne JE, Wang JJ (2014) Biology and management of psocids infesting stored products. Annu Rev Entomol 59: 279–297. [DOI] [PubMed] [Google Scholar]
- 18. Obr S (1978) Psocoptera of food-processing plants and storages, dwellings and collections of natural objects in Czechoslovakia. Acta Entomol Bohemoslov 75: 226–242. [Google Scholar]
- 19. McFarlane JA (1982) Damage to milled rice by psocids. Trop Stored Prod Inf 44: 3–10. [Google Scholar]
- 20.Sidik M, Halid H, Pranata RI (1986) Pest problems and the use of pesticides in grain storage in Indonesia. In: Proceedings of the International Seminar. Manila, Philippines: ACIAR Proceedings, pp. 37–43. [Google Scholar]
- 21. Turner BD (1986) What's moving in the muesli? New Sci 1513: 43–45. [Google Scholar]
- 22.Turner BD, Ali N (1996) The pest status of psocids in the UK. In: Proceedings of the 2nd International conference on Insect Pests in the Urban Environment. Edinburgh, Scotland: ICIPUE, pp. 515–523. [Google Scholar]
- 23. Kučerová Z (2002) Weight losses of wheat grains caused by psocid infestation (Liposcelis bostrychophila: Liposcelididae: Psocoptera). Plant Prot Sci 38: 103–107. [Google Scholar]
- 24.Kakinovic I, Rozman V, Liska A (2006) Significance and feeding of psocids (Liposcelididae: Psocoptera) with microorganisms. In: Proceedings of the 9th International Conference on Stored-Product Protection. Campinas, Brazil: ABRAPOS, pp. 1087–1094. [Google Scholar]
- 25. Dehoff TW, Stroshine R, Tuite J, Baker K (1984) Corn quality during barge shipment. Trans Am Soc Agric Eng 27: 259–264. [Google Scholar]
- 26.Ayerst G (1986) Water and the ecology of fungi in stored products. In: Proceeding of the Symposium of the British Mycological Society. New York: Cambridge University Press, pp. 359–373. [Google Scholar]
- 27.Sinha RN (1992) The fungal community in the stored grain ecosystem. In: Carrol GC, Wicklow DT, editors. The fungal community New York: Marcel Dekker. pp. 797–815. [Google Scholar]
- 28.Wicklow DT (1995) The mycology of stored grain: an ecological perspective. In: Jayas DS, White NDG, Muir WE, editors. Stored grain ecosystems. New York: Marcel Dekker. pp. 197–249. [Google Scholar]
- 29. Laca A, Mousia Z, Diaz M, Webb C, Pandiella SS (2006) Distribution of microbial contamination within cereal grains. J Food Eng 72: 332–338. [Google Scholar]
- 30. Mills JT, Sinha RN, Demianyk CJ (1992) Feeding and multiplication of a psocid, Liposcelis bostrychophilus Badonnel (Psocoptera: Liposcelidae), on wheat, grain screenings, and fungi. J Econ Entomol 85: 1453–1462. [Google Scholar]
- 31. Nayak MK, Collins PJ, Reid SR (1998) Efficacy of grain protectants and phosphine against Liposcelis bostrychophila, L. entomophila, and L. paeta (Psocoptera: Liposcelididae). J Econ Entomol 91: 1208–1212. [Google Scholar]
- 32. Athanassiou CG, Arthur FH, Throne JE (2009) Efficacy of grain protectants against four psocid species on maize, rice and wheat. Pest Manag Sci 65: 1140–1146. [DOI] [PubMed] [Google Scholar]
- 33. Athanassiou CG, Opit GP, Throne JE (2010) Influence of commodity type, percentage of cracked kernels, and wheat class on population growth of stored-product psocids (Psocoptera: Liposcelidae). J Econ Entomol 103: 985–990. [DOI] [PubMed] [Google Scholar]
- 34. Opit GP, Throne JE (2008) Population growth and development of the psocid Lepinotus reticulatus at constant temperatures and relative humidities. J Econ Entomol 101: 605–615. [DOI] [PubMed] [Google Scholar]
- 35. Hodges RJ, Sidik M, Halid H, Conway JA (1992) Cost efficiency of respraying store surfaces with insecticide to protect bagged milled rice from insect attack. Trop Pest Manag 38: 391–397. [Google Scholar]
- 36. Kleih V, Pike V (1995) Economic assessment of psocid infestations in rice storage. Trop Sci 35: 280–289. [Google Scholar]
- 37. Opit GP, Throne JE, Flinn PW (2009) Temporospatial distribution of the psocids Liposcelis entomophila and L. decolor (Psocoptera: Liposcelididae) in steel bins containing wheat. J Econ Entomol 102: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 38. Mockford EL, Krushelnycky PD (2008) New species and records of Liposcelis Motschulsky (Psocoptera: Liposcelididae) from Hawaii with first description of the male of Liposcelis bostrychophila Badonnel. Zootaxa 1766: 53–68. [Google Scholar]
- 39. Kučerová Z, Li Z, Hromádková J (2009) Morphology of nymphs of common stored-product psocids (Psocoptera: Liposcelididae). J Stored Prod Res 45: 54–60. [Google Scholar]
- 40.Mockford EL (1987) Order Psocoptera. In: Stehr FW, editor. Immature insects. Dubuque, IA: Kendall Hunt Publishing Company. pp. 196–213. [Google Scholar]
- 41.Mockford EL (1991) Psocids (Psocoptera). In: Gorham JR, editor. Insect and mite pests in food. An illustrated key. Washington, DC: United States Department of Agriculture and United States Department of Health and Human Services. pp. 371–402. [Google Scholar]
- 42.Sokal RR, Rohlf FJ (1995) Biometry, 3rd edition. New York: Freedman and Company. 887 p. [Google Scholar]
- 43.Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. New York: Cambridge University Press. 648 p. [Google Scholar]
- 44.Roesli R, Jones R (1994) The use of various insect traps for studying psocid populations. In: Proceedings of the 6th International Working Conference on Stored-Product Protection. Canberra, Australia: CAB International, pp. 448–450. [Google Scholar]
- 45. Turner BD (1994) Liposcelis bostrychophila (Psocoptera: Liposcelididae), a stored food pest in the UK. Int J Pest Manag 40: 179–190. [Google Scholar]
- 46.Rees D (1994) Distribution and status of Psocoptera infesting stored products in Australia. In: Proceedings of the 6th International Working Conference on Stored-Product Protection. Canberra, Australia: CAB International, pp. 583–587. [Google Scholar]
- 47.Rees D, Van Gerwen T, Hillier T (1994) The effect of grain movement on Liposcelis decolor (Pearman), Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelidae) and Cryptolestes ferrugineus (Stephens) (Coleoptera: Cucujidae) infesting bulk-stored barley. In: In: Proceedings of the 6th International Working Conference on Stored-Product Protection. Canberra, Australia: CAB International, pp. 1214–1219. [Google Scholar]
- 48. Gordon DM, Gurney WSC, Nisbet RM, Stewart RK (1988) A model for Cadra cautella larval growth and development. J Anim Ecol 57: 645–658. [Google Scholar]
- 49. Jones AE, Gurney WSC, Nisbet RM, Gordon DM (1990) Food degradation as a mechanism of intraspecific competition among the larvae of secondary stored-product pests. Funct Ecol 4: 629–638. [Google Scholar]
- 50. Mbata GN, Shu S, Phillips TW, Ramaswamy SB (2004) Semiochemical cues by Pteromalus cerealellae (Hymenoptera: Pteromalidae) to locate its host, Callosobruchus maculatus (Coleoptera: Bruchidae). J Econ Entomol 97: 353–360.15154455 [Google Scholar]
- 51.Tilman D (1982) Resource competition and community structure. Princeton, NJ: Princeton University Press. 296 p. [PubMed] [Google Scholar]
- 52. Rees D, Walker AJ (1990) The effect of temperature and relative humidity on population growth of three Liposcelis species (Psocoptera: Liposcelidae) infesting stored products in tropical countries. Bull Entomol Res 80: 353–358. [Google Scholar]
- 53. Wang JJ, Tsai JH, Zhao ZM, Li LS (2000) Development and reproduction of the psocid Liposcelis bostrychophila (Psocoptera: Liposcelididae) as a function of temperature. Ann Entomol Soc Am 93: 261–270. [Google Scholar]
- 54. Wang JJ, Ren Y, Wei XQ, Dou W (2009) Development, survival, and reproduction of the psocid Liposcelis paeta (Psocoptera: Liposcelididae) as a function of temperature. J Econ Entomol 102: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 55. Tang PA, Wang JJ, He Y, Jiang HB, Wang ZY (2008) Development, survival, and reproduction of the psocid Liposcelis decolor (Psocoptera: Liposcelididae) at constant temperatures. Ann Entomol Soc Am 101: 1017–1025. [Google Scholar]
- 56. Opit GP, Throne JE (2008) Effects of diet on population growth of psocids Lepinotus reticulatus and Liposcelis entomophila . J Econ Entomol 101: 616–622. [DOI] [PubMed] [Google Scholar]
- 57.Throne JE, Opit GP, Flinn PW (2006) Seasonal distribution of psocids in stored wheat. In: Proceedings of the 9th International Conference on Stored-Product Protection. Campinas, Brazil: ABRAPOS, pp. 1095–1103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.