Abstract
Rice lines genetically modified with the crystal toxin genes from Bacillus thuringiensis (Bt) have experienced rapid development, with biosafety certificates for two Bt rice lines issued in 2009. There has still been no commercial release of these lines yet due to public concerns about human health and environmental risks. Some studies confirmed that Bt rice was as safe as conventional rice to non-target organisms when pesticides were not applied, however, pesticides are still required in Bt rice to control non-lepidopteran pests. In this study, we assessed the environmental effects of two Bt rice lines expressing either the cry1Ab/1Ac or cry2A genes, respectively, by using zooplanktons as indicator species under normal field management practices using pesticides when required. In the whole rice growing season, non-Bt rice was sprayed 5 times while Bt rice was sprayed 2 times, which ensured both rice achieved a normal yield. Field investigations showed that rice type (Bt and non-Bt) significantly influenced zooplankton abundance and diversity, which were up to 95% and 80% lower in non-Bt rice fields than Bt rice fields. Laboratory rearing showed that water from non-Bt rice fields was significantly less suitable for the survival and reproduction of Daphnia magna and Paramecium caudatum in comparison with water from Bt rice fields. Higher pesticide residues were detected in the water from non-Bt than Bt rice fields, accounting for the bad performance of zooplankton in non-Bt field water. Our results demonstrate that Bt rice is safer to aquatic ecosystems than non-Bt rice, and its commercialization will be beneficial for biodiversity restoration in rice-based ecosystems.
Introduction
Rice (Oryza sativa L.) is a staple food for more than three billion people [1], and more than 90% of total rice is produced and consumed in Asia [2]. Over 100 species of insects attack and damage rice, and lepidopteran pests such as rice stem borers are the most serious with yield losses ranging from 5% to 25% [3]. Rice has been successfully modified to express the cry genes derived from the Bacillus thuringiensis (Bt) bacterium, and several Bt rice lines with high resistance to lepidopteran pests have been developed over the past 15 years in China [4]. The China Ministry of Agriculture (CMOA) has confirmed that safety certificates were issued in November 2009 for two Bt rice lines, Huahui no. 1 and Bt Shanyou 63 [5], both of which express a fusion cry1Ab/1Ac gene. However, the safety certificates did not lead to final commercial release because there were fierce public debates on the health and environmental risks of Bt rice [6]. There is therefore an urgent need to provide the public with scientifically based knowledge of the actual environmental effects of genetically modified rice.
Many field and laboratory experiments have assessed the impact of Bt rice on paddy arthropods, including parasitoids, predators and non-target herbivores. In general, Bt rice has no negative impact (in comparison to non-Bt rice) to the individual fitness, population abundance and community diversity of non-target arthropods [7]–[13]. However, almost all of the field studies have excluded pesticides from both the Bt and non-Bt rice fields. Although Bt rice is effective in reducing pesticide sprays, some pesticide application is still required to control fungal diseases and non-lepidopteran pests if full yield is to be achieved [14], [15]. To accurately assess the environmental impact of Bt rice it is therefore necessary to conduct assessments under normal agricultural practices, including pesticide sprays when required.
Previous environmental assessments of Bt rice have mainly focused on terrestrial arthropods, and have not considered the aquatic ecosystem [16]. In most growth stages of rice, a layer of water is sustained in the rice field. In tropic or subtropical areas, this paddy water layer teems with zooplankton such as rotifers, cladocerans and copepods, most of which are shared by adjacent ponds and lakes, and those organisms constitute an important food source for fish and predatory insects [17]. These organisms also carry out active functions in nutrient cycling in agroecosystems [18]. Further, pesticides readily dissolve in water, and the environmental effects of pesticide use are likely to be most acute in these ecosystems [19].
In the present study, we evaluated the environmental effects of two Bt rice lines, one of which has been granted a safety certificate by the CMOA, using paddy zooplanktons as indicator species. Bt rice and non-transgenic rice were planted in plots in a complete randomized block design. Experimental fields were inspected weekly for rice pest occurrence and sprayed with pesticides when required to guarantee that non-Bt and Bt rice achieved equal yields (Fig. 1). The diversity and abundance of three types of zooplankton were compared between Bt and non-Bt treatments. Field water was collected to conduct laboratory rearing experiments of water quality using Daphnia magna and Paramecium caudatum, two species commonly used to evaluate water pollutants [20], [21]. Pesticide residues in field-collected water were also compared between Bt and non-Bt rice fields.
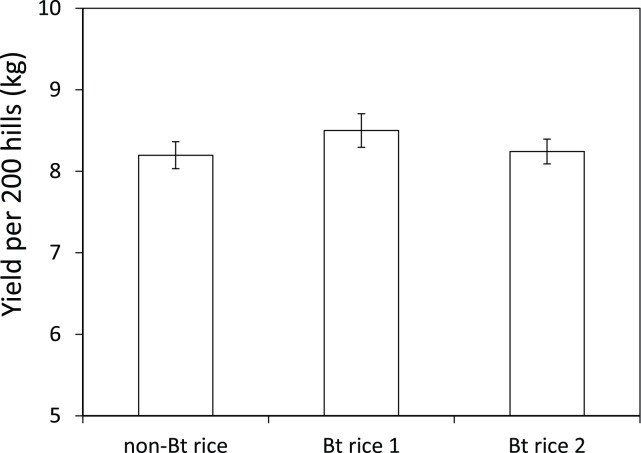
Figure 1. Yield of Bt and non-Bt rice. Non-Bt rice = Minghui 63, Bt rice 1 = Minghui 63 cry1Ab/1Ac, Bt rice 2 = Minghui 63 cry2A.
Non-Bt rice as well as Bt rice were sprayed with pesticides when insect or fungal pests reached the action threshold. Error bars indicate the standard error. Planned comparison of means showed that the yield of non-Bt rice and Bt rice did not differ significantly (d.f. = 6; t = 1.303; p = 0.240).
Materials and Methods
Ethics Statement
All field studies were carried out at an experimental field which is maintained by Huazhong Agricultural University (HZAU) and located in the campus (latitude 30°34′ N, longitude 114°47′ E). Special permits for the field studies were obtained from Science and Technology Department of HZAU which executes the function of GMO management under the supervision of China National Agricultural GMO Safety Committee (CNAGSC). All materials used during rice planting (pesticides and fertilizer etc.) were registered for legal use, and the products of Bt rice (seed and straw) were disposed according to the regulations formulated by CNAGSC. The field studies did not involve endangered or protected species.
Bt rice materials and field design
Two Bt rice lines, Bt rice 1 and Bt rice 2, and their common non-transgenic isogenic counterpart, were used as plant materials. Both the Bt rice lines are variety of Minghui 63, an elite indica rice restorer line. Bt Rice 1 was transformed with a synthetic cry1Ab/1Ac gene and Bt rice 2 was transformed with the cry2A gene [22], [23]. Bt rice 1 is also called Huahui no. 1, which was issued with a safety certificate by the CMOA in 2009 [5]. Field experiments were carried out at an experimental field at the HZAU campus in Wuhan (China) in 2013. The experimental field was constructed from a small hill in 2008, and planted with rice for 3 years without pesticide spray. According to a randomized complete block design (RCBD), the field was divided into 3 blocks, and each block divided into 3 plots which were planted with Bt rice 1, Bt rice 2 and non-Bt rice randomly. Each plot covered an area of 220 m2. Solid ridges and drainage channels were built around plots to avoid water flow between the plots. Both Bt and non-Bt rice were sowed in early May and transplanted by hand one month later. Spacing between rows was 25 cm, and hills within rows were 20 cm apart. The field was irrigated with water from a lake nearby. The soil in the experimental field was typically a yellow-brown soil consisting of 25.6% clay, 68.8% silt, 5.6% sand, and 11.2% organic matter. Field management was performed following conventional agronomic practices. Pesticides including avermectins, triazophos, difenoconazole, propiconazole and buprofezin were used when any of the rice pests exceeded action threshold (see Table S1). Action threshold refers to a point at which pest population or environmental conditions indicate that pest control action must be taken to guarantee no economic loss. A windshield was held downwind between plots when spraying.
Field investigation of zooplanktons
Five litres of water were collected from each plot in the morning before 10 a.m., and were concentrated to 50 ml using plankton net (64 µm mesh size). The concentrated samples were fixed with Lugol’s solution, and stored in amber bottles. Three kinds of zooplanktons, Rotifera, Cladocera and Copepoda, were identified down to the lowest possible taxonomic level, and were counted with the drop count method [24]. Each sample was counted twice and the mean was recorded. The zooplankton investigation was conducted twice, 5 and 12 days after the final pesticide spray on August 1st (see table S1).
Daphnia magna culturing in field-collected water
Daphnia magna is highly sensitive to toxic substances in aquatic ecosystem, and has a short generation time [25]. Daphnia magna used in the experiments were from a single clonal population cultured in laboratory. Field water was collected from each plot and was filtered by 64 µm plankton net to remove suspended particles and plankton. The filtered water was used as culture medium in the water quality tests. A newly hatched D. magna (<24 h old) was transferred to a 100-ml beaker filled with 80 ml filtered field-collected water using a broad-tipped pipette. This was replicated 30 times per plot (9 plots in total). Three millilitres of Scenedesmus obliquus solution (4∼5×105 cells/ml), cultivated according to Stein 1973 [26], was added to each beaker as food source for the D. magna [27]. Every 3 days, each D. magna was transferred to a new beaker with new filtered field-collected water and food source of S. obliquus. Each beaker was inspected daily to record survival and presence of newly born D. magna, and removal of offspring. All cultures were maintained under static conditions at 23±1°C and a controlled photoperiod at 12 h light and 12 h darkness.
Field-collected water quality test using Paramecium caudatum
Paramecium are unicellular organisms belonging to Ciliophora that are commonly found in ponds and lakes. They are often used as bioindicators of aquatic environmental pollutants [28]. Stock cultures of P. caudatum were cultured in laboratory according to Ishikawa and Hota’s procedure [29]. Twenty grams of dry rice straw (from a field without pesticide application) was boiled in 2 L tap water for 5 min and the supernatant was used as liquid food of P. caudatum. The experimental unit consisted of a 50 ml centrifuge tube with 10 ml culture medium, 3 ml P. caudatum stock culture and 37 ml filtered field-collected water. The initial density in the tubes was 54.3 cells ml. Each plot had 5 experimental replicates. The cultures were maintained in a climate chamber at 23±1°C, with a photoperiod of 12 h light and 12 h darkness. The density in each tube was determined every day in the morning using a stereo microscope and counting chamber. This experiment lasted for 8 days.
Pesticide residue test in field-collected water
Field water samples were collected from each plot on August 11, 2013 (see Table S1 for pesticide application dates). All samples were filtered through a plankton net (64 µm mesh size) and stored into 100 ml amber glass bottles at 4°C. The samples were sent to Technology Service Centre Co., Ltd (http://www.labfaster.com/) the next day, and subjected to liquid chromatography-mass spectrometry (LC-MS/MS) analysis for residues of 5 pesticides. Those pesticides had been used in the experimental field before the sampling date. Each sample was analyzed twice and the average residue was recorded.
Rice yield test
After rice grain fully ripened in September, 20 rows of rice each containing 10 hills were selected randomly in each plot. Panicles were threshed and rice grain was dried in sunlight and weighed.
Data analysis
Yield data were analyzed by one-way RCBD ANOVA, and means were compared between non-Bt rice and Bt rice by a planned comparison. Data of zooplankton in field investigation were recorded as each group abundance and total abundance, and those abundances were transformed by ln (x+1) to meet the assumption of homogeneity of variances and then were subjected to one-way RCBD ANOVA. The means of abundance were separated by Fisher’s LSD test. In order to explore the effect of overall rice type on abundance of zooplankton, multivariate analysis of variances (MANOVA) combining each group of zooplankton were also conducted. The survival rates of D. magna were arcsine-transformed and subjected to repeated measures ANOVA to estimate rice type and culturing time effect and their interaction; data of total reproduction were square root transformed and analysed by one-way RCBD ANOVA followed by a LSD test. The population growth of P. caudatum were also ln (x+1) transformed and subjected to repeated measures ANOVA. All analyses were carried out using general linear model (GLM) in SPSS package (version 16.0, SPSS Inc).
Results
Zooplankton abundance and diversity in Bt and non-Bt rice field
Thirty-three species were identified, 17 species in Rotifera, 14 in Cladocera and 2 in Copepoda (see Table S2). In the first investigation, 4, 21 and 18 species were found in non-Bt rice, Bt rice 1 and Bt rice 2 treatment, respectively, and 16, 24 and 19 species in the second investigation (see Table S2). One-way randomized complete block design (RCBD) ANOVAs detected significant rice type effect (d.f. = 2,4; F>10.659; p<0.035) and non-significant block effect (d.f. = 2,4; F<4.515; p>0.094) in both investigations on abundance of each zooplankton group (Table 1). The lowest abundance was always found in non-Bt rice plots. In the first investigation, total abundance in Bt rice treatments was around 1,500 individuals L−1, which was significantly higher than that in non-Bt rice treatment (below 50 individuals L−1) (LSD test, p<0.01); the differences between Bt rice 1 and Bt rice 2 were not significant (LSD test, p = 0.974). The second investigation found rebound in zooplankton abundance to around 1,600 and 640 individuals L−1 in Bt rice and non-Bt rice respectively, but the trend of differences between Bt and non-Bt did not change (Table 1). Multivariate analyses of variance (MANOVA) were used to assess overall rice type effect on abundance of 3 groups of zooplankton, and significant effects were detected in the first investigation (d.f. = 6,4; F = 9.049; p = 0.026), as well as in the second (d.f. = 6,4; F = 7.678; p = 0.034). Therefore the water layer in Bt rice fields harbored more abundant and diverse zooplankton than that of non-Bt rice.
Table 1. Abundance (individuals L−1) and diversity (number of species) of Rotifera, Cladocera and Copepoda in Bt and non-Bt rice plots.
| Treatment | Rotifera | Cladocera | Copepoda | Total |
| 1st investigation (abundance) | ||||
| non-Bt rice | 10.33±5.17b | 0±0b | 35.33±25.56b | 45.66±29.55b |
| Bt rice 1 | 172.66±74.99a | 29.00±7.57a | 1501.00±651.00a | 1702.66±688.26a |
| Bt rice 2 | 254.67±119.32a | 56.26±12.33a | 1031.67±335.56a | 1342.50±348.50a |
| 1st investigation (number of species) | ||||
| non-Bt rice | 1.33±0.67a | 0±0b | 1.33±0.67b | 2.67±1.33b |
| Bt rice 1 | 4.67±1.20a | 7.00±1.00a | 3.67±0.33a | 15.33±1.20a |
| Bt rice 2 | 3.67±0.33a | 6.33±2.08a | 3.63±0.33a | 15.70±3.00a |
| 2nd investigation (abundance) | ||||
| non-Bt rice | 425.33±107.33b | 4.66±2.33b | 148.00±43.39b | 638.00±69.51b |
| Bt rice 1 | 1291.33±201.17a | 34.33±8.76a | 598.00±150.89a | 1923.67±325.42a |
| Bt rice 2 | 829.67±25.69a | 38.33±10.84a | 521.33±50.11a | 1317.33±63.89a |
| 2nd investigation (number of species) | ||||
| non-Bt rice | 4.33±0.67a | 2.00±0.58b | 3.00±0.58a | 9.33±0.88b |
| Bt rice 1 | 5.67±0.33a | 7.00±1.53a | 3.67±0.33a | 16.33±1.86a |
| Bt rice 2 | 5.00±0.00a | 6.00±0.00a | 4.00±0.00a | 15.00±0.00a |
Non-Bt and Bt rice plots were sprayed with pesticides when rice pests exceeded the action threshold (Table S1). The first and the second investigation were conducted 5 days and 12 days after the final spray, respectively. The values presented in the table are untransformed means ± SE of each treatment. Means followed by the same letter within the same column in each investigation do not differ significantly at p = 0.05 (LSD post hoc test after ANOVA, n = 3).
Laboratory rearing of D. magna and P. caudatum
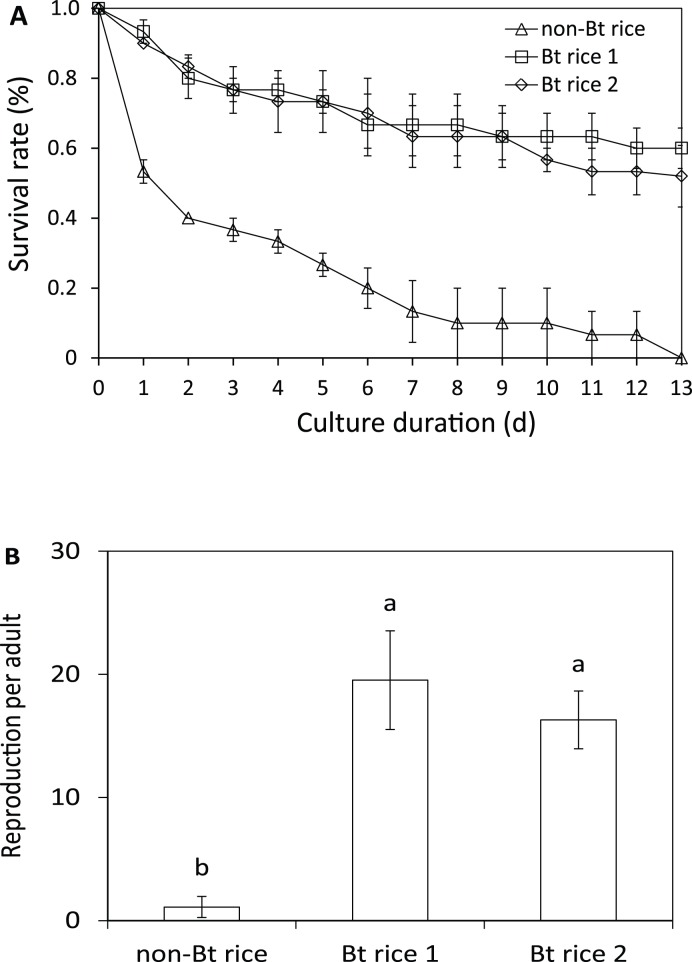
We tested the survival of D. magna and P. caudatum in water collected from Bt and non-Bt rice plots in the laboratory. Daphnids (<24 hrs old) were reared in 100 ml field-collected water with Scenedesmus obliquus as feed, and every 3 days the culture medium was refreshed with field-collected water. Differences on survival rate were found between Bt and non-Bt field-collected water one day after the onset of this experiment (Fig. 2A). In following days, survival rate in the non-Bt treatment decreased gradually to zero by the end of this 13-day experiment, while survival rates in the two Bt treatments remained relatively stable at 52% and 60% by day 13. Repeated-measures ANOVA within RCBD found significant rice type effect (d.f. = 2,4; F = 34.393, p = 0.003) and measuring time effect (d.f. = 12,52; F = 59.489; p<0.001), and no significant block effect (d.f. = 2,4; F = 1.587; p = 0.311). The survival rate did not differ between the two Bt rice treatments (LSD test, p = 0.549), but did between non-Bt rice and Bt rice 1 or Bt rice 2 (LSD test, p = 0.002 and p = 0.003). Reproduction was observed in the culture medium on the 7th day of the experiment in all treatments, which indicated rice type had no effect on sexual maturity age of D. magna. A significant effect of rice type on average reproduction rate was observed (d.f. = 2,4; F = 8.963; p = 0.033), and LSD tests revealed significant differences between non-Bt rice and Bt rice 1 (p = 0.018) or Bt rice 2 (p = 0.026) (Fig. 2B). The growth (body length) of D. magna was also recorded, but no significant differences were found.
Figure 2. Survival (A) and reproductive rate (B) of D. magna reared in water collected from non-Bt rice and Bt rice plots.
Water was collected 6 days after the final pesticide sprays and the culture medium was renewed every 3 days with field water collected on that very day. Non-Bt and Bt rice plots were sprayed with pesticides when rice pests exceeded the action threshold (see Table S1). Error bars indicated standard error. Different letters capped on the bars indicate significant difference at p = 0.05 (LSD post hoc test after ANOVA).
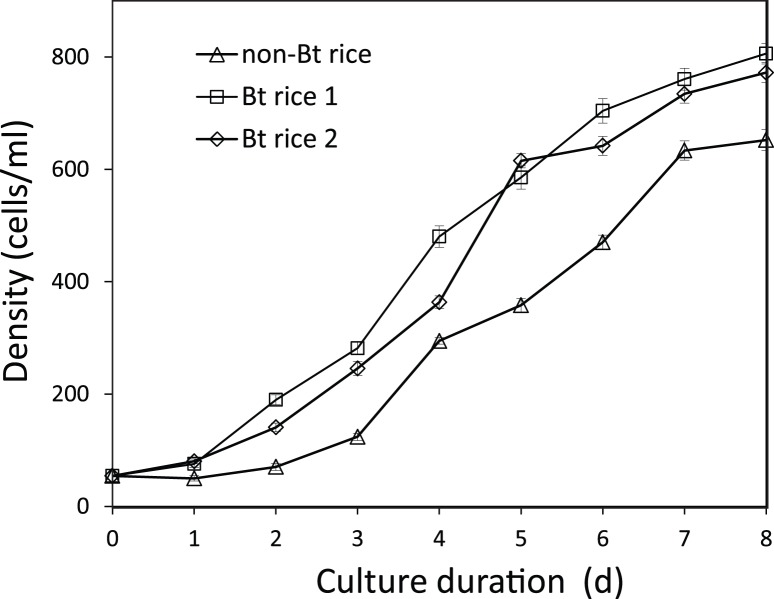
A stock of P. caudatum was cultured in the laboratory by traditional methods [29]. The stock culture was diluted to 54.3 cells ml−1 in 50 ml culture medium with 10 ml rice straw infusion and approx. 37 ml filtered field water collected from Bt and non-Bt plots. The population growth was inspected daily until the 8th day. The P. caudatum expressed lower growth rate in non-Bt rice compared to the two Bt rice treatments (Fig. 3). Repeated-measures ANOVA within RCBD detected significant effects of rice type (d.f. = 2,4; F = 195.707; p<0.001), measuring time (d.f. = 8,32; F = 1790.116; p<0.001) and the interaction of rice type×measuring time (d.f. = 16,32; F = 13.109; p<0.001). The growth rates did not differ between Bt rice 1 and Bt rice 2 (LSD test, p = 0.072). The mean density was about 300 cells/ml in non- Bt culture, and 450 and 500 in Bt rice 1 and Bt rice 2 cultures by the end of this experiment.
Figure 3. Population growth of P. caudatum in culture medium made of field water.
Field water was collected from non-Bt rice and Bt rice plots 11 days after the final pesticide spray (see Table S1). Error bars indicate the standard error.
Pesticide residue in field-collected water
In our previous study, we revealed that Bt rice (with gene cry1Ab/1Ac) in itself has no negative impact on paddy zooplanktons [30]. In order to determine what caused the differences in survival found earlier, we tested pesticide residues in the water collected from the field 10 days after the final spray (see Table S1 for pesticide application dates). We found that residues of pesticides targeting Homopteran and fungal pests were in the same order of magnitude in Bt and non-Bt rice plots, since those pesticides were used both in Bt rice and non-Bt rice (Table 2). The residues of chlorantraniliprole and triazophos, targeting lepidopteran pests, were100 times higher in non-Bt plots than Bt plots (Table 2). Theoretically, pesticides targeting lepidopteran pests would not be detected in Bt plots because they were not used in Bt rice. However, they were detected in Bt plots probably because pesticide spray drifted by wind or/and water although we had made efforts to avoid this. In short, the pesticide residues in non-Bt plots were far higher than Bt plots, which likely accounts for the poor fitness performance of zooplanktons in the non-Bt treatment.
Table 2. Pesticide residue ± SE (µg L−1) in water collected from non-Bt and Bt rice plots.
| Pesticide | Non-Bt rice | Bt rice 1 | Bt rice 2 |
| chlorantraniliprole | 15.19±1.41 | 0.17±0.04 | 0.17±0.04 |
| difenoconazole | 5.44±1.37 | 1.47±0.89 | 2.57±1.38 |
| propiconazole | 35.21±1.15 | 42.67±14.92 | 26.93±8.89 |
| triazophos | 80.45±11.91 | 0.78±0.23 | 1.19±0.52 |
| buprofezin | 0.73±0.29 | 0.16±0.08 | 0.28±0.21 |
Field water was collected 10 days after the final spray (see table S1 for the spraying date). Pesticide residues were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Discussion
Research on genetic modification has developed rapidly in China. In November 2009, COMA announced that biosafety licenses were issued to two lepidopteran-resistant Bt rice lines in designated areas. However, the decision was the subject of heated scientific and public debates in the following years. The core of these debates is the uncertainty regarding food safety and potential environmental risks.
In this study, we evaluated the environmental risk of two Bt rice lines from a new angle. Firstly, we focused on paddy aquatic organisms as the non-target organisms in the environmental assessment of Bt rice. Previous non-target effect studies of Bt crops mainly focuses on terrestrial invertebrates, such as earthworms, collembolans, mites, woodlice and nematodes [31]–[35]. Like those terrestrial invertebrates, aquatic organisms also carry out important ecological functions, such as nutrient cycling, decomposition of organic matter and maintaining aquatic food webs [36]. In many areas, the water matrix of rice fields is connected to nearby ponds and lakes which are important resources for rice farming farmers for additional income and food by fish production [37]. Secondly, we assessed Bt rice’s environmental effects in comparison with non-Bt rice under realistic agricultural conditions in which pesticides were used in both Bt and non-Bt fields as required. Former field studies demonstrated that although Bt rice (cry1Ab/Ac) reduced pesticide use by up to 60%, they were still required against fungal and non-lepidopteran pests in order to guarantee no yield loss [14], [15]. However, almost all previous studies on Bt rice’s non-target effect were conducted without considering the factor of pesticide application [4].
In this study, Bt rice was sprayed twice and non-Bt rice was sprayed 5 times throughout the whole growing season (Table S1), which resulted in equal yield between Bt and non-Bt rice (Fig. 1). The Bt rice expressed good resistance to lepidopteran pests, more specifically rice leaf folder and rice stem borers, and the two sprays only targeted a fungal disease and rice planthopper. The same results were reported in Bt cotton and Bt maize which also greatly reduced insecticide applications [38].
Our first field investigation found that the total abundance of three groups of zooplankton Rotifera, Cladocera and Copepoda in non-Bt field was about 95% lower than that in Bt rice field, and the total number of species was 80% lower (Table 1). The second sampling was conducted 7 days after the first one, and a rise in abundance and diversity was observed, however zooplanktons were still significantly more abundant and diverse in the Bt rice field. In contrast to this result, our previous two-year field study, in which we did not use pesticides, found the total population density of rotifera, cladocera and copepods did not differ significantly between Bt (cry1Ab/1Ac) and non-Bt rice fields [30]. The differences we report here are therefore not due to Bt rice itself but from the differences in pesticide application. We tested pesticide residues using LC-MS/MS in water samples collected from rice fields and found that pesticide (targeting lepidopteran pests) residues were around 100 times higher in non-Bt than Bt plots (Table 2). It is widely documented that pesticides enter into aquatic environments and accumulate to impact certain aquatic organisms. Some zooplanktons are frequently used in ecotoxicological tests of anthropogenic chemicals [39]. The qualitative and quantitative characteristics of zooplankton population depend on the degree of water pollution [40]. The high abundance of zooplankton in Bt rice fields corresponds with low water pollution from reduced pesticide application, and can be considered a positive effect of Bt rice on aquatic environment.
In addition to field investigation, we conducted laboratory experiment to rear D. magna and P. caudatum in field-collected water. The survival rate of D. magna declined to zero in non-Bt rice field-collected water by the end of the 13-days rearing experiment, while the survival rate was up to 60% in Bt rice field water (Fig. 2A). The amount of reproduction in Bt rice field water was more than 15 times higher than in non-Bt rice field water (Fig. 2B). The population of P. caudatum also developed faster in Bt rice field-collected water, and the final population density was about 20% higher in Bt rice field-collected water than non-Bt rice water (Fig. 3). Up to now, only a few studies have assessed non-target effects of Bt crops on aquatic organisms. Rosi-Marshall and colleagues reported that consumption of Bt corn (cry1Ab) debris reduced growth and increased mortality of caddisfly larvae in laboratory feeding trials [41]. Bøhn’s study demonstrated that D. magna fed on Bt maize (cry1Ab) showed significantly reduced fitness performance compared to the non-Bt control [42]. Rosi-Marshall’s study concluded that Bt δ-endotoxin in Bt maize byproducts harmed aquatic trichopterans insects which are phylogenetically close to Lepidoptera, and Bøhn’s study concluded a toxic effect rather than a lower nutritional value of Bt maize accounting for the lower fitness of D. magna. Their findings were quite different from the results of our present study. Although Bt protein is sometimes detected in Bt rice field water [43], we did not consider Bt proteins to have negative impact on paddy Daphnia because a another water flea species, Daphnia hyalina, was shown to perform as well in culture medium with high concentrations of Bt toxin as in the control medium [30]. In this study, we cultured D. magna and P. caudatum just in water collected from rice field and did not feed with Bt rice materials because water flea and paramecium typically do not feed directly on rice products or by-products [44]. In their studies, the non-target organisms were fed with high doses of Bt maize materials for a longer time than ours. Nevertheless, those aquatic organisms would not use a single food source for that long in the natural environment. Furthermore, they did not consider agricultural chemicals (such as pesticides) that probably existed in their materials, making their conclusions questionable [45]. We found that field-collected water from Bt rice fields was more suitable for survive survival and reproduction of paddy zooplanktons in short-term rearing trials, and we also detected higher pesticide residues in the water from non-Bt rice fields than Bt rice fields. Our results therefore indicate that pesticide residues in field-collected water play a more important role in zooplankton fitness than Bt toxins and Bt rice, which highlight the importance of considering pesticide application in non-target environmental assessments of Bt crops.
Conclusions
In order to settle the debate on Bt rice and realize its potential benefit as early as possible [46], scientific experiments are needed to reveal the real environmental effects of Bt rice. We assessed the influence of two Bt rice lines on aquatic organisms under normal field management which used pesticide when pests exceeded the action threshold. The results indicated that Bt rice required reduced pesticide application therefore produced lower pesticide residues in the field water body. The abundance and diversity of zooplankton were significantly higher in Bt rice fields than non-Bt rice fields, and D. magna and P. caudatum displayed far higher survival and fecundity in Bt rice field water than non-Bt rice field water. These results demonstrate that the Bt rice lines are more friendly to the aquatic ecosystem than conventional rice. Pesticide application in rice fields has long been blamed for biodiversity loss in rice-based ecosystems [47] and it is expected that biodiversity will gradually be restored following the release of Bt rice.
Supporting Information
Details of pesticide application in non-Bt and Bt rice plots.
(DOCX)
Zooplankton taxa found in non-Bt and Bt rice fields.
(DOCX)
Acknowledgments
We thank Hui Jing for identifying and counting zooplanktons and Mingyuan He for providing D. magna samples and Yongjun Lin for providing Bt rice seeds. We also thank James Hereward for valuable comments on grammar and syntax of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Major Projects of National Genetically Modified Organisms Breeding (NO. 2014ZX08012005) and the National Natural Science Funds (31370544). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Redona ED (2004) Rice Biotechnology for Developing Countries in Asia. In: A Eaglesham editors. Agricultural Biotechnology: Finding Common International Goals. National Agricultural Biotechnology Council (NABC) Report No. 16. Ithaca (New York): National Agricultural Biotechnology Council. 201–232.
- 2.Khush GS (1997) Origin, dispersal, cultivation and variation of rice. Oryza: From Molecule to Plant, (Springer), 25–34. [PubMed]
- 3.Pathak MD, Khan ZR (1994) Insect pests of rice Insect pests of rice. International Rice Research Institute, Los Banos, Philippines. 89 p. [Google Scholar]
- 4. Chen M, Shelton A, Ye GY (2011) Insect-resistant genetically modified rice in China: from research to commercialization. Annu Rev Entomol 56: 81–101. [DOI] [PubMed] [Google Scholar]
- 5. Lu C (2010) The first approved transgenic rice in China. GM Crops 1(3): 113–115. [DOI] [PubMed] [Google Scholar]
- 6.Niu S, Stanway D (8 Mar 2013) China delays GMO corn and rice to woo the public. The China Post. Available: https://www.chinapost.com.tw/china/national-news/2013/03/08/372455/China-delays.htm Accessed 15 Mar 2014.
- 7. Tian JC, Liu ZC, Yao HW, Ye GY, Peng YF (2008) Impact of transgenic rice with a cry1Ab gene on parasitoid subcommunity structure and the dominant population dynamics of parasitoid wasps in rice paddy. J Environ Entomol 1: 003. [Google Scholar]
- 8. Liu Z, Ye G, Hu C, Swapan KD (2001) Effects of Bt transgenic rice on population dynamics of main non-target insect pests and dominant spider species in rice paddies. Acta Phytophylacica Sinica 29(2): 138–144. [Google Scholar]
- 9. Jiang Y, He J, Dong B, Xu H, Fu Q, et al. (2003) Effects of transgenic Bt rice on the biological characteristics of Apanteles chilonis (Munakata) (Hymenoptera: Braconidae). Acta Entomol Sinica 47(1): 124–129. [Google Scholar]
- 10. Chen M, Zhao JZ, Ye GY, Fu Q, Shelton AM (2006) Impact of insect-resistant transgenic rice on target insect pests and non-target arthropods in China. Insect Sci 13(6): 409–420. [Google Scholar]
- 11. Chen M, Ye G, Liu Z, Fang Q, Hu C, et al. (2009) Analysis of Cry1Ab toxin bioaccumulation in a food chain of Bt rice, an herbivore and a predator. Ecotoxicology 18(2): 230–238. [DOI] [PubMed] [Google Scholar]
- 12. Bai Y, Jiang M, Cheng J, Wang D (2006) Effects of Cry1Ab toxin on Propylea japonica (Thunberg) (Coleoptera: Coccinellidae) through its prey, Nilaparvata lugens Stål (Homoptera: Delphacidae), feeding on transgenic Bt rice. Environ Entomol 35(4): 1130–1136. [Google Scholar]
- 13. Bai Y, Jiang M, Cheng J (2005) Effects of transgenic cry1Ab rice pollen on fitness of Propylea japonica (Thunberg). J Pest Sci 78(3): 123–128. [Google Scholar]
- 14. Wang Y, Zhang G, Du J, Liu B, Wang M (2010) Influence of transgenic hybrid rice expressing a fused gene derived from cry1Ab and cry1Ac on primary insect pests and rice yield. Crop Prot 29(2): 128–133. [Google Scholar]
- 15. Huang J, Hu R, Rozelle S, Pray C (2005) Insect-resistant GM rice in farmers’ fields: assessing productivity and health effects in China. Science 308(5722): 688–690. [DOI] [PubMed] [Google Scholar]
- 16. Carstens K, Anderson J, Bachman P, De Schrijver A, Dively G, et al. (2012) Genetically modified crops and aquatic ecosystems: considerations for environmental risk assessment and non-target organism testing. Transgenic Res 21(4): 813–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ali AB (1990) Seasonal dynamics of microcrustacean and rotifer communities in Malaysian rice fields used for rice-fish farming. Hydrobiologia 206(2): 139–148. [Google Scholar]
- 18. Vanni MJ (2002) Nutrient cycling by animals in freshwater ecosystems. Annu Rev Ecol Syst 33(1): 341–370. [Google Scholar]
- 19. Liess M, Ohe PCVD (2005) Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem 24(4): 954–965. [DOI] [PubMed] [Google Scholar]
- 20. Biesinger KE, Christensen GM (1972) Effects of various metals on survival, growth, reproduction, and metabolism of Daphnia magna. J Fish Board of Can 29(12): 1691–1700. [Google Scholar]
- 21. Shiny K, Remani K, Nirmala E, Jalaja T, Sasidharan V (2005) Biotreatment of wastewater using aquatic invertebrates, Daphnia magna and Paramecium caudatum . Bioresour Technol 96(1): 55–58. [DOI] [PubMed] [Google Scholar]
- 22. Tu J, Zhang G, Datta K, Xu C, He Y, et al. (2000) Field performance of transgenic elite commercial hybrid rice expressing Bacillus thuringiensis δ-endotoxin. Nat Biotechnol 18(10): 1101–1104. [DOI] [PubMed] [Google Scholar]
- 23. Chen H, Tang W, Xu C, Li X, Lin Y, et al. (2005) Transgenic indica rice plants harboring a synthetic cry2A* gene of Bacillus thuringiensis exhibit enhanced resistance against lepidopteran rice pests. Theor Appl Genet 111(7): 1330–1337. [DOI] [PubMed] [Google Scholar]
- 24. Emmanuel B, Onyema I (2007) The Plankton and fishes of a tropical creek in South-western Nigeria. Turk. J. Fish. Aquat. Sci 7(2): 105–113. [Google Scholar]
- 25. Lovern SB, Strickler JR, Klaper R (2007) Behavioral and physiological changes in Daphnia magna when exposed to nanoparticle suspensions (titanium dioxide, nano-C60, and C60HxC70Hx). Environ Sci Technol 41(12): 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stein JR (1973) Handbook of phycological methods: Culture methods and growth measurements (Cambridge University Press, New York). 472 p. [Google Scholar]
- 28. Miyoshi N, Kawano T, Tanaka M, Kadono T, Kosaka T, et al. (2003) Use of Paramecium Species in Bioassays for Environmental Risk Management: Determination of IC50 Values for Water Pollutants. J Health Sci 49(6): 429–435. [Google Scholar]
- 29. Ishikawa T, Hota M (2006) Interaction of two swimming Paramecia. J Exp Biol 209(22): 4452–4463. [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, Huang J, Hu H, Li J, Liu B, et al. (2013) Field and laboratory studies on the impact of two Bt rice lines expressing a fusion protein Cry1Ab/1Ac on aquatic organisms. Ecotox Environ Safe 92: 87–93. [DOI] [PubMed] [Google Scholar]
- 31. Zwahlen C, Hilbeck A, Howald R, Nentwig W (2003) Effects of transgenic Bt corn litter on the earthworm Lumbricus terrestris . Mol Ecol 12(4): 1077–1086. [DOI] [PubMed] [Google Scholar]
- 32. Heckmann LH, Griffiths BS, Caul S, Thompson J, Pusztai-Carey M, et al. (2006) Consequences for Protaphorura armata (Collembola: Onychiuridae) following exposure to genetically modified Bacillus thuringiensis (Bt) maize and non-Bt maize. Environ Pollut 142(2): 212–216. [DOI] [PubMed] [Google Scholar]
- 33. Griffiths BS, Caul S, Thompson J, Birch ANE, Scrimgeour C, et al. (2006) Soil Microbial and Faunal Community Responses to Maize and Insecticide in Two Soils. J Environ Qual 35(3): 734–741. [DOI] [PubMed] [Google Scholar]
- 34. Clark BW, Prihoda KR, Coats JR (2006) Subacute effects of transgenic Cry1Ab Bacillus thuringiensis corn litter on the isopods Trachelipus rathkii and Armadillidium nasatum . Environ Toxicol Chem 25(10): 2653–2661. [DOI] [PubMed] [Google Scholar]
- 35. Saxena D, Stotzky G (2001) Bacillus thuringiensis (Bt) toxin released from root exudates and biomass of Bt corn has no apparent effect on earthworms, nematodes, protozoa, bacteria, and fungi in soil. Soil Biol Biochem 33(9): 1225–1230. [Google Scholar]
- 36.Simpson I, Roger PA (1995) The impact of pesticides on nontarget aquatic invertebrates in wetland ricefields: a review. Impact of pesticides on farmer health and the rice environment, (Springer), 249–270.
- 37. Kangmin L (1988) Rice-fish culture in China: A review. Aquaculture 71(3): 173–186. [Google Scholar]
- 38. Benbrook CM (2012) Impacts of genetically engineered crops on pesticide use in the US-the first sixteen years. Environ Sci Eur 24(1): 1–13. [Google Scholar]
- 39. Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut 112(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 40. Vandysh O (2004) Zooplankton as an indicator of the state of lake ecosystems polluted with mining wastewater in the Kola Peninsula. Russ J Ecol 35(2): 110–116. [Google Scholar]
- 41. Rosi-Marshall EJ, Tank JL, Royer TV, Whiles MR, Evans-White M, et al. (2007) Toxins in transgenic crop byproducts may affect headwater stream ecosystems. Proc Natl Acad Sci USA 104(41): 16204–16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bøhn T, Primicerio R, Hessen DO, Traavik T (2008) Reduced fitness of Daphnia magna fed a Bt-transgenic maize variety. Arch Environ Contam Toxicol 55(4): 584–592. [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Hu H, Huang J, Li J, Liu B, et al. (2013) Determination of the movement and persistence of Cry1Ab/1Ac protein released from Bt transgenic rice under field and hydroponic conditions. Soil Biol Biochem 58: 107–114. [Google Scholar]
- 44.O’Sullivan P, Reynolds CS (2008) The lakes handbook: limnology and limnetic ecology, (John Wiley & Sons). 667 p. [Google Scholar]
- 45. Beachy RN, Fedoroff NV, Goldberg RB, McHughen A (2008) The burden of proof: A response to Rosi-Marshall, et al. Proc. Natl. Acad. Sci. USA 105: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James C (2009) Global Status of Commercialized Biotech/GM Crops, 2009 (ISAAA Ithaca, NY).
- 47. Halwart M (2008) Biodiversity, nutrition and livelihoods in aquatic rice-based ecosystems. Biodiversity 9(1–2): 36–40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of pesticide application in non-Bt and Bt rice plots.
(DOCX)
Zooplankton taxa found in non-Bt and Bt rice fields.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.