Abstract
Background
Cardiac tumors are rare, mostly benign with high embolic potential.
Objectives
To correlate the histological type of cardiac masses with their embolic potential, implantation site and long term follow up in patients undergoing surgery.
Methods
Between January 1986 and December 2011, we retrospectively analyzed 185 consecutive patients who underwent excision of intracardiac mass (119 females, mean age 48±20 years). In 145 patients, the left atrium was the origin site. 72% were asymptomatic and prior embolization was often observed (19.8%). The diagnosis was established by echocardiography, magnetic resonance and histological examination.
Results
Most tumors were located in the left side of the heart. Myxoma was the most common (72.6%), followed by fibromas (6.9%), thrombi (6.4%) and sarcomas (6.4%). Ranging from 0.6cm to 15cm (mean 4.6 ± 2.5cm) 37 (19.8%) patients had prior embolization, stroke 10.2%, coronary 4.8%, peripheral 4.3% 5.4% of hospital death, with a predominance of malignant tumors (40% p < 0.0001). The histological type was a predictor of mortality (rhabdomyomas and sarcomas p = 0.002) and embolic event (sarcoma, lipoma and fibroelastoma p = 0.006), but not recurrence. Tumor size, atrial fibrillation, cavity and valve impairment were not associated with the embolic event. During follow-up (mean 80±63 months), there were 2 deaths (1.1%) and two recurrences 1 and 11 years after the operation, to the same cavity.
Conclusion
Most tumors were located in the left side of the heart. The histological type was predictor of death and preoperative embolic event, while the implantation site carries no relation with mortality or to embolic event.
Keywords: Heart Neoplasms / mortality, Heart n/ surgery, Embolism / complications
Introduction
Primary cardiac tumors are uncommon and often asymptomatic with an incidence ranging from 0.0017% to 0.28%1. The differential diagnosis of cardiac masses includes vegetations, thrombi, and tumors. It may involve the endocardium, myocardium or epicardium. Secondary involvement of the heart by extra-cardiac tumors is rare. Benign tumors constitute 80% of primary cardiac neoplasms and myxomas are the prevalent type2. Angiosarcoma is the most frequent malignant cardiac tumor and is characterized by rapid growth, local invasion and distant metastasis3.
The clinical findings of cardiac tumors vary. They may mimic either cardiac or systemic diseases, and, therefore, should be considered in the differential diagnosis of distinct heart diseases. In addition, as surgical therapy is curative in most cases, early diagnosis is required to prevent complications. In patients with primary cardiac neoplasm, tumors location and cells type are important in determining clinical presentation, therapy options, and outcomes4,5.
The aim of this study is to correlate the histological type of cardiac masses with their embolic potential, implantation site and long term follow up in patients undergoing surgery.
Methods
Between January 1986 and December 2011, we retrospectively analyzed 185 consecutive patients (118 females - 64%), with age ranging from 1 month to 86 years (mean age 48 ± 20 years), who were operated on at our Institution for primary cardiac mass.
The diagnosis was established by echocardiography, magnetic resonance and histological examination.
Patients' characteristics, as well as demographic distribution, are listed in tables one to three. The most frequent primary cardiac tumors origin site was the left atrium (Table 1), most of the patients were asymptomatic (72%) and when with symptoms, dyspnea was the most common clinical presentation. Embolization was often observed (19.8%) especially to the central nervous system (Table 2).
Tabela 1.
Classificação de 185 pacientes com tumores intracardíacos, de acordo com o local de implantação
| Local de Origem | Nº de pacientes (%) |
|---|---|
| Átrio esquerdo | 143 (77,3%) |
| Átrio direito | 23 (12,4%) |
| Ventrículo esquerdo | 15 (8,1%) |
| Ventrículo direito | 4 (2,2%) |
Tabela 2.
Perfil clínico pré-operatório de 185 pacientes com tumores cardíacos
| Sintoma Cardíaco | Nº de pacientes |
|---|---|
| Dispneia | 52 (28%) |
| Fibrilação atrial | 50 (26,9%) |
| Palpitação | 17 (9,1%) |
| Síncope | 18 (9,7%) |
| Embólico | |
| SNC | 19 (10,2%) |
| IAM | 9 (4,8%) |
| Periférico | 9 (4,8%) |
| Sistêmico | |
| Febre | 9 (4,8%) |
| Anemia | 7 (3,8%) |
| Perda de peso | 3 (1,6%) |
| Artralgia | 1 (0,5%) |
SNC: Sistema nervoso central; IAM: Infarto agudo do miocárdio.
Complete patients' follow-up was collected by chart review and patients' phone interview to assess late functional status.
Statistical Analysis
All data were initially descriptively analyzed. Analysis of the minimum and maximum values, calculation of means, standard deviations and median were done for quantitative variables. For qualitative variables were calculated absolute and relative frequencies. The continuous variable studied was tumor size, the others were categorical (recurrence, embolism, origin site, histological type, atrial fibrillation, valve disease, death and hospital death).
For comparison of means of two groups (variable tumor size) it was used the Student t test (normal data distribution). The test of proportions of the categorical variables were performed using the chi-square or Fisher's exact test (when expected frequencies less than 5%). Long-term cumulative evaluations were assessed by means of Kaplan-Meier analysis and curves were compared with the log-rank test. A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 16.0 for Windows (IBM Corporation Armonk, New York).
This study was approved by our Institutional Ethics Committee / Review Board, which waived the requirement for informed patient consent due to the retrospective design of this study.
Results
One hundred seventy four patients (93.6%) had benign cardiac tumors. Their preferred implantation site was the left side of the heart (88%). 78% were in the left atrium, 8% in the left ventricle, 13% in the right atrium and 2% in the right ventricle. Myxoma was the most common histological type (72.6%), followed by fibroma (7%) and intracardiac thrombus (6.5%); the remaining types are listed in table 3.
Tabela 3.
Experiência institucional com ressecção de tumores cardíacos
| Tipo de tumor | Nº de pacientes (%) | Masculino/Feminino | Média de idade | Tamanho (média) cm |
|---|---|---|---|---|
| Mixoma | 135 (72,6%) | 45 / 90 | 52 ± 16 | 1-12,5 (4,7 ± 2,1) |
| Fibroma | 13 (7%) | 8 / 5 | 38 ± 37 | 0,6 - 11 (3,9) |
| Trombo | 11 (5,9%) | 3 / 8 | 35 ± 22 | 1,8 - 5,7 (2,9) |
| Sarcoma | 7 (3,8%) | 3 / 4 | 55 | 2,5 - 6,4 (4,6) |
| Fibroelastoma papilar | 4 (2,2%) | 2 / 2 | 46 | 0,6 - 1,8 (1) |
| Lipoma | 4 (2,2%) | 2 / 2 | 45 | 1,5 - 8,3 (4,7) |
| Rabdomioma | 4 (2,2%) | 1 / 3 | 0,03 | 2,2 - 10 (4,3) |
| Angissarcoma | 3 (1,6%) | 1 / 2 | 40 | 2,5 - 3 (2,7) |
| Osteossarcoma | 2 (1,1%) | 0 / 2 | 13,5 | 4,5 - 5,5 |
| Hemangioma | 1 (0,5%) | 1 / 0 | 67 | 2,5 |
| Meningioma | 1 (0,5%) | 1 / 0 | 19 | 9 |
Hospital mortality was 5.4%: four for patients with benign tumors (2.3%) and six malign (50%). Two infants with rhabdomyoma developed untreated low output syndrome despite high-dose inotropic support due to surgical resection and reconstruction of involved cardiac cavity. The third patient was a 12 year-old young girl who died of unknown cause during recovery of a 2,5cm left atrial thrombus and ductus arteriosus resection, and necropsy was not allowed. The fourth was a 42-year-old woman with coronary embolization prior to her myxoma resection, who died of untreated low output syndrome after surgery. The six patients with malignant tumors died early during the postoperative period due to rapidly disease progression (54.5%).
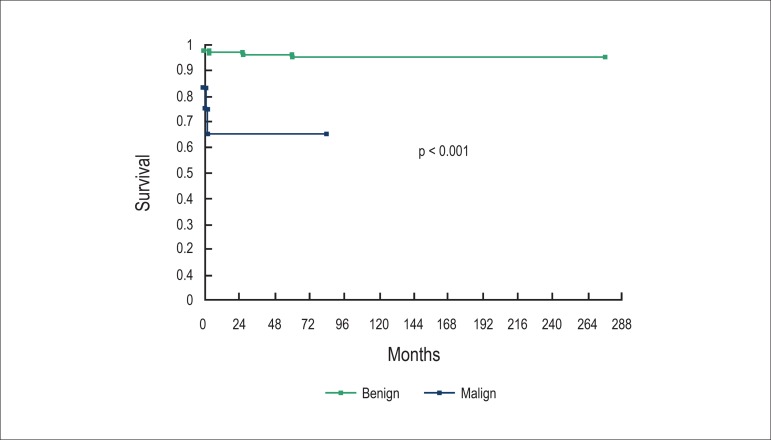
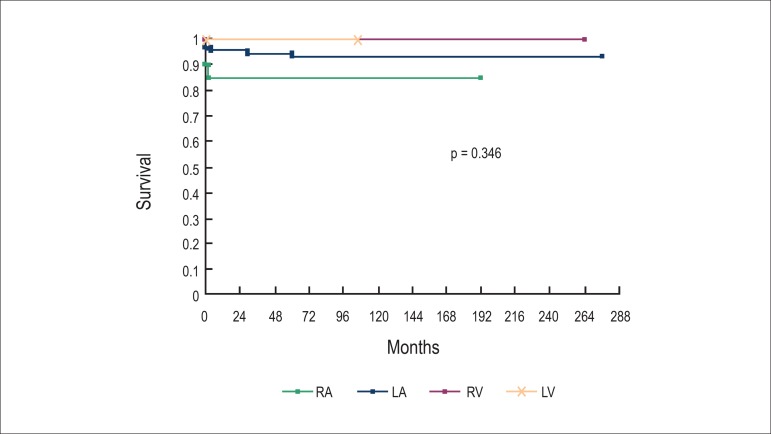
When comparing hospital mortality with cells type, more deaths related to malign tumors (p = 0.002) were observed. The histological type was predictor of mortality (rhabdomyomas, sarcomas and angiosarcomas; p < 0.001) (Figure 1) but not of recurrence (p = 0.182). There was no relation between implantation site and mortality (p = 0.346) (Figure 2).
Figure 1.
The tumors’ histological type was related with higher mortality.
Figure 2.
The tumors’ implantation site did not interfere with survival (RA: right atrium; LA: left atrium; RV: right ventricle; LV: left ventricle)
Preoperative thromboembolic event occurred in 37 patients (19.8%) (Table 2), mainly to the central nervous system (10.2%), but also to coronary arteries (4.8%), to lower limbs (4.3%) and to pulmonary territory (0.5%). The tumor size (p = 0.132), pre-operatory atrial fibrillation (p = 0.206) and implantation site (p = 0.121) were not associated with embolic event. Nevertheless, the histological type was predictor of embolic event (osteosarcoma, angiosarcoma, papillary fibroelastoma and lipoma; p = 0.006).
During follow up (mean 87 ± 51.9 months) there were two patients with prior myxoma resection who died 60 and 27 months after surgery of unknown cause and cerebrovascular accident respectively. Actuarial survival was 100%, 98.8% and 98.8% at 1, 5 and 10 years respectively. There were two recurrences to the same cavity; one left atrial mixoma recurrence 11.2 years after the surgical resection and a right atrial osteossarcoma 11 months after the operation. Both were operated on and had an eventful recovery. One neurological embolic event was observed at 27 months follow up but not related to intracardiac mass.
Discussion
In this study it was observed that the histological type was a predictor of mortality and embolic events.
Malignant tumors (sarcomas) were predictors of hospital mortality. Angiosarcoma is the most common malignant tumor of the heart and is characterized by rapid growth, local invasion, and distant metastasis with reserved prognosis. Patients with non metastatic cardiac sarcoma amenable to complete resection experienced improved survival6. However, the high overall rates of disease progression and mortality, highlight the need for more effective local and systemic treatments that may be used in conjunction with surgery to improve patient outcomes 7. Some authors recommend, in order to reducing mortality, multimodal therapy (surgical resection, radiofrequency ablation, or radiation treatment). They achieved reasonable survival for patients with resected cardiac sarcomas (mean 47 months). Patients with local tumor recurrence or metastatic disease may still benefit from aggressive treatment8.
Elbardissi et al5 in 48 years of experience in 323 patients with primary cardiac tumors found that patients with malignant tumors have a dismal prognosis and despite aggressive adjuvant chemotherapy after resection, the median survival rates of these patients were less than 1 year.
We observed a decrease in mortality over time. In the period between 1980 and 1998 we had 16% of mortality9. At that time, the great majority of patients were diagnosed belatedly and, with the advent of routine use of additional methods, such as echo complemented with MRI, the diagnosis can be performed early and avoid complications. In the subsequent period up to 2004, the mortality dropped to 6%10. In this study, the hospital mortality of 5.4% is still considered high, although mainly due to the malignant tumors aggressive behavior.
As it has been written, the majority of cardiac tumors are benign and cardiac myxomas are the most frequent ones (over 70%). Myxoma is the most prevalent cardiac tumor in the adult population, predominating in the female sex, with a gelatinous and transparent gross appearance. Myxoma is usually a single tumor, preferably located in the left atrium and inserted close to the oval fossa. They are considered benign tumors, even though they may behave in a malignant way with local relapse, invasion of the thoracic wall, and embolism11.
The clinical presentation of cardiac tumors is usually divided into cardiac, embolic and systemic manifestations, such as fever, cachexia, arthralgia, Raynaud's phenomenon, rash, and anemia, which are attributed to the production of certain cytokines, such as interleukin-612, and symptoms related to metastasis. In our series, systemic manifestations were present in 10.9% of the patients, and were reported as fever (4.8%), chronic anemia (4%), weight loss (1.6%) and arthralgia (0.5%).
Despite the fact that 72% of our patients were asymptomatic, cardiac manifestations may be related to tumor's size, because as they grow, they can progressively obstruct cardiac chambers mimicking valve disease. So, dyspnea may be followed by others symptoms of heart failure, as happened in our series.
In order to avoid complications such as intracavity obstruction or embolic events, the treatment of choice for primary cardiac tumors is resection13.
Primary cardiac tumors should be considered as a possible diagnosis for all patients with embolization, although in our casuist, we had 12 patients (6.4%) with thrombus mimicking primary cardiac tumors. Cardiac imaging has difficulty in categorizing intracardiac mass and some patients may have their mass classified as thrombus after pathological evaluation. Two of these patients were subsequently diagnosed as having antiphospholipid syndrome. There are several cases in literature of atrial and ventricular thrombus related to antiphospholipid syndrome mimicking primary cardiac tumors14. Another recent study4 found a prevalence of cardiac thrombus in 13 of 84 (15.4%) patients with cardiac tumors. They showed thrombotic masses of variable age, occasionally with features of organization and typical concentric fibrinous layers. One case showed evidence of early peripheral calcification. None of the cases contained areas suggestive of residual myxoma or other-type neoplasm.
Most patients, in whom tumor was diagnosed and later confirmed thrombus by pathology, belonged to our initial series in which the diagnosis was established only by echocardiography. We have currently used, to all cardiac masses, transthoracic echo at first, followed by transesophageal echo and resonance to confirm the diagnosis.
Recurrence develops in about 3% of tumors, although the rate is higher with familial cardiac myxomas. Our study showed tumor recurrence in two cases. One of them was a myxoma 11 years after initial surgical treatment. It was observed that the cumulative myxoma recurrence was as high as 13%, and has increased steadily up to 4 years after which there was a low hazard of tumor recurrence. There was no association between the method of surgical excision and the incidence of tumor recurrence. Patients who experienced a myxoma recurrence were younger than those who did not (mean 42 years versus 57 years). The site of recurrence was at the location of the original tumor in 81% of cases. These authors recommended, based on this finding, all patients (especially younger patients) who undergo tumor resection should be followed closely with echocardiography semiannually for 4 years after resection5.
Conclusion
Our analysis shows that most tumors were located in the left side of the heart, most of intracardiac mass is benign and myxoma is the most common histological type. And also the histological type was a predictor of death and preoperative embolic event while the implantation site carries no relation to mortality or to embolic event.
Footnotes
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author contributions
Conception and design of the research and Acquisition of data: Dias RR; Analysis and interpretation of the data: Dias RR, Fernandes F, Ramires FJA; Writing of the manuscript: Dias RR, Fernandes F; Critical revision of the manuscript for intellectual content: Mady C, Albuquerque CP, Jatene FB.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191(2):127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garatti A, Nano G, Canziani A, Gagliardotto P, Mossuto E, Frigiola A, et al. Surgical excision of cardiac myxomas: twenty years experience at a single institution. Ann Thorac Surg. 2012;93(3):825–831. doi: 10.1016/j.athoracsur.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WC. Primary and secondary neoplasm of the heart. Am J Cardiol. 1997;80(5):671–682. doi: 10.1016/s0002-9149(97)00587-0. [DOI] [PubMed] [Google Scholar]
- 4.Strecker T, Rösch J, Weyand M, Agaimy A. Primary and metastatic cardiac tumors: imaging characteristics, surgical treatment, and histopathological spectrum: a 10-year-experience at a German heart center. Cardiovasc Pathol. 2012;21(5):436–443. doi: 10.1016/j.carpath.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation. 2008;118(14) Suppl:S7–15. doi: 10.1161/CIRCULATIONAHA.107.783126. [DOI] [PubMed] [Google Scholar]
- 6.Look Hong NJ, Pandalai PK, Hornick JL, Shekar PS, Harmon DC, Chen YL, et al. Cardiac angiosarcoma management and outcomes: 20-year single-institution experience. Ann Surg Oncol. 2012;19(8):2707–2715. doi: 10.1245/s10434-012-2334-2. [DOI] [PubMed] [Google Scholar]
- 7.Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, et al. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Ann Surg Oncol. 2009;16(12):3358–3365. doi: 10.1245/s10434-009-0734-8. [DOI] [PubMed] [Google Scholar]
- 8.Bakaeen FG, Jaroszewski DE, Rice DC, Walsh GL, Vaporciyan AA, Swisher SS, et al. Outcomes after surgical resection of cardiac sarcoma in the multimodality treatment era. J Thorac Cardiovasc Surg. 2009;137(6):1454–1460. doi: 10.1016/j.jtcvs.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes F, Soufen HN, Ianni BM, Arteaga E, Ramires FJ, Mady C. Primary neoplasms of the heart. Clinical and histological presentation of 50 cases. Arq Bras Cardiol. 2001;76(3):231–237. doi: 10.1590/s0066-782x2001000300006. [DOI] [PubMed] [Google Scholar]
- 10.Dias RR, Stolf NA, Malbouisson LM, Fernandes F, Ramirez FJ, Mady C, et al. Morbidity and embolic potential of left atrial cardiac tumors. Thorac Cardiovasc Surg. 2006;54(6):400–403. doi: 10.1055/s-2006-924091. [DOI] [PubMed] [Google Scholar]
- 11.Bjessmo S, Ivert T. Cardiac myxoma: 40 years' experience in 63 patients. Ann Thorac Surg. 1997;63(3):697–700. doi: 10.1016/s0003-4975(96)01368-9. [DOI] [PubMed] [Google Scholar]
- 12.Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, et al. Embolic potential of cardiac tumors and outcome after resection: a case-control study. Stroke. 2009;40(1):156–162. doi: 10.1161/STROKEAHA.108.525709. [DOI] [PubMed] [Google Scholar]
- 13.Cianciulli TF, Saccheri MC, Lax JA, Neme RO, Sevillano JF, Maiori ME, et al. Left ventricular thrombus mimicking primary cardiac tumor in a patient with primary antiphospholipid syndrome and recurrent systemic embolism. Cardiol J. 2009;16(6):560–563. [PubMed] [Google Scholar]
- 14.Cianciulli TF, Saccheri MC, Redruello HJ, Cosarinsky LA, Celano L, Trila CS, et al. Right atrial thrombus mimicking myxoma with pulmonary embolism in a patient with systemic lupus erythematosus and secondary antiphospholipid syndrome. Tex Heart Inst J. 2008;35(4):454–457. [PMC free article] [PubMed] [Google Scholar]