Abstract
Central injections of Pituitary adenylate cyclase-activating polypeptide (PACAP) into the ventromedial nuclei (VMN) of the hypothalamus produce hypophagia that is dependent upon the PAC1 receptor, however, the signaling downstream of this receptor in the VMN is unknown. Though PACAP signaling has many targets, this neuropeptide has been shown to influence glutamate signaling in several brain regions through mechanisms involving NMDA receptor potentiation via activation of the Src family of protein tyrosine kinases. With this in mind, we examined the Src-NMDA receptor signaling pathway as a target for PACAP signaling in the VMN that may mediate its effects on feeding behavior. Under nocturnal feeding conditions, NMDA receptor antagonism prior to PACAP administration into the VMN attenuated PACAP-mediated decreases in feeding suggesting glutamatergic signaling via NMDA receptors is necessary for PACAP-induced hypophagia. Furthermore, PACAP administration into the VMN resulted in increased tyrosine phosphorylation of the GluN2B subunit of the NMDA receptor, and inhibition of Src kinase activity also blocked the effects of PACAP administration into the VMN on feeding behavior. These results indicate that PACAP neurotransmission in the VMN likely augments glutamate signaling by potentiating NMDA receptors activity through tyrosine phosphorylation events mediated by the Src kinase family, and modulation of NMDA receptor activity by PACAP in the hypothalamus may be a primary mechanism for its regulation of food intake.
Keywords: feeding, VMN, glutamate, Src kinase, PACAP
Introduction
Hypothalamic glutamate neurotransmission is crucial to energy balance [1–6], in part, through the regulation of feeding behavior [7–9]. This is in contrast to classical views of hypothalamic signaling that have focused on the importance of neuropeptides for homeostatic regulation. However, recent investigations into mechanisms of neuropeptide function have indicated that modulation of the fast-acting amino acid neurotransmitters, glutamate and GABA, may be a primary role for neuropeptide signaling [10]. For example, the neuropeptides orexin and neuropeptide Y (NPY) both potently increase feeding behavior through glutamate receptor-dependent signaling pathways in the lateral hypothalamus [11, 12] and that orexin causes enhancement of presynaptic glutamate release and postsynaptic NMDA receptor activity in the ventral tegmental area [13].
The hypothalamic ventromedial nuclei (VMN) are critical regulators of body weight and possess both high levels of glutamate and all glutamate receptor subtypes [4, 14–16]. Stimulation of these nuclei produces reductions in food intake and increased metabolic rate [8, 17–20]. Likewise, microinjection of the pleiotropic neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) into the VMN inhibits feeding behavior through activation of PAC1 receptors even after food deprivation [21, 22], however, the signaling downstream of PAC1R leading to decreased food intake in VMN neurons is currently unknown. However, previous demonstration of synergy between PACAP and glutamate [23–25] in other brain regions suggests that PACAP-PAC1R signaling in the VMN may lead to augmented glutamate neurotransmission.
Co-localization of PACAP and glutamate immunoreactivity in retinal ganglion cells, as well as in nerve terminals located in the suprachiasmatic nuclei (SCN) support a mechanism of co-release at synapses of the retinohypothalamic tract [23, 26, 27]. Functionally, PACAP application to SCN slices produces dose-dependent phase shifts in circadian rhythms through modulation of NMDA receptor activity [24]. Moreover, PACAP enhances NMDA receptor activity in the hippocampus, reportedly by two separate mechanisms involving Src tyrosine kinase signaling. The first of which involves cAMP/PKA-dependent activation of Fyn, a member of the Src tyrosine kinase family, leading to phosphorylation of multiple tyrosine residues on the GluN2B subunit of the NMDA receptor [28], while the second was shown to occur via PAC1R activation of a phospholipase C pathway leading to Src tyrosine kinase activation and augmented hippocampal NMDA receptor function [25]. Both PACAP-mediated signaling pathways reported in the hippocampus suggest that modulation of NMDA receptors can occur through Src family kinase activity, which has been implicated in the regulation of feeding behavior by lateral hypothalamic neurons [29].
In order to determine whether modulation of glutamatergic NMDA receptor signaling underlies the regulation of feeding behavior by PACAP in the hypothalamus we measured nocturnal food intake following pharmacological inhibition of NMDA receptor function within the VMN prior to PACAP injection. Moreover, we analyzed tyrosine phosphorylation of NMDA receptors following PACAP administration and examined whether activity of Src family kinases is important for PACAP-mediated alterations of feeding behavior in the VMN. Our results suggest that NMDA receptor activity is necessary for PACAP-induced hypophagia in the VMN, and PACAP may act through stimulation of Src kinases to augment NMDA receptors function.
Methods
Animals
Male Sprague-Dawley rats (Harlan; Madison, WI) weighing 225–250 g were individually housed in a climate controlled room with a 12 hr light/dark cycle. Animals had free access to Harlan standard diet (8604 formulation) and water. Food consumption was measured with a BioDAQ Food Intake Monitor (Research Diets; New Brunswick, NJ) or calculated by pre-weighing food in each bin and subtracting the weight of non-ingested and spilled food at the end of each measurement period. All procedures using animals were approved by the Marquette University Institutional Animal Care and Use Committee.
Surgery
Animals were anesthetized with a ketamine/xylazine/acepromazine (77:1.5:1.5 mg/ml/kg; ip) cocktail and placed in a stereotaxic apparatus. Bilateral guide cannulae (26 gauge; Plastics One; Roanoke VA) were placed 3 mm dorsal to the target site in all animals, and secured to the surface of the skull with an acrylic resin. The stereotaxic coordinates for the VMN were anterior/posterior, −2.5 mm from bregma; medial/lateral, ±0.6 mm from midline; dorsal/ventral, −6.2 mm from surface of the skull based on The Rat Brain in Stereotaxic Coordinates, 6th Edition [30]. Injectors extended 3 mm past the ventral tip of the cannulae reaching a VMN injection site of −9.2 mm ventral from the surface of the skull. The upper incisor bar was positioned −3.3 mm below horizontal zero. A bilateral dummy stylet placed in the guide cannulae was used to maintain patency. All animals were given at least five days to recover after cannula installation before receiving drug or vehicle injections, during which time the animals were handled and dummy stylets were removed and replaced daily in order to acclimate the animals to the physical handling necessary during experiments. Correct cannulae placements were confirmed at the conclusion of each experiment by microscopic examination of Nissl stained sections and only those with correct placement were included in the studies.
Feeding Behavior Experiments
Animals were weighed daily and acclimated to the BioDAQ Food Intake Monitor for at least 7 days before the onset of the experiment. In all experiments, approximately 1 hour prior to lights off rats received bilateral microinjections of vehicle, D-(-)-2-amino-5-phosphonopentanoic acid (AP5; 10 pmol − 1 nmol in saline/0.25 µl/side; Tocris Bioscience), or 1-(1,1-Dimethylethyl)-1-(4-methylphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP1; Src kinase inhibitor; 1 – 100 pmol in 10 % DMSO/0.25 µl/side; 1 nmol in 25% DMSO/0.25 µl/side; Tocris Bioscience) over approximately two minutes in awake animals while gently restrained. When necessary, DMSO was used as a vehicle for water insoluble substances such as PP1, as it has been previously verified to be suitable for such feeding experiments, causing no significant alterations in food intake when administered alone, even with solutions at concentrations as high as 75% [29, 31]. Upon completion of antagonist/inhibitor injections an additional minute elapsed before removing injectors to minimize backflow of injected material. Five minutes later (15 minutes for PP1 studies) rats received a second bilateral injection of either saline or PACAP (50 pmol/0.25 µl/side; PACAP38; California Peptide Research; Napa, CA) and upon completion animals were returned to their home cage for subsequent feeding measurements. The optimal injection volume of 0.25 µl to contain microinjections within VMN was determined previously [21]. Feeding measurements were collected for the next 24 hours, with the greatest emphasis placed on the first 3–5 hours after microinjections were delivered.
Western blot analysis of GluN2B tyrosine phosphorylation
Thirty minutes following saline or PACAP (50 pmol/0.25 µl/side) injections into the VMN, bilateral dissections of the ventromedial hypothalamus (VMH; including the VMN and surrounding areas) were collected. VMH tissue was homogenized by hand (10 strokes) in ice-cold homogenization buffer (320 mM sucrose, 10 mM Tris-HCl, pH 7.4, 10mM EDTA, 10mM EGTA) containing Halt protease and phosphatase inhibitor cocktail (Pierce; Rockfork, IL), followed by 3–4 seconds of sonication. Homogenates were centrifuged at 1000 X g for 2 minutes at 4° C to remove nuclei and large debris. The resulting supernatant was further centrifuged at 10,000 × g for 30 minutes at 4° C to obtain a crude membranal pellet that was resuspended in solubilization buffer (1% Triton x-100, 150 mM NaCl, 10nM Tris-HCl pH 7.4, 1 mM EDTA, 1 mM EGT, protease & phosphatase inhibitor cocktail). Protein quantification of samples was determined using a bicinchoninic (BCA) assay (Pierce). Membrane protein (20 µg) was run on an 8% gel by SDS-PAGE and transferred to a polyvinylidine fluoride (PVDF) membrane. Membranes were blocked with 5% bovine serum albumin (BSA) in tris-buffered saline containing 0.1 % Tween-20 (TBS-T). Blots were then probed with rabbit anti-pY1336 GluN2B antibody (Rockland Immunochemicals; Gilbertsville, PA) overnight at 4° C, followed by washes with TBS-T and incubation with an HRP-conjugated mouse anti-rabbit secondary antibody (Jackson Immunoresearch; West Grove, PA) at room temperature for 2 hours. Band intensities for pY1336 were developed using SuperSignal West Femto chemiluminescent substrate (Pierce) and visualized using the Kodak Image Station 4000MM. After visualization of pY1336 signal, blots were stripped and reprobed in an identical fashion for total GluN2B expression using mouse anti-GluN2B (Rockland Immunochemicals) and HRP-conjugated goat anti-mouse (Jackson Immunoresearch) antibodies. Band densities were measured using Kodak Molecular Imaging Software v4.0.
Statistics
Data are presented as means ± standard errors of the mean, and were analyzed statistically by analysis of variance (with repeated measures when appropriate). Fischer LSD analysis was used for all post-hoc group comparisons. Statistical analyses were performed using Sigma Plot 11 software (Systat Software Inc.; San Jose, CA). P < 0.05 were considered statistically significant.
Results
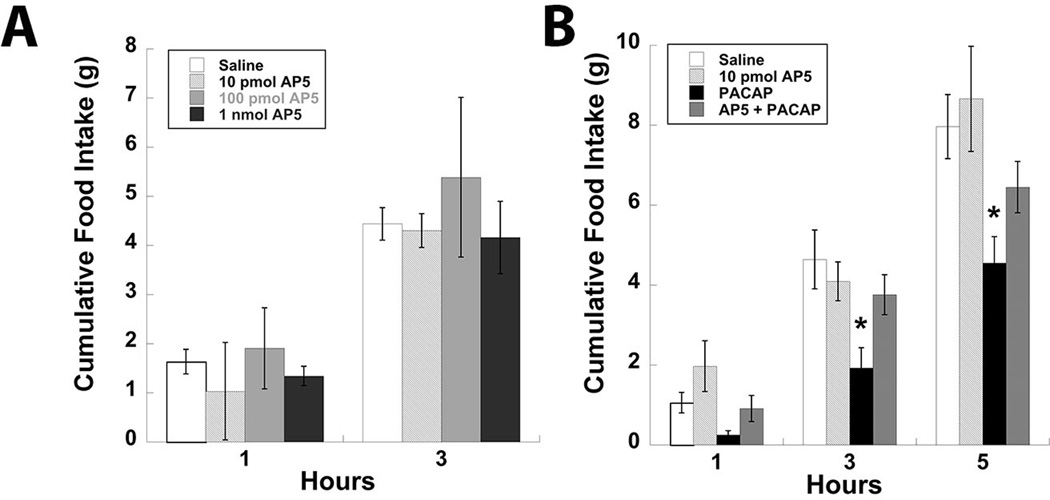
Our previous efforts to characterize the effects of PACAP signaling in the hypothalamus have demonstrated a PAC1R-dependent decrease in food intake following site-specific injections into the VMN [21, 22]. Considering previous associations between PACAP and NMDA receptor signaling [24, 25, 28, 32], as well as the potential of NMDA receptor activity to regulate feeding behavior [11, 12, 29, 33], we proceeded to test whether glutamate signaling via the NMDA receptor was necessary for PACAP-mediated reductions in food intake. Initially, the NMDA receptor antagonist AP5 was administered bilaterally into the VMN at multiple doses ranging from 10 pmol to 1 nmol to investigate its contribution to normal nocturnal feeding behavior. Surprisingly, none of the three doses tested significantly altered feeding behavior (Fig. 1A). To determine whether NMDA receptor function is necessary for PACAP-mediated decreased food intake, animals received AP5 (10 pmol/side) injections into the VMN prior to PACAP (50 pmol/side). Although AP5 treatment alone again had no effect on feeding, it did successfully block the effects of PACAP injections into the VMN on food intake (Fig. 1B; P < 0.05).
Figure 1.
NMDA receptor antagonism attenuates the hypophagic effects of PACAP administration into the VMN. (A) Feeding responses to a dose-response of bilateral AP5 microinjections into the VMN. (B) Bilateral injection of 10 pmol AP5 into the VMN blocks the inhibitory effect of PACAP on feeding behavior. Data are expressed as mean ± SEM. * = P < 0.05 compared to control group.
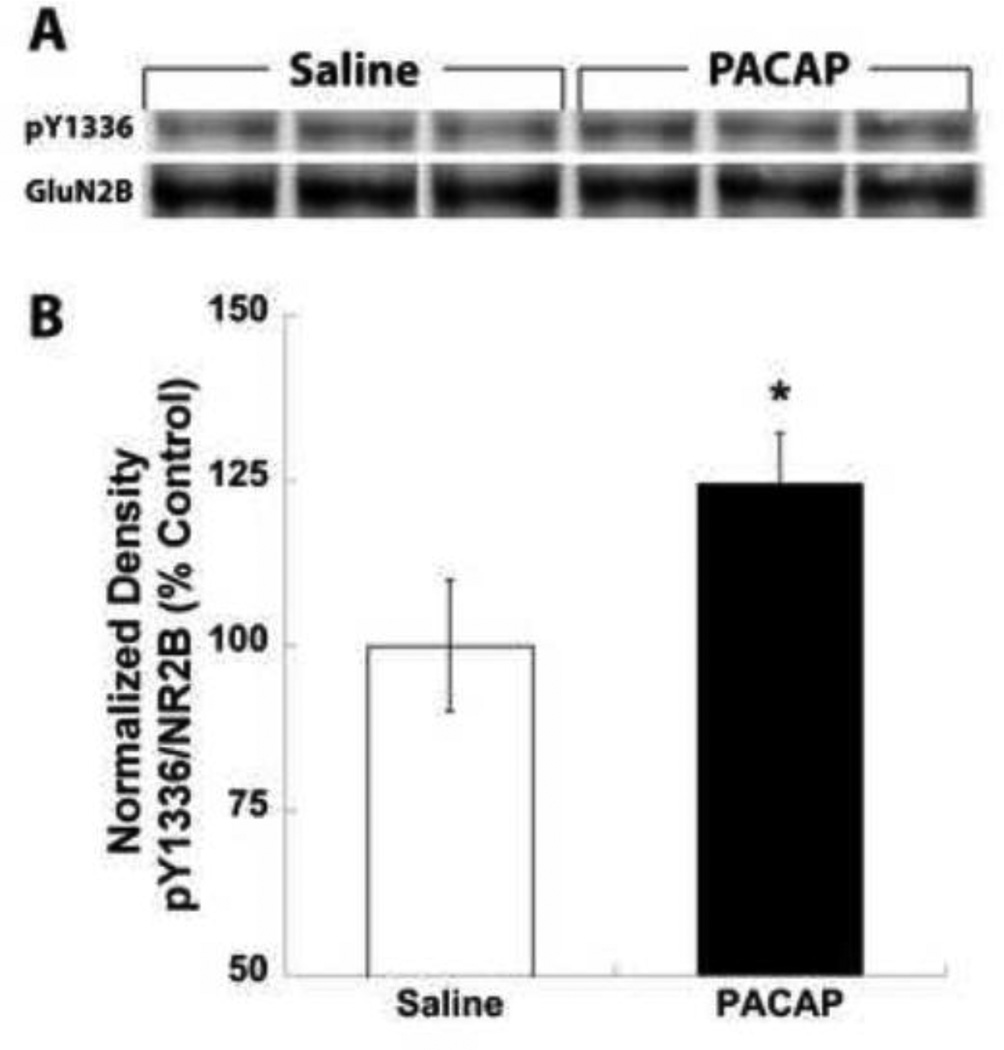
With the evidence that NMDA receptor function appears to be necessary for decreased food intake induced by PACAP, we examined whether similar mechanisms of NMDA receptor potentiation by PACAP existed within the hypothalamus as has been reported in the hippocampus [25, 28]. PACAP treatment of hippocampal slices was found to produce increased tyrosine phosphorylation specifically on the GluN2B subunit of NMDA receptors [28], therefore to test for tyrosine phosphorylation under our conditions we performed microinjections of saline or PACAP in a similar manner prior to feeding experiments but collected VMH tissue 30 minutes post-injection for GluN2B tyrosine phosphorylation analysis. In western blotting experiments we examined phosphorylation levels of tyrosine 1336 (pY1336) on the GluN2B subunit of the NMDA receptor, a previously described tyrosine residue shown to exhibit increased phosphorylation following PACAP treatment, and normalized band intensities to total GluN2B expression. Indeed, semi-quantitative analysis demonstrated that VMN PACAP treatment increased pY1336 expression by approximately 25% (Fig. 2A & B; P < 0.05).
Figure 2.
PACAP administration into the VMN results in increased tyrosine phosphorylation of the GluN2B subunit of the NMDA receptor. (A) Representative VMH protein samples from saline or PACAP (50 pmol/side) microinjections into the VMN probed for phosphorylation of tyrosine 1336 of the GluN2b subunit (pY1336) and total GluN2B protein expression. (B) Semi-quantitative analysis of band densities for pY1336 normalized to total GluN2b expression expressed as a percent of saline treatment. Data are expressed as mean ± SEM. * = P < 0.05
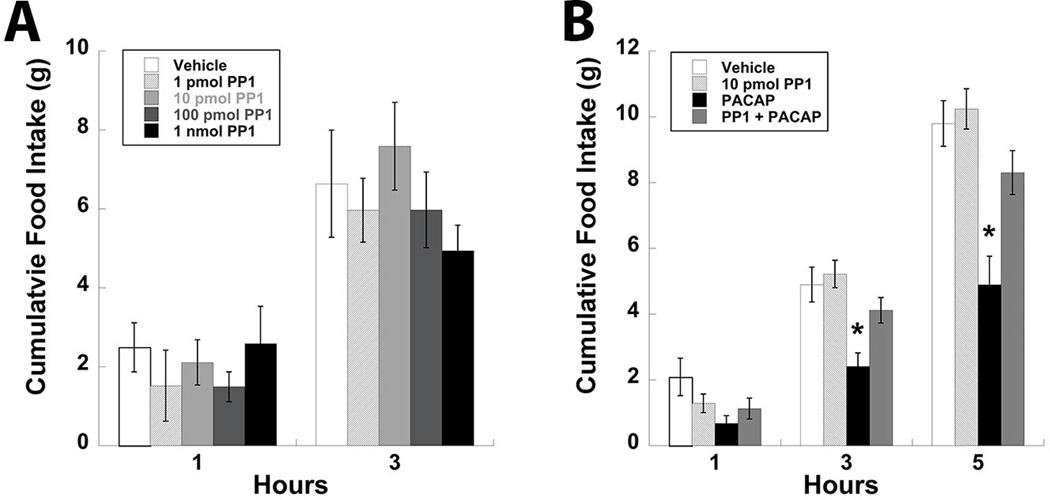
Tyrosine phosphorylation of the NMDA receptor mediated by PACAP signaling has been associated with increased Src family kinase activity [25, 28], which has also been implicated in the regulation of feeding behavior by the lateral hypothalamus [29]. Supported by the studies conducted in the hippocampus [28] as well as the data presented here, the phosphorylation appears to be specific to the GluN2B subunit of the NMDA receptor. Taken together it is probable that PACAP-PAC1R signaling results in tyrosine phosphorylation of the GluN2B subunit of the NMDA receptor in the VMN through activation of an Src kinase. To confirm this possibility the Src kinase inhibitor PP1 (1 – 1000 pmol/0.25 µl/side) was injected into the VMN followed by measurements of food intake. No concentration of PP1 used in these studies significantly altered food intake when injected into the VMN alone (Fig. 3A). However, pretreatment with PP1 (10 pmol/side) in the VMN prior to PACAP injections did significantly attenuate the hypophagic effects of PACAP (Fig. 3B; P < 0.05).
Figure 3.
Inhibition of Src kinase activity with PP1 attenuates PACAP-induced decreases in food intake in the VMN. (A) Feeding behavior following bilateral injections of PP1 into the VMN at multiple doses. (B) Pretreatment of the VMN with 10 pmol PP1 prior to PACAP injections prevents PACAP-mediated hypophagia. Data are expressed as mean ± SEM. * = P < 0.05 compared to control group.
Discussion
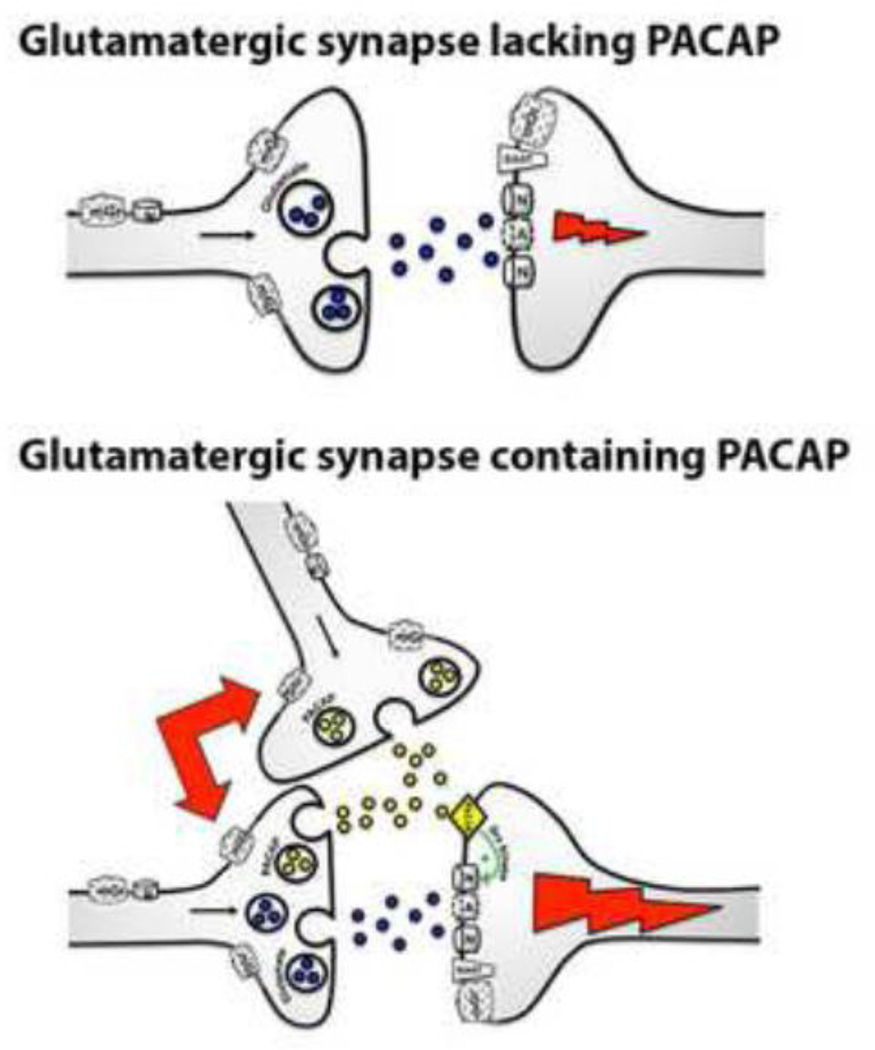
By inhibiting NMDA receptor signaling we were able to examine the contribution of its activity to PACAP-mediated decreases in food intake in the VMN. Pretreatment with the NMDA receptor antagonist AP5 successfully attenuated the hypophagic response to PACAP administration into the VMN. These data build upon previous findings indicating that PACAP-PAC1R signaling may potentiate postsynaptic NMDA receptor activity (Figure 4) [24, 25, 32], perhaps through phosphorylation of the GluN2B subunit [28]. Upon examination of GluN2B phosphorylation following PACAP injections into the VMN in vivowe detected an increase in phosphorylation at tyrosine 1336, a previously identified site of PACAP-induced tyrosine phosphorylation on the GluN2B subunit of the NMDA receptor [28]. Not only has the Src family of non-receptor tyrosine kinases been previously described to facilitate NMDA receptor phosphorylation mediated by PACAP [25, 28], but hypothalamic Src activity has been demonstrated to play a role in the regulation of feeding behavior [29]. These findings led us to test whether Src was a downstream mediator of PACAP-induced hypophagia in the VMN. Inhibition of Src family tyrosine kinases with PP1 effectively blocked the effects of intra-VMN PACAP on feeding behavior, thus supporting a mechanism for PACAP-mediated potentiation of NMDA receptors via an Src-dependent pathway.
Figure 4.
Modulation of NMDA receptor signaling by PACAP. (Upper) Conventional glutamatergic synapse showing no modulation of glutamate neurotransmission leading to lesser excitatory postsynaptic potential (EPSP). (Lower) Proposed potentiation of EPSPs following modulation of NMDA receptors by PACAP released from glutamate and PACAP co-expressing neurons or PACAPergic neurons.
Similar to our current data combining NMDA receptor antagonists with PACAP injections into the VMN, inhibition of NMDA receptors also blocks hyperphagia induced by orexin and neuropeptide Y in the lateral hypothalamus as well as refeeding following a fast [11, 12, 33]. Furthermore, AMPA/kainate and NMDA glutamate receptor agonists produce robust feeding in sated rats when administered into the lateral hypothalamus [7, 33, 34]. Although treatment with AP5 in the VMN did not produce increased feeding behavior as one might expect, AP5 was administered at a time when feeding behavior was naturally at its peak, possibly reducing our ability to detect AP5-induced alterations in feeding behavior. However, our current findings indicate that VMN PACAP injections produce NMDAR-dependent hypophagia and GluN2B tyrosine phosphorylation suggesting that interaction between PAC1R and GluN2B NMDA receptors are important in the regulation of feeding behavior. In support, PACAP is reported to induce tyrosine phosphorylation specifically on the GluN2B subunit [28] and expression of GluN2B is widespread throughout the hypothalamus including in the VMN [35]. In the lateral hypothalamus, antagonism of GluN2B containing NMDA receptors with ifenprodil attenuates the feeding response to both fasting and microinjection of NMDA into the lateral hypothalamus, although ifenprodil concentrations used in these studies may have also antagonized GluN2A containing NMDA receptors [36]. The phosphorylation state of the GluN2B subunit may be critical for augmenting NMDA receptor activity induced by PACAP signaling, and appears to be regulated by the Src family of tyrosine kinases [25, 28], a family of kinases that is critical to the function and signaling of NMDA receptors also reported to reduce GluN3A trafficking to the synaptic membrane [35–37]. Importantly, inhibition of Src family tyrosine kinases by PP1 has previously been reported to block NMDAR-dependent feeding behavior in the lateral hypothalamus [29], similar to our data regarding PACAP signaling in the VMN.
Although our investigations of PACAP-mediated feeding behavior in the VMN cannot directly demonstrate potentiation of postsynaptic NMDA receptors by PACAP signaling, extensive biochemical and electrophysiological analysis of glutamate signaling in the hippocampus has demonstrated this modulatory pathway. PACAP application yields enhancement of field excitatory postsynaptic potentials, brain derived neurotrophic factor mRNA expression, and tyrosine phosphorylation of the GluN2B subunit of the NMDA receptor by Fyn tyrosine kinase, which is a member of the Src family of tyrosine kinases [28]. Further coupling PACAP to glutamate signaling, PACAP augments NMDA currents following Schaffer collateral stimulation through a PAC1R-dependent pathway that activates Src tyrosine kinase [25]. While two different mechanisms of NMDA receptor potentiation by PACAP have been identified, both occur as a result of tyrosine phosphorylation of NMDA receptors by a member of the Src family of tyrosine kinases (Fyn and Src).
PACAP signaling may also modulate other aspects of glutamate signaling including the AMPA receptor, as PAC1R-dependent potentiation of AMPA receptors at low PACAP concentrations and VPAC2R-mediated depression of AMPA receptors at high concentrations of PACAP have been reported in the hippocampus [38]. Furthermore, excitatory transmission from the basolateral amygdala to the central amygdala is augmented through PACAP-VPAC1R increases in AMPA receptor activity [39]. However, without further behavioral pharmacology examining glutamate receptor-mediated properties of PACAP-induced hypophagia in combination with the appropriate electrophysiology, the role of other modes of glutamatergic neurotransmission cannot be ascertained at this time. Nevertheless, our experiments using AP5 and PP1 attenuated the effects of PACAP on feeding suggesting a similar mechanism of NMDA receptor modulation by PACAP likely exists in the hypothalamus as it does in the hippocampus.
Glutamate is also substantially regulated by astrocytes, which are major targets of PACAP signaling [40–42], yielding another potentially important mechanism for glutamate modulation by PACAP. Astrocytes impact glutamate neurotransmission most notably through removal of synaptic glutamate by sodium-dependent excitatory amino acid transporters (EAATs) [43–45] and glutamate release from astrocytes themselves [46–50]. Given that a single astrocyte can interact with numerous synapses [51, 52], their influence over glutamate signaling and overall network activity is immense. PACAP is already known to increase expression of both GLAST and GLT-1 (EAAT1 and EAAT2 respectively) in primary cortical astrocyte cultures [53], therefore, investigation into how astrocytic glutamate regulation is further influenced by PACAP may reveal new insights into signaling mechanisms that are important to glutamate homeostasis and may influence behavior. Despite the extensive study of how astrocytic control of glutamate affects hypothalamic function [6, 54–56] and knowledge that PACAP potently regulates feeding behavior [22, 57–59], whether or not modulation of astrocytic glutamate signaling by PACAP influences food intake has yet to be investigated.
The studies described here further our understanding of PACAP signaling in the hypothalamus with regard to feeding behavior. Previous reports have suggested that PACAP augments NMDA receptor activity [24, 25, 28, 32], and inhibition of NMDA receptor signaling is sufficient to mitigate the hypophagic response to PACAP administration in the VMN, suggesting a similar relationship exists between PACAP and NMDA receptor signaling in the hypothalamus. Furthermore, this NMDA receptor modulation by PACAP appears to be mediated through Src family kinase signaling, which is critical to both NMDA receptor function as well as the regulation of feeding behavior [29, 36, 37]. Although there is evidence for co-expression in some brain areas [23, 26, 27], we do not know if this is the case in the hypothalamus or if separate PACAPergic neurons release the neuropeptide distal to glutamatergic neurons (Figure 4). Given the identified pleiotropic actions of PACAP, further investigation is warranted to address the sources of PACAP release, other mechanisms of glutamate modulation by this neuropeptide and how such mechanisms are involved in the regulation of feeding behavior.
Highlights.
Blocking NMDA receptor activity in the hypothalamic ventromedial nuclei (VMN) prevents PACAP-induced hypophagia.
PACAP administration into the VMN promotes tyrosine phosphorylation of the NMDA receptor indicating an involvement of glutamate signaling.
Modulation of NMDA receptors via Src family tyrosine kinases is necessary for PACAP-induced hypophagia in the VMN.
Acknowledgments
This research was supported by NIH grant DK074734
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Y, Kim ER, Zhao R, Myers MG, Jr, Munzberg H, Tong Q. Glutamate release mediates leptin action on energy expenditure. Molecular metabolism. 2013;2:109–115. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y, Wu Z, Sun H, Zhu Y, Kim ER, Lowell BB, et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013;18:860–870. doi: 10.1016/j.cmet.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternson SM, Shepherd GM, Friedman JM. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 6.Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, et al. Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest. 2012;122:3900–3913. doi: 10.1172/JCI64102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley BG, Ha LH, Spears LC, Dee MG., 2nd Lateral hypothalamic injections of glutamate, kainic acid, D,L-alpha-amino-3-hydroxy-5-methyl-isoxazole propionic acid or N-methyl-D-aspartic acid rapidly elicit intense transient eating in rats. Brain Res. 1993;613:88–95. doi: 10.1016/0006-8993(93)90458-y. [DOI] [PubMed] [Google Scholar]
- 8.Takaki A, Aou S, Oomura Y, Okada E, Hori T. Feeding suppression elicited by electrical and chemical stimulations of monkey hypothalamus. Am J Physiol. 1992;262:R586–R594. doi: 10.1152/ajpregu.1992.262.4.R586. [DOI] [PubMed] [Google Scholar]
- 9.Guyenet SJ, Matsen ME, Morton GJ, Kaiyala KJ, Schwartz MW. Rapid glutamate release in the mediobasal hypothalamus accompanies feeding and is exaggerated by an obesogenic food. Molecular metabolism. 2013;2:116–122. doi: 10.1016/j.molmet.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76:98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SW, Stanley BG. NMDA receptors mediate feeding elicited by neuropeptide Y in the lateral and perifornical hypothalamus. Brain Res. 2005;1063:1–8. doi: 10.1016/j.brainres.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 12.Doane DF, Lawson MA, Meade JR, Kotz CM, Beverly JL. Orexin-induced feeding requires NMDA receptor activation in the perifornical region of the lateral hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1022–R1026. doi: 10.1152/ajpregu.00282.2007. [DOI] [PubMed] [Google Scholar]
- 13.Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- 14.Meeker RB, Greenwood RS, Hayward JN. Glutamate receptors in the rat hypothalamus and pituitary. Endocrinology. 1994;134:621–629. doi: 10.1210/endo.134.2.7905409. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–229. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- 16.Fu LY, van den Pol AN. Agouti-related peptide and MC3/4 receptor agonists both inhibit excitatory hypothalamic ventromedial nucleus neurons. J Neurosci. 2008;28:5433–5449. doi: 10.1523/JNEUROSCI.0749-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amir S. Intra-ventromedial hypothalamic injection of glutamate stimulates brown adipose tissue thermogenesis in the rat. Brain Res. 1990;511:341–344. doi: 10.1016/0006-8993(90)90181-a. [DOI] [PubMed] [Google Scholar]
- 18.Yoshimatsu H, Egawa M, Bray GA. Sympathetic nerve activity after discrete hypothalamic injections of L-glutamate. Brain Res. 1993;601:121–128. doi: 10.1016/0006-8993(93)91702-t. [DOI] [PubMed] [Google Scholar]
- 19.Ruffin M, Nicolaidis S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Res. 1999;846:23–29. doi: 10.1016/s0006-8993(99)01922-8. [DOI] [PubMed] [Google Scholar]
- 20.Beltt BM, Keesey RE. Hypothalamic map of stimulation current thresholds for inhibition of feeding in rats. Am J Physiol. 1975;229:1124–1133. doi: 10.1152/ajplegacy.1975.229.4.1124. [DOI] [PubMed] [Google Scholar]
- 21.Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1625–R1634. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resch JM, Maunze B, Gerhardt AK, Magnuson SK, Phillips KA, Choi S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am J Physiol Endocrinol Metab. 2013;305:E1452–E1463. doi: 10.1152/ajpendo.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- 24.Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, et al. Modulation of NMDA receptors by pituitary adenylate cyclase activating peptide in CA1 neurons requires G alpha q, protein kinase C, activation of Src. J Neurosci. 2005;25:11374–11384. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahrenkrug J, Hannibal J. Neurotransmitters co-existing with VIP or PACAP. Peptides. 2004;25:393–401. doi: 10.1016/j.peptides.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Engelund A, Fahrenkrug J, Harrison A, Hannibal J. Vesicular glutamate transporter 2 (VGLUT2) is co-stored with PACAP in projections from the rat melanopsin-containing retinal ganglion cells. Cell and tissue research. 2010;340:243–255. doi: 10.1007/s00441-010-0950-3. [DOI] [PubMed] [Google Scholar]
- 28.Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- 29.Khan AM, Cheung HH, Gillard ER, Palarca JA, Welsbie DS, Gurd JW, et al. Lateral hypothalamic signaling mechanisms underlying feeding stimulation: differential contributions of Src family tyrosine kinases to feeding triggered either by NMDA injection or by food deprivation. J Neurosci. 2004;24:10603–10615. doi: 10.1523/JNEUROSCI.3390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sixth Edition. San Diego, CA: Academic Press; 2007. [Google Scholar]
- 31.Blevins JE, Stanley BG, Reidelberger RD. DMSO as a vehicle for central injections: tests with feeding elicited by norepinephrine injected into the paraventricular nucleus. Pharmacol Biochem Behav. 2002;71:277–282. doi: 10.1016/s0091-3057(01)00659-1. [DOI] [PubMed] [Google Scholar]
- 32.Wu SY, Dun NJ. Potentiation of NMDA currents by pituitary adenylate cyclase activating polypeptide in neonatal rat sympathetic preganglionic neurons. Journal of neurophysiology. 1997;78:1175–1179. doi: 10.1152/jn.1997.78.2.1175. [DOI] [PubMed] [Google Scholar]
- 33.Stanley BG, Willett VL, 3rd, Donias HW, Dee MG, 2nd, Duva MA. Lateral hypothalamic NMDA receptors and glutamate as physiological mediators of eating and weight control. Am J Physiol. 1996;270:R443–R449. doi: 10.1152/ajpregu.1996.270.2.R443. [DOI] [PubMed] [Google Scholar]
- 34.Stanley BG, Willett VL, 3rd, Donias HW, Ha LH, Spears LC. The lateral hypothalamus: a primary site mediating excitatory amino acid-elicited eating. Brain Res. 1993;630:41–49. doi: 10.1016/0006-8993(93)90640-9. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury D, Marco S, Brooks IM, Zandueta A, Rao Y, Haucke V, et al. Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J Neurosci. 2013;33:4151–4164. doi: 10.1523/JNEUROSCI.2721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groveman BR, Feng S, Fang XQ, Pflueger M, Lin SX, Bienkiewicz EA, et al. The regulation of N-methyl-D-aspartate receptors by Src kinase. The FEBS journal. 2012;279:20–28. doi: 10.1111/j.1742-4658.2011.08413.x. [DOI] [PubMed] [Google Scholar]
- 37.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. The FEBS journal. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 38.Costa L, Santangelo F, Li Volsi G, Ciranna L. Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus. 2009;19:99–109. doi: 10.1002/hipo.20488. [DOI] [PubMed] [Google Scholar]
- 39.Cho JH, Zushida K, Shumyatsky GP, Carlezon WA, Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci. 2012;32:14165–14177. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatsuno I, Morio H, Tanaka T, Hirai A, Tamura Y, Saito Y, et al. Astrocytes are one of the main target cells for pituitary adenylate cyclase-activating polypeptide in the central nervous system. Astrocytes are very heterogeneous regarding both basal movement of intracellular free calcium ([Ca2+]i) and the [Ca2+]i response to PACAP at a single cell level. Ann N Y Acad Sci. 1996;805:613–619. doi: 10.1111/j.1749-6632.1996.tb17529.x. [DOI] [PubMed] [Google Scholar]
- 41.Tatsuno I, Morio H, Tanaka T, Uchida D, Hirai A, Tamura Y, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a regulator of astrocytes: PACAP stimulates proliferation and production of interleukin 6 (IL-6), but not nerve growth factor (NGF), in cultured rat astrocyte. Ann N Y Acad Sci. 1996;805:482–488. doi: 10.1111/j.1749-6632.1996.tb17508.x. [DOI] [PubMed] [Google Scholar]
- 42.Masmoudi-Kouki O, Gandolfo P, Castel H, Leprince J, Fournier A, Dejda A, et al. Role of PACAP and VIP in astroglial functions. Peptides. 2007;28:1753–1760. doi: 10.1016/j.peptides.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Asztely F, Erdemli G, Kullmann DM. Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 44.Diamond JS, Jahr CE. Synaptically released glutamate does not overwhelm transporters on hippocampal astrocytes during high-frequency stimulation. Journal of neurophysiology. 2000;83:2835–2843. doi: 10.1152/jn.2000.83.5.2835. [DOI] [PubMed] [Google Scholar]
- 45.Zheng K, Scimemi A, Rusakov DA. Receptor actions of synaptically released glutamate: the role of transporters on the scale from nanometers to microns. Biophysical journal. 2008;95:4584–4596. doi: 10.1529/biophysj.108.129874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, et al. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parpura V, Haydon PG. Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogata K, Kosaka T. Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience. 2002;113:221–233. doi: 10.1016/s0306-4522(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 53.Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- 55.Gordon GR, Iremonger KJ, Kantevari S, Ellis-Davies GC, MacVicar BA, Bains JS. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron. 2009;64:391–403. doi: 10.1016/j.neuron.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Potapenko ES, Biancardi VC, Zhou Y, Stern JE. Altered astrocyte glutamate transporter regulation of hypothalamic neurosecretory neurons in heart failure rats. Am J Physiol Regul Integr Comp Physiol. 2012;303:R291–R300. doi: 10.1152/ajpregu.00056.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morley JE, Horowitz M, Morley PM, Flood JF. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces food intake in mice. Peptides. 1992;13:1133–1135. doi: 10.1016/0196-9781(92)90019-y. [DOI] [PubMed] [Google Scholar]
- 58.Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29:14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, et al. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]