Abstract
Rickettsia is a genus of intracellular bacteria that causes a variety of diseases in humans and other mammals and associates with a diverse group of arthropods. Although Rickettsia appears to be common in ticks, most Rickettsia-tick relationships remain generally uncharacterized. The most intimate of these associations is Rickettsia species phylotype G021, a maternally and transstadially transmitted endosymbiont that resides in 100% of I. pacificus in California. We investigated the effects of this Rickettsia phylotype on I. pacificus reproductive fitness using selective antibiotic treatment. Ciprofloxacin was 10-fold more effective than tetracycline in eliminating Rickettsia from I. pacificus, and quantitative PCR results showed that eggs from the ciprofloxacin-treated ticks contained an average of 0.02 Rickettsia per egg cell as opposed to the average of 0.2 in the tetracycline-treated ticks. Ampicillin did not significantly affect the number of Rickettsia per tick cell in adults or eggs compared to the water-injected control ticks. We found no relationship between tick embryogenesis and rickettsial density in engorged I. pacificus females. Tetracycline treatment significantly delayed oviposition of I. pacificus ticks, but the antibiotic’s effect was unlikely related to Rickettsia. We also demonstrated that Rickettsia-free eggs could successfully develop into larvae without any significant decrease in hatching compared to eggs containing Rickettsia. No significant differences in the incubation period, egg hatching rate, and the number of larvae were found between any of the antibiotic-treated groups and the water-injected tick control. We concluded that Rickettsia species phylotype G021 does not have an apparent effect on embryogenesis, oviposition, and egg hatching of I. pacificus.
Introduction
Bacterial endosymbiosis is ubiquitous in arthropods and ranges from mutualism to parasitism [1]–[3]. Bacterial symbionts may exhibit multiple effects on their hosts [3] – for example, the most well studied arthropod symbiont Wolbachia is a reproductive manipulator that also increases fitness of the mosquito Aedes albopictus [4]. The most intimate association between bacteria and arthropods is heritable obligate mutualism, where the host cannot survive and/or produce viable offspring without the symbiont [5]. A classic example of obligate symbiosis is Buchnera, which provides essential amino acids to the pea aphid Acyrthosiphon pisum [6]. However, most arthropod endosymbionts appear to be facultative, i.e. not strictly required for the host’s survival and reproduction [3].
Rickettsia is a genus of gram-negative obligate intracellular bacteria within the class Alphaproteobacteria. Rickettsiae live mainly inside arthropods, but associate with a diverse range of hosts, including leeches and hydra [7]. Arthropod rickettsiae are generally facultative symbionts that display a wide range of relationships with their hosts. Rickettsia can be mutualistic, e.g. by protecting an aphid host against a fungal pathogen [8]. Rickettsia can also be a reproductive manipulator – a species of Rickettsia causes parthenogenesis in the parasitoid wasp Pnigalio soemius [9]. Reproductive manipulation that increases the female:male ratio of arthropod offspring is a common strategy among bacterial endosymbionts in arthropods, as endosymbionts’ heritability in the host lineage is usually strictly maternal [1]. Though many Rickettsia species have no reported vertebrate pathogenicity, the genus is best known as a pathogen of humans and cattle [10]. Classification of pathogenic rickettsiae is largely coincident with the diseases the bacteria cause in humans: spotted fever group (SFG) rickettsiae are known to cause spotted fevers while the typhus group is responsible for endemic typhus [11], [12]. SFG Rickettsia species are primarily vectored by the ixodid (“hard”) ticks and have been found on all continents but Antarctica [13].
The effects of rickettsiae on ticks are dependent on the specific Rickettsia and the tick species involved, but general patterns remain unavailable since few Rickettsia-tick relationships have been investigated. Natural infection with R. montanensis, R. bellii and R. rhipicephali decreases fitness of Dermacentor andersoni whereas laboratory infection with R. rickettsii is detrimental to the tick, with 97.5% of infected progeny from D. andersoni not surviving to adulthood [14]. Similarly, R. conorii induces mortality in laboratory-infected Rhipicephalus sanguineus ticks [13]. Reproductive manipulation is a possibility as endosymbiotic R. bellii universally infects a strictly parthenogenetic tick species, Amblyomma rotundum [15]. However, Rickettsia has been implicated in increased larval motility of the ticks Ixodes scapularis, Amblyomma americanum, and D. andersoni, likely making it easier for the tick species to access hosts [16].
Rickettsia bacteria have been found in several Ixodes species of ixodid ticks. Rickettsia species were recently reported in adults and nymphs of Ixodes ricinus from northern Italy [17]. In I. scapularis, 16S rDNA metagenomics revealed that Rickettsia is the most prevalent (present in 77% of all adults and nymphs studied) identified bacterial genus in Texas populations of I. scapularis, and the second most prevalent (83%) in New York populations [18]. However, the closest known association between Rickettsia and Ixodes is a recently discovered Rickettsia species (phylotype G021) that is universally present and 100% maternally transmitted in California populations of Ixodes pacificus [19], [20]. The ubiquitous prevalence and vertical transmission of the Rickettsia phylotype suggest a likely mutualistic role in the tick species.
I. pacificus transmits several bacterial pathogens of humans, dogs and cattle in western North America, and particularly in California where it has been reported in 56 out of 58 counties [21]. I. pacificus has four life cycle stages: egg, larva, nymph and adult. Larvae and nymphs require a blood meal for molting, while adult females require it for oviposition. In laboratory settings, the duration of the I. pacificus life cycle strongly depends on temperature. The length of the I. pacificus preoviposition period, for example, varies from an average of 9 days at 26°C to 70 days at 12°C [22]. Ixodes females’ egg counts are also highly variable, ranging from several thousand eggs per female [23], to less than eight hundred [24]. I. pacificus eggs can have a nearly 100% hatching rate if conditions are favorable; high humidity of at least 85% is preferred as eggs tend to die from desiccation in dry habitats [22].
Removal of symbionts using antibiotic injection has been successfully used to study functions of bacterial symbioses in ticks and aphids [25], [26]. In the lone-star tick A. americanum, injection with tetracycline and rifampin demonstrated that the tick species’ only known heritable endosymbiont, the bacteria Coxiella, are mutualists that contribute to A. americanum’s fecundity [26]. Selective antibiotic treatment is a strategy where antibiotics target specific groups of bacteria to determine how the individual groups of microorganisms affect the host. Ampicillin injection selectively removed Rickettsia in the second-generation A. pisum offspring and showed that Rickettsia decreased the aphid’s fitness by lowering the density of the aphid’s obligate symbiont Buchnera [25].
In this study, we investigated the effect of Rickettsia species phylotype G021 on reproductive fitness of I. pacificus adult females using selective antibiotic treatments. Three antibiotics were selected for the study: tetracycline, ciprofloxacin (a fluoroquinolone antibiotic) and ampicillin. The antibiotics were chosen based on their effectiveness in treating rickettsiae and on their mode of action. Tetracycline and fluoroquinolone antibiotics have been successfully used for treating spotted fever patients [27], and Rickettsia species in vitro [28]. We chose two effective antibiotics for two reasons: to control for the possibility of an antibiotic affecting I. pacificus independently of rickettsiae, and to use antibiotics with different modes of action. Fluoroquinolones kill bacteria by interfering with DNA topoisomerase IV and DNA gyrase [29], enzymes required for double-stranded DNA synthesis. Tetracyclines are, in contrast, bacteriostatic agents that inhibit protein synthesis by binding to the bacterial 30S ribosome, particularly the tRNA-binding site [29], [30]. Ampicillin was chosen as an antibiotic control in this study. Despite the antibiotic’s ability to affect Rickettsia in the second-generation offspring in A. pisum [25], ampicillin cannot be used to treat patients infected with rickettsial diseases [31] and is ineffective in vitro on tick-borne intracellular bacteria Ehrlichia and Anaplasma [32].
We assessed the effectiveness of different antibiotic treatment on the Rickettsia phylotype and determined the effects of the antibiotics on egg hatching and larval counts. Our specific research questions were as follows: (1) Which antibiotic, tetracycline or ciprofloxacin, would be more effective in treating Rickettsia phylotype G021 in I. pacificus? (2) Can ampicillin be used as an antibiotic control ineffective on Rickettsia phylotype G021 in I. pacificus? (3) Does decreasing rickettsial density in the engorged tick female affect embryogenesis, i.e. prolong the period of time before an engorged female starts ovipositing and/or have an effect on the number of offspring? (4) Does the Rickettsia phylotype affect egg hatching of I. pacificus? A broader goal of our study was to gain insights into the relationship between an important arthropod disease vector and its bacterial symbiont, with potential future implications for disease control.
Materials and Methods
Ticks and tick feeding
Adult I. pacificus were collected by flagging in mixed evergreen and oak woodland near Blue Lake in Humboldt County, California (GPS coordinate: N 40 55.200 W 123 50.400). No specific permissions were required to collect ticks from the site, and no endangered, or protected species were involved in the fieldwork conducted. The ticks were collected by three people on two trips, one in late winter and one in early spring of 2011, with roughly two hours dedicated to tick collection during each trip. Ticks were identified to the species level based on morphologic characteristics under a dissecting microscope [33] until 101 male and 101 female I. pacificus were obtained for the study. Ticks were then kept in polystyrene containers (Thermo Fisher Scientific, Waltham, MA) inside a desiccator with ambient temperature and day:night cycles, and 95% relative humidity.
Adult I. pacificus were fed on five male New Zealand white rabbits (Oryctolagus cuniculus (Figure 1A). All procedures followed protocols approved by the Institutional Animal Care and Use Committee at Humboldt State University (IACUC protocol 10/11.B.45-A), and all efforts were made to minimize discomfort. Four tin cans with seamless lids (Freund Containers, Lisle, IL) were glued to shaved spots on each rabbit’s back using cyanoacrylate glue. Up to five male and five female ticks were put in each tin can. The ticks were allowed to mate on the rabbits until engorgement.
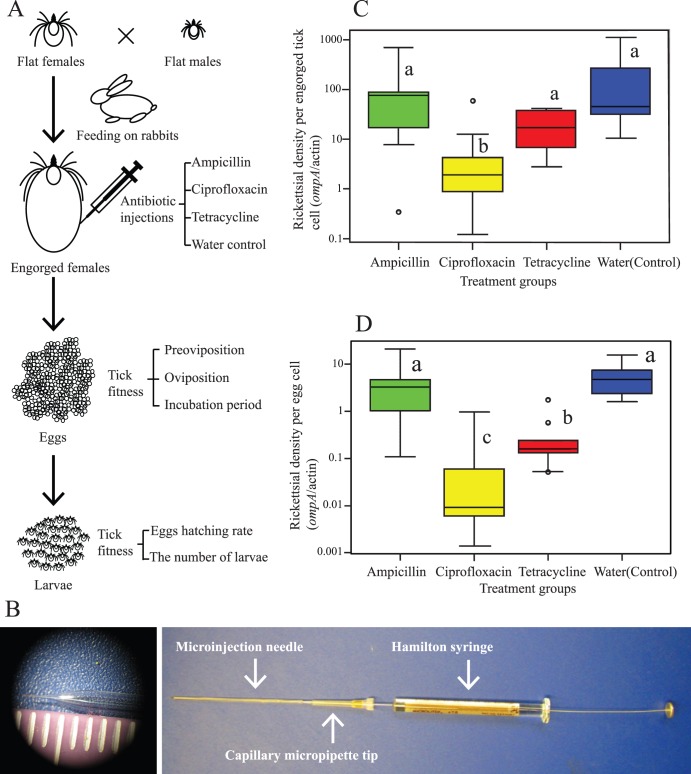
Figure 1. Illustration of the experimental design and the antibiotic injection results.
1A) Experimental design outline, represented as a flow chart. 1B) Tick injection apparatus. Solution is loaded into the microinjection needle using the capillary pipette tip until the needle is full. The tip is then left inside the glass needle and the syringe with a 23-gauge needle is fitted into the capillary pipette tip. 1C) and 1D) Box-whisker plots of rickettsial densities (gene copy ratios of Rickettsia ompA to Ixodes pacificus actin) detected by qPCR in (C) spent females and (D) eggs from the four treatment groups. In both (C) and (D), the same letter next to two plots informs of statistically insignificant difference between the treatment groups (P>0.05), meaning that lack of identical letters stipulates significance (P<0.05).
Tick antibiotic injection and determination of hatching fitness
Upon engorgement, female ticks dropped from the rabbits on their own [34], [35] and were divided into four treatment groups of roughly equal mean weight: ampicillin, ciprofloxacin, tetracycline, and water (negative control). Engorged female ticks were injected into the hemocoel between the first and second legs [26] using microinjection needles pulled by P-97 Sutter Glass needle puller (Sutter Instrument Co, Novato, CA). To inject the ticks, we designed an injection apparatus based on commercially available components (Figure 1B). The microinjection needles were loaded with antibiotics or water using a micropipette with a disposable capillary 20 µl pipette tip (Eppendorf, Hamburg, Germany) that was eventually inserted into the needle, left in place, and filled to the top with the solution. A 10 µl Hamilton syringe (Hamilton, Reno, NV) with a 23-gauge needle was then inserted into the capillary pipette tip. All three compartments were connected to each other firmly by twisting and pushing. The syringe plunger was used to pull antibiotic solution or water into the glass and to inject the contents into ticks under a dissecting microscope (Figure 1B). Three µl of 10 mg/ml antibiotic solution (or water) was injected into ticks, and the needle was left inside tick body for 30 sec before being slowly withdrawn. Injected ticks were put back into individual polystyrene containers in the desiccator and monitored for oviposition daily. Time to oviposition or the preoviposition period was recorded as the period between tick injection and the beginning of oviposition. Ticks that died shortly after injection (likely as a result of injection stress) were excluded from the study without affecting statistical analysis, as all such deaths happened before oviposition in any of the groups commenced.
After completion of oviposition and subsequent death, each spent female, and 10 randomly selected viable eggs from each female, were preserved in 70% ethanol and stored at −20°C for further molecular analysis. The remaining eggs were left in the original polystyrene containers inside the desiccators and were monitored daily until hatching was complete. The incubation period was defined as the time from the deposition of the first egg until the completion of egg hatching into larvae. After all the larvae had hatched, the containers were placed in −20°C to freeze the larvae. The larvae and the remaining unhatched eggs were then counted under a dissecting microscope. Ticks that survived injection, but died without laying eggs were not included in the analysis to determine the difference in the incubation period, hatching rate, or the number of larvae, between treatment groups.
DNA extraction and quantitative PCR (qPCR)
The spent I. pacificus females and their eggs (10 from each female) were pulverized in liquid nitrogen prior to DNA extraction. Each spent female was frozen in liquid nitrogen, and then split into four parts using a sterile scalpel; each part was treated separately for extraction purposes to improve tissue lysis and increase overall DNA yield. Each of the 10 eggs was punctured with a sterile pestle (Qiagen, Valencia, CA) before extraction. DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA), as previously described [36]. One mock DNA extraction was performed for every 10 extractions from tick samples or eggs as a contamination control. Mock extractions contained distilled nuclease-free water instead of a tick or an egg sample, and were carried out according to the protocol used for other samples during DNA extraction.
To determine rickettsial prevalence and density in ticks, an outer membrane protein (ompA) gene of Rickettsia species phylotype G021 and the beta-actin biosynthetic (actin) gene of I. pacificus were qPCR-amplified using the ABI-7300 Real-Time PCR System (Life Technologies, Foster City, CA), as previously described [20]. Primer pairs and probe were designed against a divergent sequence of the single copy ompA gene to differentiate between phylotype G021 and the rare Rickettsia phylotype G022 [37]. Rickettsial density of I. pacificus was defined as the ratio of rickettsial ompA copies per I. pacificus actin copies. All qPCR reactions were set up as follows: 4.5 µl extracted tick DNA was mixed with 7.5 µl qPCR Master Mix Plus (AnaSpec, Fremont, CA), 0.5 µl of 5 µM primers and probe, and 1.5 µl 0.4% bovine serum albumin (BSA), yielding 15 µl total reaction volume. The reactions were run in duplicates in 96-well plate qPCR plates (Life Technologies, Foster City, CA). Four negative control reactions were run on each 96-well plate to ensure that the reagents were not contaminated with template DNA.
Standard curves for the ompA gene of Rickettsia species phylotype G021 and the actin gene of I. pacificus were constructed based on gene products cloned into Psc-A-amp/kan plasmid using topoisomerase-based StrataClone™ PCR Cloning Kit (Agilent Technologies, Santa Clara, CA). For cloning purposes, amplicons of the ompA gene of phylotype G021 or the actin gene of I. pacificus were each ligated into the Psc-A-amp/kan plasmid, as specified by the manufacturer’s instructions outlined in the manual for the StrataClone PCR Cloning Kit (Agilent Technologies, Santa Clara, CA). Plasmid DNA was isolated from the transformants using the Promega Wizard Plus SV Plasmid Purification System (Promega, Madison, WI) using the manufacturer’s protocol. Concentration of the plasmid DNA was determined by measuring absorbance at the wavelength of 260 nm using the Nanodrop ND-1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The solution containing a known concentration of ompA or actin DNA was then 10-fold serially diluted to produce standards that were run in duplicates in each qPCR reaction to generate a standard curve. Based on the standard curve for each gene, experimental qPCR data were used to determine gene copy numbers for the ompA gene of phylotype G021 and the actin gene of I. pacificus in adult tick/egg DNA samples. The resultant copy numbers were used to estimate rickettsial prevalence and density of each spent female and its ten randomly selected eggs.
Statistical analyses
R Statistical Software version 3.0.2. was used to analyze data [38]. Specifically, the Anderson-Darling test [39] was used to check if the data were normally distributed, and Levene’s test [40] was performed to test for equal variances. To normalize data, log transformations were applied to copy numbers of rickettsial densities that varied by orders of magnitude; arcsine transformations were applied to hatching rates (percentages; [41]), and the square root transformation was used for the number of larvae.
Linear regression analysis was performed to determine the relationship between egg and adult rickettsial densities. Spearman correlation analyses were used to determine correlative relationships between other variables. Kaplan-Meier analysis, a common method to analyze time-to-event data [42], was employed to compare time to oviposition and incubation periods of the four treatment groups. The Hmisc package for R was used to generate confidence intervals for the medians in the Kaplan-Meier analysis [43]. The Log-rank test with Bonferroni correction was applied to make pairwise comparisons between preoviposition periods and incubation periods of different groups. One-way analysis of variance (ANOVA) with the Tukey-Kramer pairwise comparison test was used to compare the four treatment groups with respect to rickettsial densities, hatching rate, and the number of larvae.
Results
Tick feeding, injection and effects of antibiotics on Rickettsia species phylotype G021
Out of the 101 female ticks that fed on rabbits, 89 successfully fed to engorgement. Twenty-one of the 89 engorged ticks died shortly after dropping off, leaving 68 for injection. The engorged females were then split into four groups according to their mean weight of ∼170 mg in each group. Forty-four of the 68 injected ticks laid eggs that hatched into viable larvae. The 44 ticks were distributed as follows: 12 in the ampicillin group, 14 in the ciprofloxacin group, 9 in the tetracycline and the control groups. Excluding dead ticks from statistical analyses did not create a significant difference between the mean weights of the groups (Kruskal-Wallis test, P>0.80). This implies that no weight discrepancy bias (where a particular group would end up with a disproportionally larger number of heavy ticks that lay more eggs) was introduced into the analysis by eliminating the ticks.
Decrease in rickettsial density as a result of antibiotic treatment was assessed by comparing the ratio of copy numbers of the rickettsial outer membrane protein gene (ompA) to I. pacificus beta-actin biosynthetic gene (actin) in spent females and eggs between treatment groups (ompA/actin). Spent females in the water control group had the highest mean (95% confidence interval (CI)) rickettsial density of 79.4 (23.4–275.4) ompA/actin. Spent females in the ampicillin-injected group had the second highest density with 38.0 (11.5–131.8) ompA/actin, followed by the tetracycline-injected group’s density of 14.5 (6.9–30.9), and the lowest density of 2.0 (0.9–4.9) in the ciprofloxacin-injected group. Spent females in the ciprofloxacin-injected females had significantly lower rickettsial density than females in any other group (ANOVA, P<0.0001, df = 43; Tukey-Kramer Test, P<0.05) (Figure 1C). The rickettsial densities of spent females in the rest of the groups were not significantly different from each other (Tukey-Kramer Test, P>0.05).
Rickettsial density in eggs was lower than in spent females (Student’s Paired t-test, P<0.05), with the ompA/actin ratio of the control group at 4.7 (2.5–8.5) being the highest. The ampicillin treated group followed with a mean density of 2.2 (0.9–5.6), higher than that of the tetracycline group at 0.2 (0.09–0.5), which was 100 times higher than the density of 0.02 (0.007–0.05) in the ciprofloxacin group. In contrast to spent females, rickettsial densities in eggs coming from adult female ticks treated with tetracycline and ciprofloxacin were significantly lower than those in either the water control or the ampicillin group (ANOVA, P<0.0001) (Figure 1D). In addition, eggs in the ciprofloxacin group had a significantly lower rickettsial density than eggs from the tetracycline group (Tukey-Kramer Test, P<0.05). Rickettsial densities in eggs demonstrated a significant linear relationship with rickettsial densities in the respective spent females (R2 = 0.57, P<0.001).
Ciprofloxacin-injected ticks were the only group of engorged females that laid Rickettsia-free eggs. Cohorts of 10 randomly selected eggs from each of the 14 ovipositing ciprofloxacin-treated females were screened for Rickettsia species phylotype G021 by molecular analyses. Only three of the 14 egg cohorts of ciprofloxacin-injected ticks were 10 of 10 (100%) Rickettsia-positive (Table 1). Rickettsial prevalence in the remaining 11 egg cohorts varied from 0% to 90%. The prevalence of rickettsiae in the egg cohorts showed no relationship with the overall hatching rate of the respective ticks’ eggs, or the number of resultant larvae (Spearman correlation, P>0.05). The egg cohort with 0% rickettsial prevalence (meaning that not a single egg out of the 10 tested by qPCR contained Rickettsia phylotype G021) came from a tick whose eggs exhibited a hatching rate of 94.1% and yielded 1750 viable larvae. Both the hatching rate and the larval count of the cohort were above the mean values of the ciprofloxacin group as a whole (93.2% hatching rate and 1315 viable larvae, respectively). However, the three cohorts with 100% prevalence of rickettsiae had a below-average mean hatching rate of 69.3%, and a mean of 1154 larvae, also lower than the overall mean larval count of the ciprofloxacin group.
Table 1. Prevalence of infection with Rickettsia species phylotype G021 in Ixodes pacificus egg cohorts of 10 from each of the 14 ticks in the ciprofloxacin-injected group.
| Number of eggs positive forRickettsia species phylotypeG021 in a cohort | Percentage of cohorts withthe rickettsial prevalence (%) | Averagehatchingrate (%) | Averagenumber oflarvae |
| 0/10 | 7.1 | 94.1 | 1750 |
| 2/10 | 14.3 | 97.8 | 836 |
| 3/10 | 7.1 | 99.2 | 1640 |
| 6/10 | 7.1 | 99.0 | 1272 |
| 8/10 | 28.6 | 95.0 | 1513 |
| 9/10 | 14.3 | 81.6 | 1093 |
| 10/10 | 21.4 | 69.3 | 1154 |
Tick oviposition and egg hatching
Ticks in the tetracycline group took a median (95% CI) of 13 (10–16) days after injection treatment to start laying eggs. Ciprofloxacin, water control and ampicillin groups each spent a median of 9 (8–10) days after injection before commencing oviposition, (Figure 2A). Tetracycline treatment significantly delayed I. pacificus oviposition compared to all other treatment groups (Log-Rank test, P<0.05). The preoviposition periods of the ampicillin, ciprofloxacin, and control groups were not significantly different from each other (Log-Rank test, P>0.05).
Figure 2. Kaplan-Meier (time-to-event) plots of the preoviposition period and the incubation periods, and boxplots representing egg hatching rate, and the number of larvae from engorged Ixodes pacificus females.
2A) A Kaplan-Meier plot where each colored line corresponds to the fraction of adult female ticks that had not started laying eggs on a particular day after the ticks were injected with antibiotics or water (control). Vertical dashes on the colored lines represent censorship, where a tick that had not started to lay eggs died and was dropped from the study. The tetracycline group (red) took significantly longer to start laying eggs compared to the rest (P<0.05) 2B) A Kaplan-Meier plot where the colored lines represent the proportion of egg samples laid by individual ticks whose eggs had not finished hatching into larvae a certain number of days after oviposition began. 2C) A boxplot showing the hatching rate, or the fraction of tick eggs that successfully hatched into larvae, in the four treatment groups. Each individually colored box represents the hatching rate distribution of eggs laid by each female tick in the group. 2D) A boxplot specifying the distribution of the total number of larvae each female yielded. No significant difference in (C) hatching rate or (D) the number of larvae was present between treatment groups (P<0.05). Circles outside the boxes in (C) and (D) represent outliers. The colors in all the plots in the figure correspond to specific treatment groups: blue - ampicillin, yellow - ciprofloxacin, red - tetracycline, green - injection with water (control).
Several ticks did not lay eggs and were censored in the Kaplan-Meier analysis upon their deaths (Figure 2A). The three ticks that did not lay eggs in the tetracycline group were affected by a pathogen that exhibited a green, mold-like morphology and was presumably fungal. Another tetracycline-treated tick affected by the pathogen laid eggs that never hatched. Two ticks died without laying eggs in the other treatment groups – one in the ampicillin group and one in the control group. These ticks did not have any apparent fungal infection.
The mean (95% CI) duration of incubation averaged 85 (79–91) days in the eggs from ticks injected with water, 84 (81–88) days in ampicillin-injected ticks, 84 (78–89) days in the ciprofloxacin group, and 82 (74–89) days in the tetracycline group (Figure 2B). As expected from the strikingly similar incubation periods, no significant difference was present between any of the treatment groups (ANOVA, P>0.05).
Eggs in the ampicillin group had the highest hatching rate of all groups studied, with a mean (95% CI) of 96.8% (94.2%–98.7%) total eggs hatching into larvae (Figure 2C). Ticks in the control group followed, with a mean hatching rate of 94.5% (84.1%–99.5%). The ciprofloxacin group laid eggs with a slightly lower hatching rate of 93.2% (83.6%–98.5%), and only 85.2% (73.8%–94.2%) eggs from tetracycline-injected ticks hatched into larvae. However, the tetracycline group’s lower hatching rate was not significantly different from the hatching rates of other treatment groups (ANOVA, P>0.05).
Eggs from the ampicillin group yielded the highest number of larvae of all treatment groups, with a mean (95% CI) number of 1493 (1073–1847) larvae hatched. The control group followed, with an average of 1359 (780–1878) hatched larvae. The ciprofloxacin group’s eggs hatched into 1315 (790–1316) larvae, and the tetracycline group had the lowest mean number of larvae at 1223 (519–1828) (Figure 2D). As with hatching rate, no significant difference was found between any of the groups with respect to the number of larvae (ANOVA, P>0.05).
Discussion
In this study, we used antibiotics to study the effects of an endosymbiotic Rickettsia species on reproductive fitness of I. pacificus. We have concluded that (1) ciprofloxacin is a considerably more effective anti-rickettsial antibiotic than tetracycline when applied to I. pacificus in vivo. (2) Ampicillin does not clear Rickettsia species phylotype G021 from engorged females or their eggs, and can therefore be used as an antibiotic control in related studies (3) A substantial decrease in rickettsial density of I. pacificus females has no significant effect on the female’s preoviposition period or the number of offspring. Finally, (4) Rickettsia-free eggs can successfully hatch into larvae, do not take longer to do so than eggs infected with Rickettsia and do not have reduced hatching rates. This suggests that the Rickettsia phylotype is unnecessary for successful hatching of I. pacificus eggs. In summary, our findings indicate that the phylotype does not appear to have an effect on embryogenesis, oviposition and egg hatching of I. pacificus.
Effects of Antibiotics on Rickettsia
Antibiotic injections demonstrated that tetracycline and ciprofloxacin were effective antibacterial agents against Rickettsia species phylotype G021 in I. pacificus (Figure 1). Quantitative PCR using egg DNA samples showed that ciprofloxacin more effectively decreased rickettsial densities in I. pacificus than tetracycline, whereas tetracycline had much greater antibacterial activity on the Rickettsia species than ampicillin treatment and the control injection with water (Figure 1D). However, in spent females, rickettsial density in the tetracycline group was not significantly different from ticks injected with ampicillin or water (Figure 1C). The inconsistency between rickettsial densities in adults and eggs most likely resulted from tetracycline’s bacteriostatic properties. Since bacteriostatic agents operate by stopping bacterial growth, qPCR detected Rickettsia species that were no longer growing in the tetracycline-injected spent female. On the contrary, bactericidal activity of ciprofloxacin killed the Rickettsia species in I. pacificus, and we presume that much of the rickettsial DNA from lysed bacteria had been degraded in the ciprofloxacin-treated spent females.
Ticks treated with tetracycline exhibited a significant delay in oviposition compared to ticks in the other treatment groups. The tetracycline group also maintained a consistently lowest average reproductive fitness performance throughout the experiment (Figure 2). In addition, tetracycline-injected ticks were the only ones affected by a lethal mold-like infection, suggesting that the antibiotic treatment may have made I. pacificus ticks more susceptible to fungal pathogens. The reason why tetracycline affected oviposition, but ciprofloxacin did not, remains elusive. Antibiotics’ chemical toxicity on arthropods has not been conclusively established, in part because it is often impossible to separate purely chemical, direct toxicity from indirect toxicity that can result from an antibiotic affecting an arthropod’s flora [44]. However, in this study, the tetracycline-induced extension of I. pacificus’ preoviposition period was not connected to the antibiotic’s effect on Rickettsia, as delayed oviposition was not observed in the ciprofloxacin-injected ticks that had a smaller rickettsial density. This further strengthens our conclusion that ciprofloxacin is a better choice of anti-rickettsial agent to be used on I. pacificus in vivo, and potentially a more effective antibiotic to be used on other arthropods in similar studies.
Ampicillin is not effective at killing Rickettsia species phylotype G021 or stopping rickettsial growth in I. pacificus, consistent with in vitro assays that demonstrated inactivity of beta-lactam antibiotics against rickettsiae [31]. Ampicillin is also ineffective in vitro on other tick-borne intracellular bacteria Ehrlichia chaffeensis and Anaplasma phagocytophilum [32] and cannot be used to treat patients infected with rickettsial diseases [31]. Interestingly, ampicillin injection into pea aphids (Acyrthosiphon pisum) eliminates Rickettsia species in a second post-injection generation [25]. Ampicillin-treated ticks and their eggs had slightly lower rickettsial densities than did ticks and eggs in the water control group, but the difference was not statistically significant.
Rickettsia’s effect on embryogenesis, oviposition, and egg hatching of I. pacificus
Decreased rickettsial load did not reduce reproductive fitness of engorged I. pacificus females and their eggs (Figure 2), or have any other apparent consequence on fecundity, suggesting that the rickettsiae are not required for I. pacificus embryogenesis and egg hatching. The tick with the lowest reproductive fitness in the ciprofloxacin group (13% of eggs hatched into a total of 231 larvae) was one of the two ticks in the ciprofloxacin group with 100% Rickettsia prevalence in its egg cohort (Table 1). Four out of 14 egg cohorts from ciprofloxacin-injected ticks had rickettsial prevalence of less than 50%, suggesting that less than 50% of all eggs laid by those ticks contained Rickettsia. Yet ciprofloxacin-treated ticks’ egg incubation period, egg hatching rate, and the number of larvae were not significantly different from any other group, including the control. These findings strongly suggest that Rickettsia phylotype G021 is not necessary for embryogenesis, oviposition, and egg hatching of I. pacificus. A large decrease in beneficial symbionts tends to have an immediate effect on arthropod fecundity, i.e. a reduced number of progeny and significantly lower hatching rates. This has been demonstrated in Amblyomma americanum ticks containing Coxiella symbionts [26], as well as in other arthropods. An almost complete elimination of tsetse flies’ Wigglesworthia and Sodalis symbionts with rifampin- and tetracycline-laden blood led to a decrease in the number of the flies’ offspring and to a decline in the hatching rate from larva into pupa (in tsetse flies, eggs hatch inside the mother, and larvae molt into pupae without feeding; [45]). In a non-blood feeder system, rifampin treatment of secondary symbionts decreases nymphal size in two biotypes of the silverleaf whitefly Bemisia tabaci and reduces survival of eggs into adulthood in one of the whitefly’s biotypes [46]. Antibiotic treatment also decreases embryogenesis and egg hatching of a Collembolan Folsomia candida, likely because of the antibiotics’ effect on the insect species’ flora [44]. These findings provide a striking contrast with triatomine bugs (Hemiptera: Triatominae), where symbionts are passed on from one life cycle stage to the next by coprophagy. In triatomines, eggs and, therefore, newly emerged larvae do not initially contain the mutualistic symbiont Rhodococcus rhodnii; the larvae have to acquire the symbiont by ingesting contaminated feces. Although consumption of sterile feces causes mortality during later larval instars of the triatomine bug, the symbiont is clearly unnecessary for embryogenesis [47].
Our findings do not preclude the possibility that the benefit Rickettsia provide to I. pacificus may become apparent in later life cycle stages, i.e. larvae, nymphs, and flat (unfed) adults. For example, Rickettsia phylotype G021 might be a beneficial symbiont of larval, nymphal, and flat adult stages of I. pacificus in the environment by enhancing the host’s general fitness, possibly by protecting the host against stress factors that do not affect the tick in the lab. Rickettsia’s likely role in increasing larval motility of ticks I. scapularis, A. americanum, and Dermacentor andersonii, provides an example of a potential fitness benefit [16]. Concerning protection from environmental stress, Rickettsia is associated with heat-induced upregulation of structural proteins in the whitefly B. tabaci [48].
Future studies can further address the role of Rickettsia phylotype G021 in later life cycle stages of I. pacificus by creating Rickettsia-free tick lines and comparing their reproductive and general fitness to wild type infected ticks. In addition to overall fitness, these experiments can shed more light on the intriguing possibilities for Rickettsia’s specific function in the tick, such as increased motility [16]. Rickettsia has been found in I. pacificus’ relatives, I. scapularis [18] and I. ricinus [17], but almost nothing is known about those Rickettsia-tick relationships. Little is also known about other bacterial residents of I. pacificus. A metagenomic study of I. pacificus can provide insights into the biology of I. pacificus and put the Rickettsia symbiont in a broader context.
Data Availability Statement
A spreadsheet containing the data obtained and analyzed for this study is available for download on Dr. Jianmin Zhong’s laboratory webpage: http://users.humboldt.edu/jzhong/.
Acknowledgments
We would like to thank Dr. Richard N. Brown, of Humboldt State University’s Department of Wildlife, for his help with tick feeding on rabbits. We would also like to express gratitude to Dr. Yoon Kim, part of Humboldt State University’s Department of Mathematics, for providing us with a statistical consultation.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All the data are available at http://users.humboldt.edu/jzhong/.
Funding Statement
This work was supported by the National Institute of Health (http://www.nih.gov/) Grant #1R15AI082515-01. JZ received the NIH funding. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moran NA, McCutcheon JP, Nakabachi A (2008) Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42: 165–190. [DOI] [PubMed] [Google Scholar]
- 2. Dale C, Moran NA (2006) Molecular interactions between bacterial symbionts and their hosts. Cell 126: 453–465. [DOI] [PubMed] [Google Scholar]
- 3. Duron O, Hurst GD (2013) Arthropods and inherited bacteria: from counting the symbionts to understanding how symbionts count. BMC Biol 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dobson SL, Rattanadechakul W, Marsland EJ (2004) Fitness advantage and cytoplasmic incompatibility in Wolbachia single- and superinfected Aedes albopictus . Heredity (Edinb) 93: 135–142. [DOI] [PubMed] [Google Scholar]
- 5. Baumann P (2005) Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59: 155–189. [DOI] [PubMed] [Google Scholar]
- 6. Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H (2000) Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407: 81–86. [DOI] [PubMed] [Google Scholar]
- 7. Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Łukasik P, van Asch M, Guo H, Ferrari J, Charles H, et al. (2013) Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol Lett 16: 214–218. [DOI] [PubMed] [Google Scholar]
- 9. Giorgini M, Bernardo U, Monti MM, Nappo AG, Gebiola M (2010) Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pnigalio soemius (Hymenoptera: Eulophidae). Appl Environ Microbiol 76: 2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parola P, Paddock CD, Raoult D (2005) Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev 18: 719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merhej V, El Karkouri K, Raoult D (2009) Whole genome-based phylogenetic analysis of Rickettsiae . Clin Microbiol Infect 15 Suppl 2336–337. [DOI] [PubMed] [Google Scholar]
- 12. Merhej V, Raoult D (2011) Rickettsial evolution in the light of comparative genomics. Biol Rev Camb Philos Soc 86: 379–405. [DOI] [PubMed] [Google Scholar]
- 13. Socolovschi C, Matsumoto K, Brouqui P, Raoult D, Parola P (2009) Experimental infection of Rhipicephalus sanguineus with Rickettsia conorii conorii. Clin Microbiol Infec 15: 324–325. [DOI] [PubMed] [Google Scholar]
- 14. Niebylski ML, Peacock MG, Schwan TG (1999) Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl Environ Microbiol 65: 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LM, et al. (2004) Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the State of Rondonia, Western Amazon, Brazil. J Med Entomol 41: 1073–1081. [DOI] [PubMed] [Google Scholar]
- 16. Kagemann J, Clay K (2013) Effects of infection by Arsenophonus and Rickettsia bacteria on the locomotive ability of the ticks Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis . J Med Entomol 50: 155–162. [DOI] [PubMed] [Google Scholar]
- 17. Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, et al. (2011) Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS One 6: e25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan DT (2010) A metagenomic study of the tick midgut. Houston: University of Texas at Houston.
- 19. Phan JN, Lu CR, Bender WG, Smoak RM 3rd, Zhong J (2011) Molecular detection and identification of Rickettsia species in Ixodes pacificus in California. Vector Borne Zoonotic Dis 11: 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng D, Vigil K, Schanes P, Brown RN, Zhong J (2013) Prevalence and burden of two rickettsial phylotypes (G021 and G022) in Ixodes pacificus from California by real-time quantitative PCR. Ticks Tick Borne Dis 4: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dennis DT, Nekomoto TS, Victor JC, Paul WS, Piesman J (1998) Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J Med Entomol 35: 629–638. [DOI] [PubMed] [Google Scholar]
- 22. Peavey CA, Lane RS (1996) Field and laboratory studies on the timing of oviposition and hatching of the western black-legged tick, Ixodes pacificus (Acari: Ixodidae). Exp Appl Acarol 20: 695–711. [DOI] [PubMed] [Google Scholar]
- 23.Soneshine D (1991) Biology of ticks. New York: Oxford University press. [Google Scholar]
- 24. Arthur DR, Snow KR (1968) Ixodes pacificus Cooley and Kohls, 1943: its life-histor and occurrence. Parasitology 58: 893–906. [DOI] [PubMed] [Google Scholar]
- 25. Sakurai M, Koga R, Tsuchida T, Meng XY, Fukatsu T (2005) Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera . Appl Environ Microbiol 71: 4069–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong J, Jasinskas A, Barbour AG (2007) Antibiotic treatment of the tick vector Amblyomma americanum reduced reproductive fitness. PLoS One 2: e405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gudiol F, Pallares R, Carratala J, Bolao F, Ariza J, et al. (1989) Randomized double-blind evaluation of ciprofloxacin and doxycycline for Mediterranean spotted fever. Antimicrob Agents Chemother 33: 987–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rolain JM, Stuhl L, Maurin M, Raoult D (2002) Evaluation of antibiotic susceptibilities of three rickettsial species including Rickettsia felis by a quantitative PCR DNA assay. Antimicrob Agents Chemother 46: 2747–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blondeau JM (2004) Fluoroquinolones: mechanism of action, classification, and development of resistance. Surv Ophthalmol 49 Suppl 2S73–78. [DOI] [PubMed] [Google Scholar]
- 30. Thaker M, Spanogiannopoulos P, Wright GD (2010) The tetracycline resistome. Cell Mol Life Sci 67: 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rolain JM, Maurin M, Vestris G, Raoult D (1998) In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob Agents Chemother 42: 1537–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Branger S, Rolain JM, Raoult D (2004) Evaluation of antibiotic susceptibilities of Ehrlichia canis, Ehrlichia chaffeensis, and Anaplasma phagocytophilum by real-time PCR. Antimicrob Agents Chemother 48: 4822–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furman DP, LooMis EC (1984) The ticks of California (Acari: Ixodida). Bulletin of the California Insect Survey 25: 1–239. [Google Scholar]
- 34. Peavey CA, Lane RS (1995) Transmission of Borrelia burgdorferi by Ixodes pacificus nymphs and reservoir competence of deer mice (Peromyscus maniculatus) infected by tick-bite. J Parasitol 81: 175–178. [PubMed] [Google Scholar]
- 35. Troughton DR, Levin ML (2007) Life cycles of seven ixodid tick species (Acari: Ixodidae) under standardized laboratory conditions. J Med Entomol 44: 732–740. [DOI] [PubMed] [Google Scholar]
- 36. Jasinskas A, Zhong J, Barbour AG (2007) Highly prevalent Coxiella sp. bacterium in the tick vector Amblyomma americanum . Appl Environ Microbiol 73: 334–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng D, Lane RS, Moore BD, Zhong J (2013) Host blood meal-dependent growth ensures transovarial transmission and transstadial passage of Rickettsia sp. phylotype G021 in the western black-legged tick (Ixodes pacificus). Ticks Tick Borne Dis 4: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Core Team (2013) R: A language and environment for statistical computing Vienna, Austria: R Foundation for Statistical Computing.
- 39. Anderson TW, Darling DA (1952) Asymptotic theory of certain “goodness-of-fit” criteria based on “stochastic processes”. Ann Math Stat 23: 193–212. [Google Scholar]
- 40.Levene H (1960) Robust tests for equality of variances. In: Olkin I, Hotelling H, editors. Contributions to Probability and Statistics: Essays in Honor of Harold Hotelling Palo Alto, California: Stanford University Press. 278–292. [Google Scholar]
- 41. Ahrens WH, DJ Cox, Budhwar G (1990) Use of the arcsine and square root transformations for subjectively determined percentage data. Weed Sci 38: 452–458. [Google Scholar]
- 42. Rich JT, Neely JG, Paniello RC, Voelker CC, Nussenbaum B, et al. (2010) A practical guide to understanding Kaplan-Meier curves. Otolaryngol Head Neck Surg 143: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrell FE Jr (2013) Hmisc: Harrell Miscellaneous.
- 44. Giordano R, Weber E, Waite J, Bencivenga N, Krogh PH, et al. (2010) Effect of a high dose of three antibiotics on the reproduction of a parthenogenetic strain of Folsomia candida (Isotomidae: Collembola). Environ Entomol 39: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 45. Pais R, Lohs C, Wu Y, Wang J, Aksoy S (2008) The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol 74: 5965–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruan Y-M, Xu J, Liu S-S (2006) Effects of antibiotics on fitness of the B biotype and a non-B biotype of the whitefly Bemisia tabaci . Entomol Exp Appl 121: 159–166. [Google Scholar]
- 47. Durvasula RV, Gumbs A, Panackal A, Kruglov O, Aksoy S, et al. (1997) Prevention of insect-borne disease: an approach using transgenic symbiotic bacteria. Proc Natl Acad Sci U S A 94: 3274–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brumin M, Kontsedalov S, Ghanim M (2011) Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Science 18: 57–66. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All the data are available at http://users.humboldt.edu/jzhong/.
A spreadsheet containing the data obtained and analyzed for this study is available for download on Dr. Jianmin Zhong’s laboratory webpage: http://users.humboldt.edu/jzhong/.