Abstract
Purpose.
To characterize the labeling of apoptotic cells with a molecular probe of bis(zinc(II)-dipicolylamine) (Zn-DPA) conjugated with a fluorescent reporter in a rat model of retinal ganglion cell (RGC) degeneration induced by N-methyl-D-aspartate (NMDA).
Methods.
Adult Wistar rats were given unilateral intravitreal injections of 3 μL 40 mM neutralized NMDA and euthanized at 1, 2, 4, 24, and 48 hours. One hour before euthanasia, 3 μL Zn-DPA conjugated with fluorescein (Zn-DPA 480) was intravitreally injected. Prelabeling of RGC with retrograde fluorogold (FG), TUNEL, and immunohistochemistry with III β-tubulin and vimentin were performed.
Results.
Fluorescence labeling of Zn-DPA 480 was observed in the retinas from 1 hour up to 24 hours after NMDA injection, whereas the labeling was reduced at 48 hours postinjection. At both 4 and 24 hours postinjection, most Zn-DPA 480–positive cells in the RGC layer were labeled by FG and III β-tubulin. The number of TUNEL-positive cells increased from 4 to 24 hours. At 24 hours, 95.7% of Zn-DPA 480–positive cells were TUNEL positive, whereas 95.1% of TUNEL-positive cells were Zn-DPA 480 positive. The numbers of Zn-DPA 480–positive cells at 1 and 2 hours after NMDA injection were significantly higher than TUNEL.
Conclusions.
Our findings demonstrate that intravitreal injection of fluorescent Zn-DPA 480 labels retinal neurons undergoing apoptosis and that recognition of exposed phosphatidylserine appears earlier than detection of DNA fragmentation, indicating the potential of Zn-DPA as an imaging probe for tracking degenerating retinal neurons.
Keywords: fluorescent probes, ganglion cells, apoptosis, glaucoma, retina
This study demonstrates the intraocular use of Zn-DPA 480 to label apoptotic RGCs in the rat retina after NMDA-induced excitotoxicity suggesting its potential for in vivo imaging of apoptosis.
Introduction
Apoptosis, or the sequence of cellular events collectively known as programmed cell death, is an important biological process in normal development and degenerative diseases.1,2 Characteristic apoptotic events include loss of the phospholipid asymmetry on plasma membranes, activation of intracellular protease activities, loss of mitochondrial membrane permeability, and nuclear acid fragmentation, and ultimately lead to the engulfment and removal of dying cells.3–5 The detection of apoptotic events has been proposed as a diagnostic tool for identifying cell death in neurodegenerative diseases.6,7 For diagnostic purposes, several assays detecting apoptosis have been developed but many of them may have limitations or uncertainties under certain conditions.8 For instance, detection of protease activity requires access through the physical barrier of the cytosolic compartment,9,10 which may reduce the probe sensitivity. Although Annexin V is widely used to detect phosphatidylserine (PS) exposure in apoptotic cells, the storage and application of Annexin V should be undertaken with caution because the protein is susceptible to N-terminal proteolytic degradation.11–13
Glaucoma is one of the leading causes of irreversible blindness and is characterized by progressive and selective retinal ganglion cell (RGC) death. The loss of RGCs causes optic nerve cupping, nerve fiber layer thinning, and progressive and irreversible vision loss. The clinical diagnosis heavily relies on the assessment of structural and functional loss with optic nerve head imaging and perimetric evaluation of the visual field, respectively.14,15 However, for diagnosing and monitoring a chronic slowly progressive disease like glaucoma, these current techniques are limited because these tests cannot accurately diagnose a patient with early disease or accurately determine the rate of disease progression. A new method and approach to disease would be useful for an earlier preventive treatment paradigm to minimize comorbidities of this often inadequately managed neurodegenerative disease.10,16
Phosphatidylserine is one of the phospholipid components forming the bilayers of cell membrane and is normally restricted primarily to the inner membrane leaflet. Positively charged bis(zinc-dipicolylamine) (Zn-DPA) is a synthetic compound that functions as an Annexin V mimic by targeting anionic phospholipids such as PS.17–19 During apoptosis, PS is translocated to the outer membrane leaflet and Zn-DPA binds to exposed PS on the cell surfaces. When this molecule is conjugated, via a chemical linker, to a fluorescent reporter group, the binding of these compounds to cell membranes in apoptotic cells can be quantified and visualized in vitro and in vivo through fluorescence imaging.20 The compounds of Zn-DPA conjugated with various reporters have been used to track apoptotic cells in animal models of acute cell death, traumatic brain injury, stroke, myocardial ischemia-reperfusion injury, hepatic injury, chemical-induced cell death, thymic atrophy, and tumors, without any toxic effect reported.21–27 Smith et al.26 demonstrated that the in vivo imaging of traumatic brain injury using Zn-DPA 794 offered approximately two times higher target to nontarget (T/NT) ratio than the maximum T/NT ratio with Annexin-Vivo 750 (commercially available Annexin V) in a cryolesion mouse model. Palmoski et al.28 evaluated the ability of Zn-DPA to image apoptosis in a controlled preclinical tumor model during antiangiogenic therapy and showed that the in vivo imaging using a Zn-DPA probe could detect significant therapy effects but not using Annexin V. The higher accumulation of Zn-DPA probe is most probably related to its approximately 20 times smaller size, which enables better delivery of the probe to the target cells, suggesting that Zn-DPA is an alternative to Annexin V for the detection of cell death in clinical settings. This is the first study to examine whether intravitreal injection of Zn-DPA 480 (Zn-DPA conjugated with fluorescein) labels RGCs undergoing apoptosis in the retina. We previously documented that RGCs die through apoptosis in a rat model with N-methyl-D-aspartate (NMDA) injection.29 The present study aimed to compare the temporal profiles of Zn-DPA 480 labeling and DNA fragmentation and to characterize the labeling of apoptotic RGCs.
Materials and Methods
Animals
The use of animals was approved by the Animal Research Committee of the University of California, Los Angeles. The procedures were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The animals (obtained from Charles River Laboratories, Raleigh, NC, USA) were housed with standard food and water provided ad libitum. The light was turned on at 3 AM and turned off at 3 PM. For accommodation, the animals were kept at least 1 week in this environment before experiments. Animals were divided into groups randomly and the eyes were randomized for procedures. Surgical procedures were performed on one eye of each rat while the contralateral eye served as an untreated control. Topical ophthalmic ointment (tobramycin, Tobrex; Alcon, Fort Worth, TX, USA) was applied immediately after procedures and continually every 12 hours for 2 days.
Retinal Excitotoxicity Model
The previously described method was used to induce apoptotic RGC loss in rat retinas by intravitreal injection of NMDA.29 Briefly, 3-month-old male Wistar rats were anesthetized by inhalation of isoflurane (3.5%–5.0%) in oxygen. After a topical application of 0.5% procaracaine hydrochloride, an intravitreal injection of 3 μL of 40 mM (corresponding to 120 nmol) neutralized NMDA (Sigma-Aldrich Corp., St. Louis, MO, USA) in sterile 0.1 M PBS was administered unilaterally. The animals were euthanized at 1, 2, 4, 24, and 48 hours after injection by inhalation of carbon dioxide. Their eyes were enucleated for subsequent analyses (n = 4 per group).
Administration of Zn-DPA 480
Zn-DPA conjugated with a fluorescent reporter (Zn-DPA 480; MW = 1010; absorbance maximum at 480 nm, emission maximum at 519 nm) was provided by Molecular Targeting Technologies, Inc. (MTTI, West Chester, PA, USA). One millimolar Zn-DPA 480 stock solution was prepared according to manufacturer's protocol. Further dilution to 0.1 and 0.01 mM solutions of Zn-DPA 480 were made with 0.5 mM N-tris-(hydroxymethyl)-methyl-2-aminoethanesulfonic acid (TES) buffer solution. Solutions of 1 mM FITC (Acros Organics, Geel, Belgium), 1 mM Zn-DPA 480, 40 mM NMDA, 0.5 mM TES buffer, and 0.1 mM PBS were also prepared and injected into control animals. To determine the administration time of Zn-DPA 480, 3 μL Zn-DPA 480 solution was intravitreally injected 1, 4, and 24 hours before euthanasia. These animals received NMDA injection and were euthanized at 24 hours after NMDA injection (peak of TUNEL as shown previously29). For optimization of Zn-DPA 480 concentrations and durations, the retinal wholemounts were evaluated with fluorescent microscopy. The relative intensities of RGC layer cells in digital images were measured with a computer-assisted image-processing unit (Image-Pro Plus software; Media Cybernetics, Silver Spring, MD, USA) and then averaged according to a published protocol.30
Retrograde Labeling
To demonstrate whether the positive Zn-DPA 480 labeling is localized at RGCs, the published procedures for retrograde labeling with fluorogold (FG; Fluorochrome, Denver, CO, USA) were performed.31 The procedure of applying FG at the transected optic nerve was used to ensure that all RGC axons were exposed to FG, including those RGCs projecting to areas other than the superior colliculus. Briefly, the optic nerve was exposed through a lateral conjunctival incision, and a cross section of the optic nerve was made with the needle knife through the opening of the optic nerve sheath, with care taken to not damage the adjacent blood supply. A gelfoam soaked with 6% FG was applied onto the surface of the transected optic nerve. The conjunctival incision was sutured and prophylactic antibiotic ointment was applied. The procedures were performed on only one eye to avoid bilateral blindness. After 1 day from retrograde labeling procedures, the animals were given NMDA and Zn-DPA 480 injections. At 4 or 24 hours after NMDA injection, animals were euthanized by inhalation of carbon dioxide. Enucleated eyes were immediately fixed with 4% paraformaldehyde for 1 to 2 hours. In total, 16 animals were used for retrograde labeling and divided into four groups. Four retinas per group (4 and 24 hours) were used for retinal flatmounting and immunohistochemistry/TUNEL, respectively. For the preparation of flatmount, entire retinas were dissected and washed with 0.01 mM PBS. Under operating microscope, the retinas were mounted flat with several radial cuts on a glass slide. After air-drying overnight, the retinas were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted using Aqua-Mount (Thermo Scientific, Kalamazoo, MI, USA). Due to limited tissue permeability, immunohistochemistry and the TUNEL technique were performed on cryosections instead of retinal flatmounts.
Double Fluorescence Immunohistochemistry of III β-Tubulin, Vimentin, and Rbpms
The enucleated eyeballs were immersed in fixative for 1 hour, bisected, and postfixed for 3 hours. The eyecups were incubated with 30% sucrose at 4°C overnight and embedded in optimum cutting temperature compound (Sakura Finetec, Torrance, CA, USA). Ten-micrometer-thick frozen sections were obtained along the vertical meridian through the optic nerve head. Approximately 20 retinal sections containing optic nerve head were collected from one eye and these specimens were considered “whole” retinal sections and followed by quantitative analysis. Retinal sections were washed with PBS and incubated with 10% fetal bovine serum for 1 hour to block nonspecific staining. Standard immunohistochemical procedures31 were performed with primary rabbit antibodies against Rbpms (1:500), vimentin (predilute; Serotec, Oxford, UK) and mouse III β-tubulin (TUJ, 1:200, mouse; Covance, Emeryville, CA, USA), and Alexa Fluor 594 anti-rabbit and anti-mouse secondary IgG antibody (1:1000; Molecular Probes, Eugene, OR, USA). Briefly, the sections were incubated with primary antibody in PBS containing 1% Triton-X, 0.5% BSA, and 0.9% sodium chloride (PBS-T-BSA) overnight at 4°C and then the secondary antibody for 1 hour. Negative control sections were incubated without primary or secondary antibodies; DAPI was used for counterstaining. Images of the immunostained sections were captured with a digital camera (Co-olsnap; RS Photometrics, Tucson, AZ, USA) attached to a fluorescence microscope (Axioplan; Carl Zeiss, Oberkochen, Germany).
At least two alternate “whole” retinal sections from each eye were subject to each immunohistochemical procedure (III β-tubulin and vimentin). The numbers of RGC layer cells labeled with Zn-DPA 480 and III β-tubulin or vimentin, Zn-DPA 480 but no III β-tubulin or vimentin, and III β-tubulin or vimentin but no Zn-DPA 480 were counted. Only cells with DAPI-stained nuclei were included.
The TUNEL Analysis
The procedures described in the ApopTag Red In Situ Apoptosis Detection Kit (Intergen Co., Purchase, NY, USA) were followed.32 The sections were examined under a confocal laser scanning microscope (FluoView, Olympus, Japan). Microscopic images from at least three alternate retinal sections per experimental eye and four eyes per group were analyzed. For quantification, six alternate sections from each eye were included in this experiment. The numbers of TUNEL-positive cells in the RGC layer were counted manually by two examiners with a fluorescence microscope (Axioplan) in a masked fashion. The numbers of TUNEL-positive cells from six alternate sections were averaged to represent one eye and used for comparison.
Statistical Analysis
Data were presented as the mean ± SD. Differences among groups were analyzed by one-way ANOVA, followed by the Student's t-test. P less than 0.05 was considered statistically significant.
Results
Imaging of Zn-DPA 480 on Retinal Wholemount
Figure 1 depicts a generalized structure and mechanism of action of Zn-DPA probes. The Zn-DPA probe consists of an organic scaffolding that coordinates two positively charged Zn2+ ions to form a complex (the “affinity group”). The organic component is conjugated, via a chemical linker, to a reporter group that is a fluorescent moiety. Zinc ion (Zn2+) then induces a strong association of the complex through electrostatic interactions with carboxylate and phosphate anions present in the PS headgroup of dead and dying cell membranes, in turn leading to fluorescent labeling of the target-bearing exposed PS.
Figure 1.
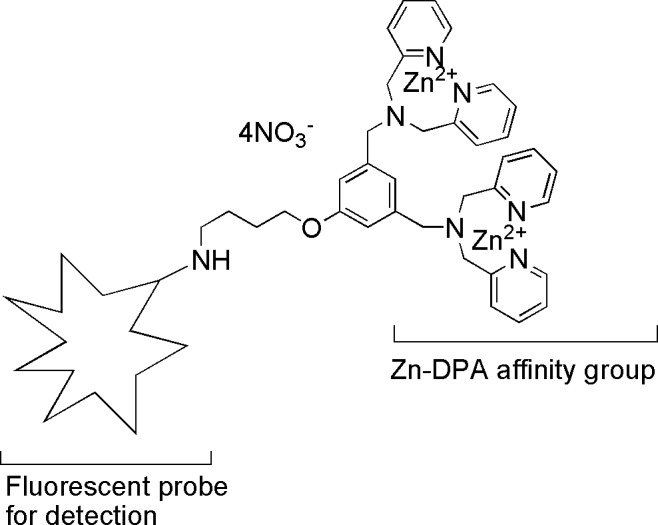
Schematic diagram of fluorescently labeled Zn-DPA probes for in vivo intravitreal injection. Zn-DPA affinity group recognizes exposed PS on cell membrane.
Intravitreal injection of NMDA solution is a common method to induce apoptosis in the retina for studying RGC degeneration.33 The fluorescent intensities of Zn-DPA 480–positive cells at 1, 4, and 24 hours after Zn-DPA 480 injection were similar (data not shown). Thus, for this entire study, the solution of Zn-DPA 480 was injected intravitreally at 1 hour before euthanasia.
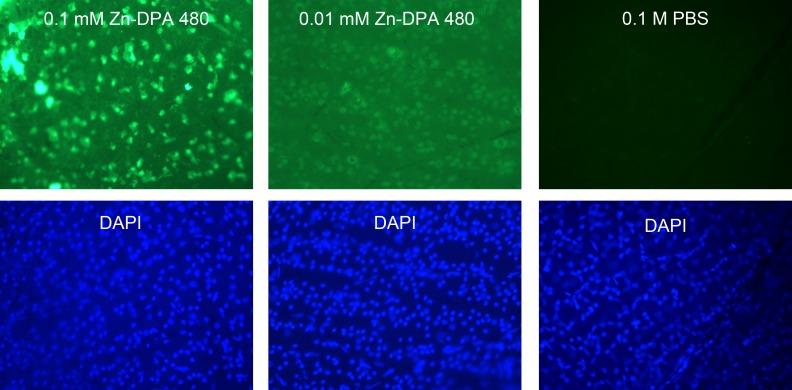
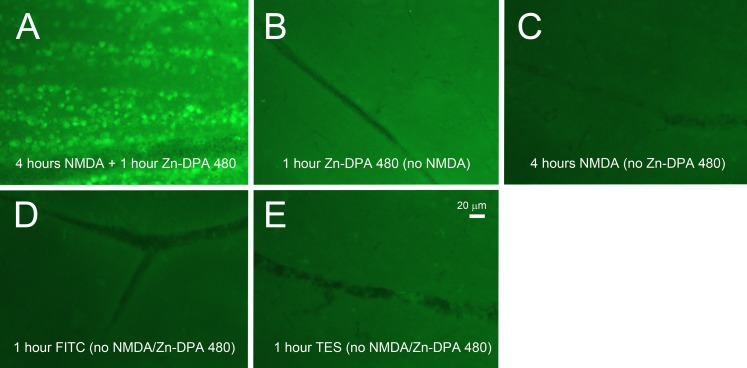
Two concentrations of Zn-DPA 480 (0.1 and 0.01 mM) were compared. Four hours after NMDA injection, the RGC layer cells were labeled with both concentrations of Zn-DPA 480 solution in the flat mount retinas, as shown in Figure 2. The intensity of labeling with 0.1 mM Zn-DPA 480 was noticeably stronger than with 0.01 mM Zn-DPA 480. An intravitreal solution of 0.1 mM Zn-DPA480 was then used in the following experiments. For negative controls, no fluorescence signal was observed in the retina after injection of PBS. To compare the background fluorescence intensity, the vehicle injections of 1 mM Zn-DPA 480 (Fig. 3B), 40 mM NMDA (Fig. 3C), 1 mM FITC (Fig. 3D), and TES buffer (Fig. 3E) were given to control animals (no NMDA injection). Their background intensities were noticeably lower than the NMDA-treated (4 hours after NMDA) retinas with positive Zn-DPA 480 labeling (Fig. 3A).
Figure 2.
Concentration effect of Zn-DPA 480 probes at 4 hours after injection of NMDA. The solutions of Zn-DPA 480 were injected 1 hour before euthanasia. Cells in the RGC layer were labeled by both 0.1 and 0.01 mM Zn-DPA 480 concentrations. Intravitreal injection of 0.1 mM Zn-DPA 480 labels the RGC layer cells with a stronger signal than 0.01 mM after NMDA injection; 1 mM Zn-DPA 480 gave similar signal intensity as 0.1 mM Zn-DPA 480 (data not shown). There was no labeling in PBS-vehicle, instead of NMDA, injected eye. To compare labeling intensity, all photographs were taken under the same magnification at a fixed 10-second exposure time.
Figure 3.
Fluorescence intensity on retinal wholemount at 1 hour postinjection of Zn-DPA 480 imaging probes. (A) At 4 hours after 40 mM NMDA injection, positive cells were labeled by 0.1 mM Zn-DPA 480 and background fluorescence was increased. There were no positive cells in the retinas after injection of (B) Zn-DPA 480, (C) NMDA, (D) FITC, and (E) TES vehicles, and the background intensities were noticeably lower than the retina treated with Zn-DPA 480 and NMDA (A). The exposure time was 10 seconds.
Temporal Profile of TUNEL and Zn-DPA 480 Labeling
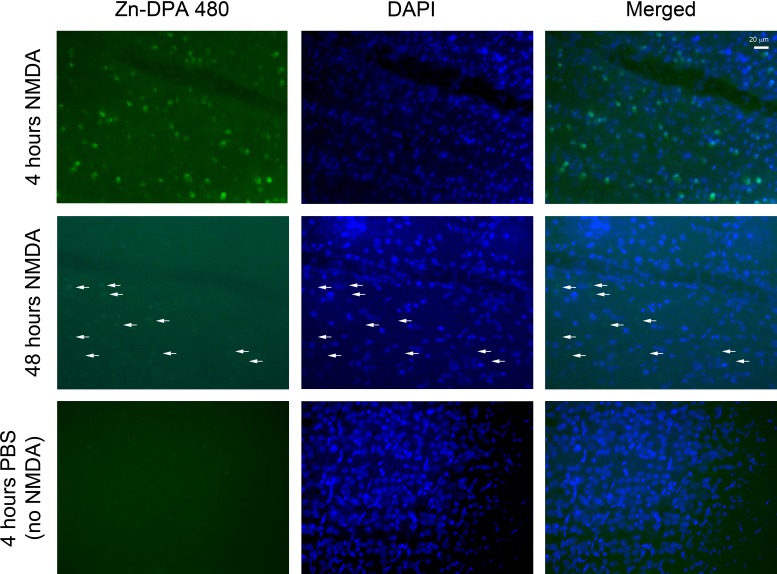
As previously demonstrated,29 the peak of DNA fragmentation appeared 18 to 24 hours after NMDA injection and disappeared before 48 hours. Therefore, the Zn-DPA 480 labeling in the retinal wholemounts was examined within this time frame. Figure 4 shows strong fluorescent labeling of the RGC layer cells at 4 hours after NMDA injection. However, mild Zn-DPA 480 labeling remained at 48 hours after NMDA injection but did not colocalize with DAPI nuclear counter staining (arrows), suggesting cellular debris.
Figure 4.
Fluorescent labeling in retinal wholemounts after intravitreal injection of NMDA and Zn-DPA 480. For all experimental groups, intravitreal injection of Zn-DPA 480 was performed 1 hour before euthanasia. At 4 hours after injection of NMDA, Zn-DPA 480–positive cells in the RGC layer were noted. At 48 hours after injection of NMDA, mild Zn-DPA 480 labeling showing negative DAPI nuclear counterstaining was detected (arrows). No positive Zn-DPA 480 cells were noted 4 hours after PBS injection (no NMDA).
To investigate whether Zn-DPA 480 tracks apoptotic cells, the retinal tissues collected at 1, 2, 4, 24, and 48 hours after NMDA injection were subjected to the TUNEL assay and quantification. Only Zn-DPA 480–positive cells or TUNEL-positive cells that were double labeled by DAPI were counted. At 1, 2, 4, and 24 hours after NMDA injection, the average number of TUNEL-positive cells (per retinal section) in the RGC layer was 0, 11, 121, and 171, respectively, and the average number of Zn-DPA 480–positive cells (per retinal section) was 65, 121, 116, and 170, respectively (Fig. 5). No TUNEL-positive cells or Zn-DPA 480–positive cells with DAPI counterstaining were found at 48 hours. The disappearance of TUNEL-positive cells at 48 hours is consistent with the dropout of nuclei in the RGC layer and the absence of DNA fragmentation using gel electrophoresis in this rat model.29
Figure 5.
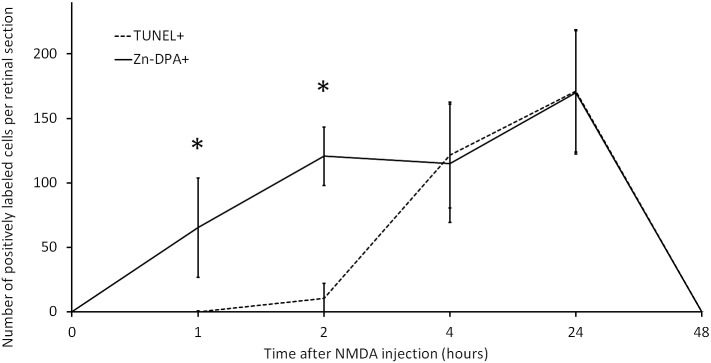
Quantitative analysis of TUNEL and Zn-DPA 480. The numbers of Zn-DPA 480– and TUNEL-positive cells were counted on retinal sections at times after NMDA injection. The number of Zn-DPA 480–positive cells was increased from 1 hour and peaked at 24 hours but declined to zero at 48 hours. Only a few TUNEL-positive cells were observed at 2 hours and more TUNEL-positive cells were noted at 4 and 24 hours but no TUNEL-positive cells were found at 48 hours. At 1 and 2 hours after NMDA injection, the numbers of Zn-DPA 480–positive cells were significantly higher than the numbers of TUNEL. *P < 0.05; n = 4 per time point. Error bars represent SD.
Zn-DPA 480 Labeling Precedes TUNEL Technique
Statistical analysis revealed that the numbers of Zn-DPA 480–positive cells at 1 and 2 hours postinjection were significantly greater than the numbers of TUNEL-positive cells (P = 0.04 and 0.005, respectively, Fig. 5). To visualize the colocalization of Zn-DPA 480 and TUNEL, the retinal sections collected at 2 and 24 hours after NMDA injection were examined using confocal laser microscopy (Fig. 6A). Strong Zn-DPA 480 labeling was shown in the cells in the RGC layer (arrowheads) within 2 hours after NMDA injection but no labeling in other retinal layers, such as inner nuclear layer and outer nuclear layer. Clearly, there was an absence of TUNEL-positive cells; however, dense DAPI nuclear staining was noted in the inner retina at this time point. Twenty-four hours after NMDA injection, there was a dramatic increase in the number of TUNEL-positive cells in both the RGC layer and inner nuclear layer. Almost all TUNEL-positive cells in the RGC layer were double labeled by Zn-DPA 480 (arrows), whereas Zn-DPA 480 did not label most TUNEL-positive cells in the inner nuclear layer. Figure 6B showed that a few RGC layer cells with condensed DAPI nuclear counterstaining were TUNEL and Zn-DPA 480 negative (arrowheads).
Figure 6.
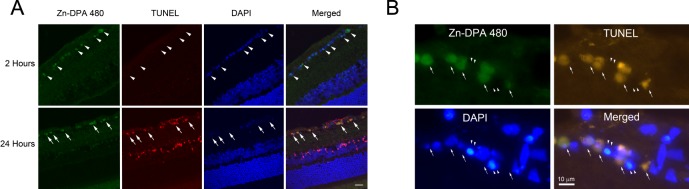
(A) Confocal laser micrographs showed RGCs with double labeling of Zn-DPA 480 and TUNEL after NMDA injection. There were Zn-DPA 480–positive cells (green) in the RGC layer (arrowheads) but no TUNEL-positive cells were noted in the retina at 2 hours postinjection (top). At 24 hours postinjection, TUNEL-positive cells were observed in both the RGC layer and inner nuclear layer, whereas colocalization of Zn-DPA 480 (green) and TUNEL (red) predominantly appeared in the RGC layer (bottom; arrows) but not in the inner nuclear layer. DAPI was used as nuclear counterstaining (blue). (B) High magnification of RGC layer cells at 4 hours after NMDA injection showed colocalization of Zn-DPA 480 (green) and TUNEL (red; arrows). However, the RGC layer cells with condensed DAPI nuclear counterstaining (blue) were TUNEL and Zn-DPA 480 negative (arrowheads).
Specificity and Sensitivity of Zn-DPA 480 for Apoptosis
To determine the specificity and sensitivity of Zn-DPA 480 compared with the TUNEL technique, the percentage of double labeling of TUNEL and Zn-DPA 480 in the RGC layer was calculated (Table 1). There were no TUNEL-positive cells at 1 and 48 hours after NMDA injection. By 2, 4, and 24 hours, more than 75.5% of TUNEL-positive cells were Zn-DPA 480 positive. As early as 1 hour after NMDA injection, there were a number of Zn-DPA 480–positive cells; however, none of them were labeled by TUNEL. Only 6.9% of Zn-DPA 480–positive cells were TUNEL positive at 2 hours after NMDA injection. At 4 and 24 hours, more than 95.7% of Zn-DPA 480–positive cells were TUNEL positive. No Zn-DPA 480–positive cells were detected in the retina 48 hours after NMDA injection.
Table 1.
Specificity and Sensitivity of Zn-DPA Labeling to TUNEL Technique in Rat Retina After Intravitreal Injection of NMDA
|
Time After NMDA Injection, h |
1 |
2 |
4 |
24 |
48 |
| % of TUNEL-positive cells are Zn-DPA positive | — | 75.5 | 89.9 | 95.1 | — |
| % of Zn-DPA–positive cells are TUNEL positive | 0 | 6.9 | 96.4 | 95.7 | — |
Dash indicates not applicable.
Labeling of RGC Apoptosis by Zn-DPA 480
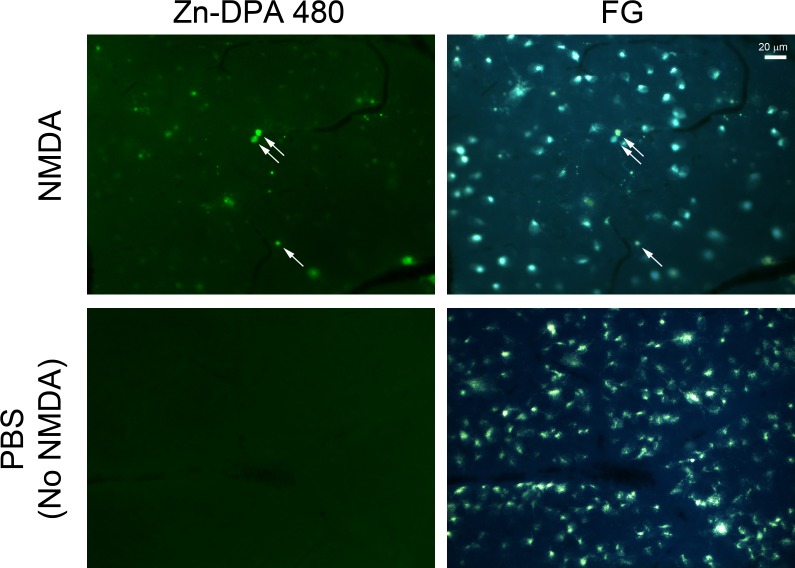
To verify whether Zn-DPA 480 labeling is specific to RGCs, the retinal sections collected at 24 hours after NMDA injection (peak of DNA fragmentation) were double labeled with RGC makers, such as Rbpms and III β-tubulin, and a Müller cell marker, such as vimentin. Most Zn-DPA 480–positive cells were labeled by Rbpms (Figs. 7A–D) and III β-tubulin (Figs. 7I–L) antibody. Most Zn-DPA 480–positive cells were not labeled by vimentin antibody. Quantitative analysis, as shown in Figure 7M, revealed that approximately 53.1% of Zn-DPA 480–positive cells were III β-tubulin positive, whereas 16.4% of Zn-DPA 480–positive cells were vimentin positive. Notably, at 24 hours after NMDA injection, most Zn-DPA 480–positive cells were labeled by FG, as shown in Figure 8. Quantitative analysis in the whole-mount retinas indicates that 82.7% and 57.8% of Zn-DPA 480–positive cells were FG positive at 4 and 24 hours postinjection, respectively (Table 2). More than 93.1% of FG-positive cells were Zn-DPA 480 positive at both 4 and 24 hours after NMDA injection. Figure 9 confirmed the colocalization of Zn-DPA 480 labeling and TUNEL in RGCs that were prelabeled by FG. In this experiment, the potential of bleed-through artifact, which is a common issue in multiple fluorescent labeling, was carefully considered.
Figure 7.
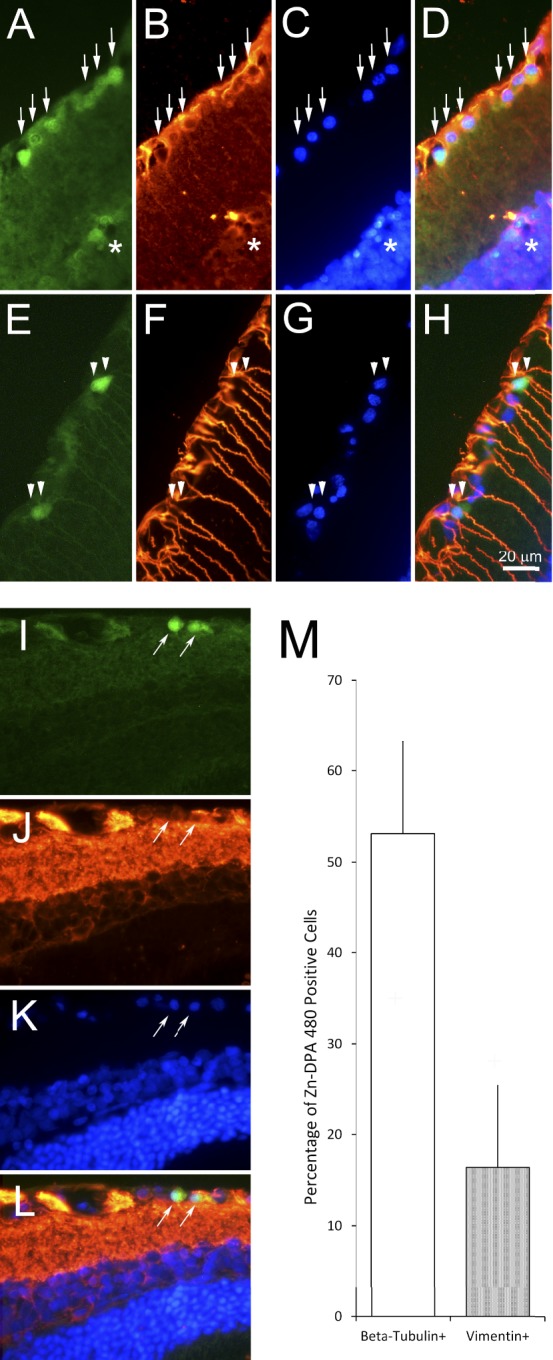
Cellular localization of Zn-DPA 480 in retina at 24 hours after NMDA injection. (A–D) Rbpms immunohistochemistry (green = Zn-DPA 480; red = Rbpms; blue = DAPI). There was colocalization of Zn-DPA 480 (A) and Rbpms (B) in the cells in the RGC layer (arrows). Very few Zn-DPA 480–positive cells were noted in the inner part of inner nuclear layer but were Rbpms negative (*). (E–H) Vimentin immunohistochemistry (green = Zn-DPA 480; red = vimentin; blue = DAPI). Zn-DPA 480–positive cells (E) were vimentin (F) immuno-negative (double arrowheads). (I–L) III β-tubulin immunohistochemistry (green = Zn-DPA 480; red = III β-tubulin; blue = DAPI). Zn-DPA 480–positive cells (I) were III β-tubulin (J) immuno-positive (arrows). (M) Quantitative analysis of Zn-DPA 480 labeling with III β-tubulin and vimentin immunohistochemistry. More than 50% of Zn-DPA 480–positive cells were labeled by a commonly used RGC marker, beta-tubulin, whereas a few Zn-DPA 480–positive cells were labeled by Müller cell marker, vimentin. There was no double labeling of Zn-DPA 480 and III β-tubulin or vimentin in the control (no NMDA) retina (data not shown). n = 4. Error bars: SD.
Figure 8.
Fluorescent labeling of Zn-DPA 480 on FG prelabeled RGCs. At 24 hours after NMDA injection, there was colocalization of FG and Zn-DPA 480 in the wholemount retina (arrows). The variable intensities of Zn-DPA 480 labeling on FG-labeled cells were noted. No Zn-DPA 480–positive labeling was observed in FG-positive cells after PBS injection (no NMDA).
Table 2.
Comparison Between Zn-DPA Labeling and Retrograde Labeling Using FG 4 and 24 Hours Postinjection
|
Time After NMDA Injection, h |
Zn-DPA–Positive Cells That Are FG Positive, % |
FG-Positive Cells That Are Zn-DPA–Positive, % |
| 4 | 82.7 ± 12.0 | 95.8 ± 0.9 |
| 24 | 57.8 ± 12.9 | 93.1 ± 6.2 |
Figure 9.
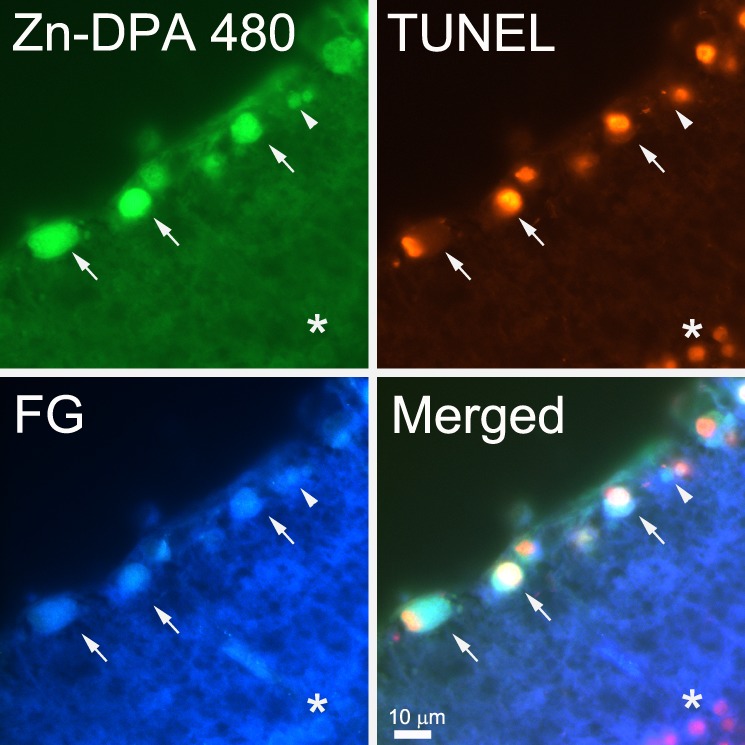
Colocalization of Zn-DPA 480 and TUNEL in FG prelabeled RGCs. At 4 hours after NMDA injection, FG-labeled RGCs were Zn-DPA 480 and TUNEL positive (arrows); some were labeled by Zn-DPA 480 but without TUNEL labeling (arrowhead). A cell in the inner nuclear layer with mild Zn-DPA 480 labeling was not labeled by FG but was TUNEL positive (*).
Discussion
Retinal ganglion cell loss is a key pathological event that leads to blindness in patients with optic nerve involvement. As apoptosis is known as the major pathway of RGC degeneration,34–36 imaging of apoptotic or dying RGCs has been proposed to be a new approach to diagnose patients with optic neuropathy, especially glaucoma.37 The addition of new approaches to current techniques may help diagnose glaucoma much earlier. Additionally, this may provide relevant information to help clinicians treat the disease.
The development of potentially new optic nerve imaging approaches relies on the advancement of molecular imaging probes and devices.38 This is the first validation of the intraocular use of Zn-DPA conjugated with a fluorescent reporter (Zn-DPA 480) for apoptosis detection in a rat model of RGC degeneration.
Assays for apoptosis are used often in cell biology research and in the drug discovery process in basic and clinical ophthalmology.39 In addition to providing insights into biological and disease processes, molecular imaging of apoptosis has the potential to evaluate and monitor in vivo disease states. Recent studies reported that intraocular application of Annexin V and TcapQ can be used to track RGC apoptosis in animal models of RGC degeneration.10,16 The binding of Annexin V to the exposed PS on the apoptotic cell membrane surface and the cleavage of TcapQ by effector caspases allow tracking of apoptotic cells with fluorescent dyes for in vivo imaging. These approaches show some promising findings from preclinical studies and are currently undergoing Phase I clinical trials. The approach of Zn-DPA may offer additional advantages. First, Zn-DPA-PS binding does not require Ca2+, so that other imaging processes can be monitored simultaneously11–13 and avoids false-positives by unwanted activation of scramblases by Ca2+, which move phospholipid nonspecifically.40 For example, in human epidermoid carcinoma xenografts, the breakdown of the vasculature in response to the antiangiogenic treatment strongly impairs the delivery of Annexin V to the tumor site.41 In the same animal model, it has been demonstrated that Zn-DPA 794, in contrast to Annexin V, showed significant therapeutic effect.28 The accumulation of Zn-DPA 794 was attributed to the smaller size of Zn-DPA 794 (MW = 1840 Da), which is 20 times smaller than Annexin V (MW = 36 kDa). Therefore, Zn-DPA not only offers a smaller molecular size but also faster kinetics and shorter incubation times. These properties suggest that Zn-DPA may be a more attractive option for in vivo imaging of apoptosis. Before achieving this goal, we believe that the present experiment characterizing the labeling of RGC apoptosis by the intraocular use of this new fluorescence probe is essential.
Since the use of Zn-DPA was first reported, a series of fluorescently labeled versions has been prepared and used for disease detection and monitoring with in vivo animal models for imaging of neurodegenerative diseases, infection, and cancers. The eye is particularly well suited for molecular imaging because of the availability of direct access and clarity of the optical media. The vitreous is an excellent route for drug administration, because the drug is directly in contact with the retinal neurons of interest and can, therefore, bypass the blood brain and retinal barrier. However, there may be a concern about in vivo imaging when excessive intravitreal concentration of fluorescent probe obstructs the view of retina. The present study demonstrates the successful tracking of RGC apoptosis with Zn-DPA 480 and warrants further investigation for human application of glaucoma and other optic nerve diseases.
Intravitreal injection of NMDA is a common method to induce RGC loss for studying molecular mechanisms and exploring protective treatments against neurodegenerative diseases such as glaucoma.34 Similar to Annexin V, Zn-DPA 480 may also label the exposed PS in the inner leaflet of broken cells during necrosis and we suspect that Zn-DPA 480 may stain necrotic RGCs as well. Propidium iodide (PI) may be helpful in distinguishing necrotic RGCs from apoptosis. However, this assay is largely used in flow cytometry and only a few laboratories could successfully detect necrotic retinal neurons in vivo by intraocular injection of PI dye.42–44 The present study used the TUNEL technique, which has been widely used to visualize apoptotic cells and showed a high correlation of Zn-DPA 480 with TUNEL at 4 and 24 hours (89.9% and 95.1%, respectively). Based on our earlier work with histology, biochemistry, and caspase inhibitor studies to confirm apoptosis in this rat model,29 we believe our statement of Zn-DPA 480 labeling of apoptotic cells in this rat model is valid.
Although apoptotic cell death predominantly occurs in RGCs in this animal model, our findings demonstrate that Zn-DPA 480 labeling is not specific to a single cell type, such as RGCs. Approximately 57.8% of TUNEL-positive cells were FG positive and 53.1% were III β-tubulin positive at 24 hours after NMDA injection, indicating that a proportion of dying cells were non-RGCs, likely displaced amacrine cells.45 To understand the involvement of amacrine cell death in glaucoma and other optic neuropathies, further investigation should address whether amacrine cell death happens after RGC death and how RGC death might contribute to amacrine cell death. Intravitreal injection of Zn-DPA 480 is useful for detecting apoptosis in the RGC layer in this animal rather than the inner and outer nuclear layers of the retina, apparently due to limited permeability (Fig. 6A). Therefore, Zn-DPA is considered as an apoptotic marker that is not limited to a specific cell type.
Apoptosis involves a series of morphological and biochemical events within a time frame. Although Zn-DPA 480 labeling colocalizes with FG-positive cells, the intensities of Zn-DPA 480–positive labeling may vary with different morphological features (Fig. 8). We found that Zn-DPA 480 is unable to detect some RGC layer cells with condensed nuclei (counterstained by DAPI; Fig. 6B). Consistent with literature showing the recognition of PS in the early phase of apoptosis,4,5 our quantitative data show that Zn-DPA 480 labels dying RGCs as soon as 1 to 2 hours after exposed to damage. This finding strongly supports the idea that Zn-DPA 480 detects cells in the early stages of apoptosis and is specific to certain morphological changes or time frame.
The present study characterizes the intraocular use of Zn-DPA 480, and both spatial and temporal profiles of Zn-DPA 480 labeling in the rat retina after NMDA-induced excitotoxicity. Intravitreal injection of Zn-DPA 480 can label apoptotic RGCs as early as 1 hour after NMDA injection and ex vivo tracking of RGC apoptosis with Zn-DPA 480 precedes the labeling of DNA fragmentation with the TUNEL technique of retinal sections (4 hours after NMDA injection). Consistently, the exposure of PS to the cell membrane occurs in the early and intermediate stages of apoptosis in NMDA-induced RGC degeneration. Therefore, our findings demonstrate that intravitreal administration of Zn-DPA 480 can be used to track apoptotic cells.
Acknowledgments
Supported by the Gerald Oppenheimer Family Foundation (JMKK), the National Institutes of Health Grant EY018644 (JMKK, JC), and unrestricted grants from Research to Prevent Blindness, Inc. (JC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosure: J.M.K. Kwong, P; C. Hoang, None; R.T. Dukes, None; R.W. Yee, P; B.D. Gray, MTTI (F, I, E), P; K.Y. Pak, MTTI (F, I, E), P; J. Caprioli, None
References
- 1. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007; 35: 495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bahr M. Live or let die—retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000; 23: 483–490 [DOI] [PubMed] [Google Scholar]
- 3. Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27: 199–214 [DOI] [PubMed] [Google Scholar]
- 4. Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001; 8: 551–563 [DOI] [PubMed] [Google Scholar]
- 5. Martin SJ, Reutelingsperger CP, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995; 182: 1545–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoenberger J, Bauer J, Moosbauer J, Eilles C, Grimm D. Innovative strategies in in vivo apoptosis imaging. Curr Med Chem. 2008; 15: 187–194 [DOI] [PubMed] [Google Scholar]
- 7. Smith BA, Smith BD. Biomarkers and molecular probes for cell death imaging and targeted therapeutics. Bioconjug Chem. 2012; 23: 1989–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanshaw RG, Smith BD. New reagents for phosphatidylserine recognition and detection of apoptosis. Bioorg Med Chem. 2005; 13: 5035–5042 [DOI] [PubMed] [Google Scholar]
- 9. Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J Med Chem. 2005; 48: 5404–5407 [DOI] [PubMed] [Google Scholar]
- 10. Barnett EM, Zhang X, Maxwell D, Chang Q, Piwnica-Worms D. Single-cell imaging of retinal ganglion cell apoptosis with a cell-penetrating, activatable peptide probe in an in vivo glaucoma model. Proc Natl Acad Sci U S A. 2009; 106: 9391–9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson P, van den Eijnde S, Schlegel RA. Phosphatidylserine exposure and phagocytosis of apoptotic cells. Methods Cell Biol. 2001; 66: 339–364 [DOI] [PubMed] [Google Scholar]
- 12. van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998; 31: 1–9 [DOI] [PubMed] [Google Scholar]
- 13. Pläsier B, Lloyd DR, Paul GC, Thomas CR, Al-Rubeai M. Automatic image analysis for quantification of apoptosis in animal cell culture by annexin-V affinity assay. J Immunol Methods. 1999; 229: 81–95 [DOI] [PubMed] [Google Scholar]
- 14. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field tests in the same persons. Invest Ophthalmol Vis Sci. 2000; 41: 741–748 [PubMed] [Google Scholar]
- 15. Nouri-Mahdavi K, Nassiri N, Giangiacomo A, Caprioli J. Detection of visual field progression in glaucoma with standard achromatic perimetry: a review and practical implications. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 1593–1616 [DOI] [PubMed] [Google Scholar]
- 16. Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci U S A. 2004; 101: 13352–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koulov AV, Stucker KA, Lakshmi C, Robinson JP, Smith BD. Detection of apoptotic cells using a synthetic fluorescent sensor for membrane surfaces that contain phosphatidylserine. Cell Death Differ. 2003; 10: 1357–1359 [DOI] [PubMed] [Google Scholar]
- 18. DiVittorio KM, Johnson JR, Johansson E, Reynolds AJ, Jolliffe KA, Smith BD. Synthetic peptides with selective affinity for apoptotic cells. Org Biomol Chem. 2006; 4: 1966–1976 [DOI] [PubMed] [Google Scholar]
- 19. Smith BA, Akers WJ, Leevy WM, et al. Optical imaging of mammary and prostate tumors in living animals using a synthetic near infrared zinc(II)-dipicolylamine probe for anionic cell surfaces. J Am Chem Soc. 2010; 132: 67–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hanshaw RG, Lakshmi C, Lambert TN, Johnson JR, Smith BD. Fluorescent detection of apoptotic cells by using zinc coordination complexes with a selective affinity for membrane surfaces enriched with phosphatidylserine. Chembiochem. 2005; 6: 2214–2220 [DOI] [PubMed] [Google Scholar]
- 21. Smith BA, Gammon ST, Xiao S, et al. In vivo optical imaging of acute cell death using a near-infrared fluorescent zinc-dipicolylamine probe. Mol Pharm. 2011; 8: 583–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith BA, Xiao S, Wolter W, Wheeler J, Suckow MA, Smith BD. In vivo targeting of cell death using a synthetic fluorescent molecular probe. Apoptosis. 2011; 16: 722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chu C, Huang X, Chen CT, et al. In vivo imaging of brain infarct with a novel fluorescent probe PSVue 794 in a rat middle cerebral artery occlusion-reperfusion model. Mol Imaging. 2013; 12: 8–16 [PubMed] [Google Scholar]
- 24. Wyffels L, Gray BD, Barber C, Woolfenden J, Pak KY, Liu Z. Synthesis and preliminary evaluation of radiolabeled bis(zinc(II)-Dipicolylamine) coordination complexes as cell death imaging agents. Bioorg Med Chem. 2011; 19: 3425–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thakur ML, Zhang K, Paudyal B, et al. Targeting apoptosis for optical imaging of infection. Mol Imaging Biol. 2012; 14: 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smith BA, Xie BW, van Beek ER, et al. Multicolor fluorescence imaging of traumatic brain injury in a cryolesion mouse model. ACS Chem Neurosci. 2012; 3: 530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wyffels L, Gray BD, Barber C, et al. Detection of myocardial ischemia-reperfusion injury using a fluorescent near-infrared zinc(II)-dipicolylamine probe and 99mTc-glucarate. Mol Imaging Biol. 2012; 11: 1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Palmowski K, Rix A, Behrendt FF, et al. Improved assessment of apoptosis in tumors during anti-angiogenic therapy using the low molecular weight phosphatidylserine-targeting ligand PSVue compared to AnnexinV. Eur Radiol. 2014; 24: 363–370 [DOI] [PubMed] [Google Scholar]
- 29. Lam TT, Abler AS, Kwong JMK, Tso MOM. N-methyl-D-aspartate (NMDA) induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999; 40: 2391–2397 [PubMed] [Google Scholar]
- 30. Ishii Y, Kwong JMK, Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone, a heat shock protein inducer, in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003; 44: 1982–1992 [PubMed] [Google Scholar]
- 31. Kwong JMK, Quan A, Kyung H, Piri N, Caprioli J. Quantitative analysis of retinal ganglion cell survival with Rbpms immunolabeling in animal models of optic neuropathies. Invest Ophthalmol Vis Sci. 2011; 52: 9694–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwong JMK, Lam TT, Caprioli J. Heat shock pre-conditioning protects the retina from N-methyl-D-aspartate (NMDA) induced apoptosis in rat retinas. Brain Res. 2003; 970: 119–130 [DOI] [PubMed] [Google Scholar]
- 33. Pang IH, Clark AF. Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007; 16: 483–505 [DOI] [PubMed] [Google Scholar]
- 34. Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995; 36: 774–786 [PubMed] [Google Scholar]
- 35. Okisaka S, Murakami A, Mizukawa A, Ito J. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. 1997; 41: 84–88 [DOI] [PubMed] [Google Scholar]
- 36. Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol. 1997; 115: 1031–1035 [DOI] [PubMed] [Google Scholar]
- 37. Galvao J, Davis BM, Cordeiro MF. In vivo imaging of retinal ganglion cell apoptosis. Curr Opin Pharmacol. 2013; 13: 123–127 [DOI] [PubMed] [Google Scholar]
- 38. Eter N. Molecular imaging in the eye. Br J Ophthalmol. 2010; 94: 1420–1426 [DOI] [PubMed] [Google Scholar]
- 39. Cordeiro MF, Migdal C, Bloom P, Fitzke FW, Moss SE. Imaging apoptosis in the eye. Eye. 2011; 25: 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamp D, Sieberg T, Haest CW. Inhibition and stimulation of phospholipid scrambling activity. Consequences for lipid asymmetry, echinocytosis, and microvesiculation of erythrocytes. Biochemistry. 2001; 40: 9438–9446 [DOI] [PubMed] [Google Scholar]
- 41. Lederle W, Arns S, Rix A, et al. Failure of annexin-based apoptosis imaging in the assessment of anti-angiogenic therapy effects. EJNMMI Res. 2011; 1: 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dong K, Zhu H, Song Z, et al. Necrostatin-1 protects photoreceptors from cell death and improves functional outcome after experimental retinal detachment. Am J Pathol. 2012; 181: 1634–1641 [DOI] [PubMed] [Google Scholar]
- 43. Huang J-F, Shang L, Zhang M-Q, et al. Differential neuronal expression of receptor interacting protein 3 in rat retina: involvement in ischemic stress response. BMC Neurosci. 2013; 14: 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosenbaum DM, Degterev A, Rosenbaum PS, et al. Necroptosis, a novel form of caspase-independent cell death, contributes to neuronal damage in a retinal ischemia-reperfusion injury model. J Neurosci Res. 2010; 88: 1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siliprandi R, Canella R, Carmignoto G, et al. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992; 8: 567–573 [DOI] [PubMed] [Google Scholar]