Abstract
Craniosynostosis is a common congenital defect caused by premature fusion of cranial sutures. The severe morphological abnormalities and cognitive deficits resulting from craniosynostosis and the potential morbidity of surgical correction espouse the need for a deeper understanding of the complex etiology for this condition. Work in animal models over the past twenty years has been pivotal in advancing our understanding of normal suture biology and elucidating pathological disease mechanisms. This article provides an overview of milestone studies in suture development, embryonic origins, and signaling mechanisms from an array of animal models including transgenic mice, rats, rabbits, fetal sheep, zebrafish, and frogs. This work contributes to an ongoing effort toward continued development of novel treatment strategies.
Keywords: Craniosynostosis, Cranial Suture, Calvarium, Animal Models
INTRODUCTION
The skull vault is a dynamic structure formed from multiple membranous flat bones connected at the edges by fibrous sutures. As the brain grows during development, free expansion of the cranium occurs at the sutures. Craniosynostosis is a common congenital defect caused by premature fusion of one or more of these sutures and results in severe deformity as well as potential cognitive impairment. While the full etiology remains largely unknown, advances in genetics and molecular biology over the past 20 years have elucidated the biology of normal suture formation. Several genetic mutations have also been implicated in craniosynostosis.1 Studies using transgenic animal models have been crucial in elucidating the normal tissue interactions and pathologic signaling mechanisms involved in abnormal suture development and recent findings have paved the way for development of future therapeutic strategies for treatment of patients with craniosynostosis.
We are honored to participate in this collection of papers dedicated to Dr. Henry Kawamoto, as two of our authors had the pleasure of being craniofacial surgery fellows under Dr. Kawamoto (MTL was fellow number 17 in 1995–1996 and DCW was fellow number 31 in 2009–2010). The experience of operating with Dr. Kawamoto and performing cranial vault remodeling procedures on children with craniosynostoses has stimulated our interest in studying this condition on animal models. This review largely focuses on models studied in the Longaker laboratory but includes investigations from other laboratories when describing models that we have not worked on.
SUTURE DEVELOPMENT AND CRANIOSYNOSTOSIS
The human calvarium is formed primarily from five bones, the paired frontal and parietal bones and the unpaired interparietal bone. The cranial sutures allow for deformation of the skull during birth and subsequent growth of the skull.2 The frontal bones are separated along the midline by the metopic suture and the parietal bones are separated by the sagittal suture. The coronal suture separates the frontal and parietal bones and the lambdoid suture separates the parietal bones from the single occipital bone posteriorly (Figure 1). These bones are formed by intramembranous ossification and growth occurs perpendicular to the orientation of the suture. In normal development, the metopic suture undergoes fusion during the first year of life while other sutures fuse at various times during adulthood.3
Figure 1.
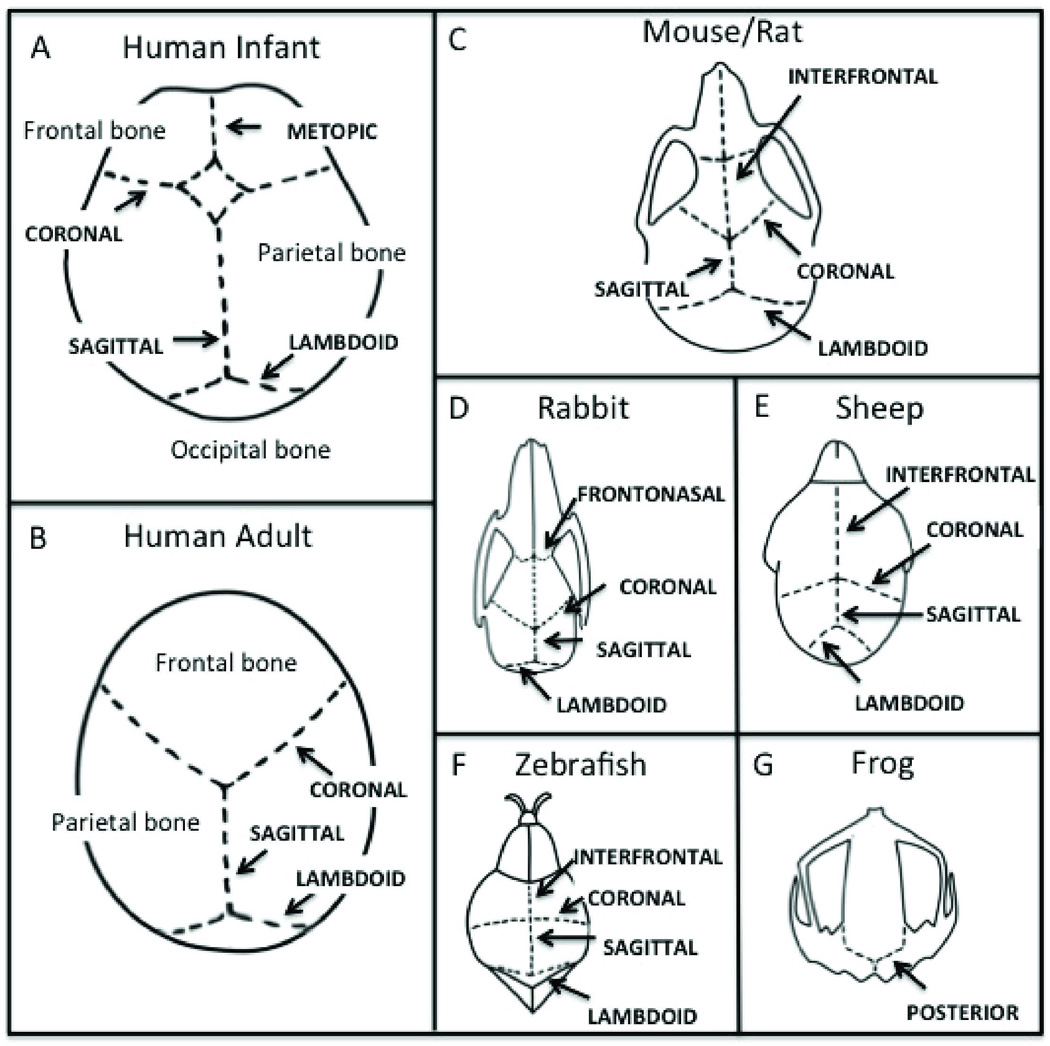
Schematic representation of cranial sutures in humans and in various animal models used for studies. Calvarial diagrams for (A) Human Infant, (B) Human Adult, (C) Mouse/Rat, (D) Rabbit, (E) Sheep, (F) Zebrafish, and (G) Frog.
Premature suture fusion can cause compensatory growth and remodeling along other sutures resulting in severe deformities in the calvarial vault, hydrocephalus, facial asymmetry, and increased intracranial pressure. In some cases, secondary visual and cognitive impairment, deafness, and airway compromise may develop.4 Surgical intervention aims to release the prematurely fused suture and normalize associated deformities. However, sutures are prone to refusion and patients often require multiple surgeries. Alternative approaches based on improved understanding of the molecular and genetic pathways of suture development and fusion could thus hold significant promise for patients with craniosynostosis.5
Craniosynostosis occurs in 1:2500 live births, most prevalently in the sagittal suture, and in up to 15% of cases multiple sutures may be involved.6 Many mutations in both syndromic and nonsyndromic craniosynostosis have been identified, the first being a rare heterozygous missense mutation in the msh homeobox 2 (MSX2) gene in patients with Boston-type craniosynostosis.7 Since this report, over 100 different craniosynostosis syndromes have been linked to specific mutations.1 A recent 10-year prospective cohort analysis of patients with craniosynostosis identified a specific genetic mutation in 21% of cases (86% single gene disorders, 15% chromosome abnormality), with most characterized by autosomal dominant inheritance8. Furthermore, these mutations were frequently found in proteins involved in regulation of cellular proliferation, differentiation, and migration during bone formation such as fibroblast growth factor receptor (FGFR)-2 (32%), FGFR-3 (25%), twist homolog 1 (TWIST1) (19%), and Ephrin-B1 (EFNB1) (7%).8,9
The complexity of molecular signaling pathways and tissue interactions involved in craniosynostosis has led to the development of many different animal models to study both normal development and pathologic mechanisms. These models have employed both genetic and physical methods of inducing suture fusion to mimic human craniosynostosis. Frequently studied models include the mouse, rat, rabbit, and sheep along with other non-mammalian animals such as zebrafish and frog (Figure 1).
MOUSE
Similarities in craniofacial development and molecular pathways between the mouse and human make this animal an excellent model to study suture biology.10 In mice, the interfrontal suture between the paired frontal bones is analogous to the metopic suture in humans. The sagittal suture between the paired parietal bones, coronal sutures between the frontal and parietal bones and the lambdoid suture between the parietal and interparietal bone are all similarly named. Like metopic suture fusion in humans, the mouse posterofrontal suture undergoes fusion in a predictable manner between postnatal days 7 and 12.11 This event occurs in an anterior to posterior direction and from the endocranial to ectocranial surface. In contrast, the sagittal, coronal, and lambdoid sutures remain patent throughout the life of the mouse. This differential suture fate allows for study of both naturally patent and physiologically fused sutures.11
Using the mouse model, investigators have identified multiple factors to play a role in suture fusion. Organ culture systems of mouse cranial sutures have allowed for the definition of transforming growth factor (TGF)-β as a potentially important signaling pathway guiding suture fate.12,13 While explanted mouse posterofrontal sutures were found to express increased levels of TGF-β during fusion, disruption of TGF-β signaling through introduction of a dominant negative receptor was found to prevent expected suture closure.12 Bone morphogenetic protein (BMP), another member of the TGF-β superfamily, has similarly been shown to significantly govern suture fate in mice. Interestingly, while BMPs are known to be potent promoters of osteogenesis, studies have localized BMP-2 and BMP-4 to sutures that both fuse and remain patent.14 Warren and colleagues, however, found that it was in fact differential expression of BMP antagonists, notably noggin, that was responsible for regulating the sutural effects of BMPs in mice.15 In sutures that remained patent (i.e. sagittal and coronal), noggin was found to be present thus limiting the signaling capacity for BMPs.15 In contrast, noggin was conspicuously absent in sutures that fused, thereby allowing unopposed BMP activity.15 Furthermore, when forced noggin expression was induced through adenoviral injection into the posterofrontal suture of three day old mice, continued abnormal patency was observed 50 days later.15 This was reflected in a widened interorbital distance and a shortened snout secondary to continued growth in a direction perpendicular to the patent suture. These findings in mice have thus shed light on some the key pathways potentially involved in suture fusion.
Recent studies in mice have also identified alternative biomolecular pathways involved differential suture fate including estrogens and Wnts. As estrogen is known to be involved in closure of growth plates in long bones, our laboratory has studied the role estrogen plays in posterofrontal suture fusion. Combining data obtained from gene expression studies with work done on estrogen receptor (ER)-α and ER-β knockout mice, we have found that signaling through ER-α and not ER-β is necessary to allow for normal suture fusion. Temporal upregulation of ER-α was noted during posterofrontal suture fusion in mice, and in ER-α knockouts, delayed suture fusion was observed.16
Wnt signaling has also been recently found to be important in regulating fusion, with activation of the canonical pathway critical for determining differential suture fate. In the posterofrontal suture of mice, expression of natural inhibitors has been noted to limit Wnt activity.17 This has been associated with the normal process of fusion. However, continuous activation of canonical Wnt signaling through the application of exogenous Wnt3A was found to inhibit endochondral ossification and thus suture closure.18 In contrast, Wnt activity has been linked to the maintenance of patency in the sagittal suture; investigators have reported inhibition of Wnts to result in endochondral ossification and abnormal sagittal suture fusion.18
The true power of the mouse for studying craniosynostosis, however, has come from the ability to study transgenic animals with mutations analogous to those described in syndromic patients. The identification of FGFR-1, FGFR-2, FGFR-3, TWIST1, EFNB1, and MSX2 genes associated with various forms of craniosynostosis has sparked the development of numerous transgenic mice with similar gain- or loss-of-function mutations mimicking each condition. Eswarakumar and colleagues generated a C342Y fgfr-2 gain-of-function mouse which demonstrated a phenotype similar to Crouzon syndrome in humans.19 The P252R FGFR-1 gain-of-function mutation associated with Pfeiffer syndrome has also been translated to a P250R fgfr-1 transgenic mouse with premature fusion noted in multiple sutures.20 And analogous to Apert syndrome patients with FGFR-2 S252W mutations, mutant mice have been created carrying the corresponding S250W amino acid substitution.21 Studies on this mouse have revealed distorted skulls with brachycephaly secondary to premature coronal suture fusion. Aside from these gain-of-function mutations in FGFRs, multiple other transgenic mice have been created to study Saethre-Chotzen (TWIST1 mutation), Boston-type craniosynostosis (MSX2), and craniofrontonasal dysplasia (EFNB1).22–24 These mice have offered a wealth of information on the etiopathogenesis of syndromic craniosynostosis. Importantly, they also serve as a potential platform to study future genetic strategies aimed at preventing premature pathologic suture fusion and all the secondary associated dysmorphologies.
RAT
The rat has been a useful model for study of craniosynostosis as the posterofrontal suture fuses in the predictable manner similar to the mouse between postnatal days 12 and 22.25 This has allowed for multiple studies to be performed identifying biomolecular mechanisms which guide suture fusion. TGF-β has been one particularly prominent factor identified in the rat model to be intimately involved in differential suture fate. Upregulation of both TGF-β receptors type I and II have been found in sutures that fuse.26 Investigations by Opperman have also suggested differential expression of TGF-β isoforms to be important for suture fate. While TGF-β2 has been noted to be increased rat posterofrontal sutures, TGF-β3 has instead been associated with sutures that remain patent.27,28 This suggests that TGF-β2 signaling may be important in guiding suture fusion while TGF-β3 may be responsible for the maintenance of patency. Subsequent studies have since been done identifying evaluating SMAD signaling secondary to differential presence various TGF-β isoforms.29
Similar to TGF-β, significant work has also been performed investigating the role of FGF signaling in the rat. Immunohistochemical studies have identified FGF-2 to be present in the dura mater during the period of suture fusion.30 Furthermore, transcript analysis demonstrated dramatic upregulation of FGF-2 expression in the posterofrontal suture relative to sutures that remain patent.31 These findings naturally led to experiments evaluating the effects of manipulating FGF-2 signaling in sutures. By injecting an adenovirus expressing FGF-2 into embryonic rats in utero, Greenwald and colleagues noted normally patent coronal sutures to fuse.32 In contrast, following an injection of an adenovirus expressing a dominant-negative FGF receptor-1 construct into the developing posterofrontal suture, inhibition of physiologic fusion was appreciated.32 These studies confirm the importance of FGF signaling in the regulation of suture fate and highlight a potential therapeutic strategy which may be one day employed to treat patients with craniosynostosis.
As the rat provides a larger animal model relative to the mouse, this has also facilitated surgical manipulations investigating the role of regional dura mater in dictating overlying suture fate. By isolating the suture complex from the underlying dura mater with a silastic sheet, Roth et al. reported a delay in normal fusion of the posterofrontal suture.33 Studies have also been performed investigating localized rotation of the calvarium. When the posterofrontal suture was re-positioned over the sagittal suture dura mater, continued patency was observed.34 Conversely, normally patent sagittal sutures placed over posterofrontal dura mater were observed to fuse.34 Taken together, these studies support the role of regional dura mater in guiding overlying suture fate.
RABBIT
Outside of murine models, a variety of other animals have also been utilized to study the process of suture fusion. In particular, a strain of New Zealand White rabbits with congenital, nonsyndromic coronal suture synostosis has been developed by Mooney et al. that mimics the pathology of human craniosynostosis.35 This model is advantageous as it demonstrates, much like humans, autosomal dominant transmission and variable phenotypic expression including unilaterally affected animals, delayed-onset suture synostosis, and animals with complete bilateral fusion.35 Characterization of this model has demonstrated that rabbits with unicoronal suture synostosis show significantly different brain growth and morphology, abnormalities in middle meningeal artery patterning and metabolic activity, and decreased mean intracranial volumes compared to controls.36,37 Methyl-methacrylate has also been used to immobilize coronal sutures and mimic pathologic fusion.38 Comparing morphologic changes of these experimental animals to rabbits with congenital bilateral coronal synostosis, similar abnormal craniofacial growth patterns were appreciated. However, rabbits with complete synostosis demonstrated craniofacial dysmorphologies more closely resembling those observed with human clinical syndromes.39
The rabbit model for craniosynostosis has also allowed for a variety of investigations on the molecular mechanisms responsible for pathologic suture fusion. Nott and colleagues used the rabbit model of delayed-onset craniosynostosis to evaluate expression of hedgehog family growth factors known to be involved in tissue development and embryologic patterning. Sonic hedgehog, Indian hedgehog, desert hedgehog, and patched-1 expression were all found to be upregulated in the synostotic rabbits.40 Similarly, perisutural tissue analysis has revealed enhanced expression for β-globin, osteopontin, osteonectin, and cathepsin K along with downregulation of collagen III and zinc finger protein 12 in rabbits with craniosynostosis.41 Collectively, these findings have not only confirmed many of the results described with other animal models, but have also revealed some novel genes not previously implicated in premature pathologic suture fusion.
One of the benefits of using the rabbit model is the relative size of animals compared to mice and rats. Surgical manipulation can be more easily performed, thus facilitating study of novel strategies to minimize risk for post-surgical refusion. In rabbits with coronal synostosis, investigators have transplanted “wild-type” dura mater allografts into suturectomy sites following removal of pathologically fused sutures.42 This resulted in a reduction of refusion rates highlighting the important role dura mater plays in dictating overlying bone formation. Alternatively, local application of anti-TGF-β2 antibodies directly into the suturectomy site has also been shown to inhibit subsequent refusion.43 Adjuvent therapies based on such a finding may be potentially translatable to minimize morbidity associated with resynostosis in patients undergoing suturectomy and remodeling of the calvarial vault.
SHEEP
The sheep model of craniosynostosis has provided a good platform for intrauterine studies. Compared to a gestation period of only 32 days in rabbits, sheep have a longer gestation of 140 days, easy intrauterine accessibility, and early calvarial bone formation.44,45 A low spontaneous abortion rate and relative ease of handling compared to primates and pigs also make them an attractive model for manipulation of the cranial sutures in utero.45 Taking advantage of these features, Stelnicki et al. developed an in utero sheep model to study unilateral coronal synostosis.45 Surgical removal of patent coronal sutures followed by implantation of demineralized bone powder, BMP-2, and TGF-β were performed on fetal lambs at 70 days gestation.45 These animals were then returned to the uterus to allow continued for continued development. By 90 days gestation, all fetuses were noted to have complete sutural fusion, and by 140 days gestation, associated craniofacial dysmorphologies could be readily appreciated.45 Description of this model has allowed for subsequent studies to be performed on timing of treatment and for development of novel therapeutic strategies. Pre-natal strip craniectomy has been performed at 91 days gestation and this has been found to mitigate many of the secondary facial morphologic abnormalities commonly seen with coronal synostosis.44 Importantly, however, as sheep do not possess any known genetic mutations associated with abnormal suture development, it is difficult to predict the level of similarity this intervention would have on all patients with craniosynostosis.
The ability to manipulate sheep prenatally has also allowed for the investigation of other theories surrounding development of pathologic suture fusion. In particular, our laboratory has evaluated the potential effects of severe intrauterine constraint, one proposed etiologic factor in the development of craniosynostosis. Compression miniplate fixation was placed in utero across the coronal suture of fetal lambs in an attempt to mimic external growth restriction at this site.46 Interestingly, evaluation of skulls four and eight weeks following delivery demonstrated mild deformation at the site of plating, but no premature pathologic fusion.46 This study therefore sheds doubt on the importance of intrauterine constraint as a major causative factor in the development of craniosynostosis.
NON-MAMMALIAN MODELS
While a variety of mammalian animal models have been used to study cranial suture biology, several non-mammalian models have also been established for the investigation of craniofacial development and patterning of the calvarial vault. The zebrafish has emerged as an attractive model organism because of their short reproductive cycle and large numbers of progeny. In addition, a high degree of genetic and development conservation exists between zebrafish and humans.47 As the entire zebrafish genome has been sequenced, genetic manipulation and screening can be performed on a whole-genome.48 Furthermore, zebrafish are known for their large and optically transparent embryos, allowing morphogenesis to be observed visually while they develop externally. Direct monitoring of the behavior of individual cells in vivo can also be performed from the earliest stages by time-lapse microscopy. And as the craniofacial elements lie just beneath a relatively thin layer of tissue into adulthood, these can be easily observed in live or fixed animals with minimal processing.49
While significant strides have been made toward understanding the genetic basis of craniofacial development, the need to extend comprehension of the developmental genetic program led our group to examine the post-embryonic developmental window of zebrafish for skull vault anatomy, calvarial osteogenesis, and cranial suture morphology.49 Different developmental stages from larvae to adult were examined and quantitative descriptions of the cranial vault phenotype and cranial suture patterning were recorded. Morphological analysis revealed the presence of frontal, parietal, and occipital cranial bone elements with associated cranial sutures similar to that in mammals. The skull vault was also found to form by intramembranous ossification, though the most anterior part of the zebrafish skull exhibited lack of suture fusion, differing from that of murine models and humans.49
The frog (Xenopus laevis) has also been used as an experimental system for investigating embryonic and early postnatal suture growth due to the high degree of conservation in the developmental processes. Several features of Xenopus make it an attractive alternative model system to study craniofacial patterning and development including ex utero development of large easily manipulated embryos, large numbers of offspring, and ease of explant culture.50 Slater and colleagues introduced Xenopus as a unique model system for examining craniofacial development and observed that these animals underwent dramatic cranial shape change during metamorphosis.51 The early larval head was found to be mostly cartilaginous and gradually ossified in a centripetal frontoparietal manner. Further morphological analysis revealed an intermediate midline cranial suture that completely fused during metamorphosis and a posterior suture that resembles the mammalian lambdoid suture.51 The Xenopus calvarium was also found to undergo significant morphological changes during cranial osteogenesis, with growth from ossification centers proceeding lateral to medial and posterior to anterior. Investigations suggested that matrix metalloproteinase-mediated restructuring of the cartilaginous cranial elements is important for cranial remodeling, akin to findings described in mice. Two sutures are seen in Xenopus, a midline suture that fuses prior to metamorphosis and a posterior suture that remains patent51. Furthermore, Xenopus calvarial vaults were noted to exhibit unique cranial suture patterning, however, with fused frontal and parietal bones and lack of metopic, coronal, and sagittal sutures which fuse early during metamorphosis.51 Interestingly, with Xenopus, cell lineage and fate maps can be generated as early as the cleavage stage, allowing for molecular and genetic analyses not feasible in zebrafish due to extensive cell mixing during the early blastula stage.52
CONCLUSION
The animal models developed over the past twenty years have advanced our understanding of normal cranial suture biology and elucidated a variety of potential pathological mechanisms. The identification of factors differentially expressed in fusing sutures has led to multiple targets for future research. However, the number of molecular pathways implicated in the etiology of craniosynostosis is indicative of the complexity involved in tissue interactions that maintain the balance between proliferation and differentiation of osteoprogenitor cells in the cranial sutures. The severe morphological abnormalities and cognitive deficits resulting from craniosynostosis and the potential morbidity of surgical correction nonetheless espouse the need for development of alternative treatment strategies. As studies have identified several potential molecular targets, novel, less invasive, and biologically based therapeutic strategies may thus one day be developed to augment or replace current surgical treatments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Morriss-Kay GM, Wilkie AO. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Weinzweig J, Kirschner RE, Farley A, et al. Metopic synostosis: Defining the temporal sequence of normal suture fusion and differentiating it from synostosis on the basis of computed tomography images. Plastic and reconstructive surgery. 2003;112:1211–1218. doi: 10.1097/01.PRS.0000080729.28749.A3. [DOI] [PubMed] [Google Scholar]
- 4.Panchal J, Uttchin V. Management of craniosynostosis. Plastic and reconstructive surgery. 2003;111:2032–2048. doi: 10.1097/01.PRS.0000056839.94034.47. [DOI] [PubMed] [Google Scholar]
- 5.Whitaker LA, Bartlett SP, Schut L, et al. Craniosynostosis: an analysis of the timing, treatment, and complications in 164 consecutive patients. Plast Reconstr Surg. 1987;80:195–212. [PubMed] [Google Scholar]
- 6.Cohen M. Epidemiology of Craniosynostosis. Oxford University Press; 2000. [Google Scholar]
- 7.Jabs EW, Muller U, Li X, et al. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell. 1993;75:443–450. doi: 10.1016/0092-8674(93)90379-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson D, Wilkie AO. Craniosynostosis. Eur J Hum Genet. 2011;19:369–376. doi: 10.1038/ejhg.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slater BJ, Lenton KA, Kwan MD, et al. Cranial sutures: a brief review. Plast Reconstr Surg. 2008;121:170e–178e. doi: 10.1097/01.prs.0000304441.99483.97. [DOI] [PubMed] [Google Scholar]
- 10.Wilkie AO, Morriss-Kay GM. Genetics of craniofacial development and malformation. Nat Rev Genet. 2001;2:458–468. doi: 10.1038/35076601. [DOI] [PubMed] [Google Scholar]
- 11.Lenton KA, Nacamuli RP, Wan DC, et al. Cranial suture biology. Current topics in developmental biology. 2005;66:287–328. doi: 10.1016/S0070-2153(05)66009-7. [DOI] [PubMed] [Google Scholar]
- 12.Mehrara BJ, Spector JA, Greenwald JA, et al. Adenovirus-mediated transmission of a dominant negative transforming growth factor-beta receptor inhibits in vitro mouse cranial suture fusion. Plastic and reconstructive surgery. 2002;110:506–514. doi: 10.1097/00006534-200208000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Slater BJ, Lenton KA, James A, et al. Ex vivo Model of Cranial Suture Morphogenesis and Fate. Cells Tissues Organs. 2009;190:336–346. doi: 10.1159/000228157. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Rice DP, Kettunen PJ, et al. FGF-, BMP- and Shh-mediated signaling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- 15.Warren SM, Brunet LJ, Harland RM, et al. The BMP antagonist noggin regulates cranial suture fusion. Nature. 2003;422:625–629. doi: 10.1038/nature01545. [DOI] [PubMed] [Google Scholar]
- 16.James AW, Theologis AA, Brugmann SA, et al. Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion. PLoS One. 2009;4:e7120. doi: 10.1371/journal.pone.0007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behr B, Longaker MT, Quarto N. Differential activation of canonical Wnt signaling determines cranial sutures fate: A novel mechanism for sagittal suture craniosynostosis. Developmental Biology. 2010;344:922–940. doi: 10.1016/j.ydbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Behr B, Longaker MT, Quarto N. Craniosynostosis of coronal suture in twist1 mice occurs through endochondral ossification recapitulating the physiological closure of posterior frontal suture. Frontiers in physiology. 2011;2:37. doi: 10.3389/fphys.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eswarakumar VP, Horowitz MC, Locklin R, et al. A gain-of-function mutation of Fgfr2c demonstrates the roles of this receptor variant in osteogenesis. Proc Natl Acad Sci U S A. 2004;101:12555–12560. doi: 10.1073/pnas.0405031101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou YX, Xu X, Chen L, et al. A Pro250Arg substitution in mouse Fgfr1 causes increased expression of Cbfa1 and premature fusion of calvarial sutures. Hum Mol Genet. 2000;9:2001–2008. doi: 10.1093/hmg/9.13.2001. [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Li D, Li C, et al. A Ser250Trp substitution in mouse fibroblast growth factor receptor 2 (Fgfr2) results in craniosynostosis. Bone. 2003;33:169–178. doi: 10.1016/s8756-3282(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 22.Winograd J, Reilly MP, Roe R, et al. Perinatal lethality and multiple craniofacial malformations in MSX2 transgenic mice. Hum Mol Genet. 1997;6:369–379. doi: 10.1093/hmg/6.3.369. [DOI] [PubMed] [Google Scholar]
- 23.El Ghouzzi V, Lajeunie E, Le Merrer M, et al. Mutations within or upstream of the basic helix-loop-helix domain of the TWIST gene are specific to Saethre-Chotzen syndrome. Eur J Hum Genet. 1999;7:27–33. doi: 10.1038/sj.ejhg.5200240. [DOI] [PubMed] [Google Scholar]
- 24.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth DA, Longaker MT, McCarthy JG, et al. Studies in cranial suture biology: Part I. Increased immunoreactivity for transforming growth factor-beta (β1, β2, β3) during rat cranial suture fusion. J Bone Miner Res. 1997;12:311–321. doi: 10.1359/jbmr.1997.12.3.311. [DOI] [PubMed] [Google Scholar]
- 26.Mehrara BJ, Steinbrech DS, Saadeh PB, et al. Expression of high-affinity receptors for TGF-beta during rat cranial suture fusion. Ann Plast Surg. 1999;42:502–508. doi: 10.1097/00000637-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Opperman LA, Nolen AA, Ogle RC. TGF-beta 1, TGF-beta 2, and TGF-beta 3 exhibit distinct patterns of expression during cranial suture formation and obliteration in vivo and in vitro. J Bone Miner Res. 1997;12:301–310. doi: 10.1359/jbmr.1997.12.3.301. [DOI] [PubMed] [Google Scholar]
- 28.Opperman LA, Moursi AM, Sayne JR, et al. Transforming growth factor-beta 3(Tgf-beta3) in a collagen gel delays fusion of the rat posterior interfrontal suture in vivo. Anat Rec. 2002;267:120–130. doi: 10.1002/ar.10094. [DOI] [PubMed] [Google Scholar]
- 29.Tholpady SS, Ogle RC. Expression of transforming growth factor-beta-responsive smads in cranial suture development and closure. The Journal of craniofacial surgery. 2011;22:324–328. doi: 10.1097/SCS.0b013e3181f7dfa0. [DOI] [PubMed] [Google Scholar]
- 30.Mehrara BJ, Mackool RJ, McCarthy JG, et al. Immunolocalization of basic fibroblast growth factor and fibroblast growth factor receptor-1 and receptor-2 in rat cranial sutures. Plast Reconstr Surg. 1998;102:1805–1817. doi: 10.1097/00006534-199811000-00001. discussion 1818–1820. [DOI] [PubMed] [Google Scholar]
- 31.Mehrara BJ, Most D, Chang J, et al. Basic fibroblast growth factor and transforming growth factor beta-1 expression in the developing dura mater correlates with calvarial bone formation. Plast Reconstr Surg. 1999;104:435–444. doi: 10.1097/00006534-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald JA, Mehrara BJ, Spector JA, et al. In vivo modulation of FGF biological activity alters cranial suture fate. Am J Pathol. 2001;158:441–452. doi: 10.1016/s0002-9440(10)63987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roth DA, Bradley JP, Levine JP, et al. Studies in cranial suture biology: Part II. Role of the dura in cranial suture fusion. Plast Reconstr Surg. 1996;97:693–699. doi: 10.1097/00006534-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Levine JP, Bradley JP, Roth DA, et al. Studies in cranial suture biology: Regional dura mater determines overlying suture biology. Plastic and reconstructive surgery. 1998;101:1441–1447. doi: 10.1097/00006534-199805000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Mooney MP, Aston CE, Siegel MI, et al. Craniosynostosis with autosomal dominant transmission in New Zealand white rabbits. J Craniofac Genet Dev Biol. 1996;16:52–63. [PubMed] [Google Scholar]
- 36.Mooney MP, Siegel MI, Burrows AM, et al. A rabbit model of human familial, nonsyndromic unicoronal suture synostosis. I Synostotic onset, pathology, and sutural growth patterns. Childs Nerv Syst. 1998;14:236–246. doi: 10.1007/s003810050219. [DOI] [PubMed] [Google Scholar]
- 37.Mooney MP, Siegel MI, Burrows AM, et al. A rabbit model of human familial, nonsyndromic unicoronal suture synostosis. II. Intracranial contents, intracranial volume, and intracranial pressure. Childs Nerv Syst. 1998;14:247–255. doi: 10.1007/s003810050220. [DOI] [PubMed] [Google Scholar]
- 38.Mooney MP, Losken HW, Tschakaloff A, et al. Congenital bilateral coronal suture synostosis in a rabbit and craniofacial growth comparisons with experimental models. Cleft Palate Craniofac J. 1993;30:121–128. doi: 10.1597/1545-1569_1993_030_0121_cbcssi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 39.Mooney MP, Losken HW, Siegel MI, et al. Development of a strain of rabbits with congenital simple nonsyndromic coronal suture synostosis. Part II: Somatic and craniofacial growth patterns. Cleft Palate Craniofac J. 1994;31:8–16. doi: 10.1597/1545-1569_1994_031_0008_doasor_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 40.Nott RL, Stelnicki EJ, Mack JA, et al. Comparison of hedgehog and patched-1 protein expression in the cranial sutures of craniosynostotic and wild-type rabbits. Plastic and reconstructive surgery. 2002;110:515–522. doi: 10.1097/00006534-200208000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Cray JJ, Jr, Gallo PH, Durham EL, et al. Molecular analysis of coronal perisutural tissues in a craniosynostotic rabbit model using polymerase chain reaction suppression subtractive hybridization. Plastic and reconstructive surgery. 2011;128:95–103. doi: 10.1097/PRS.0b013e31821740e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mooney MP, Burrows AM, Smith TD, et al. Correction of coronal suture synostosis using suture and dura mater allografts in rabbits with familial craniosynostosis. Cleft Palate Craniofac J. 2001;38:206–225. doi: 10.1597/1545-1569_2001_038_0206_cocssu_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 43.Mooney MP, Losken HW, Moursi AM, et al. Postoperative anti-Tgf-beta2 antibody therapy improves intracranial volume and craniofacial growth in craniosynostotic rabbits. J Craniofac Surg. 2007;18:336–346. doi: 10.1097/scs.0b013e3180336047. discussion 347–339. [DOI] [PubMed] [Google Scholar]
- 44.Stelnicki EJ, Vanderwall K, Harrison MR, et al. The in utero correction of unilateral coronal craniosynostosis. Plastic and reconstructive surgery. 1998;101:287–296. doi: 10.1097/00006534-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Stelnicki EJ, Vanderwall K, Hoffman WY, et al. A new in utero sheep model for unilateral coronal craniosynostosis. Plastic and reconstructive surgery. 1998;101:278–286. doi: 10.1097/00006534-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Bradley JP, Shahinian H, Levine JP, et al. Growth restriction of cranial sutures in the fetal lamb causes deformational changes, not craniosynostosis. Plastic and reconstructive surgery. 2000;105:2416–2423. doi: 10.1097/00006534-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Sheehan-Rooney K, Palinkasova B, Eberhart JK, et al. A cross-species analysis of Satb2 expression suggests deep conservation across vertebrate lineages. Dev Dyn. 2010;239:3481–3491. doi: 10.1002/dvdy.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, Wloga D, Dougan ST. Embryological manipulations in zebrafish. Methods Mol Biol. 2011;770:139–184. doi: 10.1007/978-1-61779-210-6_6. [DOI] [PubMed] [Google Scholar]
- 49.Quarto N, Longaker MT. The zebrafish (Danio rerio): a model system for cranial suture Patterning. Cells Tissues Organs. 2005;181:109–118. doi: 10.1159/000091100. [DOI] [PubMed] [Google Scholar]
- 50.Afouda BA, Hoppler S. Xenopus explants as an experimental model system for studying heart development. Trends Cardiovasc Med. 2009;19:220–226. doi: 10.1016/j.tcm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Slater BJ, Liu KJ, Kwan MD, et al. Cranial osteogenesis and suture morphology in Xenopus laevis: a unique model system for studying craniofacial development. PLoS One. 2009;4:e3914. doi: 10.1371/journal.pone.0003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beck CW, Izpisua Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]


