Abstract
Flavoprotein autofluorescence imaging, an intrinsic mitochondrial signal, has proven useful for monitoring neuronal activity. In the cerebellar cortex, parallel fiber stimulation evokes a beam-like response consisting of an initial, short-duration increase in fluorescence (on-beam light phase) followed by a longer duration decrease (on-beam dark phase). Also evoked are parasagittal bands of decreased fluorescence due to molecular layer inhibition. Previous work suggests that the on-beam light phase is due to oxidative metabolism in neurons. The present study further investigated the metabolic and cellular origins of the flavoprotein signal in vivo, testing the hypotheses that the dark phase is mediated by glia activation and the inhibitory bands reflect decreased flavoprotein oxidation and increased glycolysis in neurons. Blocking postsynaptic ionotropic and metabotropic glutamate receptors abolished the onbeam light phase and the parasagittal bands without altering the on-beam dark phase. Adding glutamate transporter blockers reduced the dark phase. Replacing glucose with lactate (or pyruvate) or adding lactate to the bathing media abolished the on-beam dark phase and reduced the inhibitory bands without affecting the light phase. Blocking monocarboxylate transporters eliminated the on-beam dark phase and increased the light phase. These results confirm that the on-beam light phase is due primarily to increased oxidative metabolism in neurons. They also show that the on-beam dark phase involves activation of glycolysis in glia resulting in the generation of lactate that is transferred to neurons. Oxidative savings in neurons contributes to the decrease in fluorescence characterizing the inhibitory bands. These findings provide strong in vivo support for the astrocyte–neuron lactate shuttle hypothesis.
Keywords: Flavoproteins, Intrinsic imaging, Parallel fibers, Oxidative metabolism, Neurometabolic coupling
Introduction
Flavoprotein autofluorescence is strongly coupled to neuronal activation and provides one approach to study neurometabolic coupling [1–6]. This intrinsic signal reflects changes in the oxidation/reduction (redox) state of flavoproteins located primarily in mitochondrial enzyme complexes [7–11]. These include, but are not limited to, non-nicotinamide adenine dinucleotide (NAD)-linked succinate dehydrogenase associated with complex II of the electron transport chain (ETC) and NAD-linked lipoamide dehydrogenase, both of which oxidize and reduce flavin adenine dinucleotide (FAD). Lipoamide dehydrogenase is in equilibrium with the mitochondrial NAD+/NADH pool and FAD is reduced as substrates are decarboxylated; for example, the decarboxylation of pyruvate to enter the tricarboxcylic acid (TCA) cycle [12–14]. Flavoproteins are also reduced by NADH generated by cytoplasmic glycolysis that can be transferred across the mitochondrial membrane via the malate–aspartate shuttle [15].
The astrocyte–neuron lactate shuttle hypothesis (ANLS) argues that neurons and astrocytes are metabolically coupled, with the oxidative metabolism of lactate dominating in neurons during glutamatergic excitatory neurotransmission [16]. The ANLS hypothesis also postulates that the glutamate released activates astrocytes via excitatory amino acid transporters (EAATs), leading to increased glycolysis and lactate generation that is shuttled to neurons to fuel oxidative metabolism. Monocarboxylate transporters (MCTs) on both the astrocytes and neurons play central roles in the shuttling of the lactate between these two cell populations [17–19]. Although the earliest formulations of the ANLS predicted lactate production from the onset of neuron activity [16], subsequent interpretations and results emphasize a temporal segregation with the first step being oxidative metabolism in neurons followed by an increase in glia glycolysis [20–22]. Considerable evidence has been marshaled in its support [22–25]; however, the ANLS hypothesis remains controversial [6, 26–28]. Tests of the ANLS hypothesis in vivo are needed [29–31]. Imaging the changes in flavoprotein or NADH fluorescence in response to neuronal activation provides one approach to evaluate this important concept in vivo.
The cerebellar cortex with its highly spatially specific and stereotypic circuitry provides an ideal system to examine the changes in flavoprotein fluorescence [32]. Parallel fiber stimulation evokes a beam-like response consisting of an initial, short-duration increase in fluorescence (on-beam light phase), followed by a longer duration decrease in fluorescence (on-beam dark phase) [1, 2, 33]. Parallel fiber stimulation also evokes parasagittal bands of decreased fluorescence that intersect the beam orthogonally [33]. These inhibitory bands are due to postsynaptic, GABAergic inhibition generated by molecular layer interneurons [33, 34]. The inhibitory bands have both on- and off-beam components. Among these three response components, on-beam light phase, on-beam dark phase, and the inhibitory bands, previous results suggest that the light phase is primarily mediated by the activation of the postsynaptic targets of the parallel fibers [1, 2, 35]. Less is known about the mechanisms underlying the dark phase and the inhibitory bands.
Therefore, this study focused on the cellular and metabolic substrates underlying the on-beam dark phase and the inhibitory bands, testing two hypotheses based on the ANLS. The first hypothesis is that the dark phase is mediated by the activation of cerebellar glial cells by glutamate released from the stimulated parallel fibers. The activation of EAATs would trigger glycolysis in glia and the production of reducing equivalents that would decrease flavoprotein fluorescence. In the hippocampal slice, the transients in NADH fluorescence due to glutamatergic excitatory synaptic transmission are consistent with this view [22]. There is some evidence from in vivo studies that support this view but each has limitations. Because blocking AMPA receptors does not completely block the dark phase, this leads to the suggestion that glia may be involved [1, 2]. However, these early studies did not separate a possible contribution to the dark phase from the inhibitory bands that are generated by molecular layer interneurons [36]. Nor did they investigate the possible contributions of other glutamate receptors including metabotropic glutamate receptors (mGluRs) and N-methyl-D-aspartic acid (NMDA) receptors [37, 38]. In addition, the glial toxin, fluoroacetate, suppresses the dark phase [35]. However, fluoroacetate also reduces the light phase, although to a lesser degree than the dark phase.
Little is known about the metabolic mechanisms underlying the fluorescence decrease characterizing the inhibitory bands [33]. Inhibitory activity can decrease metabolic demand by reducing neuronal activity or increase energy expenditure due to the release of neurotransmitters and the need to restore ionic gradients [39, 40]. Therefore, the inhibitory bands could reflect decreased oxidative metabolism as a result of neuronal hyperpolarization and/or increased glycolytic activity due to energy expenditure in neurons and/or glia. However, GABA uptake by glia does not induce a metabolic response [41], leading to the second hypothesis that the inhibitory bands reflect decreased flavoprotein oxidation and increased glycolysis in neurons. We tested these two hypotheses by examining the effects of blocking glutamate receptors and transporters, manipulating extracellular glucose, lactate and pyruvate, and blocking MCTs on the flavoprotein response to parallel fiber stimulation in vivo.
Materials and Methods
Animal Preparation
All animal experimentation was approved by the Institutional Animal Care and Use Committee of the University of Minnesota and conducted in conformity with the American Physiological Society’s Guiding Principles for the Care and Use of Animals in Research. Adult male FVB mice (Charles River Laboratories, Wilmington, MA) ages 3–8 months were anesthetized by intramuscular injection of 2 mg/kg of acepromazine as an inducing agent followed by a series of intraperitoneal injections of urethane solution totaling 1.8 mg/kg. Animals were mechanically ventilated, placed in a stereotaxic frame and body temperature was feedback-regulated through a rectal temperature probe connected to a heating pad. The electrocardiogram was continuously monitored to assess the depth of anesthesia. A craniotomy was used to expose Crus I and II, and a watertight chamber of dental acrylic was built up around the exposed cortex and filled with an artificial Ringer’s solution gassed with 95% O2 and 5% CO2. The flavoprotein signal depends on the availability of oxygen, and the 95% O2 contributes to maintaining a large and robust flavoprotein signal. Prior attempts to examine the flavoprotein signal in anesthetized animals or in vitro failed to show a sizable signal because the animals were not respirated and tissue was not bathed in oxygenated media (see [35]). We would also point out that the flavoprotein signal is present in the awake, unanesthetized animals [42, 43], demonstrating that the 95% O2 is not producing artificial or nonphysiological signals. Furthermore, prior experiments demonstrate that cerebral blood flow and cerebral blood volume changes have very little impact on the signal in this preparation, and are typically at least two orders of magnitude less than the flavoprotein signal [1]. The bathing media used in the optical chamber was the primary means of supplying or altering metabolic substrates such as glucose.
In some experiments, drugs or other compounds were added to the Ringer’s solution and perfused into the chamber. The pharmacological agent or substrate change of the chamber superfusate was typically over 20–30 min and followed by washout with control Ringer’s. This included the AMPA receptor antagonist DNQX (6,7-dinitroquinoxaline-2,3-dione disodium salt), the mGluR1 antagonist LY367385, the NMDA antagonist D-(−)-2-amino-5-phosphopentanoic acid (APV), the glutamate transport blocker threo-β-benzyloxyaspartate (TBOA) and the MCT blocker α-cyano-4-hydroxycinnamate (4-CIN). In some experiments, sodium lactate (1 to 10 mM) and sodium pyruvate (10 mM) were added to the Ringer’s solution, either as a substitute for, or in addition to, the glucose in the Ringer’s. Also, in a series of experiments, glucose was removed from the Ringer’s.
Optical Imaging and Analysis
Images of the cerebellar surface were acquired using a modified Nikon epifluorescence microscope fitted with a ×4 objective and a Quantix-cooled charge-coupled device camera with 12 bit digitization (Roper Scientific, Tucson, AZ). A 100-W mercury–xenon lamp (Hamamatsu Photonics) powered by an Opti Quip power supply was used as the light source. Flavoprotein autofluorescence imaging used a band pass excitation filter (455±35 nm), an extended reflectance diachronic mirror (500 nm), and a >515-nm-long pass emission filter [1]. The images were binned 2×2 for image frames of 265×256 pixels, resulting in a final pixel resolution of ~10 µm×10 µm. A typical acquisition protocol included a series of 20 control frames followed by series of 150 to 500 experimental frames, with an exposure time of 200 ms for each frame. A series of four sequential trials were averaged to facilitate imaging smaller amplitude inhibitory responses. Parallel fiber stimulation was initiated at the onset of the experimental frames using a paralyene-coated tungsten microelectrode (1–3 MΩ) placed just below the cerebellar surface. The stimulation parameters consisted of a train of 100 µA, 100 µs pulses at 10 Hz for 10 s [1, 33].
The first step in the analyses of the optical signals was to generate a series of difference images by subtracting the average of 18 control frames just prior to stimulation onset (referred to as the control average) from each control and experimental frame. These difference images were then divided by the control average, resulting in images in which the intensity value of each pixel reflects the ΔF/F change in fluorescence intensity in percent relative to the average of the control frames.
To quantify the amplitude and time course of the different on- and off-beam components of the autofluorescence signal, a series of regions of interest (ROI) were defined (Fig. 1). In our original characterization of flavoprotein autofluorescence changes in response to parallel fiber stimulation [1], the analysis of both the on-beam light phase and dark phase did not take into account the inhibitory bands and that these bands cross the beam [33]. In that initial study, the inhibitory bands had not been found and the entire beam was treated as an ROI, including the contributions from the inhibitory band in the on-beam light-phase response. Therefore, to dissect out the cellular and metabolic mechanisms, the present study defined separate ROIs for the off-beam component of the inhibitory bands and for segments of the on-beam response that are independent of the inhibitory bands. For both the light and dark phases of the on-beam response, the ROI consisted of region(s) on the beam that were positioned between the inhibitory bands (Fig. 1a). This was done to remove any contribution from the inhibitory bands to either the light or dark phase of the on-beam response. Next, the average ΔF/F within the ROI was calculated for each frame in the series. One or two regions in the exposed cerebellar cortex but outside of the beam/band area were used as control regions. The ΔF/F in these control regions were calculated and subtracted from the ROIs to correct for fluctuations in background fluorescence. To ensure that the regions measured did not change with brain movement, the ROIs were aligned to the blood vessel pattern rather than to precise pixel locations.
Fig. 1.
Regions of interest (ROIs) used to define on-beam and off-beam responses. a Gray-scale image showing the on-beam light phase and inhibitory bands evoked by parallel fiber stimulation. The ROI outlined in red defines the on-beam regions positioned between the inhibitory bands. Both the on-beam light phase and the on-beam dark phase were determined from the average ΔF/F in these ROI. Placing the ROI in these locations avoids contributions from the inhibitory bands. The decreases in fluorescence denoted by the arrows are the off-beam components of the inhibitory bands. The ROI in blue outlines one of these regions. b Example time courses of the average ΔF/F for the on-beam ROI (red trace) consist of both the initial light phase and later dark phase. The fluorescence change from the off-beam ROI (blue trace) shows the decrease in fluorescence characterizing the inhibitory bands
The time course of the average ΔF/F from each ROI was determined and used to define the amplitude of the response. The amplitude of the light phase was defined as the average ΔF/F over a 5-s period centered on the time of peak increase in fluorescence following stimulation. The amplitude of the on-beam, dark phase response was defined as the average ΔF/F over a 5-s period centered on the time of maximal decrease in fluorescence (typically 30–35 s after stimulation onset). To quantify the inhibitory bands, ROIs were defined for each of the patches of decreased fluorescence off the beam (Fig. 1), again determining the average ΔF/F within the ROI for each frame and averaging the 5-s period centered on the maximal decrease. If multiple ROIs of the same type were defined within a single experiment, such as the on-beam light phase or inhibitory bands, the ROIs were averaged and treated as a single value. The significance of the effects of the different drugs/ metabolic substrates on the amplitudes of the optical responses components compared to the control period was evaluated with an ANOVA using a within-subject design. Population results are expressed as mean±SD and “n” refers to the number of animals studied.
For the displayed images, a series of 25 frames were averaged together to better display the smaller amplitude inhibitory bands. To display the on-beam increase in fluorescence (light phase) and the inhibitory bands in the same image, the frames during the latter part of the light phase peak and during the early transition (typically 5–10 s after stimulation onset) were averaged together. To display the on-beam dark phase, frames around the maximum decrease in fluorescence were averaged. The images were scaled to ±1.5% ΔF/F, and presented as gray-scale or pseudocolored images. The ΔF/F time courses were low-pass-filtered (0.5 Hz) for display.
The dose – response curves for lactate were generated by fitting the amplitudes of the response in the different concentrations of lactate to the following equation:
In which Y is the vector of amplitudes normalized to the amplitude obtained in Ringer’s, X is the vector of lactate concentrations, a is the natural log of the EC50 and K is a slope factor for the function [44]. The dose – response curves were calculated for the light and dark phases and the inhibitory bands. In all cases, the p value for the fit was less than 0.01 (F test). Plots were then constructed showing the fit function as a line with the mean and standard deviation of each signal component at the different concentrations overlaid on top.
Field Potential Recordings
Field potential recordings in the molecular layer of the responses to parallel fiber stimulation provided an electrophysiological assessment of the status of the cerebellar cortical circuitry and whether the pharmacological agents altered the parallel fiber – Purkinje cell circuitry. For many of the agents used, it was important to demonstrate that the excitability was not altered (e.g., pyruvate and lactate) and for other agents that the postsynaptic response was blocked but the presynaptic activity was not effected (e.g., glutamate receptor antagonists). The field potentials were recorded using conventional techniques and glass microelectrodes (2 M NaCl, 2–5 MΩ). The recordings were digitized at 25 kHz and averaged (responses to 16 single parallel fiber stimuli at 1 Hz). The P1/N1 component was used as a measure of the presynaptic responses and the N2 component as a measure of the postsynaptic response [1, 32, 33]. As with the optical responses, the field potentials were monitored in each of the three periods (control, drug, and washout). In each period, the parallel fiber stimulation occurred four to six times and the field potentials were recorded for each. Because the amplitude of the parallel fiber volley and postsynaptic response varied among animals, for each animal, the responses in the control period were averaged and the average expressed as 100%. The P1/N1 and N2 components in the experimental and washout periods were normalized to the control period. The statistical analysis and reporting of the population results for the field potentials is as described for the optical responses.
Results
Effects of Glutamate Receptor Antagonists and Excitatory Amino Acid Transporter Blockers
The first hypothesis tested was that the on-beam light phase evoked by parallel fiber stimulation is primarily due to neuronal activation resulting in an increase in oxidative metabolism and that the later dark phase is primarily due to glial activation resulting in glycolysis. The initial experiment involved blocking the postsynaptic neuronal activation. At parallel fiber – Purkinje cell synapses, AMPARs are the primary postsynaptic ionotropic glutamate receptors and mGluR1 receptors are the primary postsynaptic metabotropic receptors on Purkinje cells [45–47]. At parallel fiber-interneuron synapses, there are AMPA, NMDA, and mGluR receptors [37, 38]. Previously, we showed that CNQX blocks 92% of the light phase but only 51% of th dark phase. However, these initial findings were based on quantifying the light and dark phases along the entire beam. This analysis inadvertently overestimated the effect of the AMPA antagonist on the dark phase due to inclusion of the on-beam component of the inhibitory bands. The CNQX markedly reduces parallel fiber activation of the inhibitory neurons as well as parallel fiber–Purkinje cell synaptic transmission [37, 38] and therefore, would have suppressed the inhibitory bands (see Fig. 4). Also, by only using an AMPA receptor blocker, the contribution of mGluR1 and NMDA receptors to either the light or dark phase was not evaluated.
Fig. 4.
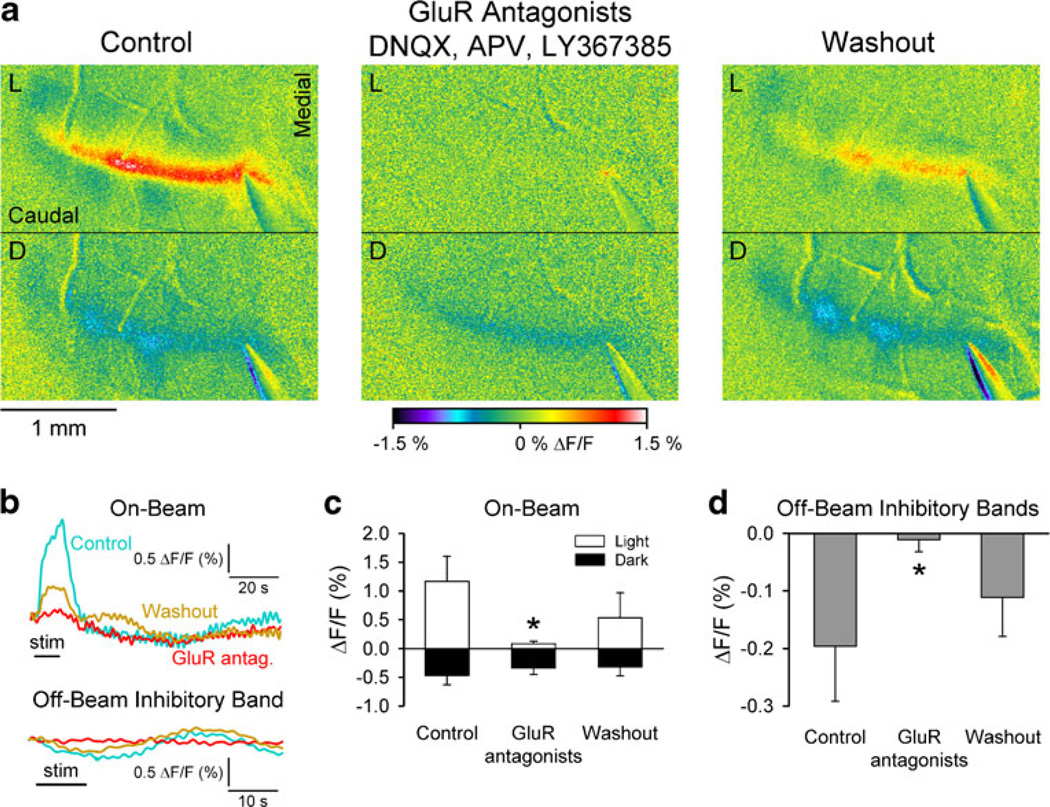
Effects of blocking ionotropic and metabotropic glutamate receptors and glutamate transporters on the response to parallel fiber stimulation. a Pseudocolored ΔF/F images taken during the light phase (L, top) and dark phase (D, bottom) before (control), during, and after adding DNQX, APV, LY367385, and TBOA to the Ringer’s. b Example time courses of the on-beam optical response for control (blue), drug cocktail (red), and washout (gold) periods. The time courses for the off-beam inhibitory bands are not shown as these are blocked by the GluR antagonists (see Fig. 2). c Bar graph showing the population data for the on-beam light phase (open) and on-beam dark phase (black). d Top traces are examples of the field potentials evoked by PF stimulation and bottom plots are the population data of the P1/ N1 and N2 components for the control (blue), during (red), and washout periods (gold). Imaging data are from five mice and the field potential data from four mice
Therefore, to determine the combined effect of AMPA, mGluR1, and NMDA receptors, we tested the effect of a cocktail of glutamate receptor blockers that included AMPA (DNQX, 100 µM), mGluR1 (LY367385, 200 µM), and NMDA antagonists (APV, 250 µM). Also, we restricted the analysis of the dark phase to the decrease in fluorescence occurring on-beam and between the inhibitory bands (see “Materials and Methods” and Fig. 1). Most importantly, the on-beam dark phase was not altered as seen in the example images (Fig. 2a) and time courses (Fig. 2b). At the population level, there was no significant effect on the dark phase (−0.47±0.16% to −0.34±0.12% ΔF/F, F (1,4)=3.3, p=0.11, n=5, Fig. 2c). Conversely, this combination of antagonists essentially eliminated the on-beam light phase (Fig. 2a–c). On average, the light phase was reduced from 1.17±0.43% to 0.09±0.04% ΔF/F (F(1,4)= 18.0, p=0.003, Fig. 2c).
Fig. 2.
Effects of blocking ionotropic and metabotropic glutamate receptors on the response to parallel fiber stimulation. a Pseudocol-ored ΔF/F images taken during the light phase (L, top) and dark phase (D, bottom) before (control), during, and after adding DNQX, APV, and LY367385 to the Ringer’s. Parallel fiber stimulation consisted of 100 µA, 100 µs pulses at 10 Hz for 10 s. b Example time courses of the on-beam optical response (top) and for the off-beam inhibitory bands (bottom) for control (blue), GluR antagonists (red), and washout (gold) periods. Stimulation denoted by the horizontal black line (stim). The data are from the experiment shown in a. cBar graph showing the mean amplitude of the on-beam light phase (open) and on-beam dark phase (black). On all figures, the error bars are ±1 standard deviation. d Bar graph showing the mean amplitude of the off-beam inhibitory response before, during, and after the GluR antagonists. Imaging data are from five mice and the field potential data from four mice. The asterisk denotes a significant change (p<0.05) during application of the drug compared to the control period. Details on the statistical tests and additional comparisons are in the text. For subsequent images, the same conventions and stimulation parameters are used unless otherwise noted
These results are consistent with the hypothesis that the increase in the on-beam flavoprotein fluorescence is due primarily to an increase in oxidative metabolism resulting from the activation of glutamate receptors on Purkinje cells [1, 35, 48]. However, the on-beam dark phase when isolated from the inhibitory bands is not dependent on the activation of postsynaptic glutamate receptors. As reasoned above, these findings suggest that our initial estimate of a large AMPA contribution to the dark phase likely reflects the inclusion of the on-beam inhibitory bands in the dark phase [1]. The results also demonstrate that the on-beam light and dark phases are generated by separate mechanisms.
The inhibitory bands were also completely suppressed by the combined antagonists, as shown in the example image (Fig. 2a), time courses (Fig. 2c), and population data (−0.20±0.10% to −0.01±0.02% ΔF/F, F(1,4)=11.4, p= 0.009, Fig. 2d). This confirms that the inhibitory bands are neuronal in origin [33, 34] and reflects the block of activation of the molecular layer interneurons [37, 38]. These results also show that the decrease in fluorescence of the inhibitory bands is independent from the on-beam dark phase and that the metabolic and/or cellular components that produce the reduction in flavoprotein fluorescence are different.
Finally, previous electrophysiological recordings demonstrate that ionotropic GluR antagonists (CNQX and DNQX) completely suppress the postsynaptic response and the presynaptic volley is unaffected (see also Fig. 4) [43, 48]. Therefore, the decreases in the on-beam light phase and the inhibitory bands are not due to changes in the presynaptic parallel fiber activity. With washout, there was substantial recovery of the on-beam light phase and the inhibitory bands. Although not complete, this degree of recovery is similar to that observed in previous studies and not unexpected with the use of three antagonists [1, 48].
The independence of the on-beam dark phase from the light phase is consistent with a glial cell origin, in which the activation of glutamate transporters increases glycolysis and the production of reducing equivalents [16, 22, 49]. To test this component of the first hypothesis further, the non-transportable EAAT blocker, TBOA [50], was added to the Ringer’s (Fig. 3). At the initial concentration used (300 µM), TBOA will block all EAAT subtypes, including the EAAT1 (GLAST) and EAAT2 subtypes on Bergmann glia and EAAT3 and EAAT4 subtypes on Purkinje cells [51, 52].
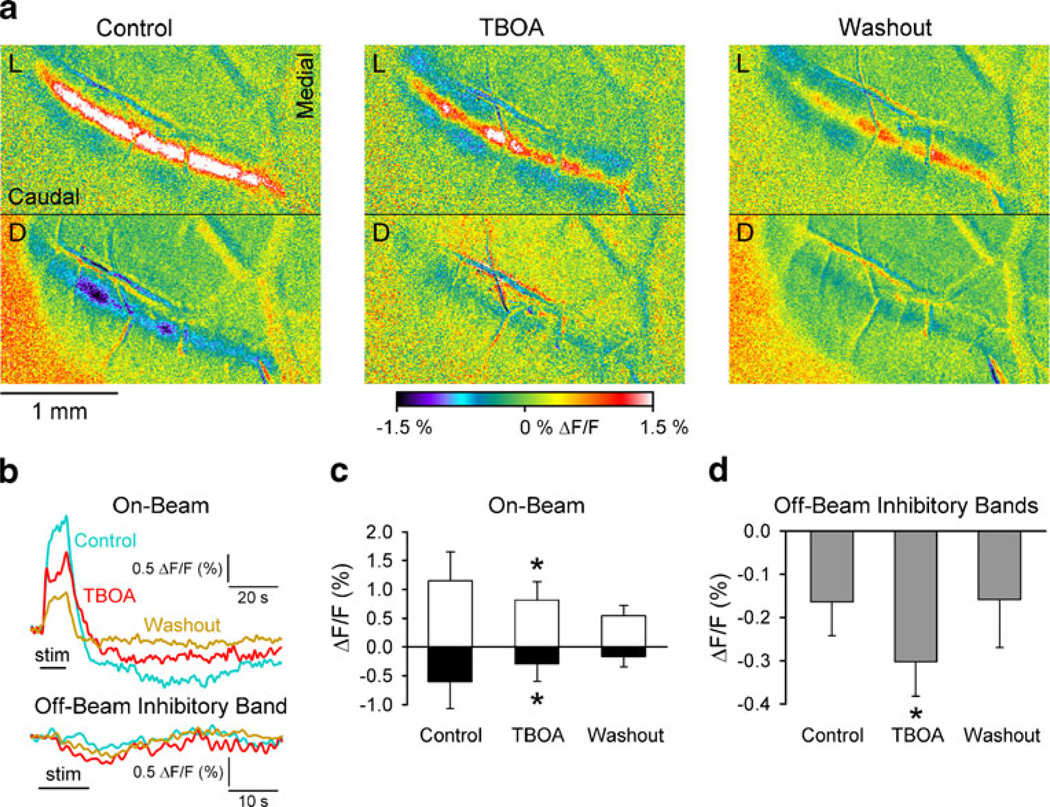
Fig. 3.
Effects of blocking EAATs on the response to parallel fiber stimulation. a Pseudocolored ΔF/F images taken during the light phase (L, top) and dark phase (D, bottom) before (control), during and after adding TBOA (300 µM) to the Ringer’s. b Example time courses of the on-beam optical responses and the inhibitory bands for control (blue), TBOA (red), and washout (gold) periods. cBar graph showing the population data for the on-beam light phase (open) and on-beam dark phase (black). d Bar graph showing the mean amplitude of the off-beam inhibitory response before, during and after the TBOA. Data are from five mice
TBOA had rather marked and complex effects on the flavoprotein responses to parallel fiber stimulation. The first and unexpected action of TBOA was to accentuate the inhibitory bands as evident from the example experiment (Fig. 3a and b). The amplitude of the inhibitory bands increased significantly by 87% (from −0.16±0.08% to −0.30±0.08% ΔF/F, F(1,4)=9.6, p=0.04, n=5, Fig. 3b). The accentuation of the inhibitory bands is likely due to TBOA-induced increase in perisynaptic glutamate that increased the activation of the inhibitory interneurons [53]. Because of the increase in molecular layer inhibition, TBOA also decreased the on-beam light phase (1.15±0.5% to 0.81±0.3% ΔF/F, F (1,4)=8.5, p=0.04). The TBOA significantly reduced the on-beam dark phase by 51% (−0.60±0.46% to −0.29± 0.3% ΔF/F, F(1,4)=12.4, p=0.02). Therefore, while the results are consistent with a component of the on-beam dark phase being generated by the activation of EAATs on glia, this conclusion needs to be tempered by the confounding increase in molecular layer inhibition and changes in the on-beam light phase in the presence of TBOA.
Therefore, we next examined the effects of TBOA after blocking the postsynaptic neuronal components with the cocktail of glutamate receptor blockers as detailed above. The goal was to block the increase in the inhibitory bands due to accumulation of the glutamate. We also increased the concentration to 1 mM TBOA to counter the increase in extracellular glutamate due to the blockade of the GluRs. In these experiments, the TBOA resulted in a 71% reduction of the dark phase (−0.73±0.38% to −0.21±0.16% ΔF/F, F (1,4)=9.9, p=0.007, n=5; Fig. 4a–c, also see Fig. 2). As expected, the glutamate receptor antagonists greatly decreased the on-beam light phase (F(1,4)=13.6, p=0.003, Fig. 4a–b). Similarly, the inhibitory bands were completely suppressed due to the GluR antagonists (data not shown, see Figs. 2 and 4a). The field potential recordings (Fig. 4d) confirm that the postsynaptic response (N2) was suppressed (8.0±10.7% of control, F(1,3)=674.7, p=0.0001, n=4). The presynaptic volley (P1/N1) increased slightly but the change was not significant (127.8±24.9% of control, F (1,3)=7.2,p=0.075), demonstrating that the change in the on-beam dark phase cannot be due to a decrease in the parallel fiber volley. The results also support the hypothesis that the dark phase is generated, at least in part, through activation of EAATs by glutamate released from parallel fiber stimulation.
Manipulation of Metabolic Substrates
The next experiment replaced glucose in the bath solution with lactate, with the goals of maintaining an energy substrate for neurons while suppressing glycolysis, a primary energy source in glia [54–57]. The increased extracellular lactate is expected to be transported into glia and neurons by MCTs following the concentration gradient [18, 19]. The lactate transported into neurons will maintain neuronal metabolic demands by fueling the TCA cycle and therefore, the hypothesis predicts that the on-beam light phase should remain intact. In the glia, the increase in lactate will suppress the conversion of pyruvate to lactate and the transport of lactate from the glia to neurons. The increase in lactate will also suppress glycolysis, causing a decrease in NAD+ regeneration that further prevents anaerobic glycolysis [58]. The suppression of glycolysis should be accentuated in astrocytes, as the peripheral processes of these cells predominantly rely on glycolysis for cellular energy (see “Discussion”). If our hypothesis is correct, the on-beam dark phase should be eliminated.
Replacing glucose with 10 mM lactate in the bath Ringer’s resulted in complete loss of the on-beam dark phase with little effect on the light phase (Fig. 5a and b). This effect is evident in the example images, time courses, and the population data. For the group averages, the dark phase of the autofluorescence signal was reduced from −0.55±0.19% ΔF/F to −0.01 ±0.06% ΔF/F after substitution of the lactate for glucose (F(1,5)=53.8, p<0.0003, n = 6). Washout resulted in complete recovery of the dark phase. There was no significant effect on the light phase 0.83±0.33% ΔF/F before lactate and 0.94±0.19% ΔF/F after lactate replacement, (F(1,5)=0.5, p=0.83). Replacing glucose with lactate reduced the inhibitory band response by 40% from −0.28± 0.09% ΔF/F to −0.17±0.05% ΔF/F (F(1,5)= 16.0, p=0.007, Fig. 5c). Lactate substitution did not alter either the parallel fiber volley (P1/N1: 103.6±8.7% of control, F (1,3)= 1.1,p= 0.37, n=4) or the postsynaptic response (N2: 111.2±15.0%, F(1,3)=2.6, p=0.21, Fig. 5e), demonstrating that the reductions in the dark phase and inhibitory bands were not due to alterations in cerebellar cortical excitability. As previously reported [6], lactate substitution reduced the background fluorescence relative to the control (93.2±7.4% of baseline, F(1,5)=23.9,p<0.0002, n=6, Fig. 5d), consistent with an overall reduction in flavoprotein oxidation.
Fig. 5.
Effects of replacing glucose with lactate in the bath Ringer’s solution. a Pseudocolored ΔF/F images taken during the light phase (L, top) and dark phase (D, bottom) before (control), during and after replacement of glucose with 10 mM lactate. b On the top are example time courses of the on-beam optical signal for control (blue), lactate (red), and washout (gold) periods. Bottom bar graphs are the population data for the on-beam light phase (open) and on-beam dark phase (black). c Top traces are example time courses of the off-beam inhibitory bands and bottom is the population data. d Background autofluorescence signal during the control period before, during and after lactate substitution. e Top traces are examples of the field potentials evoked by parallel fiber stimulation and bottom histogram are the amplitudes (mean±SD) of the P1/N1 and N2 components before (blue), during (red) and after lactate (gold) replacement. Imaging data are from 6 mice and the field potential data from 4 mice
These results support the hypothesis that the on-beam light phase can be supported by lactate alone, and is primarily generated by neuronal oxidative metabolism. Next, we investigated the effect of adding lactate (lactate supplementation) without removing the glucose from the bathing solution, testing the effects of 1, 2, 5, and 10 mM lactate supplementation (Fig. 6). The estimated EC50 of 114 mM confirms that the light phase is relatively insensitive to lactate levels as observed in the replacement experiment (Fig. 6a). Conversely, the on-beam dark phase was markedly reduced by increasing the extracellular lactate (6.3±11.1% of baseline in 10 mM lactate, F(1,3)= 22.8, p=0.0002, n=4, Fig. 6b). This high sensitivity to lactate is reflected in the 0.34 mM estimate of the EC50. The inhibitory bands were also significantly reduced by lactate addition to the bath media (51.2±16.8% of the control at 10 mM lactate, F(1,3)=11.0, p=0.0023). The EC50 of 9.34 mM for the inhibitory bands was more than an order of magnitude greater than that of the dark phase, demonstrating a lower sensitivity and providing additional evidence that the decreased fluorescence of the inhibitory band signal is generated by a different mechanism than the on-beam dark phase.
Fig. 6.
Effects of lactate supplementation and dose–response curves. a–c Dose response curves of the normalized amplitude of the flavoprotein signal and the corresponding Hill plots for the on-beam light phase (a), on-beam dark phase (b) and the off-beam inhibitory bands (c)
In addition to lactate substitution, a similar experiment tested pyruvate substitution. The prediction was that, similar to lactate substitution, pyruvate substitution for glucose would reduce the lactate shuttled to neurons and suppress glycolysis but not block oxidative metabolism. As shown in Fig. 7a and b, the on-beam light phase was not changed (0.89±0.48% versus 0.81±0.40% ΔF/F, F(1,2)= 0.9, p=0.46, n=3). The dark phase was greatly reduced (−0.50±0.15% versus −0.05±0.15% ΔF/F, F(1,2)=49.5, p=0.02). In contrast to lactate substitution, the pyruvate substitution resulted in nearly complete suppression of the inhibitory bands (−0.19±0.02% before versus −0.00± 0.069% ΔF/F after pyruvate (F(1,2)=7.3, p=0.04, n=3). This may reflect that the inhibitory bands have a glycolytic component, as the increased pyruvate would be expected to suppress neuronal glycolysis. The time course of the flavoprotein signal was essentially similar to control with an intact initial increase in fluorescence, with a greatly diminished dark phase (data not shown). Background intensity was reduced to 91.6±1.9% of baseline (F(1,2)= 37.9, p<.0025, n=3), similar to lactate substitution (Fig. 7c). There were no changes in the presynaptic (103.1±14.1% of baseline, F (1,3)=0.1, p=0.74, n=4) or postsynaptic (100.1±16.9% of control, F (1,3)=0.4, p=0.60) components of the response to parallel fiber stimulation (Fig. 7d).
Fig. 7.
Effects of replacing glucose with pyruvate in the Ringer’s solution. a Population data of the on-beam light phase (open) and onbeam dark phase (black) before, during and after replacement of glucose with 10 mM pyruvate. b Population data of the amplitude of the off-beam inhibitory bands. c Background autofluorescence signal in relation to pyruvate substitution. d Top traces are examples of the field potentials evoked by PF stimulation during the control (blue), pyruvate (red), and washout periods (gold). The bottom histogram is the population P1/N1 and N2 responses. Imaging data is from three mice and field potential data from four mice
Brain tissue is supplied with nutrients by both the blood and cerebrospinal fluid, so one can question whether manipulation of glucose levels by changing the concentration in the bathing solution in the optical chamber resulted in the desired change in metabolic substrates. Specifically, does the removal of the glucose in the chamber deprive the folium of this key metabolic substrate? Therefore, we tested the effects of 0 mM glucose on the optical responses compared to the 10 mM in the control Ringer’s solution in six additional animals. The 0 mM glucose produced approximately a 50% decrease in both the light phase (from 1.4±0.18% to 0.63±0.10% ΔF/F, F(1,5)=19.1, p=0.007, n=6) and dark phase (from −0.61±0.42% to−0.33±0.38% ΔF/F, F(1,5)= 12.4, p=0.02). There was no significant change in the amplitude of the inhibitory bands (F(1,5)=0.06, p=0.8). Therefore, removing the glucose in the chamber bathing solution produced a large reduction in the optical response, demonstrating the effectiveness of experiments in which glucose was removed and replaced with either lactate or pyruvate.
Blocking Monocarboxylate Transporters
A final test of the origins of the autofluorescence signal was based on blocking the transport of lactate into glia and neurons. Lactate transporters in both neurons and glia are blocked by 500 µM 4-CIN [59, 60]. The prediction is that blocking the transport of lactate into neurons would accentuate the light phase and suppress the dark phase. This prediction reflects that the lactate produced will be trapped in the glia suppressing both glycolysis and the dark phase. The loss of lactate transport into neurons will decrease the available fuel for the TCA cycle and prevent reduction of flavoproteins in neurons resulting in an increase in the light phase. Whether the inhibitory bands are altered will depend on whether the decreased fluorescence is due to an increase in glycolysis and/or decreased oxidative metabolism.
The application of 500 µM 4-CIN (Fig. 8) converted the on-beam dark phase into a light phase (−0.34±0.15% versus 0.80±0.25% ΔF/F, F(1,5)=39.9, p=0.0001). There was also an increase in the on-beam light phase (0.54±0.21% versus 0.78±0.37% ΔF/F, F(1,4)=7.1, p=0.02, n=5). The amplitude of the inhibitory bands increased from −0.20±0.06% ΔF/F to −0.31±0.08% ΔF/F (Fig. 8c, F(1,4)=5.0, p=0.04). The changes in the optical response due to 4-CIN were not due to alterations in the excitability as there were no changes in the presynaptic (99.7±7.5% of baseline, F(1,3)=0.02, p= 0.90) or postsynaptic (99.9±11.7% of baseline, F(1,3)=0.50, p=0.57) response to parallel fiber stimulation. It should be noted that there was a graded increase in the background fluorescence during the approximately 15 min application of the 4-CIN; however, this was not accompanied by any changes in excitability. In contrast, longer applications (>20 min) did lead to further increases in background fluorescence and loss of the flavoprotein signals. This likely reflects the loss of metabolic substrates, specifically the lack of an adequate lactate supply.
Fig. 8.
Effect of α-cyano-4-hydroxycinnamate (4-CIN) on the autofluorescence signal. a Pseudocolored ΔF/F images taken during the light phase (L, top) and dark phase (D, bottom) before (control), during and after application of 500 µM 4-CIN. b On the top are example time courses of the on-beam optical signal for control (blue), 4-CIN (red) and washout (gold) periods. Bottom bar graphs are the population data for the light (open bar) and dark (filled bar) phases. cTop traces are example time courses of the off-beam inhibitory bands and the bottom bar graph the population data for the off-beam inhibitory bands
Discussion
On-Beam Light Phase
Consistent with the hypothesis that the on-beam light phase is due primarily to oxidative metabolism in neurons, blocking ionotropic and metabotropic glutamate receptors suppressed the light phase without effect on the dark phase. The findings agree with the results from NADH fluorescence in the hippocampal slice in which the initial phase of the response to excitatory glutamatergic synaptic transmission is primarily due to oxidative metabolism in neurons [6, 22]. The time courses of the flavoprotein signals in vivo are similar to those obtained for NADH and flavoprotein imaging in vitro [4, 6, 10, 22, 61]. This supports the concept that the activation of oxidative metabolism in neurons precedes the activation of glial cells and glial glycolysis [22, 62]. The present results extend and clarify our early findings [1] by excluding the contributions of the inhibitory bands from the on-beam signal and by achieving a more complete block of neuronal activation using a cocktail of GluR antagonists.
The lactate substitution and supplementation results further support this hypothesis, as the on-beam light phase was unchanged and the dark phase was suppressed. The removal of glucose from the Ringer’s in the substitution experiment reduces the substrate availability for glycolysis in both neurons and astrocytes. Furthermore, in both the replacement and supplementation experiments, the increased lactate in the bath will inhibit glycolysis while providing lactate for use in the TCA cycle [63]. Therefore, these findings demonstrate that the vast majority of the onbeam light phase is due to oxidative metabolism and that neurons can sustain oxidative metabolism using lactate, which is in agreement with the ANLS hypothesis [16, 24, 56, 64–67]. The pyruvate substitution results have a similar interpretation.
Blocking MCTs with 4-CIN enhanced and prolonged the on-beam light phase and abolished the dark phase. These findings are likely due to 4-CIN decreasing the lactate transported into neurons and, therefore, the availability of pyruvate for the TCA cycle. This will interrupt the normal reduction of flavoproteins that occurs following their oxidation produced by neuronal activity. However, one caveat regarding the 4-CIN result is that it can decrease pyruvate entry into mitochondria [18], which would also result in augmentation of the light phase and suppression of the dark phase. We interpret the enhanced light phase as the failure to generate reducing equivalents in neurons. As noted in the “Results”, a longer duration exposure to 4-CIN leads to a loss of the flavoprotein signal. Although not thoroughly investigated, this finding is also consistent with the ANLS hypothesis. With the prolonged 4-CIN exposure, eventually, the available metabolic substrates in neurons are exhausted and without lactate there is a loss of neuronal function.
An important issue is whether Bergmann glia, the specialized astrocytes of the molecular layer, contribute to the light phase. Bergmann glia have AMPA/kainate [68], NMDA [69, 70] and mGluR receptors [71], and respond to parallel fiber stimulation with inward currents and increases in intracellular Ca2+ and Na+ [72, 73]. However, the inward currents and Na+ influx in Bergmann glia resulting from glutamate released by parallel fiber burst stimulation is caused by activation of the glutamate/Na+ transporter and is not due to activation of AMPA/kainate or NMDA receptors [73, 74]. Furthermore, NMDA receptor antagonists do not alter the light phase [48] and mGluRs could only provide a small contribution as 10–15% of the light phase is due to mGluR activation [48]. The Ca2+ influx is limited to microdomains and mediated by nitric oxide [72, 75]. Therefore, the on-beam light phase is primarily due to oxidative metabolism driven by the postsynaptic activation of neurons.
On-Beam Dark Phase
The previous and present results support the hypothesis that the on-beam dark phase is due in part to activation of EAATs on glia. The longer latency and duration of the dark phase are consistent with the time course of glial activation in response to glutamatergic stimulation [20, 22, 62]. Also, the longer latency for the dark phase is consistent with the observed delay in the increase in lactate concentration that occurs with neuronal activation [57, 76–78]. Fluoroacetate, a glia-specific metabolic toxin, suppresses the on-beam dark phase [35]. Blocking EAATs greatly suppressed the dark phase, consistent with the ANLS hypothesis that the glutamate released from parallel fibers stimulates glycolysis in astrocytes via EAATs that activate plasma membrane Na+/K+ ATPase [16, 49]. As discussed above, lactate/ pyruvate substitution or lactate supplementation will suppress glial glycolysis and production of lactate. All three manipulations eliminated the dark phase. Furthermore, 4-CIN abolished the on-beam dark phase, consistent with a build-up of lactate in astrocytes leading to a suppression of glycolysis and the production of reducing equivalents.
The TBOA results also suggest that activation of EAATs cannot be the sole mechanism responsible for the dark phase. At 300 µM and 1 mM, TBOA reduced the dark phase by 46% and 71%, respectively. Therefore, even at the highest concentration, the on-beam dark phase was not completely blocked. One possible contributor to the dark phase is the increase in extracellular K+ that occurs with parallel fiber stimulation. An increase in extracellular K+ would lead to production of reducing equivalents in neurons because extracellular K+ stimulates glycolysis in neurons and astrocytes [20, 79, 80]. It has been well established that repetitive parallel fiber stimulation results in marked increases in the extracellular K+ [81, 82]; for example, 5 Hz stimulation for 30 s raises the extracellular K+ concentration by 5 mM [81]. Another possible contribution is increased blood flow. In the cerebral cortex of the alert monkey, a large fraction of the dark phase is due to an increase in blood flow [42]. However, in the anesthetized animal, increased blood flow contributes only a small fraction of the flavoprotein signals in the cerebellar cortex [1]. Still, one cannot rule out a small hemodynamic contribution to the dark phase.
The results do not resolve whether the dark phase signal is due to the reduction of flavoproteins in glia via increased glial glycolysis or flavoproteins in neurons via glial-generated lactate that is shuttled to neurons as postulated by the ANLS. However, we propose that the major cellular source of the flavoprotein signal is neuronal [6]. In glia, oxidative metabolism is confined to the soma and large processes that harbor mitochondria [83, 84]. In contrast, glial peripheral astrocytic processes (PAPs) are too small to accommodate mitochondria and therefore, rely on anaerobic glycolysis [85, 86]. Bergmann glia PAPs cover Purkinje cell dendrites and spines [84, 87]. These appendages are small and contain few mitochondria [87]. In contrast, even the smallest dendritic branches of Purkinje cells have numerous mitochondria [88, 89]. Therefore, the flavoprotein signal likely originates predominantly in the dendrites of Purkinje cells and interneurons and not in PAPs of the Bergmann glia.
Inhibitory Bands
The decrease in fluorescence of the inhibitory bands was hypothesized to be due to decreased oxidation of flavoproteins and increased glycolysis in neurons [33, 35]. Therefore, the fluorescence decrease is postulated to be generated by different mechanisms than the decrease characterizing the dark phase. The inhibitory bands primarily originate in neurons as GluR antagonists (Fig. 2) and GABAA antagonists eliminate the inhibitory bands [33, 34] and fluoroacetate has no effect on the inhibitory bands [35]. Importantly, GABA uptake does not induce a metabolic response in astrocytes [41]. The hyperpolarization and decreased firing of Purkinje cells and molecular layer interneurons [33, 90] will result in the transient generation of pyruvate and other reducing equivalents, as well as a decrease in oxidative metabolism in already active cells, leading to a net reduction of mitochondrial flavoproteins.
While the results strongly suggest that a reduction in mitochondrial metabolism contributes to the inhibitory bands, the role of glycolysis is less clear. Both lactate and pyruvate substitution, which will reduce glycolysis, resulted in a decrease in the inhibitory bands without a change in excitability. The inhibitory bands were also diminished when lactate and glucose were supplied together (Fig. 6). However, lactate addition was expected to primarily interfere with glial glycolysis. In agreement, the dark phase was completely blocked and demonstrated a much higher degree of sensitivity to lactate addition than the inhibitory band signal. This limited sensitivity is also consistent with a glycolytic component in the inhibitory band signal. The accentuation of the inhibitory band signal on blocking MCTs is also consistent with a glycolytic component because depriving neurons of a major substrate for oxidative metabolism could enhance glycolysis as cytoplasmic NADH and pyruvate are depleted. Arguing against this view is that removing glucose from the Ringer’s did not alter the inhibitory bands. Additional experiments are needed to determine the role of glycolysis in the decreased fluorescence of the inhibitory bands.
Any interpretation of the changes in the inhibitory band signal in the experiments manipulating the lactate or pyruvate concentrations needs to take into account the decrease in the background fluorescence (i.e., redox state) [6]. The amplitude of the inhibitory bands is small and the decrease in the redox state may interfere with detecting small decreases in fluorescence. However, two observations demonstrate that this redox shift is not a major factor. First, the change in background fluorescence with lactate or pyruvate substitution did not affect the onbeam light phase. One would predict that the light phase should have increased if the change in background played a major role. Second, the larger amplitude on-beam dark phase was abolished with the lactate substitution but the smaller amplitude inhibitory bands were reduced by only 40%. Here, one would predict that the smaller inhibitory band signals would be more easily masked. These findings are not consistent with the interpretation that the fluorescence changes are due to a shift in the baseline redox state.
Summary
Neurometabolic coupling is central to brain energy metabolism and to understanding the bases of functional imaging techniques that exploit the coupling between neuronal activity, metabolism and/or blood flow [29–31]. This study was done in vivo as there is a widely acknowledged need to understand neurometabolic coupling in the intact animal [29–31]. Overall, the present findings are in agreement with the ANLS hypothesis [16]. This study shows that flavoprotein autofluorescence imaging provides a useful tool for investigating neuroenergetics, particularly in vivo, as well as mapping spatial patterns of neuronal activity [5, 33, 48, 61, 91, 92].
Acknowledgments
We wish to thank Lijuan Zhou for animal preparation, Michael McPhee for graphics, Claudia Hendrix for statistical advice, and Kathy Easthagen and Kris Bettin for manuscript preparation. This work was supported in part by NIH grant R01-NS048944 and a grant from the Bob Allison Ataxia Research Center.
Footnotes
Conflicts of Interest There are no current or potential conflicts of interest for the six authors, Drs. Kenneth Reinert, Wangcai Gao, Gang Chen, Xinming Wang, Yu-Ping Peng, and Timothy Ebner.
Contributor Information
Kenneth C. Reinert, Department of Neurobiology, University of Pittsburgh, Pittsburgh, PA 15261, USA
Wangcai Gao, Department of Neuroscience, University of Minnesota, Lions Research Building, Room 421, 2001 Sixth St. S.E., Minneapolis, MN 55455, USA.
Gang Chen, Department of Neuroscience, University of Minnesota, Lions Research Building, Room 421, 2001 Sixth St. S.E., Minneapolis, MN 55455, USA.
Xinming Wang, Department of Neuroscience, University of Minnesota, Lions Research Building, Room 421, 2001 Sixth St. S.E., Minneapolis, MN 55455, USA.
Yu-Ping Peng, Nantong University, Nantong, Jiangsu 226001, People’s Republic of China.
Timothy J. Ebner, Department of Neuroscience, University of Minnesota, Lions Research Building, Room 421, 2001 Sixth St. S.E., Minneapolis, MN 55455, USA, ebner001@umn.edu
References
- 1.Reinert KC, Dunbar RL, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging of neuronal activation in the cerebellar cortex in vivo . J Neurophysiol. 2004;92:199–211. doi: 10.1152/jn.01275.2003. [DOI] [PubMed] [Google Scholar]
- 2.Coutinho V, Mutoh H, Knopfel T. Functional topology of the mossy fibre-granule cell-Purkinje cell system revealed by imaging of intrinsic fluorescence in mouse cerebellum. Eur J Neurosci. 2004;20:740–748. doi: 10.1111/j.1460-9568.2004.03533.x. [DOI] [PubMed] [Google Scholar]
- 3.Shibuki K, Hishida R, Murakami H, Kudoh M, Kawaguchi T, Watanabe M, et al. Dynamic imaging of somatosensory cortical activity in the rat visualized by flavoprotein autofluorescence. J Physiol. 2003;549:919–927. doi: 10.1113/jphysiol.2003.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Husson TR, Mallik AK, Zhang JX, Issa NP. Functional imaging of primary visual cortex using flavoprotein autofluorescence. J Neurosci. 2007;27:8665–8675. doi: 10.1523/JNEUROSCI.2156-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- 7.Scholz R, Thurman RG, Williamson JR, Chance B, Bucher T. Flavin pyridine nucleotide oxidation-reduction changes in perfused rat liver I Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969;244:2317–2324. [PubMed] [Google Scholar]
- 8.Koke JR, Wylie W, Wills M. Sensitivity of flavoprotein fluorescence to oxidative state in single isolated heart cells. Cytobios. 1981;32:139–145. [PubMed] [Google Scholar]
- 9.Voltti H, Hassinen IE. Oxidation-reduction midpoint potentials of mitochondrial flavoproteins and their intramitochondrial localization. J Bioenerg Biomembr. 1978;10:45–58. doi: 10.1007/BF00743226. [DOI] [PubMed] [Google Scholar]
- 10.Duchen MR. Ca2+-dependent changes in the mitochondrial energetics in single dissociated mouse sensory neurons. Biochem J. 1992;283(Pt 1):41–50. doi: 10.1042/bj2830041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chance B. Optical method. Annu Rev Biophys Biophys Chem. 1991;20:1–28. doi: 10.1146/annurev.bb.20.060191.000245. [DOI] [PubMed] [Google Scholar]
- 12.Kunz WS, Kunz W. Contribution of different enzymes to flavoprotein fluorescence of isolated rat liver mitochondria. Biochim Biophys Acta. 1985;841:237–246. doi: 10.1016/0304-4165(85)90064-9. [DOI] [PubMed] [Google Scholar]
- 13.Hall CL, Kamin H. The purification and some properties of electron transfer flavoprotein and general fatty acyl coenzyme A dehydrogenase from pig liver mitochondria. J Biol Chem. 1975;250:3476–3486. [PubMed] [Google Scholar]
- 14.Hassinen I, Chance B. Oxidation-reduction properties of the mitochondrial flavoprotein chain. Biochem Biophys Res Commun. 1968;31:895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- 15.McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis—a mechanism coupling neuronal-activity to glucose-utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierre K, Pellerin L. Monocarboxylate transporters in the central nervous system: distribution, regulation and function. J Neurochem. 2005;94:1–14. doi: 10.1111/j.1471-4159.2005.03168.x. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- 19.Juel C, Grunnet L, Holse M, Kenworthy S, Sommer V, Wulff T. Reversibility of exercise-induced translocation of Na+-K+ pump subunits to the plasma membrane in rat skeletal muscle. Pflugers Arch. 2001;443:212–217. doi: 10.1007/s004240100674. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Driscoll BF, Law MJ, Sokoloff L. Role of sodium and potassium-ions in regulation of glucose-metabolism in cultured astroglia. Proc Natl Acad Sci USA. 1995;92:4616–4620. doi: 10.1073/pnas.92.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. J Cereb Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- 22.Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- 23.Magistretti PJ. Cellular bases of functional brain imaging: insights from neuron-glia metabolic coupling. Brain Res. 2000;886:108–112. doi: 10.1016/s0006-8993(00)02945-0. [DOI] [PubMed] [Google Scholar]
- 24.Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 25.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- 26.Hertz L. The astrocyte-neuron lactate shuttle: a challenge of a challenge. J Cereb Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. J Cereb Blood Flow Metab. 2003;23:1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. [DOI] [PubMed] [Google Scholar]
- 28.Mangia S, Giove F, Bianciardi M, Di Salle F, Garreffa G, Maraviglia B. Issues concerning the construction of a metabolic model for neuronal activation. J Neurosci Res. 2003;71:463–467. doi: 10.1002/jnr.10531. [DOI] [PubMed] [Google Scholar]
- 29.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 32.Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer; 1967. [Google Scholar]
- 33.Gao W, Chen G, Reinert KC, Ebner TJ. Cerebellar cortical molecular layer inhibition is organized in parasagittal zones. J Neurosci. 2006;26:8377–8387. doi: 10.1523/JNEUROSCI.2434-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, et al. Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet. 2006;38:758–769. doi: 10.1038/ng1827. [DOI] [PubMed] [Google Scholar]
- 35.Reinert KC, Gao W, Chen G, Ebner TJ. Flavoprotein autofluorescence imaging in the cerebellar cortex in vivo . J Neurosci Res. 2007;85:3221–3232. doi: 10.1002/jnr.21348. [DOI] [PubMed] [Google Scholar]
- 36.Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- 37.Grandes P, Mateos JM, Ruegg D, Kuhn R, Knopfel T. Differential cellular localization of three splice variants of the mGluR1 metabotropic glutamate receptor in rat cerebellum. Neuroreport. 1994;5:2249–2252. doi: 10.1097/00001756-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Mateos JM, Benitez R, Elezgarai I, Azkue JJ, Lazaro E, Osorio A, et al. Immunolocalization of the mGluR1b splice variant of the metabotropic glutamate receptor 1 at parallel fiber-Purkinje cell synapses in the rat cerebellar cortex. J Neurochem. 2000;74:1301–1309. doi: 10.1046/j.1471-4159.2000.741301.x. [DOI] [PubMed] [Google Scholar]
- 39.Ackermann RF, Finch DM, Babb TL, Engel J., Jr Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nudo RJ, Masterton RB. Stimulation-induced [14C]2-deoxyglucose labeling of synaptic activity in the central auditory system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- 41.Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sirotin YB, Das A. Spatial relationship between flavoprotein fluorescence and the hemodynamic response in the primary visual cortex of alert macaque monkeys. Front Neuroenergetics. 2010;2:6. doi: 10.3389/fnene.2010.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G, Popa LS, Wang X, Gao W, Barnes J, Hendrix CM, et al. Low frequency oscillations in the cerebellar cortex of the tottering mouse. J Neurophysiol. 2009;101:234–245. doi: 10.1152/jn.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton CN, Braunberg RC, Friedman L. Nonlinear statistical models for the joint action of toxins. Biometrics. 1993;49:95–105. [PubMed] [Google Scholar]
- 45.Knopfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. Cerebellum. 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- 46.Finch EA, Augustine GJ. Local calcium signaling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396:753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 47.Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Chen G, Gao W, Ebner TJ. Long-term potentiation of the responses to parallel fiber stimulation in mouse cerebellar cortex in vivo. Neurosci. 2009;162:713–722. doi: 10.1016/j.neuroscience.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, et al. DL-Threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 51.Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 53.Carter AG, Regehr WG. Prolonged synaptic currents and glutamate spillover at the parallel fiber to stellate cell synapse. J Neurosci. 2000;20:4423–4434. doi: 10.1523/JNEUROSCI.20-12-04423.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schurr A, Payne RS, Miller JJ, Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997;774:221–224. doi: 10.1016/s0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka K, Nisimaru N, Yanai S, Shimoda H, Yamada K. Characteristics of monocarboxylates as energy substrates other than glucose in rat brain slices and the effect of selective glial poisoning - a P-31 NMR study. Neurosci Res. 2000;36:215–226. doi: 10.1016/s0168-0102(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 56.Schurr A, Miller JJ, Payne RS, Rigor BM. An increase in lactate output by brain tissue serves to meet the energy needs of glutamate-activated neurons. J Neurosci. 1999;19:34–39. doi: 10.1523/JNEUROSCI.19-01-00034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- 58.Berg J, Tymoczko J, Stryer L. Maintaining redox balance: the diverse fate of pyruvate. In: Berg JM, editor. Biochemistry. New York: Freeman; 2002. [Google Scholar]
- 59.Cater HL, Benham CD, Sundstrom LE. Neuroprotective role of monocarboxylate transport during glucose deprivation in slice cultures of rat hippocampus. J Physiol. 2001;531:459–466. doi: 10.1111/j.1469-7793.2001.0459i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Matsumura K, Murata T, et al. Role of lactate in the brain energy metabolism: revealed by bioradiography. Neurosci Res. 2004;48:13–20. doi: 10.1016/j.neures.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Llano DA, Theyel BB, Mallik AK, Sherman SM, Issa NP. Rapid and sensitive mapping of long-range connections in vitro using flavoprotein autofluorescence imaging combined with laser photostimulation. J Neurophysiol. 2009;101:3325–3340. doi: 10.1152/jn.91291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 2008;320:1638–1643. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 63.Ramirez BG, Rodrigues TB, Violante IR, Cruz F, Fonseca LL, Ballesteros P, et al. Kinetic properties of the redox switch/redox coupling mechanism as determined in primary cultures of cortical neurons and astrocytes from rat brain. J Neurosci Res. 2007;85:3244–3253. doi: 10.1002/jnr.21386. [DOI] [PubMed] [Google Scholar]
- 64.Zielke HR, Zielke CL, Baab PJ. Oxidation of (14)C-labeled compounds perfused by microdialysis in the brains of free-moving rats. J Neurosci Res. 2007;85:3145–3149. doi: 10.1002/jnr.21424. [DOI] [PubMed] [Google Scholar]
- 65.Waagepetersen HS, Bakken IJ, Larsson OM, Sonnewald U, Schousboe A. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev Neurosci. 1998;20:310–320. doi: 10.1159/000017326. [DOI] [PubMed] [Google Scholar]
- 66.Schurr A, Payne RS. Lactate, not pyruvate, is neuronal aerobic glycolysis end product: an in vitro electrophysiological study. Neuroscience. 2007;147:613–619. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24:1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 68.Muller T, Moller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- 69.Muller T, Grosche J, Ohlemeyer C, Kettenmann H. NMDAactivated currents in Bergmann glial cells. Neuroreport. 1993;4:671–674. doi: 10.1097/00001756-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 70.Luque JM, Richards JG. Expression of NMDA 2B receptor subunit mRNA in Bergmann glia. Glia. 1995;13:228–232. doi: 10.1002/glia.440130309. [DOI] [PubMed] [Google Scholar]
- 71.Aguilera P, Ortega A. Stat3 participates in the metabotropic glutamate signaling pathway in Bergmann glial cells. Neurochem Res. 1999;24:981–986. doi: 10.1023/a:1021044424103. [DOI] [PubMed] [Google Scholar]
- 72.Grosche J, Matyash V, Moller T, Verkhratsky A, Reichenbach A, Kettenmann H. Microdomains for neuron-glia interaction: parallel fiber signaling to Bergmann glial cells. Nat Neurosci. 1999;2:139–143. doi: 10.1038/5692. [DOI] [PubMed] [Google Scholar]
- 73.Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch. 2007;454:245–252. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- 74.Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia. 2008;56:1138–1149. doi: 10.1002/glia.20685. [DOI] [PubMed] [Google Scholar]
- 75.Matyash V, Filippov V, Mohrhagen K, Kettenmann H. Nitric oxide signals parallel fiber activity to Bergmann glial cells in the mouse cerebellar slice. Mol Cell Neurosci. 2001;18:664–670. doi: 10.1006/mcne.2001.1047. [DOI] [PubMed] [Google Scholar]
- 76.Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, et al. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mangia S, Tkac I, Gruetter R, van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–1063. doi: 10.1038/sj.jcbfm.9600401. [DOI] [PubMed] [Google Scholar]
- 78.Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, et al. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abe T, Takahashi S, Suzuki N. Oxidative metabolism in cultured rat astroglia: effects of reducing the glucose concentration in the culture medium and of D-aspartate or potassium stimulation. J Cereb Blood Flow Metab. 2006;26:153–160. doi: 10.1038/sj.jcbfm.9600175. [DOI] [PubMed] [Google Scholar]
- 80.Peng L, Juurlink BH, Hertz L. Pharmacological and developmental evidence that the potassium-induced stimulation of deoxyglu-cose uptake in astrocytes is a metabolic manifestation of increased Na(+)-K(+)-ATPase activity. Dev Neurosci. 1996;18:353–359. doi: 10.1159/000111428. [DOI] [PubMed] [Google Scholar]
- 81.Kocsis JD, Malenka RC, Waxman SG. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol. 1983;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nicholson C, Bruggencate GT, Stockle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- 83.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- 84.Wolff J, Chao T. Cytoarchitectonics of non-neuronal cells in the central nervous system. In: Hertz L, editor. Non-neuronal Cells of the Nervous System: Function and Dysfunction. Elsevier; 2004. [Google Scholar]
- 85.Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–229. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 86.Derouiche A, Frotscher M. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 2001;36:330–341. doi: 10.1002/glia.1120. [DOI] [PubMed] [Google Scholar]
- 87.Grosche J, Kettenmann H, Reichenbach A. Bergmann glial cells form distinct morphological structures to interact with cerebellar neurons. J Neurosci Res. 2002;68:138–149. doi: 10.1002/jnr.10197. [DOI] [PubMed] [Google Scholar]
- 88.Palay SL. Structural basis for neural action. In: Brazier M, editor. Brain function. Los Angeles: University of California Press; 1964. pp. 59–107. [Google Scholar]
- 89.Bertoni-Freddari C, Fattoretti P, Casoli T, Di Stefano G, Solazzi M, Meier-Ruge W. Quantitative cytochemical mapping of mitochondrial enzymes in rat cerebella. Micron. 2001;32:405–410. doi: 10.1016/s0968-4328(00)00015-9. [DOI] [PubMed] [Google Scholar]
- 90.Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 91.Tohmi M, Kitaura H, Komagata S, Kudoh M, Shibuki K. Enduring critical period plasticity visualized by transcranial flavoprotein imaging in mouse primary visual cortex. J Neurosci. 2006;26:11775–11785. doi: 10.1523/JNEUROSCI.1643-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jongen JL, Pederzani T, Koekkoek SK, Shapiro J, Van der BJ, De Zeeuw CI, et al. Autofluorescent flavoprotein imaging of spinal nociceptive activity. J Neurosci. 2010;30:4081–4087. doi: 10.1523/JNEUROSCI.0011-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]