Abstract
Objectives
To investigate if there is a relation between the increase of bismuth oxide and the decrease of pH levels and an intensification of toxicity in the Portland cement.
Material and Methods
White Portland cement (WPC) was mixed with 0, 15, 20, 30 and 50% bismuth oxide, in weight. For the pH level test, polyethylene tubes were filled with the cements and immersed in Milli-Q water for 15, 30 and 60 days. After each period, the increase of the pH level was assessed. For the biocompatibility, two polyethylene tubes filled with the cements were implanted in ninety albino rats (n=6). The analysis of the intensity of the inflammatory infiltrate was performed after 15, 30 and 60 days. The statistical analysis was performed using the Kruskal-Wallis, Dunn and Friedman tests for the pH level and the Kruskal-Wallis and Dunn tests for the biological analysis (p<0.05).
Results
The results showed an increase of the pH level after 15 days, followed by a slight increase after 30 days and a decrease after 60 days. There were no significant statistical differences among the groups (p>0.05). For the inflammatory infiltrates, no significant statistical differences were found among the groups in each period (p>0.05). The 15% WPC showed a significant decrease of the inflammatory infiltrate from 15 to 30 and 60 days (p<0.05).
Conclusions
The addition of bismuth oxide into Portland cement did not affect the pH level and the biological response. The concentration of 15% of bismuth oxide resulted in significant reduction in inflammatory response in comparison with the other concentrations evaluated.
Keywords: Endodontics, Dental materials, Bismuth, Materials testing
INTRODUCTION
Mineral Trioxide Aggregate (MTA) is widely used in endodontics as a root-end filling material18,28,29 and has been suggested for vital pulp therapies, perforations, apexifications and numerous additional purposes22. Several studies have shown that Portland cement contains chemical, physical and biological properties similar to MTA12,13, with the exception of radiopacity10,16,25. Portland cement does not have the minimum radiopacity recommended by ISO 6876/2001 specifications15 (3 mm equivalent of Al). Thus, a radiopacifying agent is added in order to distinguish it from anatomical structures10.
Bismuth oxide (Bi2O3) is a heavy metal commonly used in pharmacological and chemical industries21. Furthermore, this element is added to different endodontic materials as a radiopacifying agent, e.g., MTA, AH 26 and Sealer 2611,27,30. The effects of bismuth oxide concentration in the biological and physicochemical properties of MTA have been questioned3,5,8. Previous studies have reported interferences with cell growth and viability5,20, with the hydration mechanisms of MTA2 and negative effects on the compressive strength of the cement6.
The quantity of bismuth oxide present in commercially-available MTAs can be variable. A previous study has verified higher amounts of bismuth oxide in ProRoot MTA in comparison with Angelus MTA26. Duarte, et al.10 (2009) suggest that this variance could be related to the method of proportion. Considering the high molecular weight of bismuth oxide (466.0 g/mol), the amount of this substance may vary, if the proportion included in the final cement is calculated by weight or volume10.
To date, it is not completely clear if the presence of bismuth oxide as a radiopacifying agent affects the pH level or biological response of Portland-based cements. Considering that Portland cement is comparable to MTA, with regards to its physical16, chemical4 and biological properties23, Portland cement can be used to evaluate if the higher amounts of bismuth oxide are deleterious to the properties of the cement. The aim of the present study was to investigate if there is a relation between the increase of bismuth oxide and changes in pH levels and an intensification of toxicity in the Portland cement.
MATERIAL AND METHODS
A portion of White Portland cement (WPC) (Irajazinho Votorantim, Cimento Rio Branco, Rio de Janeiro, RJ, Brazil) was mixed with 0, 15, 20, 30 and 50% bismuth oxide (BO) (Óxido de bismuto, Neon Comercial, São Paulo, SP, Brazil), proportioned in weight by using an electronic analytic balance (Mettler Toledo PG5002-S, Mettler Toledo, Barueri, SP, Brazil). The cements were divided into 5 groups, according to the bismuth oxide concentrations: Group 1) 100 g/WPC and 0g/BO; Group 2) 85 g/WPC and 15 g/BO; Group 3) 80 g/WPC and 20 g/BO; Group 4) 70 g/WPC and 30 g/BO; and Group 5) 50 g/WPC and 50 g/BO. The manipulation of the cements was performed using a total of 1 g of cement and 0.4 mL of distilled water.
pH assessment
A total of 0.038 g of manipulated cement, approximately, was placed into sterile polyethylene tubes (10 mm in length and 1 mm in internal diameter). Ten tubes were used for each group. After being filled and weighed, each specimen was immediately immersed into a glass flask containing 25 mL of Milli-Q water with a pH level corresponding to 6.87 (Purelab Option Elga DV25, Nova Analítica, São Paulo, SP, Brazil). To avoid any type of interference in the outcomes, all laboratory equipment was previously treated with 5% nitric acid. The measurement of the pH level was performed with a pH meter (B371, Micronal, São Paulo, SP, Brazil) and calibrated using solutions with pH levels corresponding to 4, 7, and 14. The assessments were performed after periods of 15, 30 and 60 days. The pH levels were initially (6.87) measured and compared with the proposed measurement periods.
Tissue reaction
Ninety adult male albino rats (Rattus norvegicus) with approximately 300 g of body weight each were selected. The ethics committee of the institution in which the study was carried out, approved the use of the animals for research purposes (CEP 006-2009).
Previously manipulated cements were placed in sterile polyethylene tubes (10 mm in length and 1 mm in internal diameter) and immediately implanted subcutaneously in the dorsal region of the rats. The animals were divided into 5 groups (n=6) according to the cement implanted. Each animal received 2 implants of the same material. All the materials implanted were previously sterilized with ethylene oxide.
For the surgical procedures, the rats were anesthetized with a combination of ketamine and xylazin (Vet Brands International, Miramar, FL, USA) (0.05 mL/100 g). The tissue was disinfected with povidine (Tecnofarma Ind., Campinas, SP, Brazil) and two incisions were made through the skin using a #15 scalpel blade. The polyethylene tube was carefully implanted in a pocket, immediately after being loaded with the test materials. The skin was closed with 4/0 silk. The evaluations were made at 15, 30 and 60 days after the surgical procedures. After the experimental periods, the animals were anesthetized, the dorsal skin was shaved and disinfected and the tubes with the surrounding tissue were removed in blocks. The animals were sacrificed with an overdose of anesthesia. The specimens were kept in a 10% formalin solution for 2 weeks. Sections with thickness of 5 µm were stained with hematoxylin and eosin. Four sections from each specimen were selected. Histological evaluations were carried out under a light microscope (Olympus, São Paulo, SP, Brazil) at 400x magnification, by a single examiner. For a quantitative evaluation of the inflammatory infiltrate, 30 microscopic fields were analyzed according to the classification adapted from Vosough Hosseini, et al.31 (2008): Grade 0 - Without inflammatory cells; Grade 1 - Sporadic presence of chronic inflammatory cells (<25 cells); Grade 2 - Moderate infiltration of chronic inflammatory cells (25-125 cells); Grade 3 - Dense and severe infiltration of chronic inflammatory cells (>125 cells). The measurements were repeated twice to ensure reproducibility. The median and range of the grades were calculated (Table 2).
Table 2.
Median and range of inflammatory infiltrate for groups at the evaluated periods. Different lowercase letters in rows indicate statistically significant differences among the materials in each period (p<0.05). Different uppercase letters in columns indicate statistically significant differences among the periods for each material (p<0.05)
| WPC | WPC/15% | WPC/20% | WPC/30% | WPC/50% | |
|---|---|---|---|---|---|
| 15 days | 1.33 (1.03-1.43)aA | 1.83 (1.36-2.10)aA | 1.81 (1.16-2.20)aA | 1.61 (0.83-2.13)aA | 1.73 (1.36-2.36)aA |
| 30 days | 1.25 (1.20-1.30)aA | 1.24 (0.96-1.56)aB | 1.26 (0.96-1.70)aA | 1.51 (1.33-1.73)aA | 1.61 (1.23-2.23)aA |
| 60 days | 1.50 (1.00-1.56)aA | 1.25 (0.83-1.53)aB | 1.31 (0.90-1.53)aA | 1.58 (1.26-1.80)aA | 1.31 (1.06-1.86)aA |
WPC=White Portland cement
Statistical analysis
As a result of absence of normal distribution, confirmed with the D'Agostino and Pearson test, the statistical analysis was performed using the nonparametric Kruskal-Wallis and Dunn tests to compare the pH level among the materials and the Friedman test to compare the periods for a same material (p<0.05). For the biological analysis, the Kruskal-Wallis and Dunn tests were performed (p<0.05).
RESULTS
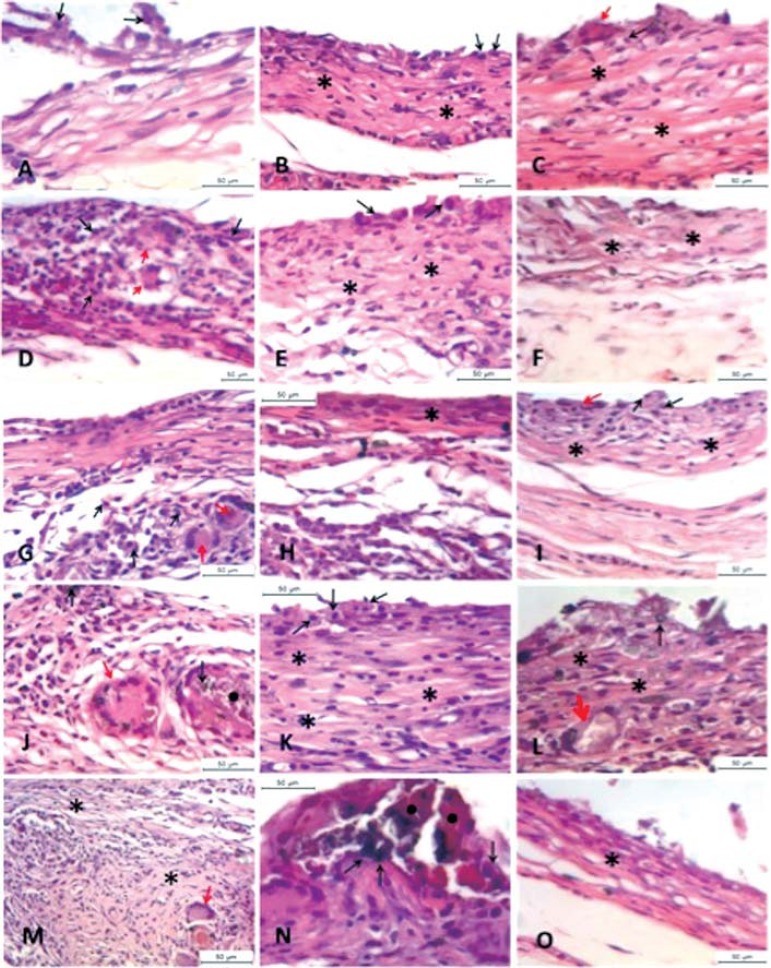
The results and significant statistical differences for the pH level and inflammatory infiltrate are represented in Tables 1 and 2, respectively. Considering the initial assessment of water, an increase of the pH level was observed after 15 days, followed by a slight increase after 30 days and a decrease after 60 days. A significant increase of the pH level was observed from 15 to 30 days for all the tested cements (p<0.05). A comparison among the groups in each period revealed no statistically significant differences (p>0.05). Representative histological images of each group (horizontally) at 15, 30 and 60 days (vertically) (hematoxylin and eosin staining, 400x magnification) are presented in Figure 1. The inflammatory infiltrate was chronic, moderate at 15 days with the predominance of macrophage-like and multinucleated giant cells for all groups. At 60 days, the chronic inflammatory response was mild with isolated macrophage-like and multinucleated giant cells. No statistically significant differences were found for the inflammatory infiltrate among the groups in each experimental period (p>0.05). The 15% WPC showed a significant decrease of the inflammatory infiltrate from 15 to 30 and 60 days (p<0.05).
Table 1.
Means and standard deviations of the assessed pH in the evaluated periods. Different lowercase letters in rows indicate statistically significant differences among the materials in each period (p<0.05). Different uppercase letters in columns indicate statistically significant differences among the periods for each material (p<0.05)
| WPC | WPC/15% | WPC/20% | WPC/30% | WPC/50% | Water | |
|---|---|---|---|---|---|---|
| 15 days | 11.16±0.15aA | 11.22±0.10aA | 11.12±0.13aA | 11.10±0.13aA | 11.04±0.14aA | 6.87 |
| 30 days | 11.83±0.13aB | 11.85±0.07aB | 11.75±0.13aB | 11.72±0.13aB | 11.70±0.07aB | 6.87 |
| 60 days | 11.65±0.90aB | 11.91±0.08aB | 11.61±0.96aB | 11.49±0.96aB | 11.39±0.92aB | 6.87 |
WPC=White Portland cement
Figure 1.
(A-C) Group 1 (0%WPC); (D-F) Group 2 (15%WPC); (G-I) Group 3 (20%WPC); (J-L) Group 4 (30%WPC); (M-O) Group 5 (50%WPC). At 15 days, a chronic inflammatory infiltrate, moderate with predominance of macrophage (black arrow) is observed, presence of giant multinuclear cell (red arrow), areas with Portland cement particles involved with giant cells (black circle) and a mild presence of fiber connective tissue (asterisk) in all groups. At 30 days, a decrease in the inflammatory infiltrate and a discrete increase in fiber connective tissue (asterisk) in comparison with the 15 days period were observed. Futhermore, at 30 days the presence of macrophage cells (black arrow) is predominant. Areas with Portland cement particles involved with giant cells are still present in this period (black circle). At 60 days, the chronic inflammatory response is mild with isolated macrophage-like cell (black arrow) and giant cells (red arrow). The presence of fiber connective tissue related to the repair process is evident (asterisk)
DISCUSSION
Bismuth oxide is added to MTA to provide radiopacity10. Variable amounts of bismuth oxide were found in different commercial brands of MTA26. It was shown that high amounts of this radiopacifying agent interfered with the physical and chemical properties of the Portland cement2,6. However, the effects of bismuth oxide concentration on the pH levels and biological properties of Portland-based cements are not completely clear at the moment. The hypothesis tested in the present study was that the increases of bismuth oxide in Portland cement could decrease the pH levels and increase the inflammatory response of this cement.
In the present study, the concentrations of bismuth oxide added to Portland cement ranged from 0-50%. The proportions were calculated in weight and were selected according to previous investigations, that evaluated various compositions in relation to other properties7,14,32. High proportions were tested to provide a critical condition of tissue exposition to bismuth oxide and interference in pH level.
High pH levels are expected for Portland-based cements due to the presence of calcium hydroxide in its composition4,16,30. The pH of calcium silicate-based cements is around 8-12, which is considered adequate for tissue repair9,13. Elevated concentrations of bismuth oxide added to Portland cement could decrease the amount of cement available to release hydroxyl ions, consequently decreasing the pH level9,24. A higher pH level alteration was observed in the initial assessment (15 days) in relation to the water pH. There was an increase of the pH level from 6.87 to approximately 11, which is considered adequate30. It can be related to the ion release that occurred during the setting time of the cements, as previously reported9. After the setting time, the increase of the pH level assessed was slight (30 days). Following 60 days, there was an insignificant increase or small decrease of the pH level, but it remained at 11, approximately. A comparison among the groups in each period showed no statistically significant differences (p>0.05). These results indicate that the addition of bismuth oxide did not interfere with the pH level of Portland cement.
Biocompatibility is desirable for root-end filling materials due to their permanent contact with periapical tissues7. Ideally, the cements should not promote irritation in the tissues or interfere with the repair process. Portland is a hydraulic cement due to its setting time and hardening by a chemical interaction with water1,17. It was previously shown that bismuth oxide added to Portland cement reacts with the calcium silicate hydrate (C-S-H) accommodated within this structure2. It is probably due to the fact that when bismuth is present in higher amounts, the C-S-H structure is not sufficient enough to react with all the molecules of the available bismuth. Camilleri, et al.5 (2004) verified that bismuth oxide interfered with and inhibited cell growth in culture, suggesting that this substance presents toxic effect. Thus, the bismuth ions that remain un-reacted could possibly contact with the adjacent tissues and affect tissue repair or intensify an inflammatory reaction.
The subcutaneous implant method is widely used to analyze inflammatory responses of root-end filling materials7,14,25,31. This method was selected to provide a dynamic system with defense cells and biotransformation19. Cell culture systems have disadvantages related to the use of one cell lineage and the lack of cell turnover19. The inflammatory cells (macrophages, lymphocytes, giant cells, plasmocytes and polymorphonuclear leukocytes) were quantified in each microscopic field for a total of 30 fields. The observed chronic inflammatory response was mild for all the groups evaluated, as previously reported7. There were no statistically significant differences among the groups for inflammatory infiltrates for all the evaluated periods (p>0.05). A significant decrease of inflammatory infiltrates was found for the 15% WPC, from 15 to 30 and 60 days (p<0.05) (Table 2). These results are in agreement with previous studies, which suggest that bismuth oxide does not promote a chronic subcutaneous tissue response, genotoxic and cytotoxic effects7,14,32. The addition of high amounts of bismuth is not appropriate due to the interference with the physical properties of the Portland cement6. Nonetheless, the quantity of bismuth oxide did not present deleterious effects with the pH level or biocompatibility of the Portland cement, denying the two hypotheses tested.
CONCLUSION
The increment of bismuth oxide did not interfere with the pH level and intensity of chronic inflammatory cells of the Portland cement. The concentration of 15% of bismuth oxide resulted in significant reduction in inflammatory response in comparison with the other concentrations evaluated.
ACKNOWLEDGMENTS
This work was supported by the Improvement of Higher Education Personnel - CAPES.
REFERENCES
- 1.American Society for Testing and Materials . ASTM C219: Standard terminology relating to hydraulic cement. West Conshohocken: ASTM; 2007. [Google Scholar]
- 2.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007;40(6):462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 3.Camilleri J. Evaluation of the physical properties of an endodontic Portland cement incorporating alternative radiopacifiers used as root-end filling material. Int Endod J. 2010;43(3):231–240. doi: 10.1111/j.1365-2591.2009.01670.x. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21(4):297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J. 2004;37(10):699–704. doi: 10.1111/j.1365-2591.2004.00859.x. [DOI] [PubMed] [Google Scholar]
- 6.Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J Endod. 2007;33(3):295–298. doi: 10.1016/j.joen.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho-Filho T, De-Deus G, Klein L, Manera G, Peixoto C, Gurgel-Filho ED. Radiopacity and histological assessment of Portland cement plus bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):e69–e77. doi: 10.1016/j.tripleo.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Cutajar A, Mallia B, Abela S, Camilleri J. Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent Mater. 2011;27(9):879–891. doi: 10.1016/j.dental.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Duarte MAH, Minotti PG, Rodrigues CT, Zapata RO, Bramante CM, Tanomaru M, Filho, et al. Effect of different radiopacifying agents on the physicochemical properties of white Portland cement and white mineral trioxide aggregate. J Endod. 2012;38(3):394–397. doi: 10.1016/j.joen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Duarte MAH, Oliveira El Kadre GD, Vivan RR, Guerreiro Tanomaru JM, Tanomaru M, Filho, Moraes IG. Radiopacity of portland cement associated with different radiopacifying agents. J Endod. 2009;35(5):737–740. doi: 10.1016/j.joen.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Ersev H, Schmalz G, Bayirli G, Schweikl H. Cytotoxic and mutagenic potencies of various root canal filling materials in eukaryotic and prokaryotic cells in vitro. J Endod. 1999;25(5):359–363. doi: 10.1016/S0099-2399(06)81172-6. [DOI] [PubMed] [Google Scholar]
- 12.Estrela C, Bammann LL, Estrela CR, Silva RS, Pécora JD. Antimicrobial and chemical study of MTA, Portland cement, calcium hydroxide paste, Sealapex and Dycal. Braz Dent J. 2000;11(1):3–9. [PubMed] [Google Scholar]
- 13.Holland R, Souza V, Murata SS, Nery MJ, Bernabe PF, Otoboni JA, Filho, et al. Healing process of dog dental pulp after pulpotomy and pulp covering with mineral trioxide aggregate or Portland cement. Braz Dent J. 2001;12(2):109–113. [PubMed] [Google Scholar]
- 14.Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, et al. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):e96–102. doi: 10.1016/j.tripleo.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.International Organization for Standardization . ISO 6876: 2012. Dental root canal sealing materials. Geneva: ISO; 2012. [Google Scholar]
- 16.Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006;32(3):193–197. doi: 10.1016/j.joen.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 17.Lamond JF, Pielert JH. Significance of tests and properties of concrete and concrete-making materials. Bridgeport: ASTM International; 2006. [Google Scholar]
- 18.Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19(11):541–544. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 19.Loushine BA, Bryan TE, Looney SW, Gillen BM, Loushine RJ, Weller RN, et al. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod. 2011;37(5):673–677. doi: 10.1016/j.joen.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Min KS, Chang HS, Bae JM, Park SH, Hong CU, Kim EC. The induction of heme oxygenase-1 modulates bismuth oxideinduced cytotoxicity in human dental pulp cells. J Endod. 2007;33(11):1342–1346. doi: 10.1016/j.joen.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Neagoe AD. Merian E, Anke M, Ihnat M, Stoeppler M. Elements and their compounds in the environment: occurrence, analysis and biological relevance. 2nd ed. Weinheim: Wiley; 2004. Bismuth; pp. 671–683. [Google Scholar]
- 22.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review - Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro DA, Duarte MA, Matsumoto MA, Marques ME, Salvadori DM. Biocompatibility in vitro tests of mineral trioxide aggregate and regular and white Portland cements. J Endod. 2005;31(8):605–607. doi: 10.1097/01.don.0000153842.06657.e2. [DOI] [PubMed] [Google Scholar]
- 24.Santos AD, Moraes JC, Araújo EB, Yukimitu K, Valério WV., Filho Physico-chemical properties of MTA and a novel experimental cement. Int Endod J. 2005;38(7):443–447. doi: 10.1111/j.1365-2591.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 25.Shahi S, Rahimi S, Lotfi M, Yavari H, Gaderian A. A comparative study of the biocompatibility of three root-end filling materials in rat connective tissue. J Endod. 2006;32(8):776–780. doi: 10.1016/j.joen.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(6):809–815. doi: 10.1016/j.tripleo.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Tanomaru-Filho M, Chaves Faleiros FB, Saçaki JN, Hungaro Duarte MA, Guerreiro-Tanomaru JM. Evaluation of pH and calcium ion release of root-end filling materials containing calcium hydroxide or mineral trioxide aggregate. J Endod. 2009;35(10):1418–1421. doi: 10.1016/j.joen.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Tanomaru-Filho M, Luis MR, Leonardo MR, Tanomaru JM, Silva LA. Evaluation of periapical repair following retrograde filling with different root-end filling materials in dog teeth with periapical lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):127–132. doi: 10.1016/j.tripleo.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21(12):603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 30.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 31.Vosoughhosseini S, Lotfi M, Shahi S, Baloo H, Mesgariabbasi M, Saghiri MA, et al. Influence of white versus gray mineral trioxide aggregate on inflammatory cells. J Endod. 2008;34(6):715–717. doi: 10.1016/j.joen.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Zeferino EG, Bueno CE, Oyama LM, Ribeiro DA. Ex vivo assessment of genotoxicity and cytotoxicity in murine fibroblasts exposed to white MTA or white Portland cement with 15% bismuth oxide. Int Endod J. 2010;43(10):843–848. doi: 10.1111/j.1365-2591.2010.01747.x. [DOI] [PubMed] [Google Scholar]