Abstract
Objective
The present study evaluated the use of a reagent to stabilize the DNA extracted from human dental tissues stored under different temperature conditions and time intervals.
Material and Methods
A total of 161 teeth were divided into two distinct groups: intact teeth and isolated dental pulp tissue. The samples were stored with or without the product at different time intervals and temperature. After storage, DNA extraction and genomic DNA quantification were performed using real-time PCR; the fragments of the 32 samples that represented each possible condition were analyzed to find the four pre-selected markers in STR analysis.
Results
The results of the quantification showed values ranging from 0.01 to 10,246.88 ng/μL of DNA. The statistical difference in the quantity of DNA was observed when the factors related to the time and temperature of storage were analyzed. In relation to the use of the specific reagent, its use was relevant in the group of intact teeth when they were at room temperature for 30 and 180 days. The analysis of the fragments in the 32 selected samples was possible irrespective of the amount of DNA, confirming that the STR analysis using an automated method yields good results.
Conclusions
The use of a specific reagent showed a significant difference in stabilizing DNA in samples of intact human teeth stored at room temperature for 30 and 180 days, while the results showed no justification for using the product under the other conditions tested.
Keywords: Forensic dentistry, Molecular biology, DNA, Teeth, Stabilization
INTRODUCTION
The application of techniques involving molecular biology in forensic dentistry represents a scientific achievement1,2,3. The DNA can be extracted from different regions of the tooth4 and this source of biological material is indicated in cases of carbonization, since the resistance and durability, associated with dental positioning, offer protection to various exogenous factors5,9.
When considering forensic samples, which generally have small DNA fragments, care must be taken in the maintenance of the material, since any loss can impair the analysis of these samples15,16,18.
One of the major difficulties encountered by experts is the storage of these samples8 because the adverse conditions found at the crime scenes may affect the stability of the material. DNA degradation is a complex phenomenon that begins with autolysis, followed by aerobic and bacterial destruction of the cell, which depends on the level of water, oxygen, and most importantly, the temperature of the local environment1,2.
The most common technique used for preserving the integrity of the samples has been freezing7. However, other methods for stabilizing the samples have been used14, and at present there are products on the market that promise to stabilize the DNA immediately after the immersion of the biological sample into the product6,10,11,13.
The aim of this study was to assess the use of AllprotectTM Tissue Reagent (Qiagen, Hilden, North Rhine-Westphalia, Germany) to stabilize the DNA from human dental tissues stored under different temperature (room temperature and under refrigeration) conditions and time intervals (1, 7, 30 and 180 days).
MATERIAL AND METHODS
161 teeth from dental extractions of healthy maxillary and mandibular third molars obtained in graduate and/or post-graduate clinics of the Ribeirão Preto School of Dentistry, University of São Paulo (FORP-USP), SP, Brazil were used. After the surgical procedure, the dental elements were cleaned with sterile gauze and saline, to later be divided into two distinct groups of study: intact teeth and pulp tissue. Prior to the division of the groups, the teeth were kept in a refrigerator (-20ºC).
Sample group - intact teeth
Each intact tooth was numbered and stored under different conditions, as shown in Figure 1 (n=5). In the samples in which AllprotectTM Tissue Reagent was used, the product was poured into a sterile centrifuge plastic bottle - Falcon type (Axygen, Inc; Tewksbury, MA, USA) using the dispensing valve present in the bottle.
Figure 1.
Description of experimental groups (n=5)
| Room temperature (24 to 37ºC) | |||||||
|---|---|---|---|---|---|---|---|
| 180 days | 30 days | 7 days | 1 day | ||||
| AllprotectTM | No product | AllprotectTM | No product | AllprotectTM | No product | AllprotectTM | No product |
| Temperature (4 to 8ºC) | |||||||
| 180 days | 30 days | 7 days | 1 day | ||||
| AllprotectTM | No product | AllprotectTM | No product | AllprotectTM | No product | AllprotectTM | No product |
Sample group - dental pulp
The extraction of the dental pulp was performed by cutting the teeth slowly in a horizontal direction in the region of the cementoenamel junction using a low speed motor with the aid of a sterile carborundum disk that was replaced after every tooth. After the tooth was sectioned, the pulp tissue was removed using sterile and individual endodontic instruments and the samples were stored in 0.5 ml microcentrifuge tubes (Axygen, Inc; Tewksbury, MA, USA). Each dental pulp was numbered and kept under different conditions, as shown in Figure 1 (n=5).
Positive control
One tooth was stored at -20ºC for 180 days. This positive control was determined to ensure the viability of the extraction from the dental pulp using a commercial kit and following the standard protocol for storage (-20ºC). This sample was not used to compare the effects of the product, and therefore the results of the quantification of the DNA extracted from this group were not used in the statistical analysis.
Extraction of DNA
The genomic DNA was extracted using the QiaampTM DNA Micro kit (Qiagen, Hilden, North Rhine-Westphalia, Germany) using the protocol for genomic DNA extraction from tissues.
Quantification of DNA by real-time PCR
Quantification by real-time PCR was performed using the 7500 Real-Time PCR System with the SDS software and the samples of the intact teeth and dental pulp were quantified at different times. To quantify the total genomic DNA present in the sample, the QuantifilerTM Human DNA Quantification kit (Applied Biosystems, Foster City, California, USA) was used in accordance with the manufacturer's protocol.
Analysis of DNA fragments - STR analysis
At the end, 32 samples were randomly selected, representing every possible condition. It was examined if amplification occurred from the use of the following four pre-selected markers with significant polymorphism: FGA (VIC - GGCTGCAGGGCATAACATTA and ATTCTATGACTTTGCGCTTCAGGA) for the 308-464 bp products; D3S1358 (ACTGCAGTCCAATCTGGGT and VIC - ATGAAATCAACAGAGGCTTGC) for the 99-147 bp products; D7S820 (VIC - ATGTTGGTCAGGCTGACTATG and GATTCCACATTTATCCTCATTGAC) for the 211-251 bp products and D16S539 (GGGGGTCTAAGAGCTTGTAAAAAG and VIC - TTTGTGTGTGCATCTGTAAGCATGTATC) for the 260-304 bp products.
These samples were standardized and diluted in TE buffer and an amplification reaction was performed so that they would contain a maximum concentration of 20 ng/μL of DNA.
The amplification of the samples was performed in a TC-412 Thermal Cycler (Techne, Stafford, Staffordshire, UK) and after an initial denaturation step at 95ºC for 2 minutes the PCR was carried out for 28 cycles using the following conditions: denaturation at 94ºC for 1 minute, annealing at 59ºC for 1 minute, extension at 72ºC for 1 minute, followed by a final extension step at 60ºC for 1 hour.
One µL of the "Amplicon" was then added to 10 µL formamide and taken to the ABI Prism 310 Genetic Analyzer (Applied Biosystems, Foster City, California, USA) using the Gene MapperTM) ID software (Applied Biosystems, Foster City, California, USA).
Statistical analysis
The experimental data were statistically analyzed by Kruskal-Wallis test (non-parametric test) to determine the influence of using AllprotectTM Tissue Reagent or not to stabilize DNA from human dental tissues under different temperature conditions and time periods. The Mann-Whitney test was used for multiple comparisons (p≤.05).
RESULTS
The presence of genomic DNA was observed in all samples. The values of the quantification by real-time PCR were expressed in ng/µL and the amount of DNA was highly variable among the samples (0.01 to 10,246.88 ng/μL). The statistical analysis was performed from the values found in each group, and the values obtained are shown in Table 1 (intact teeth) and Table 2 (pulp tissue).
Table 1.
Mean and standard deviation of the quantification of genomic DNA by real-time PCR in the group of intact teeth, samples 1-80 (ng/μL)
| Days | Intacttooth | |||
|---|---|---|---|---|
| Allprotect | No product | |||
| 4-8ºC | 23-34ºC | 4-8ºC | 23-34ºC | |
| 1 | 1,064.62±461.64aA | 481.62±52.87aA | 921.12±204.95aA | 577.06±279.95aA |
| 7 | 1,067.90±290.18aA | 598.59±372.29aA | 418.08±161.08aA | 756.42±343.70aA |
| 30 | 297.49±98.57aA | 1,592.52±616.95aA | 886.14±473.76aA | 7.26±3.23bB |
| 180 | 1,003.77±428.06abA | 2,524.82±854.32aA | 504.63±274.24bcA | 20.57±10.48cB |
(Different lowercase letters indicate statistically significant differences between columns; different uppercase lettersindicate statistically significant differences between lines)
Table 2.
Mean and standard deviation of the quantification of genomic DNA by real-time PCR in the group of dental pulp, samples 81-160 (ng/μL)
| Days | Dental pulp | |||
|---|---|---|---|---|
| All protect | No product | |||
| 4-8ºC | 23-34ºC | 4-8ºC | 23-34ºC | |
| 1 | 394.77±116.57aA | 1,110.83±467.40aA | 604.28±231.91aA | 1,130.88±189.15aA |
| 7 | 1,417.17±356.73aA | 1,345.13±516.23aA | 844.94±341.35aA | 379.41±190.53aA |
| 30 | 787.97±325.76aA | 1,279.79±686.13aA | 1,457.28±738.56aA | 1,025.09±685.44aA |
| 180 | 3,416.48±1826.41aA | 1,533.69±519.53abA | 2,260.70±454.02aA | 82.33±66.08bA |
(Different lowercase letters indicate statistically significant differences between columns; different uppercase letters indicate statistically significant differences between lines)
In this study it was observed that there was statistical difference in the amount of DNA obtained when analyzing the factors related to the time and temperature of storage. In relation to the use of the specific product AllprotectTM Tissue Reagent, it was relevant in the group of intact teeth when they were at room temperature for 30 and 180 days.
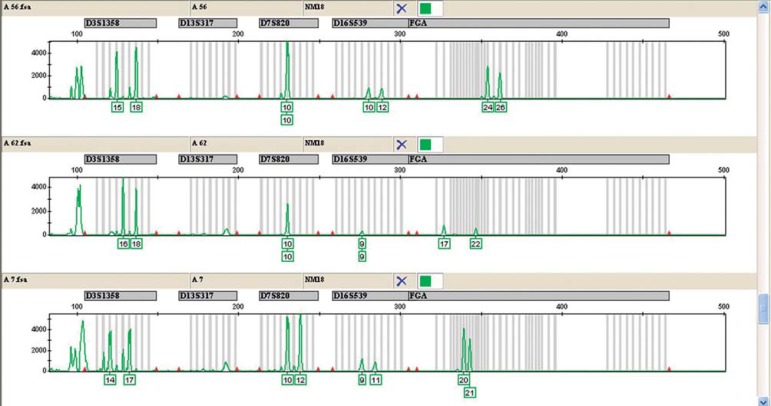
In the second laboratory stage, in which the fragments of 32 samples were analyzed, it was found that, despite the variation in the amount of DNA, the analysis of alleles of the four selected regions was possible in all samples selected. Figure 2 shows an example of how the genetic analyzer provides the results.
Figure 2.
Image of the Gene Mapper™ ID software (Applied Biosystems, USA) for the STR analysis in three differents samples
DISCUSSION
Some studies have shown how environmental factors related to time, temperature, and humidity can influence the quality and quantity of DNA1,2,12,15,16,17. Knowing this is important to define the storage protocols in order to maintain the quality and stability of the genetic material of forensic samples.
With regard to the time factor, Pfeiffer, et al.15 (1999) found that the concentration of DNA was reduced by 90% after storing teeth in the soil for a six-week time period. The results of the present study also showed that degradation is associated with the time factor when the intact teeth are stored at room temperature.
As for the variation in temperature, Schwartz, et al.16 (1991) observed that the variation of 4ºC, 25ºC and 37ºC was not decisive for obtaining DNA with high molecular weight from dental pulps despite pH, moisture, soil conditions and time of burial. In the present study, although it was possible to obtain DNA, a difference was found between the samples stored at room temperature and those refrigerated.
Because the temperature influences the amount of DNA, dry storage at low temperatures is indicated to preserve the biological material7,14. However, it is known that, in some situations the maintenance of the samples under appropriate conditions and at low temperatures may be difficult if, for example, access to the location of the crime with the necessary materials is not possible6.
Within this forensic context, using products that stabilize DNA molecules is an alternative to maintain the quality of the sample until it can be taken to the appropriate laboratories14. Accordingly, tests involving the efficiency of these products should be conducted to justify their use.
In this study the intact teeth kept under refrigeration showed no significant improvement in the amount of DNA when the product was used, as well as the storage of dental pulp, in which using the product or not showed no significant differences under the two temperature conditions. These results are similar to those found in the study of Mee, et al.11 (2011), who observed no differences in the quality of the DNA extracted from samples of breast tissues when using the product or not was compared before freezing.
In this study, it was not considered the use of the product in frozen samples since, in the forensic context, storage of the tissue in preservatives would be interesting only until the sample could be frozen and transported to the laboratory. When frozen or under refrigeration (4 to 8ºC), the results showed that the use of the product was not relevant, which does not justify the extra cost.
Furthermore, it were analyzed longer time intervals as, according to Nagy14 (2010), the tests performed so far with these stabilizers did not include this variable. It was observed that the use of AllprotectTM Tissue Reagent in intact teeth kept at room temperature for 30 and 180 days showed a significant difference in the amount of DNA (p=0.008), which indicates its use when samples need to be kept under this condition for extended periods of time.
However, despite this difference found in storage for 30 and 180 days at room temperature, the product is still considered expensive in comparison with other preservation methods, which would not justify its use in forensic context because within this time interval the samples could be sent to appropriate locations and stored following the standard freezing protocols.
In forensic investigation involving biological samples for the application of genetic testing, maintenance is important for the quality of the material. By opting for the extraction of DNA from dental tissues it is understood that other common sources, e.g., extraction of DNA from blood, are not possible and hence the material might be exposed to degradation factors.
For this reason, attention regarding the preservation of samples must be taken in order not to harm future laboratory procedures8,18. Therefore, further studies related to preservation methods should be conducted to ensure a reliable method for the preservation of the samples, particularly in relation to stabilizing agents. The results have shown that the effectiveness is limited and further investigations are required.
CONCLUSION
It may be concluded that the use of AllprotectTM Tissue Reagent showed a significant difference in stabilizing DNA in samples of intact human teeth stored at room temperature for 30 and 180 days, while the results showed no justification for using the product under the other conditions tested.
ACKNOWLEDGMENT
Financial subsidy - São Paulo Research Foundation (FAPESP): 2011/08124-5.
Funding Statement
Financial subsidy - São Paulo Research Foundation (FAPESP): 2011/08124-5.
REFERENCES
- 1.Alaeddini R, Walsh SJ, Abbas A. Forensic implications of genetic analyses from degraded DNA - a review. Forensic Sci Int Genet. 2010;4(3):148–157. doi: 10.1016/j.fsigen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Bender K, Farfán MJ, Schneider PM. Preparation of degraded human DNA under controlled conditions. Forensic Sci Int. 2004;139:135–140. doi: 10.1016/j.forsciint.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Datta P, Datta SS. Role of deoxyribonucleic acid technology in forensic dentistry. J Forensic Dent Sci. 2012;4(1):42–46. doi: 10.4103/0975-1475.99165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaytmenn R, Sweet D. Quantification of forensic DNA from various regions of human teeth. J Forensic Sci. 2003;48(3):622–625. [PubMed] [Google Scholar]
- 5.Girish K, Rahman FS, Tippu SR. Dental DNA fingerprinting in identification of human remains. J Forensic Dent Sci. 2010;2(2):63–68. doi: 10.4103/0975-1475.81284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grotzer MA, Patti R, Geoerger B, Eggert A, Chou TT, Phillips PC. Biological stability of RNA isolated from RNAlater-treated brain tumor and neuroblastoma xenografts. Med Pediatr Oncol. 2000;34(6):438–442. doi: 10.1002/(sici)1096-911x(200006)34:6<438::aid-mpo12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Höss M, Jaruga P, Zastawny TH, Dizdaroglu M, Pääbo S. DNA damage and DNA sequence retrieval from ancient tissues. Nucleic Acids Res. 1996;24(7):1304–1307. doi: 10.1093/nar/24.7.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HC, Ladd C. Preservation and collection of biological evidence. Croat Med J. 2001;42(3):225–228. [PubMed] [Google Scholar]
- 9.Manjunath BC, Chandrashekar BR, Mahesh M, Vatchala Rani RM. DNA profiling and forensic dentistry - a review of the recent concepts and trends. J Forensic Leg Med. 2011;18(5):191–197. doi: 10.1016/j.jflm.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros M, Sharma VK, Ding R, Yamaji K, Li B, Muthukumar T, et al. Optimization of RNA yield, purity and mRNA copy number by treatment of urine cell pellets with RNAlater. J Immunol Methods. 2003;279(1-2):135–142. doi: 10.1016/s0022-1759(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 11.Mee BC, Carroll P, Donatello S, Connolly E, Griffin M, Dunne B. Maintaining breast cancer specimen integrity and individual or simultaneous extraction of quality DNA, RNA, and proteins from Allprotect-stabilized and nonstabilized tissue samples. Biopreserv Biobank. 2011;9(4):389–398. doi: 10.1089/bio.2011.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muruganandhan J, Sivakumar G. Practical aspects of DNA-based forensic studies in dentistry. J Forensic Dent Sci. 2011;3(1):38–45. doi: 10.4103/0975-1475.85295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutter GL, Zahrieh D, Liu C, Neuberg D, Finkelstein D, Baker HE, et al. Comparison of frozen and RNALater solid tissue storage methods for use in RNA expression microarrays. BMC Genomics. 2004;5:88–88. doi: 10.1186/1471-2164-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagy ZT. A hands-on overview of tissue preservation methods for molecular genetic analyses. Org Divers Evol. 2010;10(1):91–105. [Google Scholar]
- 15.Pfeiffer H, Hühne J, Seitz B, Brinkmann B. Influence of soil storage and exposure period on DNA recovery from teeth. Int J legal Med. 1999;112(2):142–144. doi: 10.1007/s004140050219. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz TR, Schwartz EA, Mieszerski L, Mcnally L, Kobilinsky L. Characterization of deoxyribonucleic acid (DNA) obtained from teeth subjected to various environmental conditions. J Forensic Sci. 1991;36(4):979–990. [PubMed] [Google Scholar]
- 17.Silva RH, Quiezi R, Bertolacini CD, Carvalho SP, Gasque KC, Almeida-e-Silva CT, et al. Human identification analysis using PCR from the root portion of dental elements under different conditions of temperature and exposure time. RSBO. 2012;9(1):67–73. [Google Scholar]
- 18.Silva RH, Sales-Peres A, Oliveira RN, Oliveira FT, Sales-Peres SH. Use of DNA technology in forensic dentistry. J Appl Oral Sci. 2007;15(3):156–161. doi: 10.1590/S1678-77572007000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]