Abstract
BK virus (BKV), an ubiquitous human polyomavirus, usually does not cause disease in healthy individuals. BKV reactivation and disease can occur in immunosuppressed individuals, such as those who have undergone renal transplantation or hematopoietic cell transplantation (HCT). Clinical manifestations of BKV disease include graft dysfunction and failure in renal transplant recipients; HCT recipients frequently experience hematuria, cystitis, hemorrhagic cystitis (HC), and renal dysfunction. Studies of HCT patients have identified several risk factors for the development of BKV disease including myeloablative conditioning, acute graft-versus-host disease, and undergoing an umbilical cord blood (uCB) HCT. Although these risk factors indicate that alterations in the immune system are necessary for BKV pathogenesis in HCT patients, few studies have examined the interactions between host immune responses and viral reactivation in BKV disease. Specifically, having BKV immunoglobulin-G before HCT does not protect against BKV infection and disease after HCT. A limited number of studies have demonstrated BKV- specific cytotoxic T-cells in healthy adults as well as in post-HCT patients who had experienced HC. New areas of research are required for a better understanding of this emerging infectious disease post HCT, including prospective studies examining BK viruria, viremia, and their relationship to clinical disease, a detailed analysis of urothelial histopathology, and laboratory evaluation of systemic and local cellular and humoral immune responses to BKV in patients receiving HCT from different sources, including uCB and haploidentical donors.
Keywords: BK virus, hematopoietic stem cell transplant, BK virus immunology
BK virus (BKV) is a small non-enveloped double-stranded DNA virus in the Polyomaviridae family (1). Similar to other polyomaviruses, BKV is host-species specific and infects only humans (1). Although the exact mode of transmission has not been fully elucidated, a urine-oral route is considered the primary route of infection (2). Primary infection with BKV often occurs early in childhood and is usually asymptomatic. Thereafter, BKV remains latent in the urothelial cells (3).
With the increasing use of hematopoietic cell transplantation (HCT) as a treatment modality for hematologic malignancies, BKV DNA is frequently detected in the urine and blood of these immunocompromised patients (4). Furthermore, newer sources of hematopoietic cells, such as umbilical cord blood (uCB), are being increasingly used in HCT. Patients who undergo transplantation with these hematopoietic cells experience delayed immune reconstitution and may be more susceptible to viral reactivation (5). Deficits in cellular immunity can contribute to the immune system’s inability to control BKV replication and to prevent the resulting end-organ disease (6); however, the immune response to BKV after HCT is poorly understood.
In addition, although various interventions have been used in an attempt to treat BKV-associated hemorrhagic cystitis (HC), no treatment has established efficacy (7–12). Management is usually symptomatic and supportive, leaving clinicians and patients without definite, effective treatment options. Therefore, it is crucial to better understand the mechanisms of BKV infection and pathologic processes in HCT patients, so that effective treatments may be tested and developed to prevent and treat BKV clinical disease that can be associated with substantial morbidity.
Epidemiology in immunocompetent persons
Primary infection with BKV is thought to occur in childhood or early adulthood, as available serologic data show a seroprevalance rate of over 80% (13, 14). BKV seroprevalence rises with age, and BKV has been detected by hemagglutination inhibition tests in approximately 90% of a study population by age 40 (13). Another study reported an 82% seroprevalence of BKV immunoglobulin-G (IgG), detected by enzyme immunoassay, in 400 healthy blood donors (15). Although most initial clinical presentations are asymptomatic, some children can present with mild fever, a mild respiratory illness, or transient cystitis (3). Although no BKV polymerase chain reaction (PCR) assays are approved by the US Food and Drug Administration, and no standardization exists among current BKV PCR assays, BKV has been detected in blood and urine by PCR assays targeting different regions of the BKV genome, including those that encode the T antigen and the capsid proteins VP1 and VP2 (16). BKV has been detected by PCR of the urine in 5–44% of asymptomatic, immunocompetent adults from ages 20–89 years (15, 17–19), in 41% of pregnant women (20), and in 8–58% of human immunodeficiency virus-positive patients (21–23). Asymptomatic detection of BKV in the urine could be a consequence of periodic viral reactivation in the setting of physiologic triggers, such as a concurrent systemic illness or waning cellular immunity against BKV during pregnancy or human immunodeficiency virus infection. BKV is usually not detected in the blood of healthy individuals.
Epidemiology in immunocompromised persons
BKV particles were first identified by electron microscopy of recipient urine and donor ureteral epithelial cells from a patient who developed ureteral fibrosis and obstruction after kidney transplantion in 1971 (Fig. 1) (24). His initials, “BK,” were used to name the virus. Subsequently, BKV antigens were detected by indirect immunofluorescence in the urine of 3 patients receiving chemotherapy for underlying malignancy in 1975 (25).
Fig. 1.
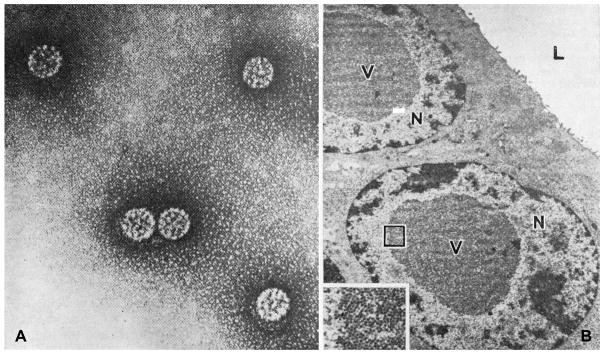
A. BK virus particles from the urine (× 180,000). B. BK virus particles (V) in nuclei (N) of epithelial cells lining the lumen (L) of the donor ureter. (× 6000). (Inset: virus particles in outlines area, × 22,500.) From (24) Gardner S, Field A, Coleman D, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1971; 297: 1253-1257, reprinted with permission from Elsevier.
Although BKV can be detected in the urine of up to 44% of asymptomatic healthy adults, it is not associated with any clinical disease in these individuals (15, 17–19). In patients who have undergone renal transplantation or HCT, BKV reactivation (active viral replication after a period of latency [26]) and disease can result in significant renal and urinary tract pathology; BKV reactivation can lead to nephropathy, ureteral stenosis, and cystitis in these patients. The prevalence of BK viruria has been reported to be 30–57% in renal transplant recipients, slightly higher than the prevalence in the healthy population. (15, 19). The frequency of BKV nephropathy is 1–10%, diagnosed at an average of 24 weeks post transplant (range, 6–270) (27), and allograft loss is thought to occur in approximately half of these cases (28–32). BKV reactivation after HCT has been associated with cystitis, HC, and renal failure (33–36), with a reported cumulative incidence of HC up to 30% at 1 year after HCT (37). Other manifestations of BKV disease after HCT are limited to case reports.
Histopathology and diagnosis
In renal transplant recipients, BKV infected kidneys show intranuclear viral inclusions in the tubular epithelial cells, with interstitial infiltrates of plasma cells and lymphocytes, interstitial fibrosis, and tubular atrophy (27).
Drachenberg et al. (38) have developed a staging system for polyomavirus nephropathy (PVN) in patients after renal transplant. This staging system, patterns A, B, and C, reflects the progression of histologic changes of PVN, with pattern C describing end-stage PVN. Although a systematic description of the histopathology of the urothelium infected by BKV in HCT recipients has yet to be reported, we have observed the presence of polyomavirus on immunohistochemical staining with SV40 antibody (which cross-reacts with BKV antigen) of the bladder mucosa in an HCT patient with persistent BK viruria. Intranuclear inclusions were present with no tissue inflammation (Fig. 2), indicating lack of an appreciable cellular allograft immune response to BKV in that case, analogous to what has been described in stage A PVN (38).
Fig. 2. Hematoxylin and eosin (H&E) and immunohistochemistry of the bladder of a patient who died after hematopoietic stem cell transplantation with asymptomatic BK virus viruria.
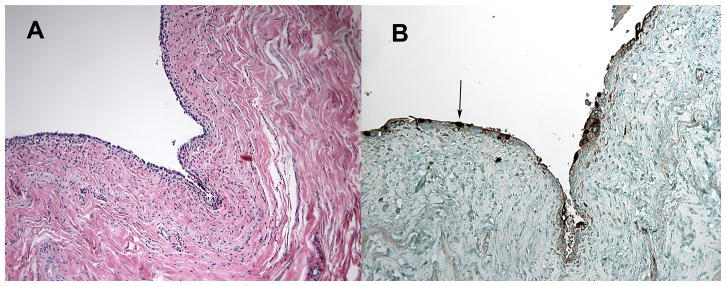
Pictures obtained during autopsy performed on a patient with asymptomatic BK viruria who had undergone double umbilical cord blood stem cell transplant and died of unrelated causes. On H&E staining (A), basophilic smudgy intranuclear inclusions are seen within the urothelium, which were positive for polyomavirus on immunohistochemistry (B, arrow). No evidence of inflammation is seen within the bladder.
Identification of large decoy cells, consisting of BKV inclusions in the nucleus of epithelial cells shed in the urine, was used in the past to diagnose BKV infection. However, it is unclear if decoy cells represent asymptomatic viral reactivation rather than a sign of BKV disease (2). More recently, urine and serum analysis using PCR has replaced detection of decoy cells in most clinical laboratories (16). However, these sensitive assays cannot discern between detection of asymptomatic viral reactivation and BKV disease.
BK virus immunology in HCT
Humoral immune response
Although BKV IgG does not prevent viral reactivation nor provide protection from disease, limited data suggest that quantitative BKV IgG may be of value in predicting BK viruria in HCT recipients. Arthur et al. (34) found significantly higher amounts of BKV in the urine of 25 of 45 (56%) HCT patients with high BKV antibody titers at the time of transplant compared with 0 of 7 HCT patients with low antibody titers at the time of transplant. A study by Wong et al. (39) examined the humoral response to BKV in 76 HCT patients before HCT, and showed that patients with an anti-BKV IgG of ≥ 1:20 pre-transplant had higher peak BKV viral loads in the urine during the post-transplant period. Thus, higher BKV IgG titers may predict higher BK viral burden (or true pre-transplant infection) and could be used to identify HCT patients with higher risk for BKV reactivation, who require closer monitoring.
Cellular immune response
A small number of studies have examined the cellular immune response in peripheral blood to BKV in healthy controls and in patients who have undergone HCT. The cellular response to BKV antigen in 13 healthy human leukocyte antigen (HLA)-A*02–positive healthy blood donors was examined by Zhou et al. (40). Nine of 13 subjects were seropositive for BKV by hemagglutination assay. Peripheral blood mononuclear cells from all donors were stimulated with BKV large T and VP1 peptides to determine CD8+ and CD4+ T-cell responses. Four of 9 (44%) BKV-seropositive patients had functional CD8+ and CD4+ T-cell responses to specific BKV antigens. This study showed that less than half of the seropositive healthy individuals in the study demonstrated detectable cellular immune responses to BKV. A recent study characterized rarely detected BKV VP1-specific CD8+ T cells in 5 HLA-A*02–positive healthy controls (41). The detected BKV VP1-specific CD8+ T cells are polyfunctional and present distinct phenotypes that differ from those CD8+ T cells specific to Epstein–Barr virus and cytomegalovirus. However, neither of these studies collected urine and blood for detection of BKV in the study subjects. Although BKV is usually not detected in the blood of healthy individuals, it is not known whether detection of host cellular immune responses against BKV correlates to viral shedding in the urine. Furthermore, additional BKV antigens may be able to elicit cellular immune responses in the remaining BKV-seropostive study subjects. Also, it is unclear if these findings can be applied to HCT patients and non-HLA-A*02–positive patients.
BKV cellular immune responses in HCT has been examined in one small study. Functional BKV-specific CD8+ T-cells were detectable in the peripheral blood in 5 of 7 (71%) HCT recipients who had experienced HC in the post-transplant period (42). This result was compared to detection of BKV-specific T cells in 7 of 26 (28%) healthy volunteers. Positive HLA-A*0201–restricted BKV-dominant epitope VP1p108 tetramer staining in all 5 BKV HC patients confirmed the presence of BKV-specific CD8+ T cells. Although this retrospective study provides some insight into the development of virus-specific CD8+ T cells in HCT, the sample size is small, and study is limited to HLA-A*0201–positive subjects. Furthermore, it is unclear at what timepoint post HCT the peripheral blood was collected, making it difficult to conclude if these functional CD8+ Tcells were present before or developed after cellular immune reconstitution in these HCT patients.
These immune response studies were performed on patients whose hematopoietic cell donors were adults. Patients who undergo uCB or haploidentical-donor HCT may have different immune responses to BKV. A better understanding of the onset and timing of BKV cellular immune response is crucial in clarifying BKV pathogenesis in HCT and if variability exists among different sources of hematopoetic cells.
Clinical manifestations and spectrum of BKV disease in adult HCT recipients
The association between BK viruria in HCT recipients and HC was first proposed by Arthur et al. (34) in 1986. Although HC has been the most frequent disease described thereafter, multiple cases have been reported of other manifestations of BKV infection and diseases, in and outside of the renal system (Table 1) (43–55). These case reports indicate that BKV reactivation can potentially cause a variety of diseases in HCT patients, but the diagnosis of BKV infection or disease may be delayed or missed because of a lack of knowledge of the broad clinical disease spectrum of BKV.
Table 1.
| Complication (reference) | Evidence of BKV infection or disease (reference) |
|---|---|
| Dysuria, hematuria, urgency/frequency, flank pain, bladder spasms (51)Obstructive nephropathy (46) | BKV urine PCR + |
|
| |
| Renal tubulointerstitial disease (47, 48, 50, 53, 54) | IHC and/or EM + |
| BK viruria | |
| BK viremia in 4 patients (48, 50, 53) | |
|
| |
| Encephalitis (44, 45, 49) | BKV CSF PCR + |
| BKV PCR brain tissue + (49) | |
| No histopathology (44, 45) | |
| No brain tissue staining | |
|
| |
| Pneumonia (43, 55) | SV40 lung tissue stain + |
| viral intranuclear inclusions (43) | |
| lung tissue cell culture BKV + (55) | |
| lung tissue BKV PCR + (55) | |
|
| |
| Pneumonitis (52) | BAL fluid with cells resembling decoy cells |
| IHC + | |
BKV, BK virus; ICH, immunohistochemistry; EM, electron micrograph; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; BAL, bronchoalveolar lavage.
Hemorrhagic cystitis (HC)
HC, defined as inflammation with subsequent bleeding of the bladder lining, can be caused by different etiologies (36, 56, 57) and has been previously divided into 2 categories according to onset in relation to neutrophil engraftment after HCT (Table 2) (33, 34, 37, 58, 59). HC is a common problem in HCT: although one study reported a 6.4% incidence of HC in 1402 patients undergoing HCT at a single center (60), other studies have reported HC cumulative incidences of 12–50% in similar study populations (6, 33, 34, 61). Several studies have reported an increased incidence of HC in patients who have undergone allogeneic HCT compared to autologous HCT (4, 34, 62–64).
Table 2.
Etiologies of hemorrhagic cystitis
| Pre-engraftment | Post-engraftment | |
|---|---|---|
| Etiologies | Coagulopathy | Adenovirus |
| Chemotherapy | BK virus | |
| Radiation therapy | Cytomegalovirus | |
| Thrombocytopenia | Coagulopathy | |
| JC virus* | ||
| Thrombocytopenia |
Pre-engraftment: before neutrophil engraftment; Post-engraftment: after neutrophil engraftment.
1 case reported.
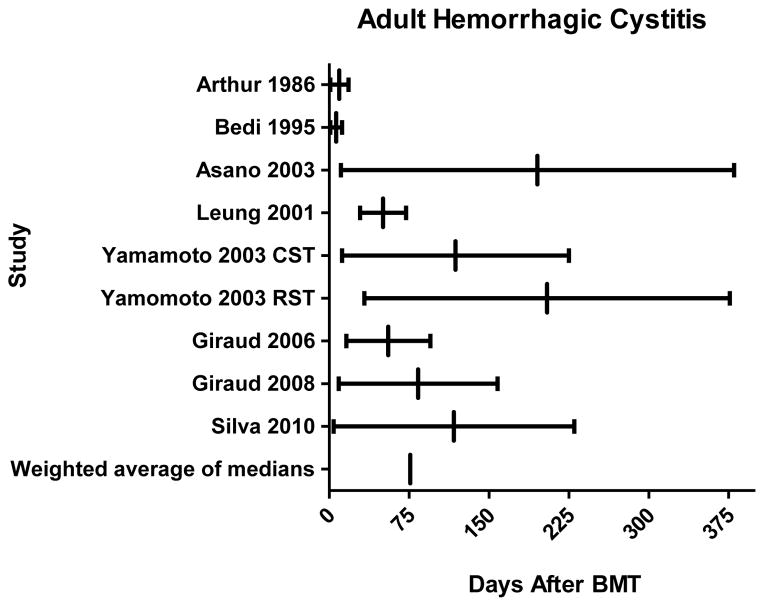
In 8 studies evaluating HC in adult HCT recipients (6, 34, 61, 65–70) patients developed HC at a median of 50 days (range, 1–380) after transplantation (Fig. 3). When comparing median days of onset of HC to neutrophil engraftment in these studies, HC was consistently a post-engraftment event in adults, occurring at an average of 76 days (range, 37–151) post HCT, whereas the median date of neutrophil engraftment ranged from 15 to 21 days (6, 34, 37, 65–70).
Fig. 3. Hemorrhagic cystitis (HC) in adults following bone marrow transplantation.
For each study the range of reported onset of HC is represented by a horizontal line and the median onset is represented by a small vertical line. Weighted average of the median of all 8 studies (6, 34, 65–70) was calculated to be 76 days after hematopoietic cell transplantation. CST, conventional stem cell transplantation; RST, reduced-intensity stem cell transplantation; BMT, bone marrow transplantation.
In 7 studies totaling 1068 adult allogeneic and autologous HCT patients, 165 (15%) cases of HC were reported, of which 23 (14%) were in recipients of autologous HCT (6, 65–70). Although several studies report a majority of post-engraftment HC cases (65, 66, 69), uniform and consistent definitions are not used to make this distinction. Among those diagnosed with HC, 103 cases (62%, 9.6% of all patients) were associated with BK viruria (6, 65–70) (Table 3). Causes of HC in the remaining 62 (38%) cases include JC virus in 1 patient (67), adenovirus in several patients (65, 70), and unknown etiologies in the remainder. Other outcomes, such as concurrent viral infections, acute graft-versus-host disease (GVHD), and death were not reported in all studies. Two of these studies report a median onset of BK viruria of 15 days (range, 2–57) and 21 days (range, 14–98) after HCT (6, 68). On the other hand, post-engraftment BK viruria is not always associated with HC. In 5 adult studies totaling 671 HCT patients (6, 66–69), 547 patients did not experience HC, 227 patients (41%) of whom had BK viruria.
Table 3.
Hemorrhagic cystitis (HC) and and BK viruria in adult hematopoietic stem cell transplantation
| Study First author (reference) | Year | Study type | Type(s) of Transplant | Overall N | BK viruria | HC & BK viruriaa (%) | HC & aGVHDb | Outcome (death) |
|---|---|---|---|---|---|---|---|---|
| Bedi (6) | 1995 | Prospective | Autologous & Allogeneic | 147 | 50 | 20 (40%) | 13 (30%)c | N/A |
| Leung (68) | 2001 | Prospective | Autologous & Allogeneic | 50 | 50 | 20 (40%) | 4 (44%) | N/A |
| Asano (65) | 2003 | Retrospective | Allogeneic | 141 | N/A | 10 | 13 (100%) | 9 |
| Yamamoto (70) | 2003 | Retrospective | Allogeneic | 256 | N/A | 2 | 13 (72%) | 3 |
| Giraud (66) | 2006 | Prospective | Allogeneic | 90 | 30 | 13 (43%) | 6 (25%) | N/A |
| Giraud (67) | 2008 | Prospective | Allogeneic | 175 | 83 | 23 (28%) | 12 (22%) | N/A |
| Silva (69) | 2010 | Prospective | Allogeneic | 209 | 96 | 25 (26%) | 8 (12%) | N/A |
% BK viruric patients who developed HC.
Grade II–IV acute GVHD (aGVHD) unless otherwise specified.
Grades of aGVHD not given.
N/A, not available; HC, hemorrhagic cystitis; aGVHD, acute graft-versus host disease.
BK viremia and HC
Although BK viruria has been detected in 5–44% asymptomatic healthy individuals (15, 19), BK viremia has only been reported in immunocompromised patients. Therefore, detection of BK viremia could be a more specific indication of severe immunosuppression. But is BK viremia a marker of active HC in HCT? Few studies have assessed BK viremia and its relationship to HC. In a retrospective case-control study using archived plasma samples from 1979–2003, Erard et al. (58) found that adult HCT patients with a plasma BK virus load >104 copies/mL had a higher risk of developing HC than patients with a viral load <104 copies/mL (odds ratio=21; P<0.001). O’Donnell et al. (50) reported that BK viremia was an independent predictor for the development of post-HCT renal impairment in 21 of 112 (19%) patients who had BK viremia. These data suggest that using BK viremia as a marker of BKV-associated HC could be of value, but evidence is limited due to small numbers of patients and a predominance of non-contemporary retrospective study designs. In studies with larger groups of patients in which BK viruria was assessed, BK viremia was not always measured (6, 34, 65–67, 69). Therefore, the significance of BK viremia and its relationship with BKV-associated HC needs to be further studied.
Risk factors for developing HC after HCT
Several risk factors for developing HC in adult HCT have been reported, including both recipient and donor factors (Table 4) (65–67, 69–71). In addition, Silva et al. (69) reported that 80% of the patients who developed HC in their cohort did so after neutrophil engraftment, leading the authors to conclude that an alloimmune response consisting of newly formed neutrophils facilitated the bleeding episodes.
Table 4.
Risk factors for hemorrhagic cystitis (HC) in adults following hematopoietic stem cell transplantation
| Study First author (reference) | Year | HC risk factors (Univariate) | HC risk factors (Multivariate) |
|---|---|---|---|
| Yamamoto (70) | 2003 | Adenovirus antibody + at time of transplant | Adenovirus antibody + at time of transplant |
| Total dose of busulfan | Total dose of busulfan | ||
| CMV infection | History of GVHD | ||
| Use of ATG and corticosteroids | |||
|
| |||
| Asano (65) | 2003 | Male gender | |
| Development of grade II–IV acute GVHD | |||
|
| |||
| Erard (71) | 2005 | BK viremia before or during HC | BK viremia before or during HC |
| Use of busulfan in conditioning regimen | |||
|
| |||
| Giraud (66) | 2006 | BK viruria | BK viruria before onset of HC |
| Unrelated donor | Unrelated donor | ||
| Myeloablative conditioning | |||
|
| |||
| Giraud (67) | 2008 | Unrelated donor | Unrelated donor |
| Myeloablative conditioning | |||
| BK viruria | |||
|
| |||
| Silva (69) | 2010 | Haploidentical or cord | Myeloablative/haploidentical or cord blood/BK virus urine PCR positive pre-transplant |
| Unrelated donor | |||
CMV, cytomegalovirus; GVHD, graft-versus-host disease; ATG, anti-thymocyte globulin; PCR, polymerase chain reaction.
Risk factors for developing BK viruria and BKV disease after HCT
Few studies have looked at specific risk factors for the development of BK viruria and BK virus disease after HCT. Myeloablative conditioning was found to be a primary risk factor for developing BK viruria and BK virus disease in several studies (51, 66, 67). In addition, use of cyclophosphamide in the conditioning regimen, mycophenolate mofetil for GVHD prophylaxis, experiencing acute GVHD grades II–IV, and undergoing uCB HCT were found to be significant risk factors for the development of BKV disease in one study (51).
How does BKV cause disease after HCT?
Studies have shown that a large percentage of the healthy population is exposed to BKV by the age of 10 (13). Clinical manifestations of infection are mild if not asymptomatic, leading to the conclusion that BKV is a pathogen of low virulence in those persons who have an intact immune system (72).
Leung et al. (73) have suggested a model of BKV disease in which cyclophosphamide-based chemotherapy and radiation cause direct injury to the epithelial cells of the bladder, which act as a “trigger” for BKV to emerge from latency and start replicating. Details on how this occurs are unknown. The host is unable to respond to replicating BKV in this setting, as innate and adaptive immune responses are weak owing to the effects of HCT conditioning. BKV replication is detected in urine, but at this timepoint patients are asymptomatic. As the new host immune system recovers, recognition of and an attempt to control replicating BKV occur, leading to host-mediated uroepithelial cell damage via an inflammatory response, and clinical BKV disease manifests in a variety of genitourinary signs and symptoms (72).
Our clinical observations and other publications (51, 74) suggest that this model should be expanded to include factors that alter the host immune response over time, as well as factors involved in host-pathogen interactions (72). Leung’s model (73) does not account for HCT patients who receive hematopoietic cells from HLA-mismatched donors, have reduced-intensity conditioning, undergo uCB HCT, or develop initial BKV disease later after transplantation in the setting of acute GVHD. Higher incidence of BKV reactivation occurs in HLA-mismatched and in uCB HCT recipients than in other allogeneic HCT patients.
These observations suggests a crucial role played by major histocompatability complex-based viral recognition as uCB HCT often can allow greater degrees of HLA mismatching. Inadequate HLA matching likely results in poor presentation of viral epitopes to BKV-specific CD8+ cytotoxic T cells and decreased viral clearance, as is demonstrated by cytomegalovirus infection in kidney transplantation patients (75, 76). Furthermore, in kidney tranplantation patients, greater degrees of HLA mismatching have been associated with increased incidences of BKV-associated nephropathy (30, 77, 78). In a small study of pediatric HCT patients (n = 40), a greater degree of HLA mismatching was associated with increased detection of viral DNA, including BKV, in blood post HCT (79). Also, HLA mismatching leads to increased risk of acute GVHD, so the higher incidence of BKV disease could be a result of the additional immunosuppression associated with GVHD and its treatment. Thus, while HLA mismatching may be important for better control of BK viral reactivation in HCT patients, the exact mechanisms needs to be further elucidated, especially in adult patients.
On the other hand, the host immune response may not be the sole factor for the development of BKV disease in HCT patients. Patients who undergo uCB HCT and others can develop BKV disease pre-engraftment, without having received cyclophosphamide-based conditioning or radiation. Patients can also develop BKV disease late in the transplantation course, during treatment of acute or chronic GVHD. These circumstances suggest that loss of immune control, without inciting cytotoxic injury, may lead to BKV reactivation and disease. These facts also suggest that clinical disease may be a result of direct viral cytotoxic effects in the setting of intense immunosuppression and not solely caused by immune reconstitution. No animal models exist to explore these questions systematically, and no histopathologic studies of BKV disease in HCT have been reported.
However, if the host gains immune control of the virus, several outcomes may ensue, including (a) resolution of BKV viruria without clinical disease, (b) resolution of genitourinary signs and symptoms without sequelae, or (c) post-BKV disease manifested by bladder and urinary tract scarring. Host and pathogen factors associated with these outcomes have not yet been studied.
BKV diagnostics in HCT
Distinguishing asymptomatic reactivation from BKV disease is important. Detection of decoy cells and BKV DNA in the urine does not confirm BKV disease, and often represents asymptomatic reactivation (2, 16). No study has evaluated the simultaneous presence of decoy cells and BKV DNA in the urine of post-HCT patients who have HC. The sensitivity of alternate diagnostic tests to confirm BKV disease, such as the detection of BKV VP1 messenger RNA in the urine (80), have not been studied in post-HCT patients. The combined sensitivity of BKV DNA detection in the blood and BKV VP1 messenger RNA in the urine of patients post HCT also have not been studied.
Management of BKV infection
No standardized screening guidelines exist for BKV infection in patients who have undergone HCT, with no effective antiviral treatment for BKV. Treatment of BKV-associated HC is currently controversial. Use of varying doses of intravenous cidofovir (8, 9, 81, 82) and intravesicular cidofovir (7, 81, 83–85) has been reported, but these have not been studied in a randomized, controlled, or prospective fashion. Ciprofloxacin prophylaxis was associated with a reduced incidence of BKV-associated HC after HCT in a small retrospective study (86). Leung et al. (12) performed a prospective study in which ciprofloxacin treatment was shown to decrease the median peak BKV urine viral loads, but ciprofloxacin did not change the rates of severe HC in treated and untreated groups. In addition, ciprofloxacin did not prevent reactivation of BKV infection in all patients. Larger randomized, placebo-controlled, prospective studies are needed to clarify the use of quinolones and other agents for treatment of BKV-associated HC.
Conclusion
BKV is often detected in post-HCT recipients, and is frequently associated with HC (6, 65–70). Although HC can be caused by several etiologies, it is strongly associated with BKV viruria and possibly, BKV viremia. In addition to HC, BKV disease can manifest with a range of symptoms (51) and affect various organ systems.
Future directions
Additional work is necessary to better understand BKV and the underlying immune response to this viral infection after HCT. Analysis of the spectrum of histopathologic and tissue-specific immunologic events in the bladder, ureters, and kidney caused by BKV in HCT patients is needed. Studies have attempted to identify risk factors for BKV infection after HCT, but currently no consensus exists, as host-pathogen interactions that lead to clinical disease have not been studied. No studies have defined the histopathology of BK disease in HCT. Furthermore, while HCT patients are often critically ill, the outcomes of BK-associated diseases are not well studied.
The host immune response to BKV also has not been well studied. Although prior exposure to BKV and development of IgG does not prevent viral replication, cellular immune responses have been evaluated in a very small group of patients with BKV disease, and this area currently remains largely unexplored. In healthy individuals, the triggers of viral shedding in urine is not well understood, and correlations of BK viruria with alterations in host immune responses or other concurrent viral infections have yet to be defined. In HCT patients, where BK-associated diseases affect several end organs, it remains unclear whether the reconstituted host cellular immune responses to the reactivating BKV are in fact destroying uroepithelial cells, resulting in HC. Futhermore, the role of host immune responses in BK-associated diseases other than HC are also not understood. In addition, studies are needed to characterize the allograft immune responses produced by the less mature uCB cells. With better understanding of host cellular immune responses, novel therapies such as cell-based immunotherapies may be beneficial in the HCT population. Therefore, prospectives studies assessing BKV viruria, BKV viremia, and clinical manifestations of infection and host immune responses are needed for a better understanding of this viral infection and the associated significant diseases.
Acknowledgments
Support: NIH grant K08 NS 064215-01 supported C.S.T.
Thanks: Vera Paulson, MD, for histopathology images of Figure 2.
Abbreviations
- BKV
BK virus
- HCT
hematopoietic cell transplantation
- HC
hemorrhagic cystitis
- uCB
umbilical cord blood
- IgG
immunoglobulin-G
- PCR
polymerase chain reaction
- PVN
polyomavirus nephropathy
- HLA
human leukocyte antigen
- GVHD
graft-versus-host disease
Footnotes
Disclosure: F.M.M. has received grant support and consulting honoraria from Chimerix and Vertex.
References
- 1.Knowles WA. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV) Advances Exper Med Biol. 2006;577:19–45. doi: 10.1007/0-387-32957-9_2. [DOI] [PubMed] [Google Scholar]
- 2.Khalili K, Stoner GL, editors. Human Polyomaviruses: Molecular and Clinical Perspectives. New York: Wiley-Liss Inc; 2001. [Google Scholar]
- 3.Dörries K. Human polyomaviruses. Principles and Practice of Clinical Virology. 2004:675–702. [Google Scholar]
- 4.Bogdanovic G, Ljungman P, Wang F, Dalianis T. Presence of human polyomavirus DNA in the peripheral circulation of bone marrow transplant patients with and without hemorrhagic cystitis. Bone Marrow Transplant. 1996;17 (4):573–576. [PubMed] [Google Scholar]
- 5.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127 (3):286–297. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedi A, Miller CB, Hanson JL, et al. Association of BK virus with failure of prophylaxis against hemorrhagic cystitis following bone marrow transplantation. J Clin Oncol. 1995;13:1103–1109. doi: 10.1200/JCO.1995.13.5.1103. [DOI] [PubMed] [Google Scholar]
- 7.Bridges B, Donegan S, Badros A. Cidofovir bladder instillation for the treatment of BK hemorrhagic cystitis after allogeneic stem cell transplantation. Am J Hematol. 2006;81 (7):535–537. doi: 10.1002/ajh.20567. [DOI] [PubMed] [Google Scholar]
- 8.Cesaro S, Hirsch HH, Faraci M, et al. Cidofovir for BK virus-associated hemorrhagic cystitis: a retrospective study. Clin Infect Dis. 2009;49 (2):233–240. doi: 10.1086/599829. [DOI] [PubMed] [Google Scholar]
- 9.Ganguly N, Clough La, Dubois LK, et al. Low-dose cidofovir in the treatment of symptomatic BK virus infection in patients undergoing allogeneic hematopoietic stem cell transplantation: a retrospective analysis of an algorithmic approach. Transpl Infect Dis. 2010;12:406–411. doi: 10.1111/j.1399-3062.2010.00513.x. [DOI] [PubMed] [Google Scholar]
- 10.Harkensee C, Vasdev N, Gennery AR, Willetts IE, Taylor C. Prevention and management of BK-virus associated haemorrhagic cystitis in children following haematopoietic stem cell transplantation--a systematic review and evidence-based guidance for clinical management. Br J Haematol. 2008;142:717–731. doi: 10.1111/j.1365-2141.2008.07254.x. [DOI] [PubMed] [Google Scholar]
- 11.Kwak EJ, Vilchez Ra, Randhawa P, Shapiro R, Butel JS, Kusne S. Pathogenesis and management of polyomavirus infection in transplant recipients. Clin Infect Dis. 2002;35:1081–1087. doi: 10.1086/344060. [DOI] [PubMed] [Google Scholar]
- 12.Leung AYH, Chan MTL, Yuen K-Y, et al. Ciprofloxacin decreased polyoma BK virus load in patients who underwent allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2005;40:528–537. doi: 10.1086/427291. [DOI] [PubMed] [Google Scholar]
- 13.Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71 (1):115–123. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 14.Viscidi RP, Rollison DE, Sondak VK, et al. Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus. Clin Vaccine Immunol. 2011;18 (10):1737–1743. doi: 10.1128/CVI.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199:837–846. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 16.Bechert CJ, Schnadig VJ, Payne DA, Dong J. Monitoring of BK viral load in renal allograft recipients by real-time PCR assays. Am J Clin Pathol. 2010;133 (2):242–250. doi: 10.1309/AJCP63VDFCKCRUUL. [DOI] [PubMed] [Google Scholar]
- 17.Husseiny MI, Anastasi B, Singer J, Lacey SF. A comparative study of Merkel cell, BK and JC polyomavirus infections in renal transplant recipients and healthy subjects. J Clin Virol. 2010;49 (2):137–140. doi: 10.1016/j.jcv.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kling CL, Wright AT, Katz SE, et al. Dynamics of urinary polyomavirus shedding in healthy adult women. J Med Virol. 2012;84 (9):1459–1463. doi: 10.1002/jmv.23319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong S, Zheng HY, Suzuki M, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45 (1):193–198. doi: 10.1128/JCM.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClure GB, Gardner JS, Williams JT, et al. Dynamics of pregnancy-associated polyomavirus urinary excretion: a prospective longitudinal study. J Med Virol. 2012;84 (8):1312–1322. doi: 10.1002/jmv.23320. [DOI] [PubMed] [Google Scholar]
- 21.Ledesma J, Munoz P, Garcia de Viedma D, et al. BK virus infection in human immunodeficiency virus-infected patients. Eur J Clin Microbiol Infect Dis. 2012;31 (7):1531–1535. doi: 10.1007/s10096-011-1474-9. [DOI] [PubMed] [Google Scholar]
- 22.Markowitz RB, Thompson HC, Mueller JF, Cohen JA, Dynan WS. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993;167 (1):13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- 23.Nali LH, de Centrone CC, Urbano PR, et al. High prevalence of the simultaneous excretion of polyomaviruses JC and BK in the urine of HIV-infected patients without neurological symptoms in Sao Paulo, Brazil. Rev Instituto Med Trop de Sao Paulo. 2012;54 (4):201–205. doi: 10.1590/s0036-46652012000400004. [DOI] [PubMed] [Google Scholar]
- 24.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K. ) isolated from urine after renal transplantation. Lancet. 1971;1 (7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 25.Reese JM, Reissing M, Daniel RW, Shah KV. Occurrence of BK virus and BK virus-specific antibodies in the urine of patients receiving chemotherapy for malignancy. Infection and Immunity. 1975;11 (6):1375–1381. doi: 10.1128/iai.11.6.1375-1381.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dörries K. Latent and persistent polyomavirus infection. In: Khalili K, Stoner GL, editors. Human Polyomaviruses: Molecular and Clinical Perspectives. New York: Wiley-Liss Inc; 2001. pp. 197–235. [Google Scholar]
- 27.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–623. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 28.Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, Imbert-Marcille BM. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant. 2005;5 (8):1926–1933. doi: 10.1111/j.1600-6143.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch HH. Polyomavirus BK nephropathy: a (re-)emerging complication in renal transplantation. Am J Transplant. 2002;2 (1):25–30. doi: 10.1034/j.1600-6143.2002.020106.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347 (7):488–496. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 31.Pires EP, Bernardino-Vallinoto CV, Alves DM, et al. Prevalence of infection by JC and BK polyomaviruses in kidney transplant recipients and patients with chronic renal disease. Transpl Infect Dis. 2011;13 (6):633–637. doi: 10.1111/j.1399-3062.2011.00614.x. [DOI] [PubMed] [Google Scholar]
- 32.Randhawa PS, Demetris AJ. Nephropathy due to polyomavirus type BK. N Engl J Med. 2000;342 (18):1361–1363. doi: 10.1056/NEJM200005043421809. [DOI] [PubMed] [Google Scholar]
- 33.Apperley JF, Rice SJ, Bishop JA, et al. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987;43:108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- 34.Arthur RR, Shah KV, Baust SJ, Santos GW, Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986;315:230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- 35.Garderet L, Bittencourt H, Sebe P, et al. Cystectomy for severe hemorrhagic cystitis in allogeneic stem cell transplant recipients. Transplantation. 2000;70:1807–1811. doi: 10.1097/00007890-200012270-00023. [DOI] [PubMed] [Google Scholar]
- 36.Chan PK, Ip KW, Shiu SY, Chiu EK, Wong MP, Yuen KY. Association between polyomaviruria and microscopic haematuria in bone marrow transplant recipients. J Infect. 1994;29 (2):139–146. doi: 10.1016/s0163-4453(94)90602-5. [DOI] [PubMed] [Google Scholar]
- 37.El-Zimaity M, Saliba R, Chan K, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: donor type matters. Blood. 2004;103:4674–4680. doi: 10.1182/blood-2003-08-2815. [DOI] [PubMed] [Google Scholar]
- 38.Drachenberg CB, Hirsch HH, Ramos E, Papadimitriou JC. Polyomavirus disease in renal transplantation: review of pathological findings and diagnostic methods. Human Pathology. 2005;36 (12):1245–1255. doi: 10.1016/j.humpath.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Wong ASY, Chan K-H, Cheng VCC, Yuen K-Y, Kwong Y-L, Leung AYH. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin Infect Dis. 2007;44:830–837. doi: 10.1086/511863. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Sharma M, Martinez J, et al. Functional characterization of BK virus-specific CD4+ T cells with cytotoxic potential in seropositive adults. Viral Immunology. 2007;20:379–388. doi: 10.1089/vim.2007.0030. [DOI] [PubMed] [Google Scholar]
- 41.van Aalderen MC, Remmerswaal EB, Heutinck KM, et al. Phenotypic and functional characterization of circulating polyomavirus BK VP1-specific CD8+ T cells in healthy adults. J Virol. 2013;87 (18):10263–10267. doi: 10.1128/JVI.01540-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneidawind D, Schmitt A, Wiesneth M, et al. Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant. Leukemia & Lymphoma. 2010;51:1055–1062. doi: 10.3109/10428191003746323. [DOI] [PubMed] [Google Scholar]
- 43.Akazawa Y, Terada Y, Yamane T, et al. Fatal BK virus pneumonia following stem cell transplantation. Transpl Infect Dis. 2012;14 (6):E142–146. doi: 10.1111/tid.12011. [DOI] [PubMed] [Google Scholar]
- 44.Bakri FG, Bahou YG, Al-Sammarrai FA, et al. Fatal encephalitis due to BK virus in a patient with common variable immunodeficiency: a case report. J Clin Virol. 2013;57 (4):363–369. doi: 10.1016/j.jcv.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Behre G, Becker M, Christopeit M. BK virus encephalitis in an allogeneic hematopoietic stem cell recipient. Bone Marrow Transplant. 2008;42(7):499. doi: 10.1038/bmt.2008.198. [DOI] [PubMed] [Google Scholar]
- 46.Khan H, Oberoi S, Mahvash A, et al. Reversible ureteral obstruction due to polyomavirus infection after percutaneous nephrostomy catheter placement. Biol Blood Marrow Transplant. 2011;17 (10):1551–1555. doi: 10.1016/j.bbmt.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84:243–246. doi: 10.1002/ajh.21358. [DOI] [PubMed] [Google Scholar]
- 48.Limaye AP, Smith KD, Cook L, et al. Case report polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5:614–620. doi: 10.1046/j.1600-6143.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- 49.Lopes da Silva R, Ferreira I, Teixeira G, et al. BK virus encephalitis with thrombotic microangiopathy in an allogeneic hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2011;13 (2):161–167. doi: 10.1111/j.1399-3062.2010.00581.x. [DOI] [PubMed] [Google Scholar]
- 50.O’Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biology of Blood and Marrow Transplantation. 2009;15:1038–1048. e1031. doi: 10.1016/j.bbmt.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rorije NMG, Shea MM, Satyanarayana G, et al. BK virus disease following allogeneic stem cell transplantation: a cohort analysis. Biol Blood Marrow Transplant. 2014;20 (4):564–570. doi: 10.1016/j.bbmt.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Sandler ES, Aquino VM, Goss-Shohet E, Hinrichs S, Krisher K. BK papova virus pneumonia following hematopoietic stem cell transplantation. Bone Marrow Transplant. 1997;20 (2):163–165. doi: 10.1038/sj.bmt.1700849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shapiro S, Robin M, Esperou H, et al. Polyomavirus nephropathy in the native kidneys of an unrelated cord blood transplant recipient followed by a disseminated polyomavirus infection. Transplantation. 2006;82 (2):292–293. doi: 10.1097/01.tp.0000226172.68372.f9. [DOI] [PubMed] [Google Scholar]
- 54.Stracke S, Helmchen U, von Muller L, Bunjes D, Keller F. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrol Dial Transplant. 2003;18 (11):2431–2433. doi: 10.1093/ndt/gfg361. [DOI] [PubMed] [Google Scholar]
- 55.Yapa HM, McLornan DP, Raj K, et al. Pneumonitis post-haematopoeitic stem cell transplant - cytopathology clinches diagnosis. J Clin Virol. 2012;55 (3):278–281. doi: 10.1016/j.jcv.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 56.Arai Y, Maeda T, Sugiura H, et al. Risk factors for and prognosis of hemorrhagic cystitis after allogeneic stem cell transplantation: retrospective analysis in a single institution. Hematology. 2012;17 (4):207–214. doi: 10.1179/1607845412Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 57.Shakiba E, Yaghobi R, Ramzi M. Prevalence of viral infections and hemorrhagic cystitis in hematopoietic stem cell transplant recipients. Exper Clin Transplant. 2011;9 (6):405–412. [PubMed] [Google Scholar]
- 58.Erard V, Storer B, Corey L, et al. BK virus infection in hematopoietic stem cell transplant recipients: frequency, risk factors, and association with postengraftment hemorrhagic cystitis. Clin Infect Dis. 2004;39:1861–1865. doi: 10.1086/426140. [DOI] [PubMed] [Google Scholar]
- 59.Gaziev J, Paba P, Miano R, et al. Late-onset hemorrhagic cystitis in children after hematopoietic stem cell transplantation for thalassemia and sickle cell anemia: a prospective evaluation of polyoma (BK) virus infection and treatment with cidofovir. Biol Blood Marrow Transplant. 2010;16:662–671. doi: 10.1016/j.bbmt.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 60.Nevo S, Swan V, Enger C, et al. Acute bleeding after bone marrow transplantation (BMT)- incidence and effect on survival. A quantitative analysis in 1,402 patients. Blood. 1998;91:1469–1477. [PubMed] [Google Scholar]
- 61.Azzi A, Fanci R, Bosi A, et al. Monitoring of polyomavirus BK viruria in bone marrow transplantation patients by DNA hybridization assay and by polymerase chain reaction: an approach to assess the relationship between BK viruria and hemorrhagic cystitis. Bone Marrow Transplantation. 1994;14:235–240. [PubMed] [Google Scholar]
- 62.Leung AYH, Chan M, Cheng VCC, Lie aKW, Yuen K-Y, Kwong Y-L. Polyoma BK viruria in patients undergoing autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:1029–1030. doi: 10.1038/sj.bmt.1704944. [DOI] [PubMed] [Google Scholar]
- 63.Seber A, Shu XO, Defor T, Sencer S, Ramsay N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999;23 (1):35–40. doi: 10.1038/sj.bmt.1701523. [DOI] [PubMed] [Google Scholar]
- 64.Sencer SF, Haake RJ, Weisdorf DJ. Hemorrhagic cystitis after bone marrow transplantation. Risk factors and complications. Transplantation. 1993;56 (4):875–879. doi: 10.1097/00007890-199310000-00020. [DOI] [PubMed] [Google Scholar]
- 65.Asano Y, Kanda Y, Ogawa N, et al. Male predominance among Japanese adult patients with late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:1175–1179. doi: 10.1038/sj.bmt.1704274. [DOI] [PubMed] [Google Scholar]
- 66.Giraud G, Bogdanovic G, Priftakis P, et al. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica. 2006;91:401–404. [PubMed] [Google Scholar]
- 67.Giraud G, Priftakis P, Bogdanovic G, et al. BK-viruria and haemorrhagic cystitis are more frequent in allogeneic haematopoietic stem cell transplant patients receiving full conditioning and unrelated-HLA-mismatched grafts. Bone Marrow Transplantation. 2008;41:737–742. doi: 10.1038/sj.bmt.1705962. [DOI] [PubMed] [Google Scholar]
- 68.Leung AYH. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood. 2001;98:1971–1978. doi: 10.1182/blood.v98.6.1971. [DOI] [PubMed] [Google Scholar]
- 69.Silva LDP, Patah Pa, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. doi: 10.3324/haematol.2009.016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamamoto R, Kusumi E, Kami M, et al. Late hemorrhagic cystitis after reduced-intensity hematopoietic stem cell transplantation (RIST) Bone Marrow Transplant. 2003;32:1089–1095. doi: 10.1038/sj.bmt.1704261. [DOI] [PubMed] [Google Scholar]
- 71.Erard V, Kim HW, Corey L, et al. BK DNA viral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood. 2005;106:1130–1132. doi: 10.1182/blood-2004-12-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casadevall A, Pirofski LA. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67 (8):3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung AYH, Yuen K-Y, Kwong Y-L. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant. 2005;36:929–937. doi: 10.1038/sj.bmt.1705139. [DOI] [PubMed] [Google Scholar]
- 74.Satyanarayana G, HS, Broge T, Viscidi R, Politikos I, Koralnik I, Cutler C, Ballen K, Boussiotis VA, Marty FM, Tan SC. BK Virus Infection After Reduced Intensity Double Umbilical Cord Blood Stem-Cell Transplantation. Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco. 2012. [Google Scholar]
- 75.Gatault P, Halimi JM, Forconi C, et al. CMV infection in the donor and increased kidney graft loss: impact of full HLA-I mismatch and posttransplantation CD8(+) cell reduction. Am J Transplant. 2013;13 (8):2119–2129. doi: 10.1111/ajt.12298. [DOI] [PubMed] [Google Scholar]
- 76.Shabir S, Kaul B, Pachnio A, et al. Impaired direct priming of CD8 T cells by donor-derived cytomegalovirus following kidney transplantation. J Am Soc Nephrol. 2013;24 (10):1698–1708. doi: 10.1681/ASN.2013040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Awadalla Y, Randhawa P, Ruppert K, Zeevi A, Duquesnoy RJ. HLA mismatching increases the risk of BK virus nephropathy in renal transplant recipients. Am J Transplant. 2004;4 (10):1691–1696. doi: 10.1111/j.1600-6143.2004.00563.x. [DOI] [PubMed] [Google Scholar]
- 78.Mengel M, Marwedel M, Radermacher J, et al. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18 (6):1190–1196. doi: 10.1093/ndt/gfg072. [DOI] [PubMed] [Google Scholar]
- 79.Schönberger S, Meisel R, Adams O, et al. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biolo Blood Marrow Transplant. 2010;16:1428–1435. doi: 10.1016/j.bbmt.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 80.Ding R, Medeiros M, Dadhania D, et al. Noninvasive diagnosis of BK virus nephritis by measurement of messenger RNA for BK virus VP1 in urine. Transplantation. 2002;74 (7):987–994. doi: 10.1097/00007890-200210150-00016. [DOI] [PubMed] [Google Scholar]
- 81.Koskenvuo M, Dumoulin A, Lautenschlager I, et al. BK polyomavirus-associated hemorrhagic cystitis among pediatric allogeneic bone marrow transplant recipients: treatment response and evidence for nosocomial transmission. J Clin Virol. 2013;56 (1):77–81. doi: 10.1016/j.jcv.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Savona MR, Newton D, Frame D, Levine JE, Mineishi S, Kaul DR. Low-dose cidofovir treatment of BK virus-associated hemorrhagic cystitis in recipients of hematopoietic stem cell transplant. Bone Marrow Transplant. 2007;39:783–787. doi: 10.1038/sj.bmt.1705678. [DOI] [PubMed] [Google Scholar]
- 83.Eisen DP, Fraser IR, Sung LM, Finlay M, Bowden S, O’Connell H. Decreased viral load and symptoms of polyomavirus-associated chronic interstitial cystitis after intravesical cidofovir treatment. Clin Infect Dis. 2009;48 (9):e86–88. doi: 10.1086/597827. [DOI] [PubMed] [Google Scholar]
- 84.Mackey MC. Intravesicular cidofovir for the treatment of polyomavirus-associated hemorrhagic cystitis. Annals Pharmacother. 2012;46 (3):442–446. doi: 10.1345/aph.1Q430. [DOI] [PubMed] [Google Scholar]
- 85.Rao KV, Buie LW, Shea T, et al. Intravesicular cidofovir for the management of BK virus-associated cystitis. Biol Blood Marrow Transplant. 2009;15 (3):391–392. doi: 10.1016/j.bbmt.2008.12.490. [DOI] [PubMed] [Google Scholar]
- 86.Miller AN, Glode A, Hogan KR, et al. Efficacy and safety of ciprofloxacin for prophylaxis of polyomavirus BK virus-associated hemorrhagic cystitis in allogeneic hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011;17:1176–1181. doi: 10.1016/j.bbmt.2010.12.700. [DOI] [PubMed] [Google Scholar]