Abstract
Background
Aromatase inhibitor (AI) therapy results in substantial survival benefits in hormone receptor-positive breast cancer. Poor adherence and discontinuation rates of AI therapy are high, primarily due to treatment-related toxicities such as musculoskeletal pain. While pain-related symptoms may worsen during AI therapy, we hypothesized that non-persistence with AI therapy was associated with symptoms which were present prior to treatment initiation.
Methods
Postmenopausal women initiating AI therapy who were enrolled in a prospective clinical trial completed questionnaires at baseline to assess sleep, fatigue, mood, and pain. Reasons for treatment discontinuation during the first year of treatment were recorded. Associations between baseline patient-reported symptoms and treatment discontinuation due to toxicity were identified using logistic regression.
Results
Four hundred forty-nine patients were evaluable. Odds of treatment discontinuation were higher in patients who reported a greater number of symptoms prior to AI initiation. Baseline poor sleep quality was associated with early treatment discontinuation, with an odds ratio (OR) of 1.91 (95% CI 1.26–2.89; p=0.002). Baseline presence of tired feeling and forgetfulness had similar odds ratios for discontinuation (OR 1.76 (95% CI 1.15–2.67, p=0.009) and OR 1.66 (95% CI 1.11–2.48, p=0.015), respectively). Increasing total number of baseline symptoms was associated with increased likelihood of treatment discontinuation, with an OR 1.89 (95% CI 1.20–2.96; p=0.006) for 3–5 symptoms versus 0–2 symptoms.
Conclusions
Symptom clusters in breast cancer survivors present prior to initiation of adjuvant AI therapy may negatively impact persistence with therapy. Interventions to manage these symptoms may improve breast cancer outcomes and quality of life.
Keywords: breast cancer, aromatase inhibitor, patient-reported outcomes, nonpersistence, symptoms
Introduction
Aromatase inhibitors (AI) are routinely used for adjuvant therapy of postmenopausal women with estrogen receptor (ER)-positive early stage breast cancer. Randomized controlled trials have demonstrated improvements in disease free (DFS) and overall survival (OS) with AI therapy compared to tamoxifen.1, 2 Early discontinuation of AI therapy however has been observed in more than a quarter of patients, primarily due to toxicity of therapy.3, 4 Non-adherence to AI therapy has been associated with increases in mortality.5
The most common toxicities reported by AI-treated patients are musculoskeletal symptoms, including arthralgias and myalgias.3 Attempts to identify the cause of these side effects have focused upon clinical and treatment factors such as time since menopause, body mass index, prior tamoxifen therapy, and prior taxane chemotherapy.3, 6–8 Despite these studies, the etiology of AI toxicity remains undefined, although it is believed to be due, at least in part, to estrogen depletion.9, 10 Vitamin D deficiency may also play a role in the development of toxicity.11
Studies of breast cancer survivors have demonstrated high rates of patient-reported symptoms, including pain, insomnia, fatigue, cognitive dysfunction, and mood disorders, which can be present during all phases of treatment and can persist into the survivorship period.12, 13 A similar constellation of symptoms is commonly reported by patients with other chronic pain conditions, including fibromyalgia and temporomandibular joint disorder.14 In patients with breast cancer, these symptoms may partly arise from the multiple treatment modalities used for disease management, including surgery, chemotherapy, radiation therapy, and/or endocrine therapy. In addition, these symptoms may be related to the stress of the diagnosis itself.15
Using data from the 503-patient Exemestane and Letrozole Pharmacogenetics (ELPh) Trial, we previously reported associations between clinical and treatment factors and early discontinuation of therapy due to toxicity.3 In that study, more than 75% of patients reported musculoskeletal pain at the time of discontinuation. Based on the literature from other chronic pain disorders, we hypothesized that some breast cancer patients who develop musculoskeletal pain might also have other symptoms seen in response to stressors such as sleep disturbances, fatigue, mood disorders, and cognitive dysfunction.16 If this were the case, then it is possible that some individuals discontinue AI therapy because of their total symptom burden at baseline, not solely because of emergence of their musculoskeletal pain.17, 18 In this manuscript we report associations between the presence of patient-reported symptoms prior to initiation of an AI and treatment discontinuation within 1 year of starting the drug in the ELPh Trial.
Methods
Study participants
Post-menopausal women with stage 0-III hormone receptor positive breast cancer who were initiating treatment with an AI were eligible for enrollment on the ELPh trial (www.clinicaltrials.gov NCT00228956). Details of the ELPh trial have been reported elsewhere.19 In brief, all indicated surgery, chemotherapy, and radiation therapy were completed prior to enrollment, and patients who previously received tamoxifen therapy were permitted to enroll. The clinical trial was approved by the Institutional Review Boards at all three participating sites, and patients were required to provide written informed consent prior to undergoing study-related procedures.
Study procedures
Patients were randomized 1:1 to treatment with exemestane (Aromasin, Pfizer, New York, USA) 25 milligrams orally daily or letrozole (Femara, Novartis, Basel, Switzerland) 2.5 milligrams orally daily. Prior to AI initiation, enrolled patients completed a battery of questionnaires and underwent phlebotomy. Patients then initiated treatment, and returned to the clinic for follow-up assessments, including phlebotomy and questionnaire completion, after 1, 3, 6, 12, and 24 months of AI therapy.
Questionnaires
At each time point, patients completed the following questionnaires: depression (Center for Epidemiologic Studies – Depression (CESD)),20 anxiety (Hospital Anxiety and Depression Scale-Anxiety (HADS-A)),21 sleep quality (Pittsburgh Sleep Quality Index (PSQI)),22 and general symptoms including joint pain, fatigue, difficulty concentrating, forgetfulness, and vaginal dryness (the Breast Cancer Prevention Trial (BCPT) symptom checklist).23
Laboratory studies
Serum samples obtained at the baseline and 3 month time points were assayed for estradiol (E2), estrone-1-sulfate (E1S), and estrone (E1) using an ultrasensitive gas chromatography tandem mass spectroscopy assay, as previously described.24 The lower limit of quantification for E2 was 0.625 pg/ml, for E1S was 2.88 pg/ml, and for E1 was 1.56 pg/ml.
Statistical plan
The primary objective of the ELPh trial was to determine the genetic predictors of change in breast density after 24 months of either an azole (letrozole) or a steroidal (exemestane) AI medication; these results are being published separately.25 The primary objective of the exploratory analysis reported in this manuscript was to investigate associations between patient-reported symptoms prior to AI initiation and discontinuation of AI therapy due to toxicity during the initial 12 months of therapy.
Validated questionnaires to evaluate fatigue or cognitive dysfunction were not included in this trial. Therefore, to investigate these symptoms, the following individual items on the BCPT questionnaire23 were analyzed: “joint pain” (pain), “forgetfulness” and “difficulty concentrating” (cognitive dysfunction), and “tired feeling” (fatigue). “Vaginal dryness” was also analyzed as a common AI-related symptom thought to be predominantly peripherally rather than centrally mediated. Presence of each symptom was defined as the patient reporting of any degree of severity of the symptom (i.e., slightly, moderately, quite a bit, or extremely).
Descriptive analyses were conducted for all baseline characteristics for the entire sample and by discontinuation status. Odds ratios and their significance comparing characteristics for those who discontinued AI therapy due to symptoms by the end of one year to those who remained on treatment at one year were calculated using logistic regression. Baseline characteristics that were associated with AI discontinuation due to symptoms by the end of the first year by a p-value <0.20 were included in a multivariable model where a stepwise procedure was utilized to find the variables significantly associated with AI discontinuation due to toxicity. Estrogen measurements were natural log transformed in all models.
Additionally, symptoms were combined to account for the total number of symptoms each patient experienced at baseline. Symptoms included in this variable were sleep quality (poor: PSQI >5 vs. good: PSQI ≤5), concentration (any severity vs. none or no severity), tired feeling (any severity vs. none or no severity), anxiety (no: HADS A ≤7 vs. borderline or definite: HADS A >7), and depression (no: CESD<16 vs. possible or probable: CESD≥16). If the baseline characteristic score was missing, it was conservatively coded as a 0 and the total number of symptoms was summed for each patient for a final number of symptoms ranging from zero to five. The total number of symptoms was dichotomized as being either 0–2 or 3–5 and association between this variable and AI discontinuation was assessed in the univariate and multivariable setting.
All analyses were performed in SAS v9.3 (SAS Institute, Inc, Cary, NC).
Results
Baseline patient characteristics and treatment discontinuation due to toxicity
The ELPh trial included 503 patients, of whom 500 were randomly assigned to letrozole or exemestane and treated for 24 months (Figure 1). Enrolled patients who discontinued therapy within 1 year of treatment initiation because of toxicity were compared to those who continued study participation beyond the 1 year time point (Table 1). During the 24 month study, a total of 51 patients discontinued therapy for reasons other than treatment-emergent toxicity, including inability to undergo phlebotomy and recovery of ovarian function, as previously reported.3
Figure 1. Consort diagram of patient flow in the ELPh trial.
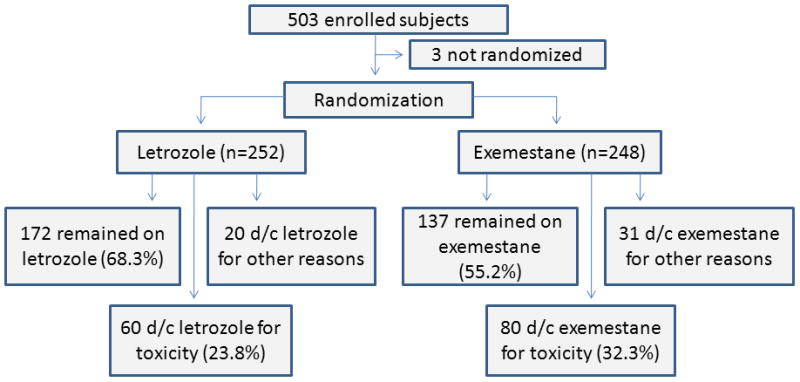
D/c = discontinued
Table 1. Baseline patient characteristics by AI treatment discontinuation status.
Baseline dichotomous characteristics of patients enrolled in the ELPh trial who discontinued AI therapy due to any symptom by or at 12 months versus all others. Comparison of cohort that discontinued versus all others was performed using logistic regression. Mean values are given in parenthesis.
| Total (n=449) | Discontinued due to Symptoms (n=140) | All Others (n=309) | Odds Ratio (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristic | N | % | N | % | N | % | ||
| Age | 491 | (59.0) | 140 | (56.0) | 309 | (60.0) | 0.97 (0.95–1.00) | |
| Body Mass Index | 490 | (29.1) | 140 | (28.7) | 308 | (29.3) | 1.00 (0.96–1.03) | 0.76 |
| Estradiol pg/mL* | 463 | (4.7) | 132 | (4.1) | 304 | (4.9) | 0.91 (0.71–1.18) | 0.50 |
| Estrone Sulfate pg/mL* | 459 | (243.5) | 128 | (275.0) | 304 | (228.0) | 1.20 (0.92–1.56) | 0.18 |
| Estrone pg/mL* | 464 | (23.0) | 133 | (21.7) | 304 | (23.8) | 0.95 (0.68–1.31) | 0.73 |
| Race | ||||||||
| White | 401 | 89.3 | 128 | 91.4 | 273 | 88.3 | 1.00 (−) | 0.33 |
| Black/Other | 48 | 10.7 | 12 | 8.6 | 36 | 11.7 | 0.71 (0.36–1.41) | |
| Breast cancer stage | ||||||||
| DCIS | 27 | 6.0 | 8 | 5.8 | 19 | 6.2 | 1.00 (−) | 0.99 |
| I | 234 | 52.3 | 73 | 52.5 | 161 | 52.3 | 1.08 (0.45–2.57) | |
| II | 143 | 32.0 | 45 | 32.4 | 98 | 31.8 | 1.09 (0.44–2.68) | |
| III | 43 | 9.6 | 13 | 9.4 | 30 | 9.7 | 1.03 (0.36–2.95) | |
| Chemo Treatment | ||||||||
| Yes | 200 | 44.5 | 64 | 45.7 | 136 | 44.0 | 1.07 (0.72–1.60) | 0.51 |
| No | 249 | 55.5 | 76 | 54.3 | 173 | 56.0 | 1.00 (−) | |
| Taxane | ||||||||
| Yes | 144 | 32.1 | 51 | 36.4 | 93 | 30.1 | 1.33 (0.87–2.03) | 0.18 |
| No | 305 | 67.9 | 89 | 63.6 | 216 | 69.9 | 1.00 (−) | |
| Radiation Therapy | ||||||||
| Yes | 355 | 79.4 | 113 | 81.3 | 242 | 78.6 | 1.19 (0.72–1.97) | 0.51 |
| No | 92 | 20.6 | 26 | 18.7 | 66 | 21.4 | 1.00 (−) | |
| Tamoxifen | ||||||||
| Yes | 163 | 36.5 | 57 | 41.0 | 106 | 34.4 | 1.33 (0.88–2.00) | 0.18 |
| No | 284 | 63.5 | 82 | 59.0 | 202 | 65.6 | 1.00 (−) | |
|
| ||||||||
| Hormone Replacement Therapy | ||||||||
| Yes | 231 | 51.8 | 65 | 47.1 | 166 | 53.9 | 0.76 (0.51–1.14) | 0.18 |
| No | 215 | 48.2 | 73 | 52.9 | 142 | 46.1 | 1.00 (−) | |
|
| ||||||||
| Drug Assignment | ||||||||
| Exemestane | 217 | 48.3 | 80 | 57.1 | 137 | 44.3 | 1.67 (1.12–2.51) | 0.012 |
| Letrozole | 232 | 51.7 | 60 | 42.9 | 172 | 55.7 | 1.00 (−) | |
| Depression (CESD) | ||||||||
| Normal | 381 | 85.0 | 113 | 81.3 | 268 | 86.7 | 1.00 (−) | 0.14 |
| Possible/ Probable Depression (>16) | 67 | 15.0 | 26 | 18.7 | 41 | 13.3 | 1.50 (0.88–2.58) | |
| Anxiety (HADS A) | ||||||||
| Non-Case | 383 | 85.7 | 118 | 84.9 | 265 | 86.0 | 1.00 (−) | 0.75 |
| Borderline or Case | 64 | 14.3 | 21 | 15.1 | 43 | 14.0 | 1.10 (0.62–1.93) | |
| Sleep Quality (PQSI) | ||||||||
| Good Sleep Quality (≤5) | 224 | 52.1 | 55 | 41.0 | 169 | 57.1 | 1.00 (−) | 0.002 |
| Poor Sleep Quality | 206 | 47.9 | 79 | 59.0 | 127 | 42.9 | 1.91 (1.26–2.89) | |
| Joint Pain Severity | ||||||||
| None or Not at All | 184 | 41.4 | 50 | 36.2 | 134 | 43.8 | 1.00 (−) | 0.14 |
| Slight - Extreme | 260 | 58.6 | 88 | 63.8 | 172 | 56.2 | 1.37 (0.91–2.08) | |
| Forgetfulness | ||||||||
| None or Not at All | 239 | 53.6 | 62 | 44.9 | 177 | 57.5 | 1.00 (−) | 0.015 |
| Slight - Extreme | 207 | 46.4 | 76 | 55.1 | 131 | 42.5 | 1.66 (1.11–2.48) | |
| Tired Feeling | ||||||||
| None or Not at All | 186 | 41.8 | 45 | 32.6 | 141 | 45.9 | 1.00 (−) | 0.009 |
| Slight - Extreme | 259 | 58.2 | 93 | 67.4 | 166 | 54.1 | 1.76 (1.15–2.67) | |
| Concentration | ||||||||
| None or Not at All | 342 | 76.7 | 100 | 71.9 | 242 | 78.8 | 1.00 (−) | 0.11 |
| Slight - Extreme | 104 | 23.3 | 39 | 28.1 | 65 | 21.2 | 1.45 (0.92–2.30) | |
| Vaginal Dryness | ||||||||
| None or Not at All | 301 | 68.1 | 87 | 63.0 | 214 | 70.4 | 1.00 (−) | 0.13 |
| Slight - Extreme | 141 | 31.9 | 51 | 37.0 | 90 | 29.6 | 1.39 (0.91–2.13) | |
Logged values were used in logistic regression
CESD: Center for Epidemiologic Studies – Depression. HADS-A: Hospital Anxiety and Depression Scale – Anxiety. PSQI: Pittsburgh Sleep Quality Index.
Of the 449 patients eligible for the analysis, 140 (31.2%) discontinued AI therapy due to symptoms by the end of the first year of treatment (Table 1). Patients who discontinued therapy were significantly younger than those who continued therapy (median age 56 vs. 60; OR 0.97, 95% CI 0.95–1.00; p=0.035). As previously reported,3 no univariate statistically significant association (at the p=0.05 level) was identified between treatment discontinuation and BMI (p=0.76), baseline serum estrogen concentration (p=0.50, 0.18, 0.73 for estradiol, estrone sulfate and estrone, respectively), race (p=0.33), previous treatment with chemotherapy (p=0.51), taxane-based chemotherapy (p=0.18), radiation therapy (p=0.51), prior tamoxifen (p=0.18), or prior hormone replacement therapy (p=0.18). Similarly, there was no statistically significant association between stage of breast cancer at diagnosis and likelihood of treatment discontinuation (p=0.99). Treatment with exemestane was significantly associated with an increased risk of treatment discontinuation compared to letrozole (OR 1.67; 95% CI (1.12–2.51); p=0.012).
Although half of the analyzed patients started AI therapy within 6 months of undergoing definitive surgery, the time interval ranged from 0 to 109 months. Time from surgery to the initiation of AI therapy was not significantly associated with AI discontinuation due to symptoms. Those who discontinued therapy had a median time since surgery of 8 months (range 1–108), whereas those who remained on AI therapy beyond 12 months had a median time since surgery of 6 months (range 0–109, p=0.11).
Patient-reported symptoms prior to AI therapy initiation and treatment discontinuation
In the ELPh trial, 67 (15.0%) patients reported being possibly or probably depressed at the time of AI initiation (using the CESD), and 64 (14.3%) reported being borderline or definitely anxious (using HADS-A, Table 1; Figure 2). Analysis of depressive symptomatology scores as a dichotomous variable (possibly or probably depressed versus not depressed) using the CESD questionnaire did not demonstrate a statistically significant association between depression prior to AI initiation and increased risk of treatment discontinuation within the first year (OR 1.50; 95% CI 0.88–2.58; p=0.14). Similarly, no significant association was identified between pre-existing anxiety assessed using the HADS-A questionnaire and discontinuation of AI therapy (OR 1.10; 95% CI 0.62–1.93; p=0.75).
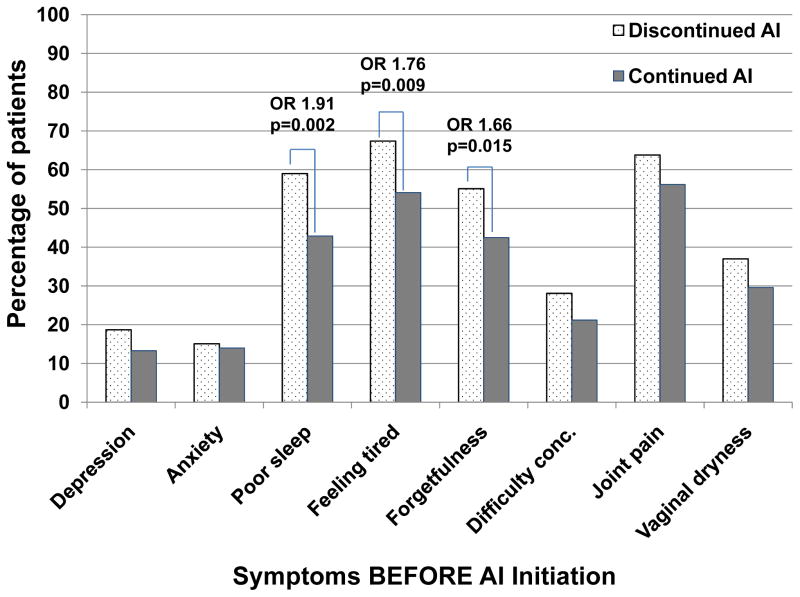
Figure 2. Percentage of evaluable patients who continued or discontinued aromatase inhibitor (AI) therapy within the first year of treatment by baseline symptoms present before AI initiation.
Dotted bars represent the percentage of total evaluable patients who discontinued AI therapy within 1 year, and solid bars represent the percentage of total evaluable patients who continued AI therapy beyond 1 year. Odds ratios (OR) and p values are given for those comparisons that were statistically significant. Conc = concentrating.
In the ELPh trial, 206 patients (47.9%) reported poor sleep quality on the PSQI questionnaire prior to initiation of AI therapy (Table 1; Figure 2). A larger percentage of patients with poor sleep quality prior to AI initiation discontinued therapy because of toxicity by 1 year compared to those with good sleep quality (59.0% vs. 42.9%; OR=1.91, 95% CI 1.26–2.89; p=0.002). Of the 206 patients who reported poor sleep quality prior to AI initiation, 139 (67.5%) reported poor sleep quality at 75% or more of their subsequent visits during AI therapy and seven discontinued after reporting sleep problems at the initial assessment. Fifty-seven of these 139 (41.0%) patients discontinued AI treatment by the end of one year.
The other patient-reported symptoms were collected using a general symptom questionnaire (Table 1; Figure 2). No statistically significant association was identified between patient-reported presence of joint pain at baseline and treatment discontinuation due to toxicity (63.8% vs 56.2%, OR 1.37, 95% CI 0.91–2.08; p=0.14). Patients who reported forgetfulness or feeling tired prior to AI initiation were more likely to discontinue therapy due to toxicity compared to those who did not report having the symptom (forgetfulness: 55.1% vs. 42.5%; OR 1.66, 95% CI 1.11–2.48; p=0.015; tired feeling: 67.4% vs. 54.1%; OR 1.76, 95% CI 1.15–2.67; p=0.009). In addition, there was no statistically significant univariate association between presence of difficulty concentrating prior to starting AI therapy and treatment discontinuation (28.1% vs. 21.2%; OR 1.45, 95% CI 0.92–2.13; p=0.11) or vaginal dryness and treatment discontinuation (37.0% vs. 29.6%; OR 1.39, 95% CI 0.91–2.13; p=0.13).
The following 5 symptoms were combined to assess if having more symptoms at baseline was associated with treatment discontinuation: poor sleep quality (PSQI>5), depression (CESD≥16), anxiety (HADS A≥7), any degree of tired feeling, and any degree of difficulty concentrating. Patients who reported a greater number of symptoms prior to treatment initiation were more likely to discontinue therapy because of toxicity (Figure 3). There was a statistically significant difference in treatment discontinuation rate among those who reported 0, 1–2, and 3–5 symptoms prior to AI initiation (p=0.006). Of the 117 patients who did not report any of these symptoms at baseline, 26 (22%) discontinued AI therapy within 1 year because of side effects. Of the 225 patients who reported one or two symptoms before AI initiation, 69 (31%) discontinued AI therapy. In contrast, of the 107 patients with 3 or more of these symptoms, 45 (42%) discontinued AI therapy. Compared to those patients who reported none of the symptoms at baseline, those patients with 1 or 2 symptoms at baseline had an increased likelihood of AI discontinuation during the first year with an odds ratio of 1.55 (95% CI 0.92–2.60), and those with 3–5 symptoms had an increased likelihood with an odds ratio of 2.54 (95% CI 1.42–4.54; p=0.007). Furthermore, we found, on average, that individuals who discontinued AI therapy within 1 year because of toxicity reported having 3 or more symptoms at 37% of their subsequent visits, compared with 22% for who did not discontinue therapy (p=0.0009).
Figure 3. Percentage of patients who discontinued aromatase inhibitor (AI) therapy according to the number of symptoms present prior to AI initiation.
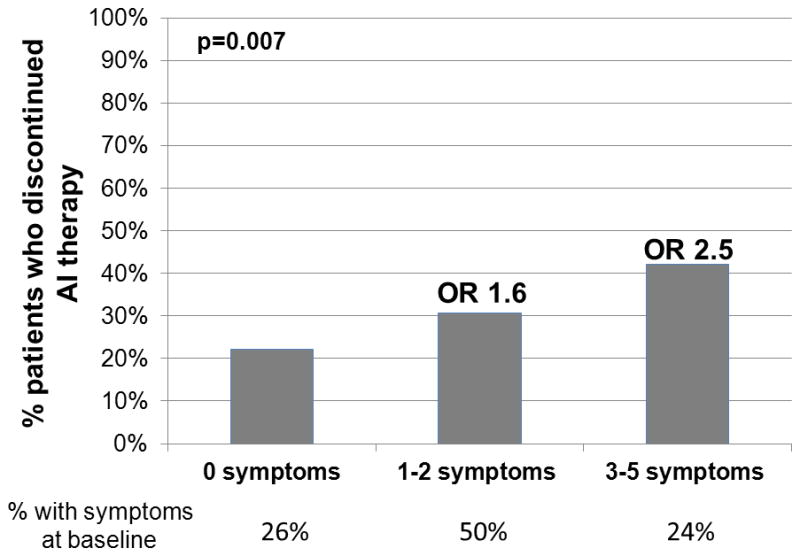
The number of patients in each group is given below the x axis. OR: odds ratio.
Multivariable analysis of predictors of treatment discontinuation due to toxicity
Multivariable logistic regression was used to evaluate predictors of treatment discontinuation due to toxicity. Including all variables univariately related to discontinuation from Table 1 we sought a more parsimonious model using a stepwise approach. Thus we evaluated a model with age, AI medication, sleep quality and concentration (Table 2). E1S at baseline (p=0.14) and pain score (p=0.12) were the last variables to be removed from the model. With all 5 variables included, the area under the curve (measure of predictive power in logistic regression) was 0.68. In either the full model or the reduced model that excluded E1S and pain score, both poor sleep quality and difficulty concentrating remained statistically significant. In the reduced model shown in Table 2, those with poor sleep quality had 1.79 times the odds of discontinuing AI medication by the end of the first year of treatment, holding age, AI medication, and concentration severity constant (p=0.01). Those with moderate to extreme difficulty concentrating had 2.62 times the odds of discontinuing treatment, holding all other variables constant (p=0.017). The area under the curve of this reduced model was 0.65.
Table 2. Multivariable logistic regression analysis of predictors of AI treatment discontinuation, including individual symptoms.
Multivariable logistic regression resulting from step-down analysis of treatment discontinuation during the first year of AI therapy due to baseline sleep and concentration difficulties before AI initiation and patient characteristics (n=428, area under the curve = 0.65)
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Age | 0.97 (0.95–1.00) | 0.028 |
| Drug (exemestane vs. letrozole) | 1.63 (1.07–2.49) | 0.024 |
| Sleep quality (PSQI >5 vs. ≤5) | 1.79 (1.15–2.79) | 0.010 |
| Concentration (vs. none) | 0.017 | |
| Not at all or slight | 0.75 (0.42–1.33) | |
| Moderate-extreme | 2.62 (1.22–5.65) |
A second model was evaluated to specifically analyze the effect of total number of symptoms at baseline (3–5 vs. 0–2; Table 3). In this model, age (OR 0.97; p=0.03), AI medication (OR 1.66; p=0.02), and 3 or more symptoms (OR 1.68; p=0.03) were statistically significantly associated with treatment discontinuation due to toxicity. This model also included pain score (VAS) where each 1 point increase in pain increased the odds of discontinuation by 1.09 (p=0.07). This model had an area under the curve of 0.64. When baseline E1S (p=0.17) was also included in this base model the area under the curve increased slightly to 0.65.
Table 3. Multivariable logistic regression analysis of predictors of AI treatment discontinuation including symptom clusters.
Multivariable logistic regression resulting from step-down analysis of treatment discontinuation during the first year of AI therapy due to number of symptoms present before AI initiation and patient characteristics (n=447, area under the curve = 0.64)
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Age | 0.97 (0.95–1.00) | 0.025 |
| Drug (exemestane vs. letrozole) | 1.66 (1.10–2.50) | 0.016 |
| VAS | 1.09 (0.99–1.20) | 0.067 |
| Total Number of Symptoms (vs. 2 or less) | ||
| 3 or more | 1.68 (1.05–2.70) | 0.031 |
Discussion
Early discontinuation of AI therapy, which is associated with worse breast cancer outcomes, is frequently due to the development of side effects, especially musculoskeletal toxicity.3, 5 Numerous prior studies of AI therapy have reported increased risk of developing musculoskeletal symptoms during AI treatment with factors such as age, body mass index, pre-existing pain or arthritis, prior chemotherapy, and prior tamoxifen.3, 6–8 For this analysis, we instead focused on associations between patient-reported non-pain symptoms present before initiation of AI therapy, such as poor sleep quality, fatigue, depression, and anxiety, and increased rates of premature treatment discontinuation because of toxicity. In the relatively large ELPh trial, we found that pre-existing poor sleep quality and difficulty concentrating were strongly associated with early treatment discontinuation due to toxicity. In addition, increased symptom burden prior to AI therapy initiation was associated with both increased symptom burden during AI treatment and increased likelihood of treatment discontinuation within 1 year.
Our findings are consistent with those reported in the MA.27 trial of exemestane versus anastrozole.26 In that study, the hazard ratio of early treatment discontinuation due to bother from side effects from prior treatment that were present at the time of AI initiation was 1.29 (95% CI 1.08–1.55; p=0.006). The contributions of specific side effects were not described.
The symptom cluster of mood disorders, fatigue, and difficulty sleeping is frequently identified in patients across diseases, including cancer and chronic pain syndromes.27 Many of the published reports evaluating symptom clusters in breast cancer patients have focused on patients undergoing therapy with short-term treatment modalities, including chemotherapy and/or radiation therapy. For example, in one study patients who reported a greater number of symptoms prior to the start of chemotherapy were more likely to report worse symptoms during the treatment.27 Other studies have identified specific patterns of change in symptoms over time during chemotherapy and radiation. These patterns ultimately impact functional status and quality of life and may influence patient management.17, 28 In contrast, few published reports have focused on patterns of specific symptoms that occur during long-term adjuvant endocrine therapy. Analogous to what has previously been seen with chemotherapy, in the ELPh trial we identified an association between greater number of symptoms prior to treatment and decreased persistence with AI therapy due to toxicity. The symptoms of poor sleep and difficulty concentrating stood out as clinically important contributors to this symptom cluster.
Knowledge of these associations is clinically relevant, since these symptoms are not frequently recognized as problematic and are not typically carefully assessed or aggressively managed by oncologists. Indeed, since difficulty sleeping and complaints of fatigue are common in patients with breast cancer29 and are often thought to be self-limited side effects of prior therapies including chemotherapy and radiation therapy, oncologists may not appreciate that the presence of these symptoms may compromise future treatments. These results raise the possibility that asking patients about these symptoms and addressing them at the time of AI initiation, or even prophylactically, could identify patients at risk, and allow the implementation of measures to improve adherence/persistence with subsequent adjuvant endocrine therapy.
Although management of these symptoms is essential for improving quality of life, these data suggest that it may also impact breast cancer outcomes if improvement in symptoms led to increased adherence to and persistence with therapy. There is a paucity of effective treatment options for poor sleep, cognitive problems, and fatigue in cancer survivors. Numerous clinical trials have been conducted to test various pharmacologic therapies for fatigue, although few studies of pharmacologic treatments have been conducted specifically for sleep disturbance in cancer patients.18 Meta-analyses have demonstrated a statistically significant benefit from methylphenidate compared to placebo for cancer-related fatigue, although the clinical benefit is modest.30 There are also considerable data to support use of cognitive behavioral therapy (CBT) for treatment of both insomnia and fatigue in cancer patients.30–32 Use of CBT is recommended in the National Comprehensive Cancer Network (NCCN) guidelines for management of Cancer-Related Fatigue,33 although it is unknown how often these behavioral techniques are actually used in clinical practice. Improvements in fatigue have also been noted in cancer patients treated with other non-pharmacologic interventions, including physical activity.30 Although a number of treatment options are listed in the currently available national guidelines, no individual modality is preferred.33
Our study has multiple strengths. The findings were derived from a large, prospective clinical trial in which reasons for discontinuation were prospectively recorded. Validated questionnaires were used to assess patient-reported sleep, pain, and mood disorders, although fatigue and cognitive function data elements had to be obtained from a more general symptom questionnaire. The study medication was provided to the patients by the study, so cost of the medication was not a factor in persistence with therapy.
In this analysis we identified a numerically greater but not statistically significant increased risk of treatment discontinuation within 1 year in patients treated with a taxane-based chemotherapy regimen, which we and others have previously reported. One important difference between the prior analysis of the ELPh trial and this one is the focus on discontinuation specifically within the first year of AI therapy. This 1 year limitation was intended to restrict analysis to symptoms likely due to the AI medication, and less likely to changes that can occur over time with the natural aging process, such as worsening osteoarthritis. Our findings suggest that pre-existing symptoms are more strongly associated with AI discontinuation due to toxicity than clinical factors such as prior treatment with chemotherapy. However, it remains possible that prior treatments such as chemotherapy contributed to the symptoms reported by patients at the time of the baseline study visit.
Our findings demonstrate the importance of symptom clusters that include poor sleep and difficulty concentrating in patients with breast cancer, and the potential detrimental effects of these symptoms not just on quality of life but also on AI treatment adherence and breast cancer outcomes. Therapies to improve the constellation of symptoms rather than those that target individual symptoms should be considered to allow optimal patient care. Clinical trials are warranted to evaluate the impact of management of symptom clusters on adherence to potentially life-saving adjuvant endocrine therapies.
Acknowledgments
Funding sources: NLH is a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (grant number CI-53-10). This study was also supported in part by Pharmacogenetics Research Network Grant no. U01-GM61373 (to DAF) and Clinical Pharmacology training grant 5T32-GM08425 (to DAF) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), Bethesda, MD, and by Grant Numbers M01-RR00042 (UM), M01-RR00750 (IU) and M01-RR00052 (JHU) from the National Center for Research Resources (NCRRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. In addition, these studies were supported by grants from Pfizer, Inc (to DFH), Novartis Pharma AG (to DFH), and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (to DFH). Study medication was provided by Pfizer (exemestane) and Novartis (letrozole).
Footnotes
Financial disclosures: SEH serves as a consultant to Analgesic Solutions and receives research funding from Forest and Cerephex. DFH received research funding from AstraZeneca, Novartis, and Pfizer. AMS received research funding from Novartis and Pfizer. DAF received research funding from Novartis and Pfizer and serves on the Scientific Advisory Board for Quest Diagnostics, and as a consultant to Boehringer-Ingelheim. VS received research grants from Abbott, Celgene, Medimmune, Merck, Novartis, and Pfizer. DJC serves as a consultant for Pfizer, Lilly, Forest, Cerephex, Tonix, Purdue, Theravance, and Nuvo and receives research funding from Pfizer, Forest, Cerephex, Nuvo and Merck. DAW serves as a consultant to Pfizer and to Health Focus Inc. NLH received research funding from AstraZeneca, Eli Lilly, BioMarin Pharmaceuticals, and Sanofi Aventis. KMK and JC have no disclosures to report.
References
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 3.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation due to treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 7.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–3639. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 9.Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum. 2005;52:2594–2598. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- 10.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22:1401–1408. [PubMed] [Google Scholar]
- 11.Niravath P. Aromatase inhibitor-induced arthralgia: a review. Ann Oncol. 2013;24:1443–1449. doi: 10.1093/annonc/mdt037. [DOI] [PubMed] [Google Scholar]
- 12.Bower JE. Behavioral symptoms in patients with breast cancer and survivors. J Clin Oncol. 2008;26:768–777. doi: 10.1200/JCO.2007.14.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz PA, Coscarelli A, Fred C, Kahn B, Polinsky ML, Petersen L. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 14.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia, and temporomandibular disorder. Arch Intern Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 15.Kangas M, Henry JL, Bryant RA. Posttraumatic stress disorder following cancer. A conceptual and empirical review. Clin Psychol Rev. 2002;22:499–524. doi: 10.1016/s0272-7358(01)00118-0. [DOI] [PubMed] [Google Scholar]
- 16.Henry NL, Clauw DJ. Thinking beyond the tumor to better understand chronic symptoms in breast cancer survivors. Breast cancer research and treatment. 2012;133:413–416. doi: 10.1007/s10549-011-1804-8. [DOI] [PubMed] [Google Scholar]
- 17.Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. Eur J Oncol Nurs. 2010;14:101–110. doi: 10.1016/j.ejon.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorentino L, Rissling M, Liu L, Ancoli-Israel S. The symptom cluster of sleep, fatigue and depressive symptoms in breast cancer patients: severity of the problem and treatment options. Drug Discovery Today: Disease Models. 2011;8:167–173. doi: 10.1016/j.ddmod.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–372. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 21.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 23.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 24.Santen RJ, Demers L, Ohorodnik S, et al. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Henry NL, Chan HP, Dantzer J, et al. Aromatase inhibitor-induced modulation of breast density: clinical and genetic effects. British J Cancer. 2013;109:2331–2339. doi: 10.1038/bjc.2013.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner LI, Zhao F, Chapman JA, et al. Patient-reported predictors of early treatment discontinuation: NCIC JMA.27/E1Z03 quality of life study of postmenopausal women with primary breast cancer randomized to exemestane or anastrozole. Cancer Res. 2011;71:Abst S6–2. doi: 10.1007/s10549-018-4713-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Fiorentino L, Natarajan L, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psychooncology. 2009;18:187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HJ, McDermott PA, Barsevick AM. Comparison of Groups With Different Patterns of Symptom Cluster Intensity Across the Breast Cancer Treatment Trajectory. Cancer Nurs. 2013 doi: 10.1097/NCC.0b013e31828293e0. epub Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 30.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 31.Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: Sleep and psychological effects. J Clin Oncol. 2005;23:6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 32.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 33.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]