SUMMARY
The adipocyte-derived hormone adiponectin promotes metabolic and cardiovascular health. Circulating adiponectin increases in lean states such as caloric restriction (CR), but the reasons for this paradox remain unclear. Unlike white adipose tissue (WAT), bone marrow adipose tissue (MAT) increases during CR, and both MAT and serum adiponectin increase in many other clinical conditions. Thus, we investigated if MAT contributes to circulating adiponectin. We find that adiponectin secretion is greater from MAT than from WAT. Notably, specific inhibition of MAT formation in mice results in decreased circulating adiponectin during CR, despite unaltered adiponectin expression in WAT. Inhibiting MAT formation also alters skeletal muscle adaptation to CR, suggesting that MAT exerts systemic effects. Finally, we reveal that both MAT and serum adiponectin increase during cancer therapy in humans. These observations identify MAT as an endocrine organ that contributes significantly to increased serum adiponectin during CR, and perhaps in other adverse states.
INTRODUCTION
White adipose tissue (WAT) is a major endocrine organ that exerts diverse systemic effects. One of the most extensively studied adipocyte-secreted factors is the hormone adiponectin, which promotes insulin sensitivity, fat oxidation, anti-atherogenic and anti-cancer effects (Ye and Scherer, 2013). Serum adiponectin is also a well-established biomarker for insulin resistance and cardiovascular disease; indeed, circulating adiponectin is low in obese, insulin-resistant individuals and in other adverse metabolic states (Ye and Scherer, 2013). Conversely, serum adiponectin increases in lean, insulin-sensitive states such as with calorie restriction (CR) in animals and anorexia nervosa (AN) in humans (Combs et al., 2003; Dolezalova et al., 2007; Pannacciulli et al., 2003). Reduced circulating adiponectin in obesity likely derives from decreased adiponectin expression and secretion, which may result from mitochondrial dysfunction or aberrantly increased inflammation, hypoxia or endoplasmic reticulum stress (Ye and Scherer, 2013). Far less is known about why serum adiponectin increases in lean states. Although some studies report increased adiponectin expression in WAT during extensive CR (Qiao et al., 2011), most studies in mice and humans find that prolonged CR or extensive weight loss increases serum adiponectin without affecting adiponectin expression or secretion from WAT (Behre et al., 2007; Combs et al., 2003; Kovacova et al., 2009; Wang et al., 2006). Indeed, adiponectin expression in WAT decreases in human subjects with AN (Dolezalova et al., 2007). Adiponectin clearance is also unaltered during CR (Qiao et al., 2011). Thus, in lean states such as CR or AN, the paradoxical increase in serum adiponectin can occur without greater expression or secretion from WAT, or decreased adiponectin clearance.
Our knowledge of adiponectin derives from extensive study of WAT biology over the past generation. In comparison, metabolic research has largely neglected another adipose depot: bone marrow adipose tissue (MAT). Bone marrow (BM) adipocytes were identified over a century ago, and MAT accounts for approximately 70% of BM volume in adult humans (Fazeli et al., 2013). In striking contrast to WAT, MAT markedly increases during CR in animals, including humans (Devlin, 2011). Thus, in this manuscript we investigate the hypothesis that MAT is a source of circulating adiponectin in states of leanness. We provide direct evidence that MAT is required for maximal increases in serum adiponectin during CR.
RESULTS
Anorexia nervosa is associated with increased serum adiponectin and MAT
Previous studies have not assessed both serum adiponectin and BM adiposity in a single cohort of AN subjects. Thus, we completed both analyses in a group of AN subjects and healthy controls (HC). Body mass index (BMI), adiposity and bone mineral density (BMD) were significantly lower in AN compared to HC subjects (Table 1). AN subjects had significantly higher MAT of the L4 vertebrae, femoral metaphysis and femoral diaphysis, and increased total and HMW serum adiponectin, despite decreased total fat mass (Table 1). Further calculations revealed that MAT comprised 13.0% of total adipose mass in HC subjects and 31.5% in AN subjects. This striking increase strongly suggests that, during CR, MAT exists in an amount that has clear potential to exert systemic effects through secretion of endocrine factors such as adiponectin.
Table 1. MAT, WAT, BMD, and serum adiponectin in subjects with Anorexia Nervosa and healthy controls.
Subjects with Anorexia Nervosa (AN; n = 12) and healthy controls (HC; n = 21) were characterized as described in Experimental Procedures and in Supplemental Information. Data are mean ±SD.
| HC | AN | p-value | |
|---|---|---|---|
| Age (years) * | 27.8 ±4.6 | 30.3 ±6.8 | 0.46 |
| Body mass (kg) | 61.7 ±6.1 | 49.0 ±5.2 | <0.0001 |
| % IBW | 100.4 ±5.4 | 79.2 ±4.9 | <0.0001 |
| BMI (kg/m2) | 22.3 ±1.6 | 17.3 ±1.3 | <0.0001 |
|
| |||
| % Body fat | 27 ±4.7 | 18.8 ±5.5 | <0.001 |
| Total fat mass (kg) | 17.1 ±3.6 | 9.9 ±3.2 | <0.001 |
|
| |||
| Total body BMD * | 1.11 ±0.10 | 0.97 ±0.07 | <0.001 |
| L4 MAT * | 0.53 ±0.26 | 0.86 ±0.36 | <0.01 |
| Metaphysis MAT | 2.20 ±0.87 | 3.93 ±2.38 | 0.04 |
| Diaphysis MAT | 4.08 ±2.76 | 6.76 ±2.95 | <0.02 |
| Estimated MAT mass (kg) | 2.16 ±0.24 | 2.90 ±0.21 | <0.0001 |
| MAT as % Total fat mass | 13.0 ±2.7 | 31.5 ±9.2 | <0.0001 |
|
| |||
| Total adiponectin (ng/mL) * | 6396 ±1864 | 9573 ±5291 | <0.03 |
| HMW adiponectin (ng/mL) * | 3735 ±1476 | 6406 ±4139 | <0.01 |
Asterisks indicate that a Wilcoxon rank sum test was used to compare these data because they were non-normally distributed. BMI, body mass index; IBW, ideal body weight; BMI, body mass index; BMD, bone mineral density; MAT, marrow adipose tissue; HMW, high molecular weight.
The relative expression and secretion of adiponectin is greater from MAT than from WAT
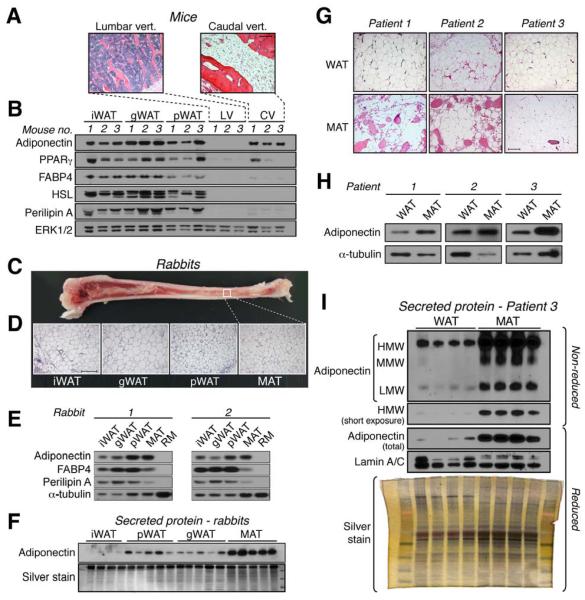
The ability of MAT to express or secrete adiponectin has not been addressed. To do so, we took advantage of the fact that, in mice, BM of lumbar vertebrae (LV) contains few adipocytes, whereas BM of caudal vertebrae (CV) is almost entirely MAT (Fig 1A). We found that adiponectin expression in CV of C57BL/6J mice was similar to that in inguinal WAT (iWAT), gonadal WAT (gWAT), and perirenal WAT (pWAT) (Fig 1B). In contrast, expression of peroxisome proliferator-activated receptor-γ (PPARγ) was similar between WAT and CV of some mice, but much lower in CV of other mice. Other adipocyte proteins, such as fatty acid-binding protein 4 (FABP4) and perilipin A, were also markedly decreased in CV compared to WAT, while hormone-sensitive lipase (HSL) expression was undetectable in CV (Fig 1B). Similar results were observed in C3H/HeJ mice (Fig S1A). However, because CV also contain many non-adipocyte populations, expression of adipocyte proteins in CV lysates might be diluted compared to that in WAT. Therefore, we next analyzed pure MAT from rabbit tibiae, in which there is a gradient of increasing adiposity from proximal to distal (Fig 1C). This pattern of MAT distribution, which is also typical of that in humans (Scheller and Rosen, 2014), allowed us to isolate intact pieces of pure MAT or red marrow (RM). No trabecular bone was observed in any of the MAT samples (Fig 1D). As in mice, adiponectin protein was robustly expressed in rabbit MAT relative to FABP4 and perilipin A (Fig 1E); this was also observed for Adipoq and Fabp4 transcripts (Fig S1B). Expression of Pparg was similar between MAT and WAT, whereas Cebpa and Lep were lower in MAT (Fig S1B). These observations show that, compared to WAT, MAT expresses adiponectin at disproportionately high levels relative to other typical adipocyte transcripts and proteins.
Figure 1. Relative expression and secretion of adiponectin is greater from MAT than from WAT.
(A,B) iWAT, gWAT, pWAT, lumbar vertebrae (LV; for red marrow) and caudal vertebrae (CV; for MAT) were isolated from male C57BL/6J mice. (A) Micrographs of H&E-stained tissue sections. (B) Immunoblots of total protein lysates from tissues of three mice; ERK1/2 is a loading control. Phosphorylated forms of HSL and Perilipin A appear as multiple bands. (C-F) WAT, red marrow (RM) and MAT were isolated from New Zealand White rabbits. (C) Image of a bisected tibia showing the typical distribution of MAT. (D) Micrographs of H&E-stained tissue sections. (E) Immunoblots of total protein lysates from two rabbits, representative of four rabbits; α-tubulin is a loading control. (F) Immunoblots and silver stain of conditioned media from WAT and MAT explants from one rabbit, representative of seven rabbits. (G-I) Tibial MAT and scWAT were isolated from patients undergoing lower limb amputation. (G) Micrographs of H&E-stained tissue sections. (H) Immunoblots of total protein lysates of each tissue; -tubulin is a loading control. (I) Immunoblots and silver stain of conditioned media from explants of scWAT and MAT from patient 3. Lamin A/C was analyzed as an estimate of explant breakdown. Similar results were obtained for explants from patients 1 and 2 (Figure S1). In (F) and (I), silver staining was used to assess total protein content. Images in (A-B) and (C-D) are representative of ten mice or rabbits. For micrographs, scale bars = 200 μm. See also Figure S1.
To investigate the ability of MAT to secrete adiponectin, we cultured rabbit MAT and WAT explants and analyzed adiponectin secretion into conditioned media. We found that MAT secreted adiponectin at levels far higher than WAT, despite similar media total protein content (Fig 1F; Fig S1C).
To test if these observations extend to humans, we characterized WAT and MAT from three patients undergoing below-the-knee amputation. Unlike rabbit MAT, bone fragments were interspersed with MAT in two of the three samples (Fig 1G). Nevertheless, adiponectin expression was greater in MAT than in WAT (Fig 1H). Strikingly, adiponectin secretion was also markedly higher from explants of MAT than from WAT, despite a similar degree of explant breakdown and total protein content of the conditioned media (Fig 1I; Fig S1D). These results demonstrate that human MAT has a greater capacity than WAT to both express and secrete adiponectin.
MAT directly contributes to increased serum adiponectin during CR
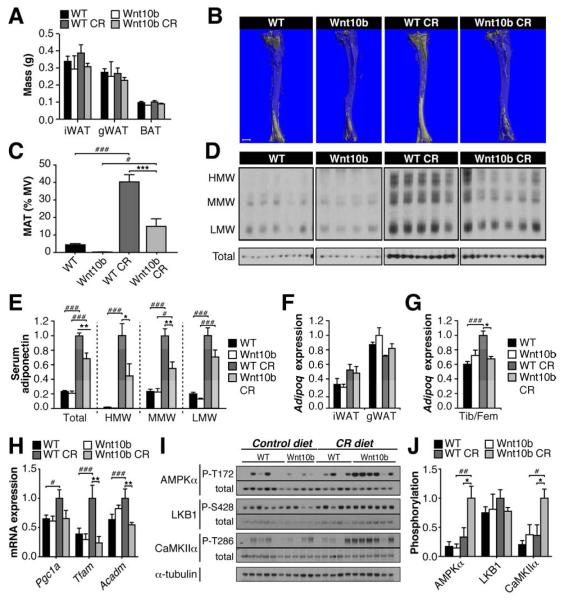
The above results are consistent with our hypothesis that MAT is a source of circulating adiponectin. However, our observations in AN subjects are correlative; therefore, we next directly tested if MAT expansion is required for increased serum adiponectin with CR. To do so we used Ocn-Wnt10b mice, which overexpress Wnt10b from osteoblasts (Bennett et al., 2007). Wnt10b potently inhibits adipogenesis; hence, we hypothesized that Ocn-Wnt10b mice would resist MAT expansion with CR, allowing us to test if increased MAT is required for elevated serum adiponectin in this context. We fed wild-type (WT) and Ocn-Wnt10b mice a control or 30% CR diet for 6 weeks. In female mice of both genotypes, CR significantly decreased body mass, blood glucose, lean mass, and liver mass, but not fat mass (Fig S2A-C; Fig 2A). Though not intuitive, this preservation of fat mass in female C57BL/6J mice is consistent with previous CR studies (Li et al., 2010; Varady et al., 2010). Other expected effects of CR on WAT were also noted, including increased expression of fatty acid synthase protein (FAS) and transcripts (Fasn) (Fig S2D-F) (Bruss et al., 2010). Importantly, neither body mass nor fat mass differed between WT and Ocn-Wnt10b mice on either diet. In contrast, Ocn-Wnt10b mice had markedly increased bone mass (Fig S2G; Table S1), consistent with our previous studies (Bennett et al., 2007). We next used osmium tetroxide staining to assess BM adiposity (Fig 2B-C). On a control diet, MAT did not significantly differ between WT and Ocn-Wnt10b mice. CR markedly increased MAT in WT mice; however, this increase was significantly blunted in Ocn-Wnt10b mice. Further analysis of whole tibiae and femurs revealed that CR increased expression of the adipocyte markers Fabp4, Pparg and Lep in bones of WT mice, but not in Ocn-Wnt10b mice (Fig S2H). Thus, both osmium tetroxide staining and qPCR demonstrate that Ocn-Wnt10b mice resist MAT expansion during CR. Strikingly, CR-associated increases in serum adiponectin were also significantly blunted in Ocn-Wnt10b mice: HMW adiponectin was over two-fold lower in CR-fed Ocn-Wnt10b mice, and total, MMW and LMW adiponectin were similarly decreased (Fig 2D-E). These differences are unlikely to be caused by differential hemoconcentration (Naruse et al., 2005), because silver staining revealed similar total serum protein content across all four groups (data not shown). We cannot exclude the possibility that adiponectin secretion from WAT is lower in Ocn-Wnt10b mice; however, WAT expression of Gstk1 and Erp44, which encode regulators of adiponectin secretion (Ye and Scherer, 2013), was unaffected by CR or transgenic Wnt10b (Fig S2F). These data are consistent with the finding that adiponectin secretion from WAT is unaltered during CR (Kovacova et al., 2009). Importantly, neither CR nor transgenic Wnt10b affected expression of Adipoq transcripts or protein in WAT (Fig 2F; Fig S2D-E). Thus, altered adiponectin expression in WAT does not account for the observed differences in serum adiponectin. In contrast, Adipoq expression in tibiae and femurs mirrored the changes in serum adiponectin (Fig 2G), suggesting that circulating adiponectin levels are directly related to adiponectin production from MAT. These results therefore provide direct evidence that MAT expansion is necessary for maximal production of serum adiponectin during CR.
Figure 2. Blocking MAT expansion directly prevents increased circulating adiponectin during CR.
WT and Ocn-Wnt10b mice were fed ad libitum or a 30% CR diet from 9-15 weeks of age. (A) Masses of iWAT, gWAT and BAT in 15-week-old mice. (B,C) BM adiposity was assessed by osmium tetroxide staining of tibiae followed by μCT analysis. (B) Representative images of stained tibiae; scale bar = 1 mm. (C) MAT as % marrow volume (MV) was determined from μCT scans. (D) Analysis of HMW, MMW, LMW and total adiponectin in sera of 15-week-old mice. Immunoblots are from the same exposure of film. (E) Quantitation of serum adiponectin from (D). (F,G) qPCR analysis of Adipoq expression in iWAT, gWAT, or combined tibiae and femurs (Tib/Fem). (H-J) Total RNA and protein was isolated from quadriceps muscle. Expression of Pgc1a, Tfam and Acadm was determined by qPCR (H). Protein phosphorylation was determined by immunoblotting (I) and quantified by densitometry (J). CaMKII phosphorylation is a marker of Ca2+/calmodulin signaling. Data are mean ±SEM of the following numbers of mice: WT, n = 6; Wnt10b, n = 4; WT CR, n = 5; Wnt10b CR, n = 6. Similar results were observed in a second mouse cohort. For each diet group, significant differences between WT and Wnt10b mice are indicated by * (P <0.05), ** (P <0.01) or *** (P <0.001). Within each genotype, significant differences between ad libitum and CR diets are indicated by ## (P <0.01) or ### (P <0.001). See also Figure S2, Figure S3, and Table S1.
Impaired MAT expansion alters skeletal muscle adaptations to CR
We next investigated if limiting the increases in MAT or adiponectin during CR has wider metabolic consequences. In obese states, lack of adiponectin causes glucose intolerance (Maeda et al., 2002; Ye and Scherer, 2013). However, glucose tolerance did not differ between WT and Ocn-Wnt10b mice (Fig S2I). This is consistent with adiponectin deficiency not affecting glucose tolerance in lean mice (Maeda et al., 2002). Expected effects of adiponectin or CR on liver transcript expression, including increased Pparg, Ppara, and Hnf4a, and decreased Fabp5 (Liu et al., 2012; Nogueira et al., 2012), also did not differ between WT and Ocn-Wnt10b mice (Fig S3). Therefore, we next analyzed skeletal muscle, a major metabolic target of adiponectin. Here, adiponectin stimulates Ca2+ influx and LKB1 activation, thereby enhancing AMPK activity, PPARγ coactivator-1α (PGC-1α) expression, and mitochondrial biogenesis (Ye and Scherer, 2013). Consistent with a previous report (Finley et al., 2012), in WT mice CR increased expression of Pgc1a and its downstream targets, Tfam and Acadm (Fig 2H), which encode proteins with important mitochondrial functions. Strikingly, these effects of CR were absent in Ocn-Wnt10b mice (Fig 2H). In contrast, hepatic expression of Pgc1a and Tfam did not differ between genotypes (Fig S3), suggesting that consequences of impaired MAT expansion are specific to skeletal muscle. As reported elsewhere (Gonzalez et al., 2004), CR in WT mice did not affect steady-state AMPK activity in muscle (Fig 2I-J). Two pathways upstream of AMPK activation, LKB1 and Ca2+/calmodulin signaling, were also unaffected by CR in WT mice (Fig 2I-J). In contrast, CR markedly activated AMPK and CaMKII in skeletal muscle of Ocn-Wnt10b mice, without affecting LKB1 activity (Fig 2I-J). These observations demonstrate that blocking MAT expansion during CR alters local homeostatic adaptations in skeletal muscle.
Both MAT and circulating adiponectin increase during cancer therapy in humans
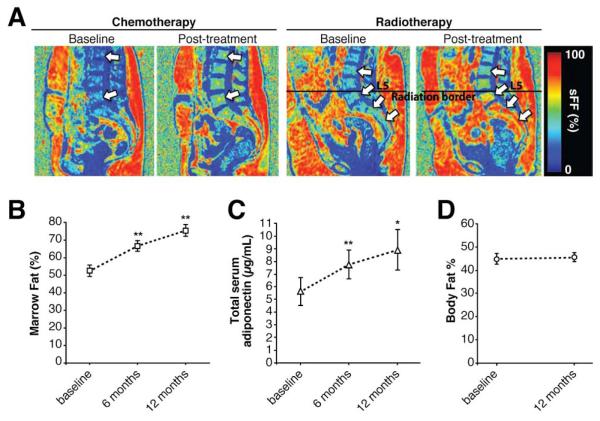
The above findings in humans, rabbits, and mice identify MAT as a key source of circulating adiponectin during CR. We next began to explore this relationship in other clinical conditions. BM adiposity increases during radiotherapy or chemotherapy for cancer; hence, we hypothesized that serum adiponectin might also increase during such treatments. To address this possibility, we analyzed MAT and serum adiponectin in patients undergoing therapy for ovarian or endometrial cancer. We found that both lumbar vertebral MAT and total serum adiponectin increased significantly at 6 and 12 months after onset of chemotherapy or radiotherapy, despite no change in total body fat (Fig 3A-D). Thus, both MAT and serum adiponectin increase during cancer therapy, without changes in total adiposity outside of the BM. This suggests that MAT might contribute to circulating adiponectin in contexts beyond CR.
Figure 3. Both MAT and serum adiponectin increase during cancer therapy in humans.
MAT, serum adiponectin and % body fat were assessed in patients undergoing radiotherapy for endometrial cancer or chemotherapy for ovarian cancer. (A) Representative MRI images of two patients before and at six months post-treatment. Arrows highlight increased vertebral MAT (signal fat fraction; sFF) post-treatment. (B) MAT was determined by water-fat MRI at the indicated time points. Data are mean ±SEM of 11-15 patients. (C) Total serum adiponectin concentrations were determined by ELISA and are mean ±SEM of 8-11 patients, with 11 patients assessed at baseline and 6 months, and 8 of these patients also assessed at 12 months. (D) Body fat %, as determined by DXA, shown as mean ±SEM of 11 patients. For (B-D), statistically significant differences between baseline and 6 or 12 months post-treatment are indicated by * (P < 0.05) or ** (P < 0.01).
DISCUSSION
Previous studies have investigated BM adipocytes as local regulators of skeletal remodeling (Scheller and Rosen, 2014); however, the present study is the first to identify MAT as an endocrine organ. We show that compared to WAT, MAT of mice and rabbits expresses adiponectin more highly than other adipocyte markers. Moreover, we reveal that secretion of adiponectin is far greater from MAT than from WAT of rabbits and humans. Our observations in AN subjects show that MAT can make up over 30% of total body fat, underscoring the notion that, during CR in humans, MAT exists in amounts sufficient to make a major contribution to circulating adiponectin. Conclusive evidence comes from our use of Ocn-Wnt10b mice as an unprecedented model of specific MAT ablation. We show that with CR, these mice resist increases in both MAT and serum adiponectin, without differences in WAT mass or adiponectin expression in WAT. It should be noted that, in some contexts, CR can increase adiponectin expression in WAT (Qiao et al., 2011); however, our findings are consistent with previous studies (Behre et al., 2007; Combs et al., 2003; Dolezalova et al., 2007; Kovacova et al., 2009), which collectively suggest that elevated circulating adiponectin during CR can occur without increased adiponectin production from WAT. Notably, our results in Ocn-Wnt10b mice greatly extend these earlier studies by providing direct evidence that MAT is a key source of circulating adiponectin during CR. Thus, adiponectin production from MAT may account, at least in part, for the adiponectin paradox.
By producing adiponectin, MAT has the potential to exert systemic effects on metabolic homeostasis, immune responses, vascular function or cancer risk. Indeed, we find that impaired MAT expansion during CR leads to altered metabolic adaptations in skeletal muscle, suggesting that MAT has effects beyond the skeleton. However, whether these effects are driven by changes in circulating adiponectin remains unclear. For example, the CR-associated increase in Pgc1a, Tfam and Acadm in muscle of WT, but not Ocn-Wnt10b mice, is consistent with the differences in circulating adiponectin, whereas increased AMPK and CaMKIIα activation with CR in Ocn-Wnt10b, but not WT mice, is highly unexpected. One possibility is that skeletal muscle of Ocn-Wnt10b mice adapts metabolically to a relative adiponectin deficiency by sustaining Ca2+/calmodulin and AMPK activation. However, even in WT mice, skeletal muscle AMPK activity does not increase during CR (Fig 2I-J) (Gonzalez et al., 2004), despite increased circulating adiponectin. This suggests that, during CR, adiponectin does not stimulate AMPK in muscle. Indeed, existing knowledge of adiponectin action is largely limited to the context of obesity and insulin resistance (Ye and Scherer, 2013); the role of adiponectin during CR is poorly understood. In addition to adiponectin, we show that rabbit MAT expresses leptin at similar levels to iWAT and pWAT, and silver staining of human MAT- and WAT-conditioned media indicates that these tissues have distinct secretory profiles. These observations suggest that unique endocrine functions of MAT extend beyond adiponectin.
Finally, we reveal that both MAT and circulating adiponectin increase in patients undergoing cancer therapy. Based on these findings, it is tempting to speculate that the increased adiponectin derives from MAT expansion. Given that low circulating adiponectin is associated with increased cancer risk, and that adiponectin can limit tumor growth (Dalamaga et al., 2012), the consequences of elevated adiponectin during cancer therapy clearly warrant further investigation. Beyond cancer, many other conditions are associated with increases in both MAT and circulating adiponectin, including aging, estrogen deficiency, type 1 diabetes, and treatment with pharmacologic agents such as thiazolidinediones or fibroblast growth factor-21 (Combs et al., 2003; Fazeli et al., 2013; Isobe et al., 2005; Kharitonenkov et al., 2007; Scheller and Rosen, 2014; Wei et al., 2012; Ye and Scherer, 2013). This suggests that MAT might impact upon circulating adiponectin in other clinically relevant conditions. In addition, both circulating adiponectin and MAT volume inversely correlate with BMD in human populations (Richards et al., 2007; Shen et al., 2012). This suggests that MAT might positively associate with circulating adiponectin in non-pathological contexts, as recently reported in Caucasian girls (Newton et al., 2013).
In summary, we have found that MAT expansion contributes significantly to increased serum adiponectin and skeletal muscle adaptation during CR. These findings suggest that MAT is a major source of circulating adiponectin in states of leanness, and show that, through endocrine functions, MAT can act beyond the skeleton to exert systemic effects. However, the consequences of adiponectin production from MAT are yet to be fully established, and much about MAT biology remains unknown. Thus, future research in this area is clearly warranted if we are to better understand this understudied, clinically relevant tissue.
EXPERIMENTAL PROCEDURES
Additional Experimental Procedures are described in Supplemental Information.
Human subjects
All work was done as approved by the Institutional Review Board (IRB) of the relevant institutions, as follows: AN study, Partners IRB; cancer therapy study, University of Minnesota IRB; human MAT studies, University of Michigan IRB.
Animals
All procedures were approved by the University of Michigan Committee on the Use and Care of Animals. C57BL/6J (000664) and C3H/HeJ (000659) mice were from The Jackson Laboratory (Bar Harbor, ME). Ocn-Wnt10b mice (C57BL/6J background) were described previously (Bennett et al., 2007). Mice were housed on a 12 h light/dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan, with free access to water and, as indicated, food. Random-fed blood glucose, body fat, lean mass, and free fluid were assessed as described previously (Mori et al., 2012). For serum adiponectin, blood was taken from the tail vein of mice or the marginal ear artery of rabbits using Microvette CB 300 capillary tubes (Sarstedt, Newton, NC). Male New Zealand White rabbits, used at 11-18 weeks in age, were from Harlan Laboratories (Haslett, MI)..
Caloric restriction
Mice were fed a control diet (Research Diets D12450B) or 30% CR diet (Research Diets D10012703) from 9 to 15 weeks of age, as described previously (Devlin et al., 2010). Food was administered daily. The CR diet restricts macronutrient intake while maintaining mineral and vitamin levels. Mice were single housed from 8-9 weeks of age to determine average daily ad libitum food intake prior to CR.
Tissues
Tissues were fixed in 10% neutral-buffered formalin. Bones were decalcified in 14% EDTA for 14 days. Paraffin-embedded tissue sections were processed and stained with H&E or Toluidine blue, as indicated.
Statistical Analysis
Statistical analysis was done using JMP 9.0 (SAS Institute, Carry, NC), SPSS (IBM, Armonk, NY), or GraphPad Prism 6 software (GraphPad Software, La Jolla, CA), with means of normally distributed data compared using a two-tailed Student’s t-test and means of non-normally distributed data compared using the Wilcoxon test. Significant differences in transcript expression of rabbit tissues were assessed using a paired t-test. Significant differences between WT and Ocn-Wnt10b mice were assessed using a two-sample t-test or ANOVA with post-tests, as appropriate. Significant differences between MAT, serum adiponectin and body fat percentage of human subjects undergoing cancer therapy were assessed using a Wilcoxon matched-pair signed rank test, with comparisons made between baseline and 6 months or 12 months post-treatment. Error bars in figures represent SEM. For all comparisons, a P-value of < 0.05 was considered statistically significant.
Supplementary Material
HIGHLIGHTS.
Secretion of adiponectin is greater from MAT than from WAT
Blocking MAT expansion during CR suppresses increased serum adiponectin
Blocking MAT expansion alters skeletal muscle adaptation to CR
Both MAT and circulating adiponectin increase in humans undergoing cancer therapy
ACKNOWLEDGEMENTS
This work was supported by the NIH (R24 DK092759 to O.A.M., A.K., M.C.H. and C.J.R.; R01 DK62876 to O.A.M.; K99-DE-024178 to E.L.S.; R01 DK090262 to C.N.L.; 1R03AR055333, 1K12-HD055887 to S.K.H.; P30 DK089503 to the Michigan Nutrition Obesity Research Center). S.K.H. is also supported by the Translational Science Institute of University of Minnesota (8UL1TR0001114). O.A.M. holds a Fulbright Scholar’s Award. W.P.C. holds a Lilly Innovation Fellowship Award, and previously a Postdoctoral Research Fellowship from the Royal Commission for the Exhibition of 1851 (UK). H. M. was supported by a mentor-based postdoctoral fellowship from the American Diabetes Association. D.T.B. and S.S.S. were supported by the UM Molecular & Integrative Physiology Department SURF program.
W.P.C. holds a postdoctoral fellowship funded by Eli Lilly and Company; V.K. is employed by Eli Lilly and Company; O.A.M. holds stock in MRK and ESRX, and has received research funding from Eli Lilly and Company. E.L.S. and O.A.M. have received research funding from Biomet Biologics. A.K. has received research funding from Rhythm Pharmaceuticals.
Footnotes
AUTHOR CONTRIBUTIONS W.P.C. and E.L.S. made equal contributions to the conceptualization, design, performance, and analysis of experiments, and to writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Behre CJ, Gummesson A, Jernas M, Lystig TC, Fagerberg B, Carlsson B, Carlsson LM. Dissociation between adipose tissue expression and serum levels of adiponectin during and after diet-induced weight loss in obese subjects with and without the metabolic syndrome. Metabolism. 2007;56:1022–1028. doi: 10.1016/j.metabol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Bennett CN, Ouyang H, Ma YL, Zeng Q, Gerin I, Sousa KM, Lane TF, Krishnan V, Hankenson KD, MacDougald OA. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22:1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- Bruss MD, Khambatta CF, Ruby MA, Aggarwal I, Hellerstein MK. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298:E108–116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocr Rev. 2012;33:547–594. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23:577–585. doi: 10.1002/ajhb.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–2088. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezalova R, Lacinova Z, Dolinkova M, Kleiblova P, Haluzikova D, Housa D, Papezova H, Haluzik M. Changes of endocrine function of adipose tissue in anorexia nervosa: comparison of circulating levels versus subcutaneous mRNA expression. Clin Endocrinol (Oxf) 2007;67:674–678. doi: 10.1111/j.1365-2265.2007.02944.x. [DOI] [PubMed] [Google Scholar]
- Fazeli PK, Horowitz MC, Macdougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98:935–945. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley LW, Lee J, Souza A, Desquiret-Dumas V, Bullock K, Rowe GC, Procaccio V, Clish CB, Arany Z, Haigis MC. Skeletal muscle transcriptional coactivator PGC-1alpha mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc Natl Acad Sci U S A. 2012;109:2931–2936. doi: 10.1073/pnas.1115813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. American journal of physiology Endocrinology and metabolism. 2004;287:E1032–1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Isobe T, Saitoh S, Takagi S, Takeuchi H, Chiba Y, Katoh N, Shimamoto K. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91–98. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007;148:774–781. doi: 10.1210/en.2006-1168. [DOI] [PubMed] [Google Scholar]
- Kovacova Z, Vitkova M, Kovacikova M, Klimcakova E, Bajzova M, Hnevkovska Z, Rossmeislova L, Stich V, Langin D, Polak J. Secretion of adiponectin multimeric complexes from adipose tissue explants is not modified by very low calorie diet. Eur J Endocrinol. 2009;160:585–592. doi: 10.1530/EJE-08-0727. [DOI] [PubMed] [Google Scholar]
- Li X, Cope MB, Johnson MS, Smith DL, Jr., Nagy TR. Mild calorie restriction induces fat accumulation in female C57BL/6J mice. Obesity (Silver Spring) 2010;18:456–462. doi: 10.1038/oby.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Yuan B, Lo KA, Patterson HC, Sun Y, Lodish HF. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci U S A. 2012;109:14568–14573. doi: 10.1073/pnas.1211611109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, Cawthorn WP, Susulic VS, Krishnan V, Greenfield A, Macdougald OA. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122:2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Newton AL, Hanks LJ, Davis M, Casazza K. The relationships among total body fat, bone mineral content and bone marrow adipose tissue in early-pubertal girls. BoneKEy Rep. 2013;2 doi: 10.1038/bonekey.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira LM, Lavigne JA, Chandramouli GV, Lui H, Barrett JC, Hursting SD. Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med. 2012;1:275–288. doi: 10.1002/cam4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G, De Giacomo P, Giorgino R, De Pergola G. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88:1748–1752. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- Qiao L, Lee B, Kinney B, Yoo HS, Shao J. Energy intake and adiponectin gene expression. American journal of physiology Endocrinology and metabolism. 2011;300:E809–816. doi: 10.1152/ajpendo.00004.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014 doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Chen J, Gantz M, Punyanitya M, Heymsfield SB, Gallagher D, Albu J, Engelson E, Kotler D, Pi-Sunyer X, et al. MRI-measured pelvic bone marrow adipose tissue is inversely related to DXA-measured bone mineral in younger and older adults. Eur J Clin Nutr. 2012;66:983–988. doi: 10.1038/ejcn.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21:188–195. doi: 10.1016/j.jnutbio.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.