Abstract
Purpose
The discovery of new, effective non-anthracycline-based re-induction regimens for children with relapsed acute myeloid leukemia (AML) is critical. In this Phase I/II study, we investigated the tolerability and overall response rate of clofarabine in combination with cytarabine in children with relapsed/refractory acute myeloid leukemia (AML).
Methods
AAML0523 enrolled 49 children with AML in first relapse or refractory to induction therapy. The study consisted of a dose finding phase (N=9) and an efficacy phase (N=40). Two children received clofarabine at 40 mg/m2/day and 47 at 52 mg/m2/day.
Results
Toxicities typical for intensive chemotherapy regimens were observed at all doses of clofarabine. The recommended pediatric phase II dose (RP2D) of clofarabine in combination with cytarabine was 52 mg/m2/day for 5 days. Of 48 evaluable patients, the overall response rate (ORR) (CR + CRp) was 48%. Four patients met conventional criteria for complete remission with incomplete count recovery (CRi). Twenty-one of 23 responders subsequently received hematopoietic stem cell transplantation (HSCT). Overall survival at 3 years was 46 % for responders compared to 16% for non-responders (p value <0.001). Patients with no minimal residual disease at end of first cycle by flow cytometric analysis had superior overall survival after 1 year (100% vs. 38%, p=0.01).
Conclusion
The combination of clofarabine and cytarabine yielded an acceptable response rate without excess toxicity in children with relapsed AML. The nearly 50% survival rate in responders is highly encouraging in these high risk patients and suggests that this combination is an effective bridge to HSCT.
Keywords: clofarabine, cytarabine, acute myeloid leukemia, pediatric
Introduction
Cure rates for children with acute myeloid leukemia (AML) have dramatically improved with the advent of anthracycline-based chemotherapy regimens and improvements in supportive care1, 2. Nevertheless, approximately 50% of children still relapse with resistant disease. It is critical to develop effective non-anthracycline containing re-induction regimens to avoid further risk of cardiotoxicity while allowing for curative hematopoietic stem cell transplantation (HSCT). Clofarabine is a second-generation purine nucleoside analogue designed to integrate the mechanistic properties of fludarabine and cladribine3. Clofarabine requires intracellular phosphorylation by deoxycytidine kinase (dCK) to the active triphosphate form (clo-CTP) prior to inhibition of DNA polymerase4, 5. Clofarabine has shown single agent activity in Phase I and II studies in pediatric patients with relapsed or refractory ALL and AML6, 7.
Clofarabine is a potent inhibitor of ribonucleotide reductase, leading to increased accumulation of the cytotoxic triphosphate form of cytarabine (ara-CTP) in leukemia cells. This biochemical modulation is well established in vitro and has been studied in clinical trials in adults with AML8-10.
Here we describe the findings of the AML stratum of Children's Oncology Group (COG) Phase I/II study AAML0523. The primary objective of this study was to define the overall response rate to clofarabine in combination with cytarabine in children with relapsed or refractory AML or acute lymphoblastic leukemia (ALL). This manuscript reports only results for children with relapsed AML; the findings for children with ALL have been published11.
Methods
Patients
AAML0523 was open to accrual between March 12, 2007 and January 5, 2012. Data analyses for AML patients are current as of September 30, 2012. AML patients were required to be between 1 and 21 years of age, in first relapse or refractory to reinduction. Patients were required to have histologically proven AML according to the French- American-British (FAB) classification system and ≥ 5% bone marrow blasts. Other requirements included adequate liver (serum bilirubin ≤1.5 times upper limit of normal (ULN) for age, ALT ≤ 2.5 times ULN for age), renal (derived from the Schwartz formula), cardiac (echocardiogram with shortening fraction ≥ 27%), and pancreatic function (serum amylase and lipase ≤ 1.5 times ULN), and adequate performance Status (Karnofsky or Lansky ≥50%). Exclusion criteria included uncontrolled systemic infection and active central nervous system involvement (CNS3). Due to severe hepatotoxicity observed in a concurrent pediatric clinical trial in relapsed ALL using concurrent clofarabine, cyclophosphamide and etoposide12, AAML0523 was amended to exclude patients that had received HSCT within 12 months of study entry. Institutional review boards at participating centers approved the study, and participating patients or their parents signed written informed consent. The original clinical trial was registered at www.clinicaltrials.gov as NCT00372619.
Study Design
The study was conducted in 2 phases: a dose finding phase and an efficacy phase. The dose finding phase consisted of a single dose escalation/de-escalation of clofarabine in combination with a fixed dose of cytarabine (1 gram/m2/day for 5 days). For each dose level, 10 patients (both ALL and AML) were enrolled. The first cohort received clofarabine at 40 mg/m2/day for 5 days. Based on safety data on the first cohort, the dose of clofarabine would either be escalated to 52 mg/m2/day or de-escalated to a dose of 30 mg/m2/day. The recommended phase II dose (RP2D) was used in the efficacy portion of the study with separate ALL and AML cohorts. An optimal two stage Simon design was implemented to test the null hypothesis that the ORR (CR + CRp) is ≤ 40% versus the alternative hypothesis that ORR is ≥ 60% based on overall response rate from Children's Cancer Group (CCG) 2951 study.13 Eighteen patients were to be enrolled in stage 1; if at least 8 overall responses were observed, an additional 28 patients would be enrolled in stage 2; the null hypothesis would be rejected if at least 23 overall responses were observed in patients treated at 52 mg/m2/dose. Patients who received therapy at recommended phase II dose in the first phase were included in the efficacy phase.
Treatment Plan
Induction therapy consisted of up to 2 cycles. If a bone marrow aspirate (BMA) performed between day 14 and 21 of cycle 1 revealed ≥5% blasts, Cycle 2 was administered without waiting for count recovery. BMA was repeated at least once every 14 days, until response assessment was possible. If the day 14-21 BMA revealed < 5% blasts, patients received therapy once attaining adequate peripheral blood count recovery, defined as absolute neutrophil count (ANC) > 1000/μl and platelet count > 100,000/μl. Patients without ANC recovery by day 42 were to proceed to Cycle 2 if there was no bone marrow aplasia. In the dose finding phase, Cycle 1 consisted of clofarabine on days 2-6 and cytarabine on days 1-5 for correlative study purposes. Correlative biology studies will be described in a separate manuscript. In the efficacy phase of the study, both clofarabine and cytarabine were administered intravenously (IV) over 2 hours on days 1-5. Cytarabine was administered beginning 4 hours after the start of clofarabine. As systemic inflammatory response is a known side effect of clofarabine6, 7, patients experiencing respiratory distress, unexplained hypotension or tachycardia were to receive intravenous hydrocortisone pretreatment (50-100 mg/m2/day) before each clofarabine dose for remainder of that cycle. Prophylactic intrathecal cytarabine (IT) was administered to all patients at the time of diagnostic lumbar puncture or on Day 0 of Cycle 1 (at least 24 hours prior to administration of IV cytarabine). On day 1 of Cycle 2 patients were allowed to receive intrathecal cytarabine dosed according to age, at the discretion of the treating physician.
Response Criteria
The overall response rate (ORR) consisted of patients who achieved a complete remission (CR) and complete remission with partial recovery of platelet count (CRp). Criteria did not include those with CR with incomplete hematologic recovery (CRi). Response was considered as the best response after up to 2 induction cycles. CR was defined as attainment of an M1 bone marrow (<5% blasts) with an absolute neutrophil count (ANC) > 1000/μl and platelet count > 100,000/μl; CRp as attainment of an M1 bone marrow (<5% blasts) with recovery of ANC > 1000/μl and platelet transfusion independence defined as no platelet transfusions for one week; Stable Disease (SD) as present when a patient did not qualify for either a CR, CRp or progressive disease (PD); Progressive Disease (PD) as an increase in the extent of bone marrow infiltration by leukemic cells of ≥ 20% blasts or extramedullary disease.
Minimal Residual Disease (MRD) Detection
Response to initial therapy was assessed by multidimensional flow cytometry (MDF) as previously described14. Samples were to be collected at study entry, end of induction 1 and end of induction 2. Detection threshold of residual disease has been demonstrated to be 0.01%.14
Statistical Analysis
Data were analyzed through September 30, 2012. If therapy was associated with a 40% ORR after up to 2 cycles of therapy, the null hypothesis (40% ORR) would be rejected in the two-stage design with probability 0.10. If therapy was associated with a 60% ORR, the null hypothesis would be rejected in the two-stage design with probability 0.90. Kaplan-Meier method was used to estimate overall survival (OS, defined as time from study entry to death) and log-rank test was used to compare OS. Patients alive at last contact were censored for OS analyses at the date of last contact. Patients defined as responders (best response of CR or CRp) were compared with non-responders. The significance of observed difference in proportions was tested using the Chi-squared test and Fisher's exact test when data were sparse comparing groups of patients. The Kruskal-Wallis test was used to determine the significance between differences in medians of groups.
Results
Study Population
As outlined in Table I, 51 patients were enrolled on AAML0523, of whom 49 were eligible for response assessment. Of the 49 eligible patients, 44 were in first morphologic relapse and 5 were refractory to induction chemotherapy. Patients in first relapse had median length of initial CR of 306 days (range 55-2212), with 27 (61%) patients having relapsed within 1 year of achieving initial CR. Of 40 patients with cytogenetic data, 3 patients (7.5%) had high risk cytogenetics (Monosomy 7 and Del 5q), 11 had CBF AML (27.5%), 4 had 11q23 abnormalities and 4 had extra copies of chromosome 8.
Table I. Patient Characteristics.
| AAML0523 (AML) | AAML0523 (Dose: 40 mg/m2) | AAML0523 (Dose: 52 mg/m2) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |
| AML patients enrolled | 51 | 2 | 4% | 49 | 96% | ||
| ineligible | 2 | 0 | 2 | ||||
| Gender | |||||||
| Male | 27 | 55% | 2 | 100% | 25 | 53% | 0.495 |
| Female | 22 | 45% | 0 | 0% | 22 | 47% | |
| Race | |||||||
| White | 36 | 80% | 1 | 50% | 35 | 81% | 0.364 |
| Black or African American | 6 | 13% | 0 | 0% | 6 | 14% | 1.000 |
| Asian | 2 | 4% | 0 | 0% | 2 | 5% | 1.000 |
| American Indian or Alaska Native | 1 | 2% | 1 | 50% | 0 | 0% | 0.044 |
| Unknown | 4 | 0 | 4 | ||||
| Patients in First Relapse | 44 | 90% | 2 | 100% | 42 | 89% | 1.000 |
| Refractory to Re-induction | 5 | 100% | - | 5 | 100% | ||
| Prior Hematopoietic Stem Cell Transplant | 4 | 8% | 0 | 0% | 4 | 9% | 1.000 |
| Age at study entry in years (median, range) | 14.1 | 1.4 - 23.0 | 15.1 | 10.6 - 19.6 | 14.1 | 1.4 - 23.0 | 0.419 |
| WBC (× 103/μl) (median, range) | 3.3 | 0.5 - 1400 | 1.65 | 1.5 - 1.8 | 3.6 | 0.5 - 1400 | 0.111 |
| Length of CR1 in days (median, range) (n=44) | 306 | (35 - 2212) | 361.5 | (213 - 510) | 306 | (35 - 2212) | 0.955 |
| Cytogenetics | |||||||
| Normal | 8 | 20% | 1 | 50% | 7 | 18% | 0.364 |
| Inv(16) | 7 | 18% | 1 | 50% | 6 | 16% | 0.323 |
| t(8;21) | 4 | 10% | 0 | 0% | 4 | 11% | 1.000 |
| Monosomy 7 | 1 | 3% | 0 | 0% | 1 | 3% | 1.000 |
| Del7q | 1 | 3% | 0 | 0% | 1 | 3% | 1.000 |
| Del5q | 1 | 3% | 0 | 0% | 1 | 3% | 1.000 |
| 11q23 | 4 | 10% | 0 | 0% | 4 | 11% | 1.000 |
| t(6;9) | 1 | 3% | 0 | 0% | 1 | 3% | 1.000 |
| +8 | 4 | 10% | 0 | 0% | 4 | 11% | 1.000 |
| Other | 9 | 23% | 0 | 0% | 9 | 24% | 1.000 |
| Unknown | 9 | 0 | 9 | ||||
Toxicity
Non-hematologic toxicities grade 3 and higher as defined in Common Terminology Criteria for Adverse Events (CTCAE), version 3 were collected on all patients. Those occurring in more than one individual at clofarabine doses of 40 mg/m2 and 52 mg/m2 are included in Table II. Toxicity data on 20 patients (including 2 AML patients treated at 40 mg/m2 and 7 AML patients treated at 52 mg/m2) treated in the dose-finding portion of the study have been published11. There were no DLT's for the 2 AML patients treated at 40 mg/m2, while 1 patient with AML treated at 52 mg/m2 experienced a DLT (bone marrow aplasia, Grade 4 hypokalemia, Grade 3 nausea, and Grade 3 dehydration). Therefore, the recommended dose of clofarabine for the efficacy portion (RP2D) of AAML0523 was 52 mg/m2. The most common toxicities were infection, nausea/diarrhea/anorexia, fever/neutropenia, transaminitis, hyperglycemia, and hypokalemia, consistent with prior studies on clofarabine. No deaths occurred in AML patients within 30 days of protocol therapy.
Table II. Toxicities Grade 3 or Higher Observed in > 10% of Patients during Induction cycles I and II.
| Adverse Event ≥ Grade 3 | Induction, Cycle I (N=49) | Induction, Cycle II (N=31) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Febrile neutropenia | 20 | 41% | 9 | 29% |
| Infections and infestations | 23 | 47% | 13 | 42% |
| Hypokalemia | 10 | 20% | 10 | 32% |
| Anorexia | 7 | 14% | 2 | 6% |
| Diarrhea | 6 | 12% | 3 | 10% |
| Nausea | 6 | 12% | 2 | 6% |
| Hyperglycemia | 6 | 12% | 2 | 6% |
| Alanine aminotransferase increased | 6 | 12% | 6 | 19% |
| Aspartate aminotransferase increased | 4 | 8% | 3 | 10% |
Of 47 AML patients treated at the RP2D, 12 had ANC recovery (> 1000/ μl) and 16 had platelet recovery (≥100,000/ μl) after cycle 1 at a median of 29.5 and 26.5 days, respectively. Of those that received a second cycle, the median time to ANC recovery (15 patients) and platelet recovery (16 patients) were 35 and 27.5 days, respectively. The remainder of the patients who did not have ANC (35 patients) or platelet recovery (31 patients) after one cycle of therapy had a median cycle length of 23 and 29 days, respectively. Of 31 patients that received cycle 2 at the RP2D, 18 patients did not have ANC recovery (4 SD), platelet recovery (3 CRp), or both (6PD, 5SD). Of these 18 patients, 7 received more chemotherapy and HSCT, 4 HSCT only, 6 chemotherapy only, and 1 did not receive additional therapy.
Response
Table IIIa shows the response of eligible patients at the RP2D. A sufficient number of responses were observed at the RP2D in stage 1 of the two-stage design to proceed to stage 2. Forty-six evaluable patients were treated at the RP2D, of which 16 achieved CR and 5 achieved a CRp for an overall response rate of 45.7% (95% CI: 30.9-61.0%). The 2 AML patients treated on the dose-finding portion of the study at a dose of 40 mg/m2 responded (1 CR, 1 CRp), thus increasing the response rate for all AML patients to 48%. Of 23 responders, 11 (48%) were SD after course 1, then achieved CR or CRp after course 2. However, 6 patients were removed from protocol therapy by their physician after Course 1 with SD. Not included amongst responders were four patients with SD who achieved CRi, defined as having fulfilled criteria for marrow CR, but without recovery of ANC (< 1.0 × 109/L) and platelet count (<100 × 109/L). All 4 patients with CRi proceeded to HSCT and only 1 patient was alive at last follow up. There were 30 patients who received cycle 2 at the RPD2. Fifteen patients received a bone marrow evaluation between days 14-21 and 16 patients at day 23 or later. Response by length of first remission (CR1) is reported in Table IIIb and is consistent with previous studies demonstrating CR1< 1 year predicts poor response. 15
Table IIIa. Overall Response for eligible patients at the 52 mg/m2 dose level.
| N | % | |
|---|---|---|
| Course 1 response (N=47) | ||
| CR | 7 | 15% |
| CRp | 3 | 7% |
| SD | 30 | 65% |
| PD | 6 | 13% |
| Not evaluable | 1 | 2% |
| Course 2 response (N=30) | ||
| CR | 12 | 40% |
| CRp | 2 | 7% |
| SD | 10 | 33% |
| PD | 6 | 20% |
| Best response | ||
| CR | 16 | 35% |
| CRp | 5 | 11% |
| SD | 14 | 30% |
| PD | 11 | 24% |
| Not evaluable | 1 * | 2% |
One patient not evaluable for response because therapy was withdrawn before response assessment
Table IIIb. Overall Response for eligible patients at the 52 mg/m2 dose level (patients in first relapse only, n=42) by duration of CR1.
| Duration of CR1 ≤365 days | Duration of CR1 >365 days | ||||
|---|---|---|---|---|---|
| N | % | N | % | p | |
| Course 1 response (N=42) | |||||
| CR | 2 | 8% | 5 | 31% | 0.085 |
| CRp | 3 | 12% | 0 | 0% | 0.275 |
| SD | 16 | 62% | 10 | 63% | 0.950 |
| PD | 5 | 19% | 1 | 6% | 0.380 |
| Not evaluable | 0 | 0% | 0 | 0% | - |
| Course 2 response (N=29) | |||||
| CR | 4 | 27% | 7 | 50% | 0.196 |
| CRp | 1 | 7% | 1 | 7% | 1.000 |
| SD | 4 | 27% | 6 | 43% | 0.450 |
| PD | 6 | 40% | 0 | 0% | 0.017 |
| Best response | |||||
| CR | 6 | 23% | 9 | 56% | 0.029 |
| CRp | 4 | 15% | 1 | 6% | 0.633 |
| SD | 6 | 23% | 5 | 31% | 0.720 |
| PD | 10 | 38% | 1 | 6% | 0.030 |
| Not evaluable | 0 | 0% | 0 | 0% | |
CR - Complete Response; CRp - Complete Response with partial platelet recovery; SD - Stable Disease; PD - Progressive Disease; CR1 - first remission
Characteristics of Responders
The clinical features of all responders are listed in Table IV. Responders generally had lower risk features including a longer median duration of CR1 (374.5 days vs. 258 days p=0.085) and a higher percentage of favorable cytogenetics (40% vs. 15%, p=0.077). There were responses in higher-risk patients, as 4 of 12 patients with an initial CR < 6 months achieved CR (1 patient) or CRp (3 patients). Also, there were responses in a patient with Monosomy 7, and 2 with 11q23 rearrangement. Twenty-one of 23 responders proceeded to HSCT (10 after 1 cycle, 11 after 2 cycles) compared to 12 of 25 of non-responders. For these patients responses were durable with a 3-year OS of 46% ± 27% compared to 16 ± 16% in non-responders. (p <0.001)(Figure1).
Table IV. Characteristics of responders and non-responders for eligible patients *.
| Responders (CR + CRp) | Non-responders | |||
|---|---|---|---|---|
| N | % | N | % | |
| Total | 23 | 47% | 25 | 51% |
| Gender | ||||
| Male | 14 | 61% | 12 | 48% |
| Female | 9 | 39% | 13 | 52% |
| Patients in first relapse | 22 | 96% | 22 | 88% |
| Patient refractory to induction | 1 | 4% | 3 | 12% |
| Patients with prior hematopoietic stem cell transplant | 2 | 9% | 2 | 8% |
| Cytogenetics (N=40) | ||||
| Normal | 4 | 20% | 4 | 20% |
| Inv(16)/t(16;16) | 6 | 30% | 1 | 5% |
| t(8;21) | 2 | 10% | 2 | 10% |
| Monosomy 7 | 1 | 5% | 0 | 0% |
| Del7q | 0 | 0% | 1 | 5% |
| Del5q | 0 | 0% | 1 | 5% |
| 11q23 | 2 | 10% | 2 | 10% |
| t(6;9) | 1 | 5% | 0 | |
| +8 | 0 | 0% | 4 | 20% |
| Other | 4 | 20% | 5 | 25% |
| Age (y) at study entry (median, range) | 14.7 | 1.4 - 21.5 | 9.0 | 1.7 – 23.0 |
| WBC (× 103/μl) (median, range) | 2.7 | 1.1 - 129.6 | 4.2 | 0.5 - 1400 |
| Length of CR1 in days (median, range) | 374.5 | 42 – 2212 | 258 | 35 - 1066 |
| Received HSCT in follow-up | 21 | 91% | 12 | 48% |
| Number of received courses to best | ||||
| 1 cycle | 12 | 52% | 11 | 44% |
| 2 cycles | 11 | 48% | 14 | 56% |
One patient not evaluable for response because therapy was withdrawn before response assessment
CR = Complete Response
CRp = Complete Response with partial recovery of platelet count
CR1 - First remission
HSCT - Hematopoietic Stem Cell Transplant
Figure 1.
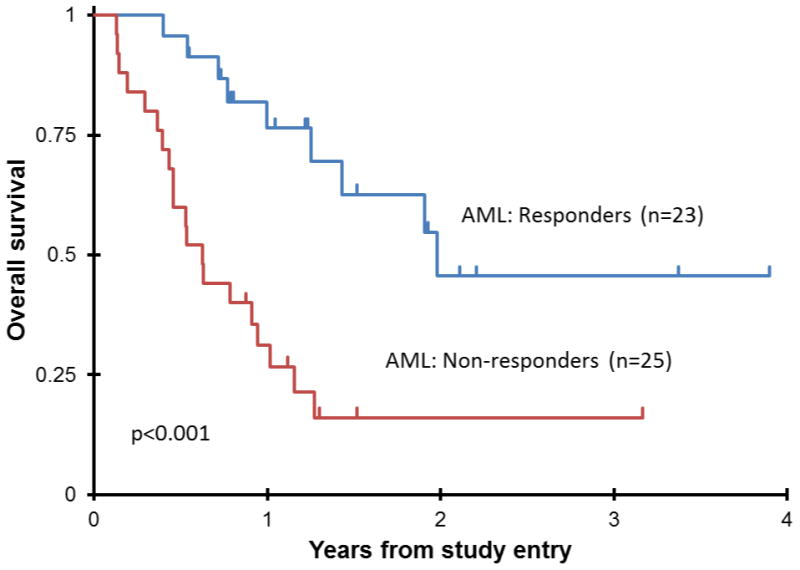
Overall Survival from study entry for all AML responders vs. non-responders on AAML0523.
Minimal Residual Disease
Of 46 evaluable patients at the RP2D, specimens for MRD evaluation after end of induction I (EOI1) was submitted in 13 patients, of whom 3 were in morphologic CR/CRp and 10 had SD or PD. In these 13 patients, 8 had disease detectable by MDF after course I (62%) (Table V). Six of 8 MRD positive patients at EOI1 have died, while all patients without MRD are alive to date. Of 10 patients with morphologic SD or PD at EOI1, 3 were MRD negative, all of which are long-term survivors compared to only 1 of 7 with MRD (14%). This suggests that MDF may provide clinically relevant data beyond morphology. MDF data at the EOI2 was available for 10 patients with an MRD rate of 40%. All patients without evidence of MRD at the EOI2 are long term survivors whereas two of the four patients with MRD at EOI2 have died.
Table V. MRD Status for the evaluable patients at the 52 mg/m2 dose level*.
| Patient # | EOI 1 Response | EOI 1 Bone Marrow Blast Percentage (%) | MRD at EOI1 | EOI2 Response | EOI2 Bone Marrow Blast Percentage (%) | MRD at EOI2 | Best response | Life status |
|---|---|---|---|---|---|---|---|---|
| 1 | CRp | 4 | Negative | Not available | Not available | CRp | Alive | |
| 2 | SD | 25 | Positive | PD | 36 | Positive | PD | Dead |
| 3 | CR | 0.2 | CR | 0 | Negative | CR | Alive | |
| 4 | SD | 61 | Positive | SD | 1 | SD | Dead | |
| 5 | SD | 0 | Negative | CR | 0 | Negative | CR | Alive |
| 6 | SD | 0 | Positive | SD | 2 | SD | Dead | |
| 7 | SD | 18 | Negative | CR | 4 | Negative | CR | Alive |
| 8 | SD | 63.3 | CR | 3 | Negative | CR | Alive | |
| 9 | SD | 53 | Positive | SD | 9 | Positive | SD | Dead |
| 10 | CR | 2 | Positive | CR | 0 | Positive | CR | Alive |
| 11 | SD | 66 | Positive | Not available | Not available | Not available | SD | Dead |
| 12 | PD | 94 | Positive | Not available | Not available | Not available | PD | Dead |
| 13 | SD | . | Negative | CRp | Not available | Negative | CRp | Alive |
| 14 | SD | 8 | Positive | PD | 27 | Positive | PD | Alive |
| 15 | CR | 3 | Negative | CR | 0 | Negative | CR | Alive |
MRD = Minimal Residual Disease
EOI1 = End of Induction one
EOI2 End of induction two
CR - Complete Response; CRp - Complete Response with partial platelet recovery; SD - Stable Disease; PD - Progressive Disease
Patient 3 and 8 had MRD measured at EOI2 only
Discussion
Cure rates in children with AML in first relapse are poor, especially those that recur within one year of therapy13, 16. An important factor directing post-relapse therapy is prior anthracycline exposure, as most children treated on current COG AML protocols have a cumulative anthracycline exposure of approximately 440 mg/m2. Emerging data reveal a significant risk for cardiovascular death in pediatric AML survivors.17, 18 Therefore, effective non-anthracycline reinduction regimens are desperately needed to decrease treatment related morbidities.
AAML0523 demonstrated that clofarabine and cytarabine is an active non-anthracycline chemotherapy regimen for children with AML in first relapse. The combination proved to be an effective bridge to more definitive therapy, with 21 of 23 responders proceeding to HSCT. The significance of this “bridge to transplant” is demonstrated by the 3-year OS of 46 ± 27% for responders versus 16± 16% for non-responders (p <0.001) (Figure1). The ORR in our study (45.7% at RP2D, 48% overall) was suboptimal, however, non-adherence to protocol recommendations may have affected the results. Since single-agent clofarabine trials demonstrated that many patients required more than one cycle to achieve best response,6, 7 the protocol recommended that patients receive 2 cycles of protocol therapy. Of those patients in compliance with this recommendation, 11 (48% of responders) with stable disease after cycle 1 achieved CR or CRp after cycle 2. However, 6 patients (29%) with SD after course 1 were removed from protocol therapy by treating physicians without receiving a second cycle. In addition, 4 patients met conventional criteria for CRi, but came off study before meeting parameters for CR or CRp. These 4 patients proceeded to HSCT but only 25% are alive to date. It is possible that improved protocol compliance may have resulted in an improved response rate. Of note, two patients with acute leukemia of ambiguous lineage were treated on study and both responded. The toxicity profile of this combination was consistent with other cytotoxic AML reinduction regimens, without unexpected Grade 3 or 4 toxicities. After 2DLT's identified as fungal infections in the Phase I portion of the study (both in ALL patients), the supportive care guidelines were amended to include anti-fungal prophylaxis. Veno-occlusive disease, a concern with the combination of clofarabine/etoposide/cyclophosphamide, was not observed on this study. Results from (CCG) 2951 study showed that a combination of mitoxantrone/cytarabine in children with relapsed/refractory AML achieved an overall response rate of 58%.13 (Personal communication, Todd Alonzo) Patients generally received less intensive therapy and less cumulative anthracyclines compared to those treated on AAML0523. Also, most AAML0523 patients had already received mitoxantrone/cytarabine, so the response rate in our study is not surprising. The combination of fludarabine and cytarabine has recently demonstrated efficacy in relapsed childhood AML15. Kaspers et al. recently published their results on a randomized study of fludarabine, cytarabine, and GCSF (FLAG) vs. FLAG with liposomal daunorubicin (Daunoxome/DNX). Randomizing 394 patients over 10 years who relapsed after a variety of de novo therapeutic regimens, the CR rate (ANC>1000 and Platelets > 50K) after two courses was 69% with FLAG/DNX, and 59% with FLAG (p=0·07). In this trial every responder received 2 cycles while many patients on AAML0523 did not and re-emphasizes an important potential confounding factor in our patient's response rates to clofarabine/cytarabine.
We demonstrate that the quality of remission achieved by salvage chemotherapy, as defined by MDF, impacts long term survival. The use of MDF for assessment of response is well-established in predicting outcome in children with de novo AML14, 19, 20. In COG study AAML03P1, nearly a third of patients in morphologic CR had residual disease by MFD and had a significantly higher relapse rate. Conversely, this same study revealed that nearly 25% of the patients in morphologic failure (> 5% blasts) had no evidence of disease by MDF. This lack of immunophenotypic disease correlated with favorable outcome, highlighting the difficulty of distinguishing normal, regenerating cells from leukemic blasts by morphology alone14. Given the paucity of available data, the utility of MRD by MFD in the setting of relapsed AML is unclear. All submitted samples were evaluated for MRD. However, the number of optional biology samples was limited because most patients had received a bone marrow examination to confirm relapse prior to consent for trial participation. There were no significant differences in response when comparing those that did and did not receive MRD testing. Despite the limited sample size, there was a statistically significant difference in 1-year OS from the end of the first cycle between patients with negative MRD (100%) versus patients with positive MRD (38%) (p=0.01). This suggests that MDF may provide a valuable tool in response assessment in relapsed patients, and further supports the need for accurate and sensitive response assessment beyond morphologic response in the setting of retrieval regimens. This will allow for improved patient care and facilitate more rapid evaluation of new agents under study based on MDF data as surrogate marker.
In conclusion, this study shows that the non-anthracycline combination of clofarabine and cytarabine has clinically important activity in pediatric patients with refractory/relapsed AML. The durability of these responses and the relatively high 3-year OS of 46% indicate that this combination is an effective therapy as a bridge to HSCT in this patient population.
Acknowledgments
Funding Sources: The work was supported by National Institute of Health Grants: Children's Oncology Group Chair's grant NIH U10 CA98543 and SDC U10 CA98413. Clofarabine was supplied by Genzyme Oncology/Sanofi
Footnotes
There are no financial disclosures from any authors
References
- 1.Kersey JH. Fifty years of studies of the biology and therapy of childhood leukemia. Blood. 1997;90(11):4243–51. [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–65. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA., 3rd Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35(2):397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 4.Mansson E, Flordal E, Liliemark J, et al. Down-regulation of deoxycytidine kinase in human leukemic cell lines resistant to cladribine and clofarabine and increased ribonucleotide reductase activity contributes to fludarabine resistance. Biochem Pharmacol. 2003;65(2):237–47. doi: 10.1016/s0006-2952(02)01484-3. [DOI] [PubMed] [Google Scholar]
- 5.Parker WB, Shaddix SC, Rose LM, et al. Comparison of the mechanism of cytotoxicity of 2-chloro-9-(2-deoxy-2- fluoro-beta-D-arabinofuranosyl)adenine, 2-chloro-9-(2-deoxy-2-fluoro- beta-D-ribofuranosyl)adenine, and 2-chloro-9-(2-deoxy-2,2-difluoro- beta-D-ribofuranosyl)adenine in CEM cells. Mol Pharmacol. 1999;55(3):515–20. [PubMed] [Google Scholar]
- 6.Jeha S, Gandhi V, Chan KW, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004;103(3):784–9. doi: 10.1182/blood-2003-06-2122. [DOI] [PubMed] [Google Scholar]
- 7.Jeha S, Gaynon PS, Razzouk BI, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24(12):1917–23. doi: 10.1200/JCO.2005.03.8554. [DOI] [PubMed] [Google Scholar]
- 8.Cooper T, Ayres M, Nowak B, Gandhi V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55(4):361–8. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- 9.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105(3):940–7. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 10.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30(20):2492–9. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper TM, Razzouk BI, Gerbing R, et al. Phase I/II trial of clofarabine and cytarabine in children with relapsed/refractory acute lymphoblastic leukemia (AAML0523): A report from the Children's Oncology Group. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijiya N, Thomson B, Isakoff MS, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118(23):6043–9. doi: 10.1182/blood-2011-08-374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells RJ, Adams MT, Alonzo TA, et al. Mitoxantrone and cytarabine induction, high-dose cytarabine, and etoposide intensification for pediatric patients with relapsed or refractory acute myeloid leukemia: Children's Cancer Group Study 2951. J Clin Oncol. 2003;21(15):2940–7. doi: 10.1200/JCO.2003.06.128. [DOI] [PubMed] [Google Scholar]
- 14.Loken MR, Alonzo TA, Pardo L, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children's Oncology Group. Blood. 2012;120(8):1581–8. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved Outcome in Pediatric Relapsed Acute Myeloid Leukemia: Results of a Randomized Trial on Liposomal Daunorubicin by the International BFM Study Group. J Clin Oncol. 2013;31(5):599–607. doi: 10.1200/JCO.2012.43.7384. [DOI] [PubMed] [Google Scholar]
- 16.Webb DK, Wheatley K, Harrison G, Stevens RF, Hann IM. Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial. MRC Childhood Leukaemia Working Party. Leukemia. 1999;13(1):25–31. doi: 10.1038/sj.leu.2401254. [DOI] [PubMed] [Google Scholar]
- 17.Orgel E, Zung L, Ji L, Finklestein J, Feusner J, Freyer DR. Early Cardiac Outcomes Following Contemporary Treatment for Childhood Acute Myeloid Leukemia: A North American Perspective. Pediatr Blood Cancer. 2013 doi: 10.1002/pbc.24498. [DOI] [PubMed] [Google Scholar]
- 18.Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525–33. doi: 10.1136/hrt.2007.136093. [DOI] [PubMed] [Google Scholar]
- 19.Yin JA, O'Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826–35. doi: 10.1182/blood-2012-06-435669. [DOI] [PubMed] [Google Scholar]
- 20.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11(6):543–52. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


