Abstract
Dopaminergic neurotransmission in the nucleus accumbens is important for various reward-related cognitive processes including reinforcement learning. Repeated cocaine enhances hippocampal synaptic plasticity, and phasic elevations of accumbal dopamine evoked by unconditioned stimuli are dependent on impulse flow from the ventral hippocampus. Therefore, sensitized hippocampal activity may be one mechanism by which drugs of abuse enhance limbic dopaminergic activity. In the present study, in vivo microdialysis in freely moving adult male Sprague-Dawley rats was used to investigate the effect of repeated cocaine on ventral hippocampus-mediated dopaminergic transmission within the medial shell of the nucleus accumbens. Following seven daily injections of saline or cocaine (20 mg/kg, ip), unilateral infusion of N-methyl-D-aspartate (NMDA, 0.5 μg) into the ventral hippocampus transiently increased both motoric activity and ipsilateral dopamine efflux in the medial shell of the nucleus accumbens, and this effect was greater in rats that received repeated cocaine compared to controls that received repeated saline. In addition, repeated cocaine altered NMDA receptor subunit expression in the ventral hippocampus, reducing the NR2A:NR2B subunit ratio. Together, these results suggest that repeated exposure to cocaine produces maladaptive ventral hippocampal-nucleus accumbens communication, in part through changes in glutamate receptor composition.
Keywords: cocaine, NMDA, mesolimbic, microdialysis, sensitization
Introduction
Dopaminergic activity within the nucleus accumbens is thought to be a significant mediator of psychostimulant reinforcement. Drugs of abuse and drug-predictive environments preferentially increase extracellular dopamine in the nucleus accumbens shell (Di Chiara et al., 2004; Wheeler et al., 2011). The ventral hippocampus sends afferents to the medial shell of the nucleus accumbens which also receives input from dopaminergic terminals originating in the ventral tegmental area (VTA; Kelley and Domesick, 1982; Groenewegen et al., 1987; Gracy and Pickel, 1996). Unilateral N-methyl-D-aspartate (NMDA) stimulation of the ventral hippocampus induces a large equivalent increase in c-fos expression in the ipsilateral and contralateral nucleus accumbens shell (Zornoza et al., 2005). In addition, it has been demonstrated that release of dopamine in the nucleus accumbens shell is significantly increased, along with parallel increases in locomotor behavior, as a result of NMDA stimulation of the ventral hippocampus (Brudzynski and Gibson, 1997; Peleg-Raibstein and Feldon, 2006). The increase in locomotor activity produced by ventral hippocampal NMDA infusion is blocked by lesions of the VTA as well as by systemic administration of D2 (haloperidol), D1 (SCH-23390) receptor antagonists, or the vesicular monoamine transporter blocker reserpine (Wu and Brudzynski, 1999; Bardgett and Henry, 1999) demonstrating the dependence of this effect on dopamine . Electrical stimulation of the ventral hippocampus reinstates drug-seeking for cocaine (Vorel et al., 2001), while ventral hippocampal inhibition reduces cocaine- or context-induced reinstatement of drug-seeking behavior (Sun and Rebec, 2003; Atkins et al., 2008; Lasseter et al., 2010). Therefore, induction of accumbal dopamine release by the ventral hippocampus is important in drug reinforcement. The influence of environmental context of the reward experience is essential for subsequent reward-seeking behaviors, and communication between the hippocampus and the nucleus accumbens shell is vital for the formation of place-reward associations (Floresco et al., 1997; Ito et al., 2008). Therefore, activation of the hippocampus, by contextual cues for instance, may engage reinforcement-related neurotransmission mediated by the nucleus accumbens.
Long-term potentiation (LTP), a sustained increase in the efficacy of synaptic transmission, is accepted as a substrate for learning and memory formation in the hippocampus (Daoudal and Debanne, 2003). Repeated exposure to cocaine has been shown to enhance LTP in the hippocampus (Thompson et al., 2002; del Olmo et al., 2006). Re-exposure to a drug-associated context also alters hippocampal synaptic plasticity and increases expression of immediate early genes associated with memory retrieval (Monti et al., 2010). Threshold to generate hippocampal LTP is reduced in rats sensitized to cocaine, and these animals have enhanced retention of an avoidance memory compared to non-sensitized rats (Perez et al., 2010). Recent studies have shown that ventral hippocampal input to the nucleus accumbens shell is selectively potentiated after repeated cocaine exposure and withdrawal (Britt et al., 2012). However, previous studies recording from the shell of the nucleus accumbens demonstrated that tetanic stimulation of the hippocampus, which induced LTP at hippocampal inputs in control animals, failed to induce any persistent changes in cocaine-sensitized rats (Goto and Grace, 2005). Since an excitatory drive to the nucleus accumbens shell produces reinstatement to cocaine seeking, the present experiments tested the hypothesis that repeated cocaine alters the ventral hippocampus in a way that would enhance hippocampal output to the medial shell of the nucleus accumbens.
Ionotropic NMDA glutamate receptors are critical for the induction of LTP (Bliss and Collingridge, 1993). NMDA receptors are tetramers composed of NR2 subunits which bind glutamate, and an obligatory NR1 subunit, which bind the co-agonist glycine. Multiple studies have investigated the effect of cocaine on hippocampal NMDA receptor protein expression, with a majority of reports indicating no significant effects on subunit expression or NMDA receptor binding (Loftis and Janowsky, 2002; Turchan et al., 2003; Kaminski et al., 2011). However, no study to date has investigated cocaine-induced changes in hippocampal NMDA receptor protein expression with respect to its dorso-ventral axis. Differences between dorsal and ventral regions of the hippocampus have been demonstrated with respect to behavioral regulation (Bannerman et al., 2004), LTP induction (Maggio and Segal, 2007), and NMDA receptor subunit expression (Pandis et al., 2006). Consequently, the hypothesis that repeated cocaine would increase NMDA receptor protein expression in the ventral hippocampus and further that cocaine pre-treatment would enhance dopaminergic activity in the nucleus accumbens following acute NMDA stimulation of the ventral hippocampus was investigated in freely moving rats using in vivo microdialysis.
Material and Methods
Animals
Male Sprague-Dawley rats (Charles River, Inc., 250–275 g at the start of the experiment), were maintained on a 12 h light/dark cycle (lights on at 7am), and provided food and water ad libitum. Animals were allowed to acclimate to the animal facility for five days prior to the start of the experiment and were weighed and handled daily. Animal use procedures were conducted in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th edition, 2011) and approved by the Institutional Animal Care and Use Committee of Temple University.
Guide cannulae implantation
Rats underwent surgery to implant chronic indwelling cannulae targeting the medial shell of the nucleus accumbens for later insertion of a microdialysis probe and ipsilateral ventral hippocampus for later insertion of a drug infusion cannula. Rats were anesthetized with a mixture of ketamine hydrochloride (80 mg/kg, ip) and xylazine (10 mg/kg, ip) and placed within a small mammal stereotaxic frame (Kopf, Tujunga, CA, USA). Sterilized stainless steel guide cannulae for microdialysis and drug infusion (21 and 26 gauge, respectively, Plastic One, Roanoke, VA) were implanted using stereotaxic coordinates of (in mm): +1.4 anterior and 1.0 lateral to bregma, and 5.8 ventral from dura for the shell of the nucleus accumbens; −5.5 posterior and +5.0 lateral to bregma, and 6.0 mm ventral from dura for the ventral hippocampus, based on Paxinos and Watson (2009). Dummy cannulae that extended 1 mm and 2 mm beyond the tip of the infusion and microdialysis guide cannula were inserted immediately after surgery. Following surgeries, rats were housed individually and drug administration began 2 days postoperatively.
Drugs
Cocaine hydrochloride, generously provided by the NIDA drug supply program, was dissolved in 0.9% saline and administered (20 mg/kg/day, ip) for seven days. N-methyl-d-aspartate (NMDA; Sigma) was freshly prepared and dissolved in saline on the day of infusions.
Locomotor quantification
Behavioral activity was evaluated between 9 am and 11 am each day during administration of saline or cocaine for 7 days in automated monitors containing 16 infrared light emitters and sensors mounted on a frame within which a standard plastic animal cage was positioned (45 cm × 20 cm × 20 cm; AccuScan Instruments, Inc., Columbus, OH). The number of photocell beam breaks was recorded throughout a 90 min session (30 min pre- and 60 min post - injection) by a computer equipped with the Digiscan DMicro software system (AccuScan Instruments).
Microdialysis
Microdialysis was conducted twenty four hours following the last drug administration. Microdialysis was performed in freely moving rats placed in the same monitors used for locomotor measurements. The dummy cannulae were removed from the guide cannulae and a laboratory-made concentric microdialysis probe (2 mm exposed membrane length, typical recovery 19.6%; Barr and Forster, 2011) was inserted into the guide cannula targeting the medial shell of the nucleus accumbens. An infusion cannula (33 gauge) was inserted into the guide cannula targeting the ventral hippocampus. A two-channel liquid swivel (Instech Lab. Inc., Plymouth Meeting, PA, USA) guided the inlet and outlet tubing of the probe, and inlet tubing of the infusion cannula connected to a microinfusion pump (CMA/102). Artificial cerebrospinal fluid (aCSF; containing 147 mM NACl, 2.7 mM KCl, 1mM NaH2PO4, 1.4 mM Na2HPO4, 2.1 mM MgCl2 and 1.6 mM CaCl2, pH 7.4) was continuously perfused through the probe at a rate of 0.5 μl/min. Dialysate collection began 4 hrs following probe insertion at 15 min intervals. Following collection of 4 baseline samples, NMDA was infused into the ventral hippocampus at a concentration of 0.5 μg/ 0.5μl over 1 min (Peleg-Raibstein and Feldon., 2006). Following infusion, dialysates were collected for an additional 90 minutes.
Histology
Upon completion of experiments, brains were removed and fixed in 4% paraformaldehyde for three days. Brains were then sectioned at 60 μm on a Vibratome (Leica Biosystems, Buffalo Grove, Il) stained with cresyl violet and examined under a light microscope to determine the placement of the probe and infusion cannula for each animal. Only data from rats with correct placements in both areas were included in the analyses.
High-performance liquid chromatography measurement of dopamine
The mobile phase (containing per 500 ml: 340 mg EDTA, 250 mg sodium octanesulfonate, 4.7 g NaH2PO4, 250 μl triethylamine and 85 ml methanol, pH 5.75; all obtained from Sigma, St Louis, MO, USA) was pumped through a UniJet 3 μm C18 microbore column (Bioanalytical Systems; West Lafayette, IN, USA) under nitrogen gas pressure (2000 psi). Dialysates were injected onto the chromatographic system using a rheodyne injector via a 5 μl loop (Bioanalytical Systems). Following separation by the column, dopamine was detected by a glassy carbon electrode (Bioanalytical Systems), which was maintained at +0.5 V with respect to an Ag AgCl2 reference electrode using an LC-4C potentiostat (Bioanalytical Systems). The voltage output was recorded by Clarity v2.4 Chromatography Station for Windows (DataApex, Prague, Czech Republic). Dopamine peaks were identified by comparison to a dopamine standard (9.2 pg/ 5 μL DA). The 2:1 signal to noise detection limit for dopamine using this system was 0.07 +/− 0.02 pg.
Western immunoblotting
A separate cohort of rats were administered saline (1ml/kg, ip, n=9) or cocaine (20 mg/kg, ip, n=10) once daily for 7 days, killed 30 hrs after the last injection (this time point corresponds to when NMDA was infused in the previous experiment) and ventral hippocampus was dissected rapidly on ice. Samples were prepared by homogenizing tissue from individual animals in boiling 1% (gm/100 ml) sodium dodecyl sulfate. Homogenized samples were boiled for 5 minutes and stored at −80°C. Equal amounts of protein (40 μg, determined using a Thermo Scientific NanoDrop 2000 spectrometer) from all samples were loaded on 7.5% mini-Protean TGX gels (Bio Rad) for separation and transferred onto nitrocellulose membranes. After blocking for 60 minutes at room temperature, the membranes were incubated overnight at 4 °C with rabbit anti-NR1, NR2B or NR2A primary antibodies (1:1000; mAb #5704; #4205; #4212; Cell Signaling, Danvers, MA). After washing, the membranes were further incubated with IRDye 800 or 680-conjugated goat anti-rabbit IgG second antibody (Li-Cor Biosciences, Lincoln, NE 1:10,000) for 2 hr at room temperature. After incubation, membranes were washed three times with TBST before visualization. Control for protein loading was achieved by using primary antibodies to β-tubulin (1:800,000; Cell Signaling) and IRDye 800-conjugated affinity purified anti-mouse IgG as secondary antibodies (1:20,000). Proteins were detected using the Odyssey infrared imaging system (LI-COR Biosciences). Optical density of each individual sample was corrected against the optical density of β-tubulin.
Statistical analyses
All statistical tests were conducted with Sigmaplot 12.5 with the alpha level set at 0.05. Cumulative hyperactivity counts were analyzed by two-way ANOVA with drug (cocaine or saline) and day as main factors. Significant ANOVAs were followed by Student-Newman-Keul's (SNK) post-hoc for multiple comparisons. Activity and dopamine levels from microdialysis are expressed as a percent change from baseline levels. Separate Grubb's tests were applied to the microdialysis data to remove outliers, which resulted in removal of 3 data points from possible 190 data points of dopamine levels over time. Activity counts during dialysis collection were collated into 15 min time bins to correspond with DA sample collection periods. Separate Grubb's tests were applied to remove outliers from the behavioral data, which resulted in removal of 5 from possible 171 data points. Separate two-way repeated measures ANOVA (drug × time) were then used to compare motoric activity or dopamine levels across time between saline- and cocaine-pretreated rats following NMDA infusion. Significant main effects were followed by Student-Newman-Keul's (SNK) post-hoc for multiple comparisons at each time point. Significant main effects of time were followed by separate one-way repeated measures ANOVA for each group, with significant effects across time identified by post-hoc Holme-Sidak tests for multiple comparisons, with time point zero serving as the control comparison. The quantification of immunoblots was analyzed by one-way ANOVA with pretreatment (saline or cocaine administration) as main factor.
Results
Histology and baseline dopamine levels for microdialysis experiments
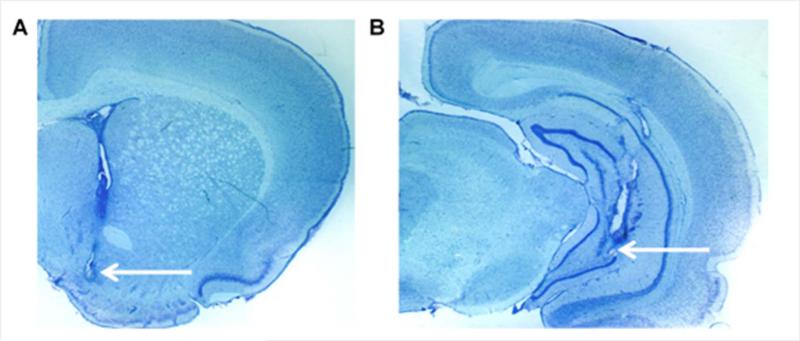
The placement of microdialysis probes ensured that the 2 mm length of dialysis membrane sampled from the medial shell nucleus accumbens [1.4 mm anterior from bregma (Paxinos & Watson, 2009; Fig. 1A)], and were similarly distributed in saline- and cocaine-administered animals. The placement of the infusion cannulae ensured that NMDA infusions occurred within the ventral hippocampus [5.5 mm posterior from bregma (Paxinos & Watson, 2009; Fig. 1B)], and were similarly distributed in saline- and cocaine-administered animals. A total of 5 rats out of 24 were excluded from further analysis due to probe placements outside of the accumbens or cannula placements outside of the ventral hippocampus. Baseline levels of dopamine for saline-pre-treated rats were 2.08 ± 0.68 pg/ 5μL, and for cocaine-pre-treated rats were 1.97± 0.68 pg/ 5μL (uncorrected for recovery). There was no statistical difference in baseline dopamine levels between groups (P > 0.05).
Figure 1.
Representative photomicrographs of coronal brain coronal sections of (A) microdialysis probe membrane placement in the nucleus accumbens and (B) infusion cannula placement in the ventral hippocampus.
Motor activity is enhanced by repeated cocaine administration
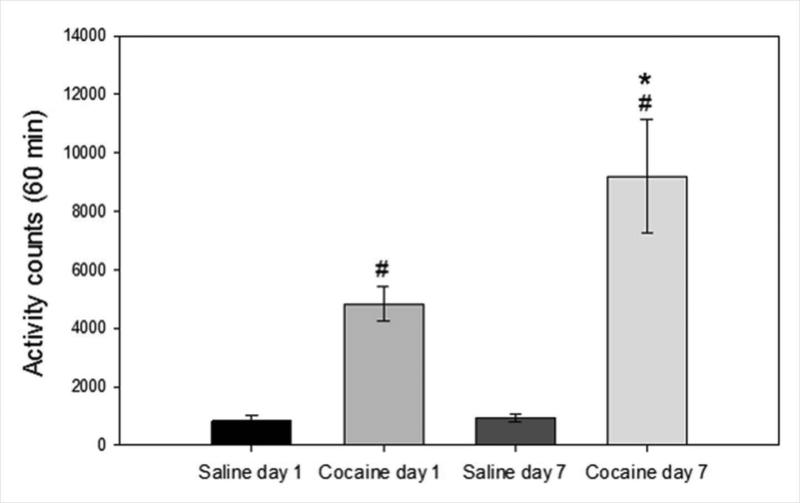
Rats were injected with saline or cocaine (20 mg/kg, ip) once daily for seven days and activity was measured for 60 minutes post injection. Cumulative activity counts over 60 minutes on treatment days one and seven are shown in Fig. 2. Two-way ANOVA of the data showed significant interaction, drug, and day effects (Interaction: F 1, 37= 5.323, P=0.030; drug: F 1, 37= 4.926, P=0.036; day: F 1, 37= 33.621, p<0.001). Post-hoc analysis revealed that cocaine significantly increased motor activity compared to saline injected controls on day one (P=0.018) and day seven (P<0.001), and that within the cocaine administered group there was a significant difference in activity between day one and day seven (P=0.005). Thus seven daily injections of cocaine resulted in the development of sensitization to the locomotor-stimulating effects of cocaine.
Figure 2. Cocaine administration increased motoric activity and produced locomotor sensitization.
Cumulative activity counts for 60 minutes post-cocaine (20 mg/kg ip) and saline injection are shown. Rats administered cocaine had greater activity than rats administered saline on both day one and day seven (# significantly different from saline on the same day, P<0.05). On the seventh day of administration, rats injected with cocaine showed a greater increase in activity than on the first day of cocaine administration (*significantly different between day one and seven within cocaine administered rats, P < 0.05). Data are expressed as mean ± SEM beam breaks/60 minute period. (N=9–10/group)
Cocaine pretreatment enhances ventral hippocampal NMDA-stimulated motor activity and extracellular dopamine in the nucleus accumbens
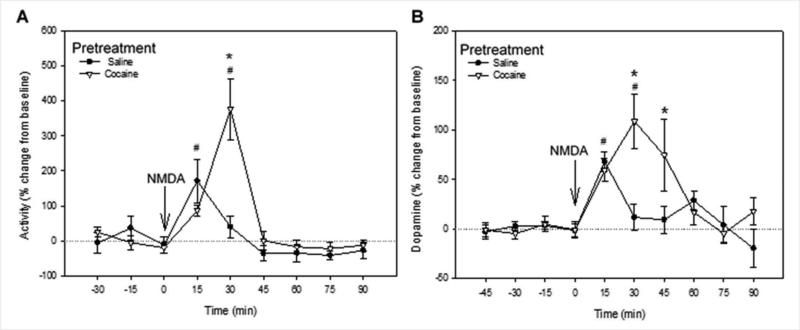
These experiments aimed to establish whether NMDA infusion into the ventral hippocampus enhances behavioral and accumbal dopaminergic responses in cocaine-sensitized rats compared to saline-pretreated controls. During collection of baseline dialysates, behavioral activity was low. Following infusion of NMDA into the ventral hippocampus, the activity counts transiently increased in both the saline and cocaine pretreated groups (Fig. 3A). There was a significant effect of time (F8, 79=9.698, P <0.001), a trend toward a significant effect of drug pretreatment (F9, 79 = 4.993, P = 0.053) and an interaction between drug and time (F8, 79 = 4.991, P < 0.001). Post hoc tests revealed that activity at 30 min after NMDA infusion was significantly higher in the cocaine- than in the saline-pretreated group (SNK, P < 0.05). One-way repeated measures ANOVA revealed an effect of NMDA over time for control rats (F8,38 = 2.820; P =0.021) and for cocaine pretreated rats (F8,40= 10.2; P < 0.001) at 15 and 30 min post-NMDA injection respectively (Holm–Sidak; P < 0.05).
Figure 3. Cocaine pretreatment enhanced ventral hippocampal NMDA-stimulated motor activity and extracellular dopamine in the nucleus accumbens.
Effects of ventral hippocampal NMDA infusion on (A) motoric activity and (B) extracellular DA in the nucleus accumbens of saline (N=9) and cocaine (N=10) pre-treated rats, expressed as percentage of baseline. Arrow indicates time of NMDA infusion. Data represent mean ± SEM. # significantly different from pre-infusion levels in both saline- and cocaine-pre-treated rats. * Significant difference between saline- and cocaine-pretreated groups (P < 0.05).
Analysis of dopamine levels (Fig 3B), showed a significant effect of time (F9, 160 = 5.450; P <0.001), no significant effect of drug (F1, 160 = 1.961; P= 0.178), and a significant interaction between drug and time (F12, 245 = 2.529; P = 0.011). One-way repeated measures ANOVA revealed an effect of NMDA over time for saline pretreated rats (F8,73 = 4.713; P < 0.001), apparent at 15 min post-infusion, and an effect over time was also observed for cocaine pretreated rats (F9,86 = 4.235; P <0.001), at 30 min post-infusion (Holm– Sidak; P <0.05). Ventral hippocampal infusion of NMDA evoked an increase in extracellular dopamine in the nucleus accumbens of rats previously exposed to cocaine that was greater than in saline pretreated rats from 30 to 45 min post-infusion (SNK; P < 0.05; Fig 3B). These data demonstrate that NMDA infusion in the ventral hippocampus produced hyperactivity and larger increases in extracellular dopamine in the nucleus accumbens and these responses were greater in rats sensitized to repeated cocaine than in non-sensitized saline controls.
The effects of repeated cocaine on NMDA receptor subunit expression in the ventral hippocampus
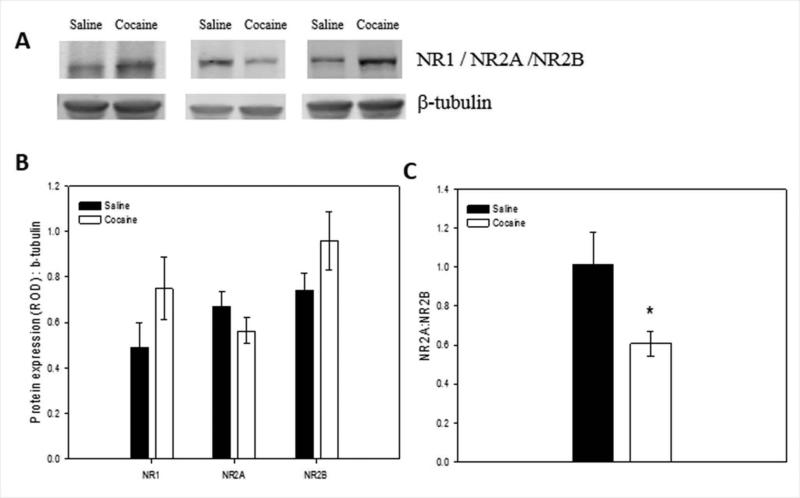
Since NMDA produced greater effects in rats pretreated with cocaine, this experiment examined whether NMDA subunit protein levels in the ventral hippocampus was altered following administration of cocaine for 7 days and acute withdrawal for 30 h (at the same time point of NMDA administration in the previous experiment). NR1, NR2A, and NR2B were quantified in ventral hippocampus by immunoblotting. There was no significant effect of cocaine on the levels of the obligatory NR1 subunit (Fig. 4A, F1,19 = 1.111, P > 0.05), the NR2A subunit (Fig. 4B, F1,18 = 2.920, P > 0.05) or the NR2B subunit (Fig. 4C, F1,18 = 2.948, P > 0.05), in the ventral hippocampus when compared with saline-administered controls. However, when NR2A:NR2B ratio was calculated for each animal, cocaine-administered rats had significantly lower ratios than the saline-administered rats (Fig. 4D; F1, 18 = 5.824, P =0.039).
Figure 4. Effect of repeated cocaine on NMDA receptor subunit expression in the ventral hippocampus.
(A) Representative protein bands on immunoblots for NR1 (120 kD), NR2A (180 kD), and NR2B (190 kD) in the ventral hippocampus (examples are from single animals in each treatment group). (B) NMDA subunit protein levels in ventral hippocampal tissue of saline- and cocaine-administered rats. (C) Calculated NR2A:NR2B ratios of rats injected with saline or cocaine for seven days. Repeated cocaine resulted in a reduced NR2A:NR2B ratio in the ventral hippocampus compared with repeated saline (*significant difference between saline and cocaine groups, P < 0.05). Means + SEM are shown. (N=9–10/group)
Discussion
The major results of this study demonstrate that repeated intraperitoneal administration of cocaine (20 mg/kg/day for 7 days) induced sensitization to the locomotor stimulating effects of cocaine. Also, these same rats displayed increased motoric activity and extracellular dopamine in the nucleus accumbens following NMDA stimulation of the ventral hippocampus, which may result from altered NMDA receptor subunit composition in this brain region.
Behavioral sensitization is suggested to be related to drug craving observed in human psychostimulant abusers (Robinson and Berridge, 1993) and can facilitate acquisition of cocaine self-administration (Schenk and Partridge, 2000). The expression of behavioral sensitization to psychostimulants is greatly influenced by environmental context (Bonate et al., 1997; Uslaner et al., 2001) and the ventral hippocampus is involved in contextual processing that involves a motivational component, such as drug-paired environmental stimuli (Lasseter et al., 2010). Therefore, repeated engagement of the ventral hippocampus during cocaine administration may produce adaptations that make the ventral hippocampus sensitive to drug-associated environmental stimuli. However, short-term withdrawal from repeated amphetamine increases action potential threshold in the ventral subiculum (Cooper et al., 2003), and LTP in the ventral hippocampal-nucleus accumbens pathway following long-term withdrawal from repeated cocaine administration in rats is attenuated (Goto and Grace, 2005). These results suggest that following psychostimulant administration and withdrawal, the activity of the ventral hippocampus is reduced. Conversely, Lodge and Grace (2008) demonstrated that behavioral sensitization to amphetamine is associated with enhanced ventral hippocampal modulation of dopamine neuronal activity. Additionally, Britt et al. (2012) demonstrated that, in mice, protracted withdrawal from repeated cocaine administration produces an increase in synaptic strength selectively in the ventral hippocampal input to the nucleus accumbens shell and that inactivation of the ventral hippocampal input to the nucleus accumbens during administration of cocaine attenuates cocaine-induced locomotion in a specific environment (Britt et al., 2012). Results from the present study demonstrate that NMDA stimulation of the ventral hippocampus of behaving rats produced a significantly greater increase in extracellular dopamine in the nucleus accumbens of rats undergoing acute withdrawal from repeated cocaine than in control saline-injected rats. These results support the hypothesis that in behaving animals, hippocampal output is enhanced following repeated cocaine administration. Phasic dopaminergic neurotransmission is associated with reward prediction and cue-reward learning (Schultz et al., 1997) and the hippocampus-nucleus accumbens shell pathway is involved in place conditioning and context-dependent cue-reward retrieval (Floresco et al., 1997; Ito et al., 2008). Therefore, altered phasic dopamine resulting from enhanced ventral hippocampal activation of the VTA (Legault et al., 2000; Lodge and Grace, 2006) would likely result in maladaptive reward processing within the nucleus accumbens. In support of this, experimental activation of VTA dopamine neurons can enhance cue-reward learning or strengthen previously learned associations (Steinberg et al., 2013).
Synaptic plasticity requires complicated cellular processes involving multiple signaling pathways in different brain regions and subregions. Nonetheless, NMDAR are vital mediators of excitatory synaptic transmission in the brain. Subunit composition of NMDAR has been proposed to control NMDAR activity by controlling Ca2+ entry and intracellular signaling by the receptor. NR2B-containing receptors produce prolonged channel opening and greater Ca++ currents compared to NR2A-containing receptors (Yashiro, and Philpot, 2008), whereas NR2A-containing channels have higher opening probability and faster deactivation. Therefore, NR2A-containing receptors are more likely to open earlier but also close more quickly than NR2B-containing receptors. Over-expression of NR2B in mouse forebrain is associated with larger hippocampal LTP and enhanced learning in multiple behavioral tests (Tang et al., 2001; Cao et al., 2007; Wang et al., 2009). In the hippocampus, the NR2B subunit is vital for LTP and is involved in recruiting molecules such as CAMKII that are important for LTP (Foster et al., 2010). No significant changes in the mean expression of individual subunits following repeated cocaine are observed in the current study. However, cocaine pretreated animals exhibited a reduced NR2A/NR2B expression ratio, suggesting a greater contribution of NR2B-containing receptors in the ventral hippocampus of cocaine sensitized animals. The properties of the NR2B subunit, such as longer channel open times and greater Ca2+ entry per opening (Cull-Candy and Leszkiewicz, 2004), suggest that a greater contribution of ventral hippocampal NR2B (due to decreased NR2A:NR2B ratio) in cocaine pretreated rats may produce enhanced responses to NMDA infusion in these animals.
Previous studies demonstrate that repeated cocaine administration results in heightened anxiety-like behaviors (Hayase et al., 2005; Perrine et al., 2008; El Hage et al., 2012). An intriguing possibility is that the altered NMDAR composition observed in the ventral hippocampus following repeated cocaine in the present study is also involved in these behaviors. Infusion of the NMDA-receptor antagonist, AP5, into the ventral hippocampus produces an anxiolytic-like effect in drug naïve rats (Nascimento Häckl and Carobrez, 2007). Furthermore, rats selected for high anxiety show elevated NR2B in the hippocampus following re-exposure to a fear-conditioned context (Lehner et al., 2011). Together, our finding of reduced NR2A:NR2B ratio may be related to increased output to other structures besides the accumbens, such as the basolateral amygdala (Felix-Ortiz et al., 2013) or prefrontal cortex (Adhikari, 2010), involved in the regulation of anxiety-like behaviors. This possibility of greater general activity of the ventral hippocampus following repeated cocaine exposure in relation to emotional behaviors requires further investigation.
Taken together, the present study supports the hypothesis that enhanced behavioral responsiveness to psychomotor stimulants is associated with enhanced dopaminergic activity in the accumbens due to activation of the ventral hippocampus. This is extended by the finding that adaptation to glutamatergic signaling within the ventral hippocampus may produce sensitivity to activation due to changes in glutamatergic receptor composition. A more complete understanding of how drugs of abuse affect reward processing modulated by the ventral hippocampus will result in a better understanding of the pathophysiology of drug abuse, and possibly indicate novel therapeutic avenues.
Acknowledgements
This work was supported in part by P30 DA013429, T32 DA007237 and R01 DA019921. We would like to thank Ms. Mary McCafferty for her valuable assistance, Ms. Jamie Scholl for assistance with HPLC analysis of dopamine, and the NIDA Drug Supply Program for supplying cocaine hydrochloride for our studies.
Footnotes
The authors have no conflicts of interest to report.
References
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Henry JD. Locomotor activity and accumbens Fos expression driven by ventral hippocampal stimulation require D1 and D2 receptors. Neuroscience. 1999;94(1):59–70. doi: 10.1016/s0306-4522(99)00303-6. [DOI] [PubMed] [Google Scholar]
- Barr JL, Forster GL. Serotonergic neurotransmission in the ventral hippocampus is enhanced by corticosterone and altered by chronic amphetamine treatment. Neuroscience. 182:105–114. doi: 10.1016/j.neuroscience.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bonate PL, Swann A, Silverman PB. Context-dependent cross-sensitization between cocaine and amphetamine. Life sciences. 1997;60:l1–7. doi: 10.1016/s0024-3205(96)00591-7. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM, Gibson CJ. Release of dopamine in the nucleus accumbens caused by stimulation of the subiculum in freely moving rats. Brain research bulletin. 1997;42:303–308. doi: 10.1016/s0361-9230(96)00290-0. [DOI] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin Z, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Cooper DC, Moore SJ, Staff NP, Spruston N. Psychostimulant-induced plasticity of intrinsic neuronal excitability in ventral subiculum. J Neurosci. 2003;23:9937–9946. doi: 10.1523/JNEUROSCI.23-30-09937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Science's STKE : signal transduction knowledge environment. 2004:re16. doi: 10.1126/stke.2552004re16. 2004. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learning & memory (Cold Spring Harbor, N.Y.) 2003;10:456–465. doi: 10.1101/lm.64103. [DOI] [PubMed] [Google Scholar]
- del Olmo N, Miguéns M, Higuera-Matas A, Torres I, García-Lecumberri C, Solís JM, Ambrosio E. Enhancement of hippocampal long-term potentiation induced by cocaine self-administration is maintained during the extinction of this behavior. Brain Res. 2006;1116:120–126. doi: 10.1016/j.brainres.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, et al. Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology. 2004;47(Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- El Hage C, Rappeneau V, Etievant A, Morel AL, Scarna H, Zimmer L, Berod A. Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PloS one. 7:e43535. doi: 10.1371/journal.pone.0043535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron. 79:658–664. doi: 10.1016/j.neuron.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural immunocytochemical localization of the N-methyl-D-aspartate receptor and tyrosine hydroxylase in the shell of the rat nucleus accumbens. Brain Res. 1996;739:169–181. doi: 10.1016/s0006-8993(96)00822-0. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behavioural pharmacology. 2005;16:395–404. doi: 10.1097/00008877-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski RM, Nunez-Taltavull JF, Budziszewska B, Lason W, Gasior M, Zapata A, Shippenberg TS, Witkin JM. Effects of cocaine-kindling on the expression of NMDA receptors and glutamate levels in mouse brain. Neurochemical research. 36:146–152. doi: 10.1007/s11064-010-0284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Sub-region specific contribution of the ventral hippocampus to drug context-induced reinstatement of cocaine-seeking behavior in rats. Neuroscience. 171:830–839. doi: 10.1016/j.neuroscience.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Rompre PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20:1635–1642. doi: 10.1523/JNEUROSCI.20-04-01635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Skorzewska A, Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Krzascik P, Plaznik A. Expression of N-methyl-D-aspartate (R)(GluN2B) - subunits in the brain structures of rats selected for low and high anxiety. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 62:473–482. [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. Cocaine treatment- and withdrawal-induced alterations in the expression and serine phosphorylation of the NR1 NMDA receptor subunit. Psychopharmacology (Berl) 2002;164:349–359. doi: 10.1007/s00213-002-1209-9. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus. 2007;17:10–25. doi: 10.1002/hipo.20237. [DOI] [PubMed] [Google Scholar]
- Maggio N, Segal M. Differential modulation of long-term depression by acute stress in the rat dorsal and ventral hippocampus. J Neurosci. 2009;29:8633–8638. doi: 10.1523/JNEUROSCI.1901-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Monti MC, Almirón RS, Bignante EA, Ramírez OA. Changes in hippocampal arc protein expression and synaptic plasticity by the presentation of contextual cues linked to drug experience. Synapse. 64:39–46. doi: 10.1002/syn.20700. [DOI] [PubMed] [Google Scholar]
- Nascimento Häckl LP, Carobrez AP. Distinct ventral and dorsal hippocampus AP5 anxiolytic effects revealed in the elevated plus-maze task in rats. Neurobiol Learn Mem. 2007;88:177–185. doi: 10.1016/j.nlm.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140:163–175. doi: 10.1016/j.neuroscience.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Peleg-Raibstein D, Feldon J. Effects of dorsal and ventral hippocampal NMDA stimulation on nucleus accumbens core and shell dopamine release. Neuropharmacology. 2006;51:947–957. doi: 10.1016/j.neuropharm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Perez MF, Gabach LA, Almiron RS, Carlini VP, De Barioglio SR, Ramirez OA. Different chronic cocaine administration protocols induce changes on dentate gyrus plasticity and hippocampal dependent behavior. Synapse. 64:742–753. doi: 10.1002/syn.20788. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Sensitization to cocaine's reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav. 2000;66:765–770. doi: 10.1016/s0091-3057(00)00273-2. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nature neuroscience. 16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wang H, Feng R, Kyin M, Tsien JZ. Differential effects of enrichment on learning and memory function in NR2B transgenic mice. Neuropharmacology. 2001;41:779–790. doi: 10.1016/s0028-3908(01)00122-8. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42:1039–1042. doi: 10.1016/s0028-3908(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Turchan J, Maj M, Przewlocka B. The effect of drugs of abuse on NMDAR1 receptor expression in the rat limbic system. Drug and alcohol dependence. 2003;72:193–196. doi: 10.1016/s0376-8716(03)00193-5. [DOI] [PubMed] [Google Scholar]
- Uslaner J, Badiani A, Day HE, Watson SJ, Akil H, Robinson TE. Environmental context modulates the ability of cocaine and amphetamine to induce c-fos mRNA expression in the neocortex, caudate nucleus, and nucleus accumbens. Brain Res. 2001;920:106–116. doi: 10.1016/s0006-8993(01)03040-2. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang D, Cui Z, Zeng Q, Kuang H, Wang LP, Tsien JZ, Cao X. Genetic enhancement of memory and long-term potentiation but not CA1 long-term depression in NR2B transgenic rats. PloS one. 2009;4:e7486. doi: 10.1371/journal.pone.0007486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Brudzynski SM. Mesolimbic dopamine terminals and locomotor activity induced from the subiculum. Neuroreport. 1995;6(12):1601–4. doi: 10.1097/00001756-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zornoza T, Cano-Cebrián MJ, Martínez-García F, Polache A, Granero L. Hippocampal dopamine receptors modulate cFos expression in the rat nucleus accumbens evoked by chemical stimulation of the ventral hippocampus. Neuropharmacology. 2005;49(7):1067–76. doi: 10.1016/j.neuropharm.2005.06.005. [DOI] [PubMed] [Google Scholar]