Abstract
Small penetrating brain artery thickening is a major feature of cerebral autosomal dominant arteriopathy with subcortical infacts and leukoencephalopathy (CADASIL). Though affected fibrotic arteries of CADASIL have been shown to accumulate collagen, other components that compose pathological arterial walls remain incompletely characterized. We investigated the expression of decorin (DCN), the first collagen-binding small leucine rich proteoglycan identified, in CADASIL. DCN was markedly upregulated in pathologically affected leptomeningeal and small penetrating arteries in CADASIL and notably weaker in normal arteries from control brains. DCN protein was localized principally to the media and adventitia and only occasionally expressed in the intima. Immunoblotting of brain lysates showed a 3-fold increase of DCN in CADASIL brains (compared to controls). Messenger RNA encoding DCN was 5-fold increased in CADASIL. We conclude that DCN is the first identified proteoglycan to be identified in CADASIL arteries and may accumulate through transcriptional mechanisms. Additional studies are warranted to determine whether DCN localizes broadly to pathological small vessels in other cerebrovascular disorders.
Keywords: CADASIL, small leucine rich proteoglycans, decorin, collagen, Notch, arteries
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is caused by mutations in conserved residues of NOTCH3 [1]. Brain arteries affected in CADASIL are markedly thickened and exhibit significant smooth muscle cell loss and fibrosis in the vascular media [2,3]. The role of protein pathology in CADASIL is underscored by the striking molecular genetics of CADASIL mutations (which nearly invariably involve cysteine residues) and marked hyalinization of brain arteries that includes complex macromolecules such as NOTCH3 [4], multiple forms of collagen[5], von Willebrand factor [6], TIMP3 [7], and vitronectin [7]. Staining of CADASIL brains demonstrates intense periodic acid Schiff (PAS) reactive arteries [3], suggesting the accumulation of glycosylated molecules within thickened vessel walls. Although proteoglycans have been implicated in peripheral artery disease, their role in brain arterial thickening has not been examined.
Decorin (DCN) was the first small leucine rich proteoglycan (SLRP) to be described [8]. It is known to bind collagen [9–12], and hence can colocalize with areas of fibrosis. In addition to a potential structural role in tissue, DCN also modulates a wide array of key signal transduction pathways with relevance to inflammation and fibrosis [13]. Since DCN binds to collagen and CADASIL features thickened PAS reactive arteries with extensive collagen deposition, we examined the distribution of DCN in a cohort of genetically characterized CADASIL brains.
Materials and Methods
Brain histology
Control brains were obtained from the Alzheimers Disease Research Core at the University of Michigan and the Brain Bank of the National Institute for Developmental and Childhood Disorders at the University of Maryland. Six rains from CADASIL patients with cysteine-altering NOTCH3 mutations have been previously described[5,6]. Two additional CADASIL brains with mutations R141C and R153C in NOTCH3 were also studied. The average age of CADASIL patients was 66 (n=8, range 46–83). For controls, the average age was 63 (n=6, range 47–82). Five micron sections from frontal cortex were analyzed by conventional immunohistochemical staining after antigen retrieval using microwave-assisted heating in citrate buffer. Sections were counterstained with hematoxylin. Mouse monoclonal antibody BRIC231 (anti-H; Santa Cruz) was used in parallel experiments to confirm antigen integrity in sections.
Protein and RNA quantification
Two monoclonal antibodies against DCN were used to detect protein distribution by immunohistochemistry. 3B3 and 6D6 were used separately at 1:100 dilution for staining. For Western blotting, electrophoretically separated proteins blotted to nitrocellulose were probed with 1:100 dilutions of both 3B3 and 6D6. Secondary antibodies labeled with infrared chromophores were detected using a Licor Odyssey scanner. Expression levels were normalized to tubulin content assessed on a parallel Western blot.
For mRNA quanitification assays, we analyzed wedges of frozen brain tissue that included meninges and an equal volume of gray and white matter. We converted RNA purified from frozen brain tissue by reverse transcription. cDNA was quantified by real time PCR, using HPRT, as a control to assess target gene regulation; the primer sequences were: Human DCN sense: 5′-CGGATTAAAAGGTTCCCTGGT-3′ and antisense: 5′-GACCACTCGAAGATGGCATT-3′. Human HPRT: Sense: 5′-TGGCGTCGTGATTAGTGATG-3′ and antisense: 5′-AATCCAGCAGGTCAGCAAAG -3′.
Statistical analysis
Results are displayed with standard deviations. All experiments were performed three times with the similar results. T-tests were applied with statistically significant differences considered for p<0.05.
Results
DCN protein in CADASIL
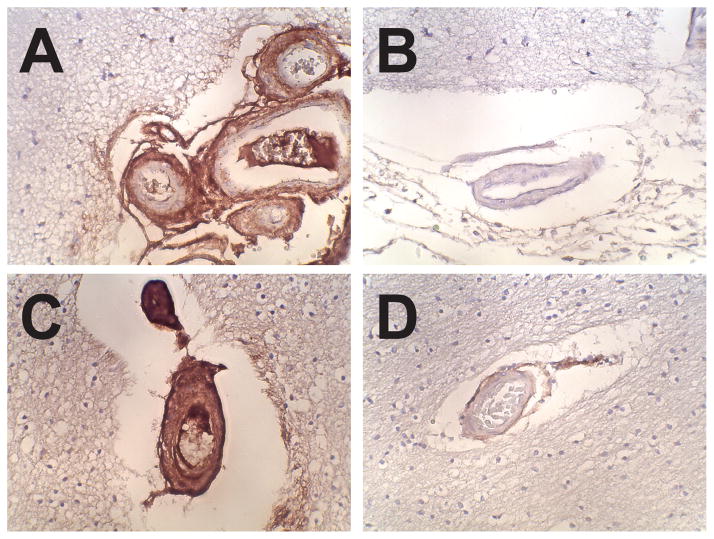
We localized DCN expression in the brain by immunohistochemistry using two independent monoclonal antibodies. Eight CADASIL brains from autopsies of individuals with NOTCH3 mutations were examined (Figure 1). Leptomeningeal arteries in CADASIL exhibit strong DCN reactivity in adventitia and degenerating vascular media. Much less reactivity was seen in regions of intimal hyperplasia. In penetrating small arteries of the cortical white matter, we observed intense staining that frequently extended through nearly the entire thickness of the artery, sparing the endothelium in most cases.
Figure 1.
Localization of DCN in CADASIL and control brain. Representative images of frontal lobe sections from a CADASIL brain (A, C) and control brain (B, D) stained with the 6D6 monoclonal antibody against DCN. Leptomeningeal arteries (A, B) and small penetrating arteries from the white matter (C, D) were photographed and demonstrate upregulated deposition of DCN. Images were taken at 400x magnification. Staining patterns using the independent antibody 3B3 was nearly identical (not shown).
In control brains, DCN was largely confined to the adventitia around leptomeningeal vessels. Penetrating vessels of the white matter were lightly stained in the adventitia. Finally, similar to CADASIL, there was light, sporadic capillary staining.
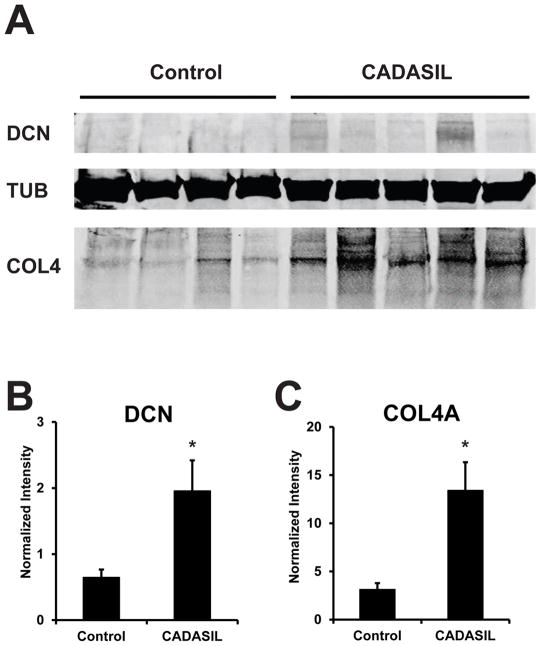
Western blot analysis was used to assess DCN protein levels in CADASIL brains compared to controls. DCN was significantly increased in CADASIL brain lysates compared to controls (Figure 2A and B). A parallel increase in COL4A protein was found on analysis of the same samples (Figure 2A and C). DCN mRNA was quantified using real time RT-PCR in samples examined in Figure 3. We found a significant increase in DCN mRNA in CADASIL brain compared to controls.
Figure 2.
DCN protein levels in CADASIL and control brain. (A) Cortical protein lysates from frontal lobes of CADASIL and control brains were analyzed by immunoblotting for the indicated proteins. Band intensities for DCN (B) and COL4A (C) were normalized to tubulin content and displayed. For DCN, we show quantification for the top band (approximately 160kDa.) A significant difference between groups is marked (*p=0.04 and p=0.02, respectively).
Figure 3.
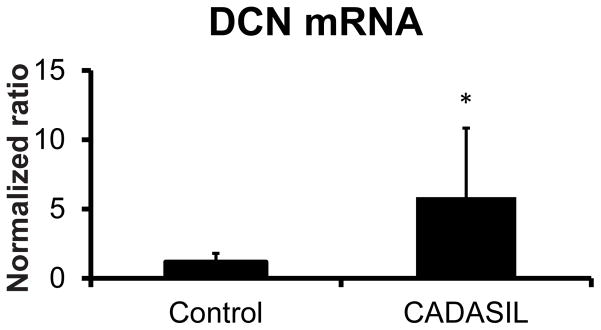
DCN mRNA levels in CADASIL and control brain. RNA prepared from CADASIL and control brain samples (frontal lobe sections) were analyzed by quantitative reverse transcriptase PCR. Ct-Ct analysis, using the HPRT as a reference, was employed to calculate relative mRNA expression levels. A significant difference between groups is marked (*p=0.001).
Discussion
CADASIL is the most common and well-investigated hereditable cause of small vessel disease [14], but the identity of molecules expressed in arteries is still evolving. In particular, proteoglycans, a class of glycoproteins suspected to contribute to tissue hyalinosis, have not been examined in CADASIL. We report here that DCN, the first small leucine rich proteoglycan to be described, accumulates in the media and adventitia of CADASIL arteries.
What stimulates the accumulation of DCN in cerebral vessels in CADASIL? The regulation of DCN has not been defined in the vasculature. In this study, we identify activation of DCN mRNA, which makes it likely that transcriptional regulation plays at least a partial role in activating DCN expression. Additional studies may shed light on whether DCN activation could result from transcriptional programs that are known to increase DCN transcript levels in diverse cell types [15–19]. Prior studies have suggested a complex mode of positive regulation of DCN transcripts, including an IL-1 responsive promoter element and post-transcriptional stabilization of DCN mRNA [20,21]. Until now, transcriptional activation mechanisms have not been implicated as a cause of protein accumulation in CADASIL.
A second, non-mutually exclusive mechanism of protein accumulation could be post-translational. DCN was first described as a collagen binding protein, and it is certainly feasible that anchoring of the protein stabilizes it and prevents normal clearance. The collagen expression patterns in CADASIL have been systematically studied, and the staining pattern of type VI collagen [5] most resembles that of DCN, which exhibits an “outside in” gradient of deposition; in contrast, the basement membrane predominant type IV collagen follows an “inside out” pattern [5]. In penetrating vessels, a number of collagen subtypes could bind to DCN, including types IV, VI, and XVIII collagen [5,22]. Of additional interest, LRP1 has been demonstrated as a receptor for DCN [23]. An interaction between NOTCH3 (which accumulate in CADASIL) and LRP1 has also been demonstrated [24]. The known function of LRP1 as a clearance receptor could implicate protein clearance deficits in DCN accumulation in diseased vessels.
What is the potential role of DCN in CADASIL? Previous studies imply that a potential role of DCN could include homeostatic downregulation of fibrosis. Prior studies in other tissues demonstrate a potent anti-fibrotic function of DCN via sequestration and inactivation of TGF-beta. Small vessel disease from TGF-beta inhibiting pathway mutations has recently been described. Therefore, if DCN binds to TGF-beta in arteries, it could serve a stabilizing role within the vessel. Furthermore, DCN has been shown to regulate pathways that participate in angiogenesis and vascular remodeling, including the generalized inhibition of receptor tyrosine kinases, IGF1, and integrin signaling [25–28]. DCN inhibition of HGF/Met signaling has been shown to increase expression of TIMP3, which has been shown to accumulate in CADASIL vessels. A structural role of DCN in CADASIL could also involve alterations of fibril formation within vessels. Multiple previous studies have demonstrated and DCN binds to collagen [10] and alters collagen properties both in vitro [12] and in vivo [29].
In summary, we demonstrate for the first time that DCN, a small leucine rich proteoglycan, localizes to subregions of pathologically thickened arteries affected by CADASIL. We have not addressed whether DCN is a general feature of small vessel disease or is specific for CADASIL. Miners et al has demonstrated transmural deposition of DCN in amyloid angiopathy of Alzheimers disease [30], suggesting that DCN could be a common core component of small vessel pathology. As a factor that is regulated at the mRNA level in CADASIL, DCN may provide a toe-hold to discovering transcriptional mechanisms in cerebral arteriopathy, including sporadic and hypertensive small vessel disease.
Acknowledgments
We thank the multiple reviewer for constructive feedback. We have incorporated the critiques into the revised manuscript, shown as “Track Changes” in Microsoft Word. The specific replies to the recent review are summarized below (in bold).
The National Institutes of Health and NINDS (NS052681, NS054724, and NS062816) and the Department of Veterans Affairs (5I01BX000375) provided funding for these studies. Tissue was also obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, contract HHSN275200900011C, Ref. No N01-HD-9-0011. DCN monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank, an entity created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
List of Abbreviations and Acronyms
- DCN
decorin
- mRNA
messenger ribonucleic acid
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
References
- 1.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 2.Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56:947–964. [PubMed] [Google Scholar]
- 4.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Blaivas M, Wang MM. Bidirectional encroachment of collagen into the tunica media in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain research. 2012;1456:64–71. doi: 10.1016/j.brainres.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Meng H, Blaivas M, Rushing EJ, Moore BE, Schwartz J, et al. Von Willebrand Factor permeates small vessels in CADASIL and inhibits smooth muscle gene expression. Translational stroke research. 2012;3:138–145. doi: 10.1007/s12975-011-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monet-Lepretre M, Haddad I, Baron-Menguy C, Fouillot-Panchal M, Riani M, Domenga-Denier V, et al. Abnormal recruitment of extracellular matrix proteins by excess Notch3 ECD: a new pathomechanism in CADASIL. Brain : a journal of neurology. 2013;136:1830–1845. doi: 10.1093/brain/awt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krusius T, Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. The Journal of biological chemistry. 1992;267:5250–5256. [PubMed] [Google Scholar]
- 10.Scott JE, Orford CR. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. The Biochemical journal. 1981;197:213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thieszen SL, Rosenquist TH. Expression of collagens and decorin during aortic arch artery development: implications for matrix pattern formation. Matrix biology : journal of the International Society for Matrix Biology. 1995;14:573–582. doi: 10.1016/s0945-053x(05)80006-x. [DOI] [PubMed] [Google Scholar]
- 12.Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. The Biochemical journal. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. The American journal of pathology. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 15.Gouveia RM, Connon CJ. The effects of retinoic acid on human corneal stromal keratocytes cultured in vitro under serum-free conditions. Investigative ophthalmology & visual science. 2013;54:7483–7491. doi: 10.1167/iovs.13-13092. [DOI] [PubMed] [Google Scholar]
- 16.Hahne JC, Okuducu AF, Fuchs T, Florin A, Wernert N. Identification of ETS-1 target genes in human fibroblasts. International journal of oncology. 2011;38:1645–1652. doi: 10.3892/ijo.2011.981. [DOI] [PubMed] [Google Scholar]
- 17.He F, Zhang Q, Kuruba R, Gao X, Li J, Li Y, et al. Upregulation of decorin by FXR in vascular smooth muscle cells. Biochemical and biophysical research communications. 2008;372:746–751. doi: 10.1016/j.bbrc.2008.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi E, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, Hayashi M, et al. Hepatocyte growth factor regulates proteoglycan synthesis in interstitial fibroblasts. Kidney international. 2003;64:1179–1188. doi: 10.1046/j.1523-1755.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 19.Qin P, Haberbusch JM, Zhang Z, Soprano KJ, Soprano DR. Pre-B cell leukemia transcription factor (PBX) proteins are important mediators for retinoic acid-dependent endodermal and neuronal differentiation of mouse embryonal carcinoma P19 cells. The Journal of biological chemistry. 2004;279:16263–16271. doi: 10.1074/jbc.M313938200. [DOI] [PubMed] [Google Scholar]
- 20.Mauviel A, Korang K, Santra M, Tewari D, Uitto J, Iozzo RV. Identification of a bimodal regulatory element encompassing a canonical AP-1 binding site in the proximal promoter region of the human decorin gene. The Journal of biological chemistry. 1996;271:24824–24829. doi: 10.1074/jbc.271.40.24824. [DOI] [PubMed] [Google Scholar]
- 21.Pearson D, Sasse J. Differential regulation of biglycan and decorin by retinoic acid in bovine chondrocytes. The Journal of biological chemistry. 1992;267:25364–25370. [PubMed] [Google Scholar]
- 22.Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, et al. Hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E128–135. doi: 10.1073/pnas.1101964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabello-Verrugio C, Brandan E. A novel modulatory mechanism of transforming growth factor-beta signaling through decorin and LRP-1. The Journal of biological chemistry. 2007;282:18842–18850. doi: 10.1074/jbc.M700243200. [DOI] [PubMed] [Google Scholar]
- 24.Meng H, Zhang X, Lee SJ, Strickland DK, Lawrence DA, Wang MM. Low density lipoprotein receptor-related protein-1 (LRP1) regulates thrombospondin-2 (TSP2) enhancement of Notch3 signaling. The Journal of biological chemistry. 2010;285:23047–23055. doi: 10.1074/jbc.M110.144634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiedler LR, Schonherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, et al. Decorin regulates endothelial cell motility on collagen I through activation of insulin-like growth factor I receptor and modulation of alpha2beta1 integrin activity. The Journal of biological chemistry. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 26.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, et al. Decorin is a novel antagonistic ligand of the Met receptor. The Journal of cell biology. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan GA, Girish GV, Lala N, Di Guglielmo GM, Lala PK. Decorin is a novel VEGFR-2-binding antagonist for the human extravillous trophoblast. Molecular endocrinology. 2011;25:1431–1443. doi: 10.1210/me.2010-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moscatello DK, Santra M, Mann DM, McQuillan DJ, Wong AJ, Iozzo RV. Decorin suppresses tumor cell growth by activating the epidermal growth factor receptor. J Clin Invest. 1998;101:406–412. doi: 10.1172/JCI846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. The Journal of cell biology. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miners JS, Ashby E, Van Helmond Z, Chalmers KA, Palmer LE, Love S, et al. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer’s disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathology and applied neurobiology. 2008;34:181–193. doi: 10.1111/j.1365-2990.2007.00885.x. [DOI] [PubMed] [Google Scholar]