Abstract
Failures in cervical cancer screening include non-participation, under-screening, and loss-to-follow up of abnormal results. We estimated the long-term health benefits from and maximum investments in interventions targeted to improving compliance to guidelines while remaining cost-effective. We employed a mathematical model empirically calibrated to simulate the natural history of cervical cancer in Norway. A baseline scenario reflecting current practice using cytology-based screening was compared to scenarios that target different sources of non-compliance: 1) failure to follow-up women with abnormal results, 2) screening less frequently than recommended (i.e., under-screening), and 3) absence of screening. A secondary analysis included human papillomavirus (HPV)-based screening as the primary test. Model outcomes included reductions in lifetime cancer risk and incremental net monetary benefit (INMB) resulting from improvements with compliance. Compared to the status quo, improving all sources of non-compliance leads to important health gains and produced positive INMBs across a range of developed-country willingness-to-pay thresholds. For example, a 2% increase in compliance could reduce lifetime cancer risk by 1-3%, depending on the targeted source of non-compliance and primary screening method. Assuming a willingness-to-pay threshold of $83,000 per year of life saved and cytology-based screening, interventions that increase follow-up of abnormal results yielded the highest INMB per 2% increase in coverage ($19 ($10-21)). With HPV-based screening, recruiting non-screeners resulted in the largest INMB ($23 ($18-32)). Considerable funds could be allocated towards policies that improve compliance with screening under the current cytology-based program or towards adoption of primary HPV-based screening while remaining cost-effective.
Keywords: Mass screening, compliance, cost-effectiveness, cervical cancer, Pap smear, human papillomavirus
BACKGROUND
Organized cervical cancer (CC) screening programs are credited with significant reductions in cancer risk and death, but areas for improvement nonetheless exist. Surveillance of three Nordic screening programs has indicated that at least half of all CCs are diagnosed among women who are noncompliant with screening guidelines, identifying potential areas for improvement in program goals (1-3). Specifically in Norway, roughly 65% of eligible women attend cytology-based screening every three years in compliance with national guidelines, but the remaining never attend (i.e., non-screeners) or attend less frequently than the recommended interval (i.e., under-screeners). In addition, at least 35% of women with abnormal results fail to return within one year for follow-up testing as recommended (4).
Interventions to increase screening participation and adherence to guidelines, such as mass-media campaigns, pre-scheduling appointments (5), reminder letters (6, 7) and telephone reminders (6) have been explored. Surveillance of the Norwegian screening program indicates that after repeated reminder letters, screening coverage rates increased to nearly 80% within a 5-year period (4). Conceivably, more intensive interventions aimed at improving screening compliance could yield even greater benefits. In addition, primary human papillomavirus (HPV) testing for women over age 30, not yet adopted in Norway, could improve CC prevention in a cost-effective manner (8). Importantly, primary HPV testing may also facilitate improved coverage rates through the use of patient-collected (i.e., “self”) sampling (9).
Studies that assess interventions to increase participation are often limited to reporting outcomes in terms of the percent-increase in coverage since longer-term health gains, such as cancer reduction or life expectancy gains, are not readily observable (10). In addition, few studies evaluate whether the gains in coverage justify the additional cost associated with programs to decrease noncompliance. Studies that can translate the surrogate endpoint of coverage into a meaningful clinical benefit and measure costs are able to assess the value of the intervention. In the absence of trials, decision-analytic models can help estimate the downstream impact of reducing failures in the screening program and determine whether investing in interventions to increase participation is a valuable use of scarce resources. We therefore conducted a model-based analysis to estimate the additional health benefits associated with programs to improve compliance to cervical cancer screening, as well as the maximum amount that can be invested for these programs to remain cost-effective.
METHODS
Analytic overview
We employed a model-based approach, using an existing individual-based (i.e., first-order) Monte Carlo model that simulates the natural history of CC. The model was empirically-calibrated to reflect the burden of HPV and cervical disease in Norway and was previously used to evaluate HPV-based screening strategies (8). In the current analysis, we compared a baseline scenario representing status quo cytology-based screening and participation to scenarios that target three different failures in screening: 1) absence of screening, 2) under-screening, and 3) loss to follow-up after an abnormal result. Model outcomes included reductions in lifetime risk of cancer, discounted life expectancy, and lifetime costs under different scenarios of improved screening compliance. Cost-effectiveness results were expressed in terms of incremental net monetary benefits (INMB), which translate the additional benefit and costs of an intervention into a single unit of monetary cost for a given willingness to pay (WTP) threshold. The WTP, or cost-effectiveness, threshold signifies the amount society is willing to pay for a unit of health benefit, such as a year of life saved (YLS), and can be regarded as the amount below which an intervention may be considered ‘good value for money.’ Multiplying the incremental benefit accrued from one intervention compared to another by the WTP threshold allows the monetization of the benefit, which can then be compared against the intervention's incremental cost. Interventions with a positive INMB are considered cost-effective. We used the INMB, expressed on a per-woman basis, as a proxy for the maximum cost that could be additionally incurred before the incremental cost-effectiveness ratio associated with the intervention exceeds the WTP threshold, or in other words, the maximum added cost that could be spent to improve screening. In the base case, we assumed a WTP threshold of 500,000 Norwegian Kroner (NOK) ($83,000 U.S. dollars) per YLS in Norway (11); however, we also explored a threshold range often cited in the U.S. (i.e., $50,000 to $100,000 per YLS) to reflect the lack of consensus around a single threshold and to facilitate international comparisons.
Model
Individual girls enter the model at age nine (prior to sexual initiation) with a healthy cervix, and at each month, face age-dependent probabilities of type-specific HPV incidence and clearance, progression and regression of precancerous lesions, and progression to cancer. The model tracks and records outcomes for a cohort of women, such as the number of detected cancers and the discounted (4% per year) life expectancy and cost associated with each strategy. The model allows for complex pathways (i.e., screening, treatment, expenditures) between individual women to be recorded. This accounts for a large amount of heterogeneity between individual women with respect to screening compliance and history, arguably the most important risk modifier of cervical cancer risk. As reported previously (8), the model was calibrated, using a likelihood-based algorithm, to empirical epidemiological outcomes observed in Norway including age-specific prevalence of HPV-16, -18 (8) and high-grade precancerous lesions (12,13), type distribution of HPV-16, -18 in high-grade precancerous lesions (14), and pre-screening age-specific cancer incidence rates from the Cancer Registry of Norway (1953-1969). Observational data from Northern European (15-17) and Canadian (18) cancer registries suggest that an elevated (10-60% higher) baseline risk of CC may exist among non-participants. Therefore, we calibrated baseline input values for two populations: 1) a higher-risk population for the 10% of non-screeners, and 2) a lower-risk population for the 90% of women who either under-screen or who are lost to follow-up. The calibration process resulted in multiple parameter sets that fit well to the empirical data; we elected to use 50 “good-fitting” sets for each population in analyses to reflect uncertainty in input values (see supplementary Figure 1). To estimate the population-level outcomes we calculated the weighted average of the expected costs and benefits between these two populations. Credible bounds (CB) for the INMB represent the minimum and maximum values across the good-fitting parameter sets. To reflect decision-making from a societal viewpoint, we included all direct medical and non-medical costs (e.g., transport costs) as well as patient time costs associated with screening, management, and treatment (Table 1). All costs were measured in 2010 NOK and converted to US dollars (US $) using the average annual 2010 exchange rate (US $1=NOK6.05) (19). Total lifetime costs associated with the scenarios of increased participation included the upfront screening costs, as well as any downstream costs incurred (or averted) for diagnosis and/or treatment of precancer and invasive cancer. Details of the calibration process and cost estimation methods have been reported elsewhere (8, 20-22).
Table 1.
Selected model inputs
| Costs | Baseline ($) | Range ($) |
|---|---|---|
| Screening | ||
| Cytology | 49 | 8b |
| hr-HPV DNA testinga | 62 | 54b |
| Office visitc | 160 | 80 - 320 |
| Colposcopy with biopsyc | 340 | 170 - 670 |
| Treatmentc | ||
| High-grade precancer | 2,200 | |
| Local | 25,800 | 12,900 - 51,500 |
| Regional | 51,600 | 25,800 - 100,200 |
| Distant | 59,600 | 29,800 - 119,300 |
| Test Characteristics | Baseline (%) | Range (%) |
|---|---|---|
| HPV DNAd | ||
| Probability of HR-HPV given high-grade precancer + | 85% | 62-99% |
| Probability of no HR-HPV given no high-grade precancer | 89% | 85-94% |
| Cytologye | ||
| Probability of abnormal cytology given low-grade precancer | 70% | 50-70% |
| Probability of abnormal cytology given high-grade precancer + | 80% | 50-80% |
| Probability of normal cytology given normal histology | 95% |
*hr-HPV: high-risk human Papillomavirus; DNA: Deoxyribonucleic acid; Local: Stage Ia-IIa, Regional: Stage IIb-IIIb, Distant: IVa-IVb. All costs are expressed in 2010 U.S. dollars (US$=NOK6.05).
Shares co-collection fee for cytology.
Based on published reimbursement fees.
Includes patient time and transport, rounded. Surveillance of women after treatment for high-grade precancer varies in clinical practice but was assumed to involve three negative primary screening results before returning to routine screening. The model records all costs associated with post-treatment surveillance.
Probability of hr-HPV DNA positivity given hr-HPV is assumed to be 100%, but lowered to 90% in sensitivity analysis.
Abnormal cytology is defined as atypical squamous cells of undetermined significance (ASCUS) or worse.
Scenarios
Published screening rates in Norway (4) were used to estimate a distribution of screening frequency and compliance across the simulated cohort of women. We assumed 10% were nonscreeners, 65% complied with triennial screening and the remaining 25% were under-screeners (i.e., 10%, 5% and 10% of women were screened every four, five and eight years, respectively). Of those women requiring triage testing or diagnostic colposcopy, we assumed that 64% complied. We compared this baseline “status quo” scenario to three scenarios that improve different sources of non-compliance: 1) increasing follow-up after an abnormal result, 2) increasing screening frequency among the under-screened, and 3) increasing recruitment of previously unscreened women to attend triennial screening (but assuming the same 64% compliance after an abnormal result). For a woman who does not comply, the model requires the individual to wait until her next primary screening month where she will have an opportunity to be rescreened (i.e., the individual in not permanently lost to follow-up). We also considered a fourth scenario that improves all three issues simultaneously, essentially mimicking perfect compliance with guidelines. We assessed each scenario assuming primary cytology-based testing (current practice) but repeated all analyses assuming primary HPV testing for women over age 30. For scenarios 1-4, we calculated the INMB of reaching full compliance and interpreted this value as an upper bound of the amount that could be spent to improve adherence for each source of non-compliance. To allow for comparison and prioritization between scenarios, we then recalculated the INMB assuming a common absolute increase (i.e., 2%) in participation in each scenario. In sensitivity analysis, we explored the impact of varying input costs, screening test characteristics and lengthening (to five years) the primary HPV screening interval (assuming the same distribution of compliance) on results.
RESULTS
Cancer risk reduction
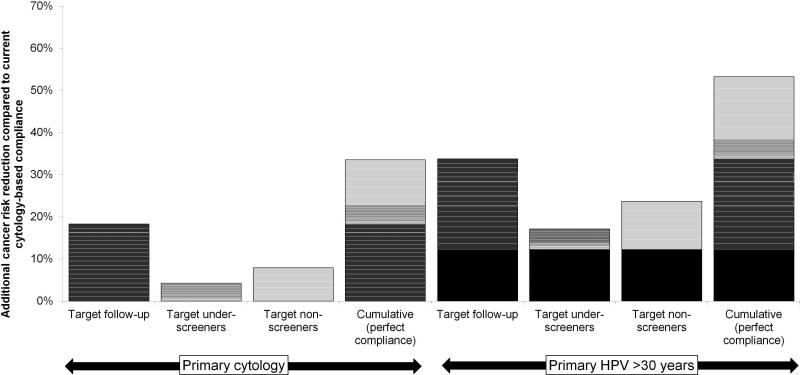
After undergoing calibration for the two risk groups, the model estimated a 28% higher background risk of CC for the high-risk population than the low-risk population. Assuming the status quo screening program in Norway, the model projected a population-level lifetime CC risk of 1.0% (CB: 0.7%, 1.3%) and a risk prior to age 65 of 0.7% (CB: 0.5%, 0.9%). These model estimates are consistent with the empirical data from the Cancer Registry of Norway that reports a 0.9% cumulative risk of CC by age 75 (4). Following up women who are currently noncompliant after an abnormal test (i.e., Scenario 1) would reduce lifetime cancer risk by 18% compared to the status quo (Figure 1, left). Resolving all screening failures simultaneously (i.e., Scenario 4) was projected to reduce cancer risk by 34% compared to the status quo. Even without improvements in screening compliance rates, switching to an HPV-based program in women over age 30 was expected to reduce cancer risk by 12%, compared to current cytology-based screening (Figure 1, right). Achieving perfect compliance to guidelines using primary HPV testing increased this reduction to nearly 51% compared to status quo participation using cytology. Per 2% increase in the follow-up rate, the expected cancer risk reduction was 1% under the current cytology, based screening program and nominally higher under a program that adopts HPV testing for older women. Every 2% increase in uptake by non-screeners reduced cancer risk by 1.6% using cytology and by 2.3% using HPV testing. When screening recruitment was accompanied by perfect follow-up compliance after an abnormal result, the cancer risk reduction increased to 2.3% and 3.0%, respectively.
Figure 1.
Additional cancer risk reduction and net monetary benefit (INMB) associated with targeted interventions to improve compliance to screening guidelines. The height of each bar corresponds to the y-axis (additional cancer risk reduction compared to status quo screening using cytology) for increasing compliance to100% within each scenario or for an incremental (2%) increase in screening adherence, designated by white lines. The INMBs associated with human papillomavirus (HPV)-based scenario are inclusive of the benefit (designated by the darkest solid box) and costs associated with switching current screeners to primary HPV testing for women age 30 years or older. For the primary HPV testing strategy, we assumed that women with a positive HPV test underwent additional cytology testing. Those who were both HPV and cytology-positive (i.e., atypical cells or worse) were referred directly to colposcopy; for women HPV-positive but cytology-negative, two additional persistent HPV-positive, cytology-negative results were required (each 12 months apart) prior to prompting referral to colposcopy.
Incremental net monetary benefit with maximized compliance
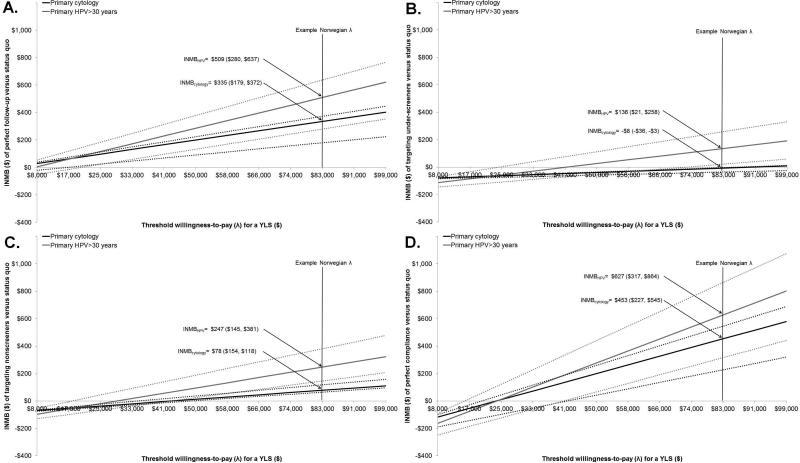
Given a willingness-to-pay threshold of $83,000 per YLS, all but one scenario produced positive incremental net monetary benefits compared to the status quo. That is, the incremental value of increasing participation exceeded the added lifetime costs in each scenario (Figure 2). For example, we interpreted the INMB for perfect follow-up of abnormal results of $335 (CB: $179, $372) as the maximum amount that could be spent on average per woman over her lifetime before an intervention to improve follow-up is no longer cost-effective. For a birth cohort of approximately 30,000 Norwegian females eligible for screening, this equates to nearly $10 million that could be allocated to achieve perfect follow-up over their lifetime. By comparison, the INMBs of improving screening frequency for under-screeners and recruiting previously unscreened women to screening were far lower (Figure 2B and 2C). Simultaneously improving all screening failures (i.e., perfect compliance) yielded an INMB of $453 (CB: $227, $545) per woman (Figure 2D). Under this best case scenario, nearly $14 million could be allocated across a birth cohort of 30,000 screen-eligible women and still remain cost-effective. The INMB and uncertainties around these values as a function of different WTP threshold values are shown for each of the four scenarios in Figure 2.
Figure 2.
Incremental net monetary benefit (INMB) of A) targeting follow-up, B) targeting under-screeners, C) targeting non-screeners and D) perfect compliance. Point estimate (solid line) and credible bounds (dotted lines) for the INMB assuming perfect compliance compared to status quo participation using cytology and are shown as a function of the willingness to pay (WTP) threshold (λ) per year of life saved (YLS). Credible bounds reflect uncertainty in input values across the good-fitting parameter sets.
Simply switching to HPV testing without improvements in compliance resulted in an INMB of $147 (CB: $69, $234), compared to current cytology-based screening and participation under the base case WTP threshold of $83,000 per YLS. Switching test method as well as maximizing compliance for follow-up, under-screeners and non-screeners resulted in INMBs of $509, $136 and $247 per woman, respectively. If, however, we assumed an organized screening program was operating in a situation where primary HPV testing for women over age 30 was already the current standard (and therefore the baseline comparator), targeting under-screeners would not provide any additional value (i.e., INMB<0), while eliminating loss-to-follow-up would have a INMB of $362 (CB: $211, $403) per woman. The INMB would be maximized at $627 (CB: $317, $864) under the best-case scenario of adopting HPV testing and perfect compliance across all failures in screening (Figure 2D).
Incremental net monetary benefit per 2% increase in participation
Although perfect compliance provides an upper-bound INMB, nominal improvements in follow-up and screening are more likely. To portray more realistic increases in compliance and to facilitate head-to-head comparison across scenarios, we expressed the INMB per 2% improvement in each scenario (Table 2). Under a WTP threshold of $83,000 per YLS, increasing follow-up by 2% yielded an INMB of $19 (CB: $10, $21) for cytology and comparable values for the primary HPV testing strategies. For primary cytology, the INMB increased by approximately $3 assuming a WTP of $100,000 per YLS and decreased to $11 (CB: $5, $12) at a WTP of $50,000 per YLS. When the under-screeners were disaggregated by screening interval, we found it was generally not cost-effective to increase participation among those women who attend screening every four or five years, though more attractive for women who seldom attend screening (i.e., every eight years), particularly at higher WTP thresholds. Under a WTP threshold of $83,000 per YLS, improving follow-up of women with abnormal results (i.e., Scenario 1) yielded the highest INMB under primary cytology-based screening, while recruiting previously unscreened women assuming imperfect follow-up (i.e., Scenario 3) resulted in the highest INMB with primary HPV-based testing. Moreover, recruiting nonscreeners accompanied by complete follow-up of abnormal results yielded higher INMB than any of the failures considered separately, except when WTP was low ($50,000 per YLS).
Table 2.
Average change in incremental net monetary benefit (INMB) per 2% increase among different subgroups of non-compliant women (credible bounds)
| Cytologyb |
HPV >30 yearsb |
|||||
|---|---|---|---|---|---|---|
| INMBa per 2% increase in: | λ=$50,000 | λ=$83,000 | λ=$100,000c | λ=$50,000 | λ=$83,000 | λ=$100,000c |
| Scenario 1 (imperfect follow-up) | $11 (5,12) | $19 (10,21) | $22 (12,25) | $12 (6,13) | $20 (12,22) | $24 (14,27) |
| Scenario 2 (under-screeners) | ||||||
| Attend every 4th or 5th year | −$3 (−5,−2) | −$1 (−4,0) | $0 (−3,2) | −$3 (−4,−1) | −$1 (−3,1) | $0 (−2,2) |
| Attend every 8th yeard | −$4 (−6,−4) | $0 (−3,0) | $2 (−1,2) | −$5 (−8,−2) | $0 (−5, 2) | $2 (−4,7) |
| Scenario 3 (non-screeners) | $2 (0,7) | $16 (12,22) | $22 (18,30) | $5 (1,11) | $23 (18,32) | $32 (26,42) |
| Non-screeners with perfect follow-upe | $8 (3,13) | $25 (18,34) | $34 (26,44) | $12 (4,19) | $34 (23,45) | $45 (32,58) |
HPV: Human papillomavirus, INMB: Incremental net monetary benefit; λ: Willingness to pay per year of life saved.
INMB=λ*Δeffect-Δcost
Estimates are the average difference across 2% increments in uptake, and rounded to nearest dollar. Credible bounds are based on average change in minimum and maximum of the 50 good-fitting parameter sets
Rounded from $99,000
No published coverage rates exist for 8-yearly screening; however, we selected this interval to represent the group of women who attend screening but very infrequently
Involves improvements in the higher-risk group of non-screeners (Scenario 3), but assumes compliance after an abnormal result is perfect.
Sensitivity analysis
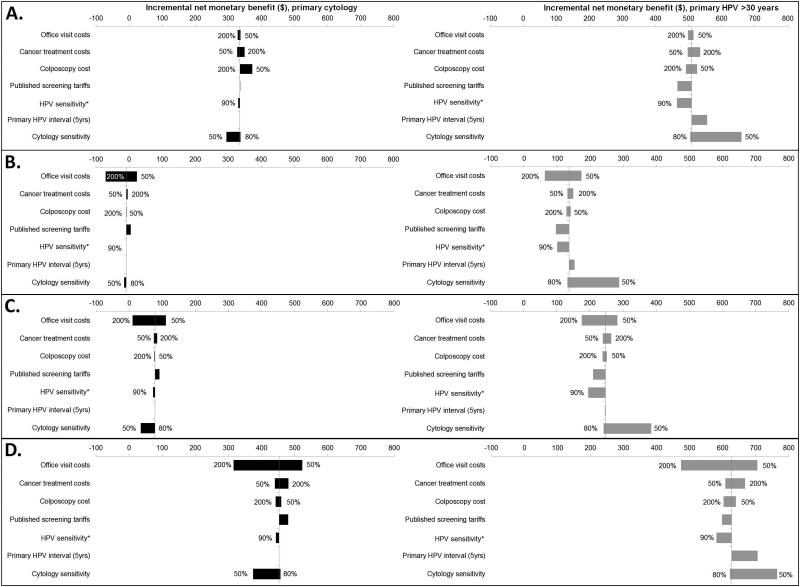
For all scenarios, the INMBs decreased (strategies became less attractive) when office visit costs doubled compared to status quo participation using cytology (Figure 3). Altering cytology test characteristics also had considerable impact on results. For instance, with a lower sensitivity, the INMB of any cytology-based scenario decreased, and the INMB of any HPV-based strategy became correspondingly more attractive. Under a situation of lower HPV test sensitivity, less money could be invested for improvements in all scenarios. For example, $0-$10 less could be invested in the cytology-based scenarios that use HPV testing in triage, while $36-$50 less could be invested in the scenarios that utilize primary HPV screening. Extending the primary HPV screening interval to five years primarily affected those women already participating in screening. For example, 5-yearly HPV-based screening under status quo participation yielded an expected reduction in lifetime cancer risk of 1.3% compared to status quo participation under 3-yearly cytology. We also found that health authorities could generally spend more to increase compliance among women who are lost to follow-up or under-screened, while lengthening the primary interval had a nominal impact on the amount that could be invested in interventions that target non-screeners. Varying cancer treatment costs, colposcopy costs and screening costs had a smaller impact on INMB.
Figure 3.
Tornado plot of influential parameters on the incremental net monetary benefit (INMB) of increasing screening among different subgroups of noncompliant women for primary cytology (black bars) and primary human papillomavirus (HPV) testing for women > age 30 (grey bars). A) targeting follow-up, B) targeting under-screeners, C) targeting non-screeners and D) perfect compliance. *HPV sensitivity defined as the probability of high-risk HPV DNA positivity given high-risk HPV.
DISCUSSION
Our results suggest that improving common failures in CC screening can lead to important health gains. Furthermore, our analysis indicates that a substantial amount of money could be invested in improving compliance to guidelines while remaining cost-effective across a broad range of willingness-to-pay threshold values. Moreover, as we did not specify the type of intervention, the information that we provide can be applied to a wide range of interventions.
Assuming 100% compliance, while overly optimistic, gives an upper bound of the expected health benefit and amount that could be spent to improve compliance; any investment beyond this is unlikely to be a good use of resources. We estimated the average INMB for a 2% increase in participation to allow for direct comparisons between the scenarios and to allow consideration of different rates of improvement in follow-up and uptake. Realistically, the hardest to reach individuals will likely require the most investment (e.g., increasing follow-up from 98 to 100% may require more resources than increasing it from 64 to 66%); however, by reporting the average maximum amount that could be spent allows this quantity to be divided (not necessarily equally) amongst relatively easier- or harder-to-reach groups. Interestingly, the primary screening test impacted which improvements to prioritize. With cytology testing, reducing imperfect follow-up (Scenario 1) yielded the highest INMB per 2% increase in coverage, whereas with HPV testing, recruiting non-screeners (Scenario 3) generally resulted in the highest INMB. Due to the lower sensitivity but higher specificity of a cytology test, the disease severity among the women identified and in-need of follow-up testing is greater than those identified by the more sensitive but less specific HPV test. Greater health benefits per dollar spent are achieved by ensuring the group of women identified by cytology complies with follow-up recommendations as opposed to targeting non-screeners. Conversely, with a more sensitive HPV test, greater value is achieved through targeting non-screeners than increasing follow-up rates.
Irrespective of test method, the INMB values associated with improving the frequency of under-screeners were primarily driven by targeting the women who screen least frequently (at >5-year intervals). Conversely, increasing compliance among women who attend screening more frequently was unattractive except at higher willingness-to-pay threshold values (Table 2). When we considered lengthening the primary screening interval to five years for HPV testing in sensitivity analysis, the value of improvements in compliance were greater for women lost to follow-up or under-screened. This finding underscores that compliance is relatively more important with less intensive screening intervals. On the other hand, the value of targeting nonscreeners to comply with 5-yearly screening remained comparable to encouraging them to comply with 3-yearly testing.
To our knowledge, this analysis represents the most comprehensive attempt to enumerate the benefits and value of targeting different screening short-comings in terms of meaningful health benefits (YLS, as opposed to simply increased coverage). In 1990, Koopmanschap and colleagues (23) conducted a model-based analysis to demonstrate that resources may be better spent increasing coverage rates compared to increasing the screening intensity among a well-screened population; a similar finding was reiterated in Denmark (24). However, these studies neither applied a distribution of screening compliance nor evaluated improvements beyond uptake of the primary screening visit. Beyond modeling analyses, few clinical studies have assessed the effectiveness of interventions using health outcomes (10), but were unable to report on long-term endpoints such as life expectancy, given the considerable time lag between detection and cancer-related death. A recent Swedish randomized controlled trial registered upfront costs of a telephone-based intervention and reported resource use in terms of total cost per additional detected case of high-grade precancer (25). By applying a “rule of thumb” for the ratio of treated high-grade lesions to averted CC case, the authors concluded that the telephone-based intervention would likely be cost-saving. While such empirical studies are needed, only a relatively short time horizon and a limited number of scenarios are feasible.
Limitations
Our study has important limitations. After decades of screening for cervical cancer, it is difficult to directly observe the comparative baseline risk between participants and non-participants. Universal health coverage in Norway coupled with an organized screening program may mitigate some extreme socio-economic disparities, often used as a proxy for high-risk individuals, reported in other settings. A Norwegian analysis of non-attendance (women with no cytology exam within four years) did not find lower education, number of lifetime sexual partners, or age of sexual debut to be significant predictors of non-attendance in a multivariable model (26). Yet, as shown in a recent paper by Dugue and colleagues (27) for a neighboring Nordic country, differences in non-cervical mortality between those who participate in the national screening program and those who do not, may still exist. After calibrating input parameters for two risk populations, our model predicted that the odds of CC among those who never attend were 2.9 times higher than those who attended and followed-up according to guidelines. It would be expected that this relative odds is higher than estimates published in a recent audit of the Swedish screening program that found a relative odds among those women who had not been screened in the last 6 years of 2.5 (3). Therefore, our assumptions of differential background risk may be reasonable, at least in settings similar to Norway and Sweden, but may not less generalizable to settings without universal health coverage. In settings in which noncompliance may have a greater correlation with higher background cervical cancer risk and non-attendance, our results may be a conservative estimate of the value of increasing compliance. We also did not consider whether a higher background risk among non-screeners would also translate into greater excess mortality. These factors are likely correlated (27), and may result in less attractive INMBs as those women who avoid cervical cancer death would face a higher mortality from other causes compared to women who face lower mortality from other causes. Alternatively, if improvements in screening behavior impacted utilization of other health services, greater screening participation may have positive effects on other areas of health. However, such analyses were considered to be outside the scope of the paper.
Simplifying assumptions were inherently necessary due to model constraints. We assumed that increasing participation occurs instantaneously which may have overestimated the benefit and subsequently the INMB of each scenario. However, due to the long interval between detected abnormalities and malignant behavior, a brief lag-time should have little impact on findings. Norwegian authorities have yet to decide on a screening interval for primary HPV testing; therefore in the base case, we elected to compare the frequency of cytology and HPV screening head-to-head; however, in sensitivity analysis, we assessed the impact of extending primary screening intervals. Analyses surrounding improvements in participation should be revisited as primary screening interval decisions are finalized and actual compliance to the new recommendations are observed. We did not consider over-utilization of screening, as it is not specifically reported in Norway; identification of over-screening may help to reduce total screening costs without compromising on health outcomes. Norwegian-specific cervical cancer utility values have not been elicited. Accounting for utility decrements would likely yield more attractive INMBs and, therefore, we expect our results (expressed in unadjusted life-years) to be a more conservative estimate. Lastly, the introduction of the HPV vaccination would be expected to reduce the risk of developing CC. Consequently, the value of increasing compliance to screening (assuming no changes to guidelines) would decrease. This scenario was not explicitly explored because it is not known how screening behavior and vaccination status may be correlated. In addition, national guidelines may well recommend different screening strategies for vaccinated women. These are important questions for future analyses, as studies addressing compliance with guidelines that reflect cost-effective interventions will continue to be of value.
CONCLUSION
Improving compliance to cervical cancer screening guidelines increases health benefits in terms of cancer reduction and life expectancy compared to current practice. In addition, a considerable investment could be allocated towards programs to improve compliance to screening while still remaining cost-effective under the current cytology-based program or towards adoption of primary HPV-based screening.
Supplementary Material
Novelty.
This study is among the first to quantify the value of improving failures in current cervical cancer screening practice. Our study translates the surrogate endpoint of improvements in coverage and compliance into long-term benefits and suggests that the largest gains in health from cervical cancer screening involve improvements in participation among women lost to follow-up after an abnormal test result, as well as recruiting previously unscreened women; targeting under-screened women provides modest health benefits.
Acknowledgements
We would like to thank Ivar Sønbø Kristiansen and Brian Potter for helpful comments during manuscript revisions.
Funding Sources
EAB is supported in part by the Norwegian Cancer Society [634201-2012]; JJK is supported by the U.S. National Cancer Institute of the National Institutes of Health [U54CA164336, R01CA160744].
Abbreviations
- CC
Cervical cancer
- HPV
Human papillomavirus
- INMB
Incremental net monetary benefit
- WTP
Willingness-to-pay
- YLS
Years of life saved
Footnotes
Details of Contributors
EAB and JJK contributed to the conception and design of the study; the analysis and interpretation of data; drafting and critically revising the manuscript for important intellectual content; and final approval of the version to be published. EAB is the guarantor for the study.
Conflicts of Interest
Authors have no conflicts of interest to declare
References
- 1.Nygard JF, Nygard M, Skare GB, Thoresen SO. Screening histories of women with CIN 2/3 compared with women diagnosed with invasive cervical cancer: a retrospective analysis of the Norwegian Coordinated Cervical Cancer Screening Program. Cancer Causes Control. 2005;4:463–474. doi: 10.1007/s10552-004-6295-z. [DOI] [PubMed] [Google Scholar]
- 2.Ibfelt E, Kjaer SK, Johansen C, et al. Socioeconomic position and stage of cervical cancer in Danish women diagnosed 2005 to 2009. Cancer Epidemiol Biomarkers Prev. 2012;5:835–842. doi: 10.1158/1055-9965.EPI-11-1159. [DOI] [PubMed] [Google Scholar]
- 3.Andrae B, Kemetli L, Sparen P, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100(9):622–629. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Registry of Norway . [2008 Annual Report Population-based Screening against Cervical Cancer] Oslo: 2009. [Google Scholar]
- 5.Segnan N, Senore C, Giordano L, Ponti A, Ronco G. Promoting participation in a population screening program for breast and cervical cancer: a randomized trial of different invitation strategies. Tumori. 1998;3:348–353. doi: 10.1177/030089169808400307. [DOI] [PubMed] [Google Scholar]
- 6.Eaker S, Adami HO, Granath F, Wilander E, Sparen P. A large population-based randomized controlled trial to increase attendance at screening for cervical cancer. Cancer Epidemiol Biomarkers Prev. 2004;3:346–354. [PubMed] [Google Scholar]
- 7.Morrell S, Taylor R, Zeckendorf S, Niciak A, Wain G, Ross J. How much does a reminder letter increase cervical screening among under-screened women in NSW? Aust N Z J Public Healt. 2005;1:78–84. doi: 10.1111/j.1467-842x.2005.tb00753.x. [DOI] [PubMed] [Google Scholar]
- 8.Burger EA, Ortendahl JD, Sy S, Kristiansen IS, Kim JJ. Cost-effectiveness of cervical cancer screening with primary human papillomavirus testing in Norway. Br J Cancer. 2012;9:1571–1578. doi: 10.1038/bjc.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snijders PJ, Verhoef VM, Arbyn M, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer. 2012;10:2223–36. doi: 10.1002/ijc.27790. [DOI] [PubMed] [Google Scholar]
- 10.Everett T, Bryant A, Griffin MF, Martin-Hirsch PP, Forbes CA, Jepson RG. Interventions targeted at women to encourage the uptake of cervical screening. Cochrane Database Syst Rev. 2011;5:CD002834. doi: 10.1002/14651858.CD002834.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norwegian Directorate of Health . [Health Effects of Socio-Economic Analyses] Oslo: 2007. [Google Scholar]
- 12.Molden T, Kraus I, Karlsen F, Skomedal H, Nygard JF, Hagmar B. Comparison of human papillomavirus messenger RNA and DNA detection: a cross-sectional study of 4,136 women >30 years of age with a 2-year follow-up of high-grade squamous intraepithelial lesion. Cancer Epidemiol Biomarkers Prev. 2005;2:367–372. doi: 10.1158/1055-9965.EPI-04-0410. [DOI] [PubMed] [Google Scholar]
- 13.Molden T, Kraus I, Karlsen F, Skomedal H, Hagmar B. Human papillomavirus E6/E7 mRNA expression in women younger than 30 years of age. Gynecol Oncol. 2006;1:95–100. doi: 10.1016/j.ygyno.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 14.Tjalma WA, Fiander A, Reich O, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;4:854–867. doi: 10.1002/ijc.27713. [DOI] [PubMed] [Google Scholar]
- 15.Magnus K, Langmark F, Andersen A. Mass-Screening for Cervical-Cancer in Ostfold County of Norway 1959-77. International Journal of Cancer. 1987;39:311–316. doi: 10.1002/ijc.2910390308. [DOI] [PubMed] [Google Scholar]
- 16.Hakama M, Rasanenvirtanen U. Effect of A Mass Screening-Program on Risk of Cervical-Cancer. American Journal of Epidemiology. 1976;103:512–517. doi: 10.1093/oxfordjournals.aje.a112253. [DOI] [PubMed] [Google Scholar]
- 17.Lonnberg S, Nieminen P, Luostarinen T, Anttila A. Mortality audit of the Finnish cervical cancer screening program. International Journal of Cancer. 2013;132:2134–2140. doi: 10.1002/ijc.27844. [DOI] [PubMed] [Google Scholar]
- 18.Fidler H, Boyes D, Worth A. Cervical Cancer Detection in British Columbia. J. Obstet. Gynaec. Brit. Cwlth. 1968;75:392–404. doi: 10.1111/j.1471-0528.1968.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 19.Federal Reserve [June 13, 2011];Historical Rates for the Norwegian Krone. 2011 http://www.federalreserve.gov/RELEASES/H10/Hist/dat00_no.htm.
- 20.Goldhaber-Fiebert JD, Stout NK, Salomon JA, Kuntz KM, Goldie SJ. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst. 2008;5:308–320. doi: 10.1093/jnci/djn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;8:821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JJ, Kuntz KM, Stout NK, et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol. 2007;2:137–150. doi: 10.1093/aje/kwm086. [DOI] [PubMed] [Google Scholar]
- 23.Koopmanschap MA, van Oortmarssen GJ, van Agt HM, van Ballegooijen M, Habbema JD, Lubbe KT. Cervical-cancer screening: attendance and cost-effectiveness. Int J Cancer. 1990;3:410–415. doi: 10.1002/ijc.2910450305. [DOI] [PubMed] [Google Scholar]
- 24.Gyrd-Hansen D, Holund B, Andersen P. A cost-effectiveness analysis of cervical cancer screening: health policy implications. Health Policy. 1995;1:35–51. doi: 10.1016/0168-8510(95)00720-d. [DOI] [PubMed] [Google Scholar]
- 25.Broberg G, Jonasson JM, Ellis J, et al. Increasing participation in cervical cancer screening: Telephone contact with long-term non-attendees in Sweden. Results from RACOMIP, a randomized controlled trial. Int J Cancer. 2012;1:164–71. doi: 10.1002/ijc.27985. [DOI] [PubMed] [Google Scholar]
- 26.Hansen BT, Hukkelberg SS, Haldorsen T, Eriksen T, Skare GB, Nygard M. Factors associated with non-attendance, opportunistic attendance and reminded attendance to cervical screening in an organized screening program: a cross-sectional study of 12,058 Norwegian women. BMC Public Health. 2011;11:264. doi: 10.1186/1471-2458-11-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dugue PA, Lynge E, Rebolj M. Mortality of non-participants in cervical screening: Registry-based cohort study. Int J Cancer. 2013 doi: 10.1002/ijc.28586. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.