Summary
The melanocortin system regulates metabolic homeostasis and inflammation. Melanocortin agonists have contradictorily been reported to both increase and decrease metabolic rate and body temperature. We find two distinct physiologic responses occurring at similar doses. Intraperitoneal administration of the nonselective melanocortin agonist MTII causes a melanocortin-4 receptor (Mc4r) mediated hypermetabolism/hyperthermia. This is preceded by a profound, transient hypometabolism/hypothermia that is preserved in mice lacking any one of Mc1r, Mc3r, Mc4r, or Mc5r. Three other melanocortin agonists also caused hypothermia, which is actively achieved via seeking a cool environment, vasodilation, and inhibition of brown adipose tissue thermogenesis. These results suggest that the hypometabolic/hypothermic effect of MTII is not due to a failure of thermoregulation. The hypometabolism/hypothermia was prevented by dopamine antagonists and MTII selectively activated arcuate nucleus dopaminergic neurons; these neurons may contribute to the hypometabolism/hypothermia. We propose that the hypometabolism/hypothermia is a regulated response, potentially beneficial during extreme physiologic stress.
Keywords: hypothermia, hypometabolism, hypotension, blood pressure, melanocortin, dopamine, thermoregulation, body temperature, metabolic rate, MTII
Introduction
Tight and flexible control of energy homeostasis is essential for survival, both of the individual and of the species. The evolving epidemics of obesity and type 2 diabetes are likely the result of environmental changes interacting with genetic variation, causing an imbalance between energy assimilation and energy expenditure. A more complete understanding of the physiologic control mechanisms should lead to improved therapies for obesity and diabetes.
The melanocortin system helps control energy homeostasis (Cone, 2005, 2006; Tao, 2010). Of the five melanocortin receptors, Mc4r has received the most attention: mice become obese when they express agouti, a protein inhibitor of Mc4r (Yen et al., 1994), when Mc4r function is disrupted (Huszar et al., 1997), or when the endogenous ligands (e.g. α-melanocyte stimulating hormone, α-MSH) derived from pro-opiomelanocortin are lost (Yaswen et al., 1999). Similarly, human obesity can be caused by reduced MC4R function (Vaisse et al., 1998; Yeo et al., 1998) or by loss of agonist ligand (Krude et al., 1998). MC4R mutations account for ~1–5% of extreme human obesity (Alharbi et al., 2007; Stutzmann et al., 2008) and common variants near MC4R are also associated with obesity (Loos et al., 2008). MC4R agonists are being considered for the treatment of obesity, as they reduce food intake, increase metabolic rate, and increase insulin sensitivity (e.g. (Kievit et al., 2013)). However, MC4R agonists also elevate blood pressure (Greenfield et al., 2009; Silva et al., 2006) and increase erectile activity (Van der Ploeg et al., 2002).
α-MSH also binds to and activates MC1R, MC3R, and MC5R, but not MC2R. MC1R agonism causes darkening of skin and hair (Robbins et al., 1993) and reduces inflammation (Leoni et al., 2010; Li and Taylor, 2008) while loss of MC1R function reduces sensitivity to certain painful stimuli (Mogil et al., 2005; Mogil et al., 2003). MC3R contributes to the control of energy homeostasis (e.g., null mice are mildly obese (Butler et al., 2000; Chen et al., 2000a)), natriuresis (Ni et al., 2003), and inflammation, acting at least partially on macrophages (Getting et al., 2008). Genetic variation in MC3R may contribute to human obesity (Feng et al., 2005; Renquist et al., 2011). MC5R regulates exocrine secretion (Chen et al., 1997) and inflammation (Lee and Taylor, 2013).
With the focus on melanocortins in obesity, an older, sometimes contradictory, literature investigating stress, inflammation, and core body temperature (Tb) has received less attention. Lipton reported that α-MSH reduced rabbit rectal temperature (Lipton and Glyn, 1980) and found that low doses of α-MSH prevented lipopolysaccharide-induced fever (Catania and Lipton, 1993; Murphy et al., 1983). In contrast, α-MSH increased Tb in rats (Raible and Knickerbocker, 1993; Resch and Simpson, 1991). Much of this Tb work was performed using non-selective ligands, such as α-MSH and MTII (Haskell-Luevano et al., 1997), before the identification of all five melanocortin receptors. MC4R-selective agonists can both reduce and increase Tb, although a non-MC4R contribution is also suspected (Metzger et al., 2010; Nicholson et al., 2007; Sinha et al., 2003, 2004).
The mouse, due to its small body size, exhibits amplified changes in Tb and responses to manipulation of environmental temperature (Gordon, 1993; Gordon, 2012). The large Tb changes and available genetic variants make the mouse an ideal system for studying the thermal biology of melanocortins. While melanocortin agonists typically increase metabolic rate (Chen et al., 2000b), there is also a report of a metabolic rate reduction (Wisse et al., 2006). Here we study the effects of melanocortin agonism on metabolic rate and Tb, finding divergent effects with importance for both energy homeostasis and the control of inflammation.
Results
Biphasic effect of treatment with MTII on energy expenditure and Tb
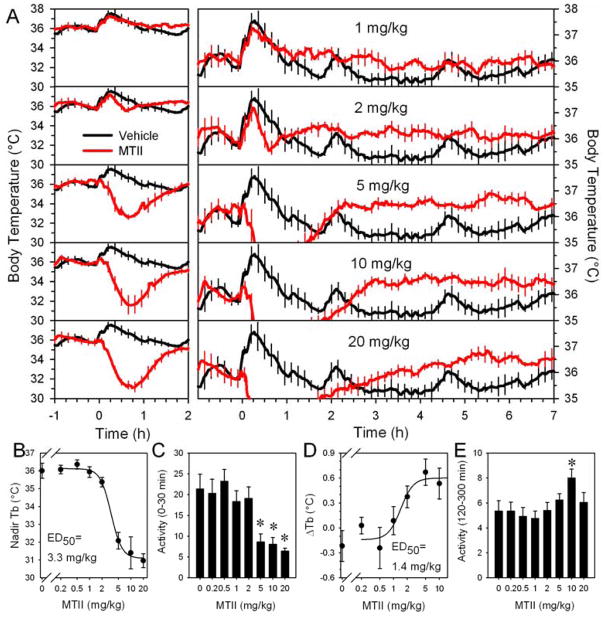
The nonselective melanocortin agonist, MTII, has a biphasic effect, first decreasing and then increasing Tb in chow-fed C57BL/6J mice (Figure 1A). These effects are in addition to the stress of handling, which initially increases both Tb and physical activity in mice treated with either vehicle or MTII. The ED50 for the hypothermic effect of MTII was 3.3 ±0.5 mg/kg (Figure 1 B). The hypothermia effect reached a plateau at the highest doses (5, 10, and 20 mg/kg), with these doses exhibiting a similar nadir Tb, time to nadir Tb (35 ±1, 38 ±3, and 37 ±3 minutes), and maximum cooling rate (−1.33 ±0.08, −1.37 ±0.08, and −1.50 ±0.12 °C/5 minutes at ~8–14 minutes after dosing). During hypometabolism/ hypothermia, physical activity was reduced (Figure 1C). The late Tb increase produced by MTII had an ED50 of 1.4 ±0.5 mg/kg (Figure 1D) and was sometimes accompanied by slight increases in physical activity (Figure 1E).
Figure 1.
MTII Tb dose response in lean C57BL/6J mice. (A) Tb response to the indicated MTII dose (red) or vehicle (black, repeated in each panel) injected into chow-fed mice (mean of n=6/group, body weight 27.1 ±0.3 g) studied at 21–22 °C. Left and right panels show the same data with different Tb axis scales to allow visualization of both effects. Every fifth SEM is shown for visual clarity. Dose response curves for the nadir in Tb (B), physical activity (C) (mean, 0 to 30 minutes), peak increase in Tb (D) (difference between the mean of −150 to −30 minutes and the mean of 120 to 300 minutes) and (E) physical activity (mean, 120 to 300 minutes) from the analyses of the data shown in (A). Activity is in arbitrary units via Mini Mitter. * indicates P<0.05 vs pooled vehicle, 0.2, and 0.5 mg/kg MTII data. Data are mean ±SEM.
MTII’s hypothermic and hyperthermic effects in diet-induced obese (DIO) mice (46.1 ±0.8 g) were similar to those in the chow-fed mice. The hypothermia ED50 was 3.3 ±0.6 mg/kg and the hyperthermia ED50, 2.0 ±0.8 mg/kg. The nadir Tb was 32.7 ±0.5, 31.7 ±0.3, and 31.4 ±0.8 °C at 5, 10, and 20 mg/kg, respectively, occurring at 41 ±3, 43 ±3, and 42 ±4 minutes.
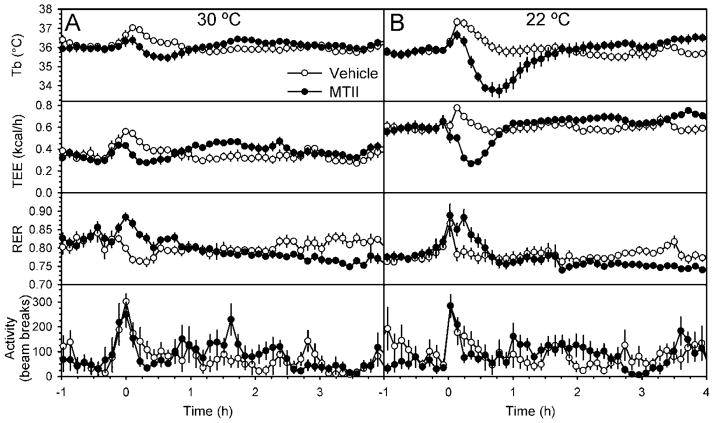
The effects of pharmacologic agents on metabolic rate are typically studied at thermoneutrality to neutralize the contribution of facultative thermogenesis. In DIO mice, the MTII-induced hypothermia at thermoneutrality (30 °C) was truncated compared the effect at 22 °C (Figure 2). Like its effect on Tb, MTII decreased and then increased total energy expenditure (TEE), while it had the opposite effect (increase then decrease) on respiratory exchange ratio (RER, indicating a decrease then increase in fractional fat oxidation). In DIO mice at 22 °C the Tb reduction was ~4 °C and the TEE reduction ~50% (Figure 2B). The nadir in TEE precedes that for Tb (at 23.8 ±1.6 minutes for TEE vs 39.9 ±2.8 minutes for Tb; p<0.001). The late increase in Tb and TEE and decrease in RER was seen at both 22 °C and 30 °C.
Figure 2.
Thermogenic effects of MTII treatment in diet-induced obese C57BL/6J mice housed at thermoneutrality or room temperature. (A) Tb, TEE, RER, and activity in DIO mice (mean body weight, 45.6 g) treated with MTII (black circles) or vehicle (white circles) in a randomized cross-over design studied at 30 °C (n=6/group). (B) Tb, TEE, RER, and activity in the same mice studied at 22 °C (n=9/group). Data are mean ±SEM.
Interestingly, hypothermia was not observed with every MTII treatment—it was not seen after 9% of 155 injections in 65 mice. All eleven non-responding mice that were tested more than once did show hypothermia with previous or subsequent MTII treatment. The hypothermic response to MTII was preserved in the setting of a cage switch (Lee et al., 2004) (Figure S1). This suggests that stress, as caused by cage switch, does not prevent the hypothermic response.
Hypothermia generation is an active process with reduced heat generation and ongoing heat loss
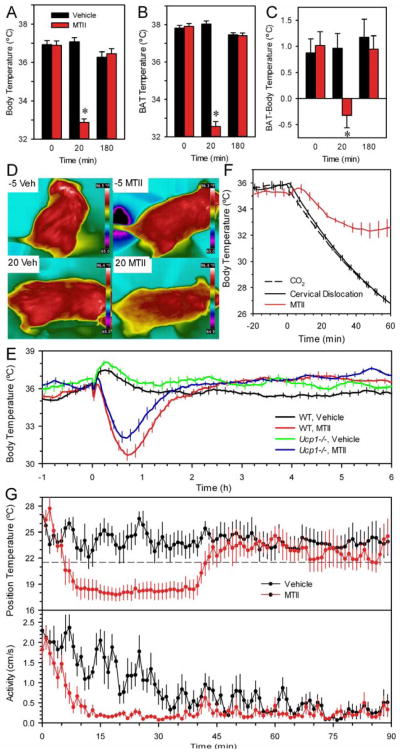
The physiological basis for the hypometabolism/ hypothermia was investigated next. BAT temperature is ~1 °C higher than Tb, but at 20 minutes after MTII dosing, this BAT-Tb difference was abolished. By 50 and 180 minutes after dosing, the BAT-Tb differential was re-established (Figure 3A–C). The mice were cooler over their full surface area; no preferred sites for heat loss were detected using infrared thermography (Figure 3D). These data demonstrate that BAT inactivation contributes to the hypothermic effect of MTII and suggest that BAT reactivation contributes to rewarming. However, rewarming occurred normally in Ucp1−/− mice (Figure 3E), suggesting that other heat-generating mechanisms can substitute for the lack of BAT functionality.
Figure 3.
Physiology of the hypothermic response to MTII. (A) Tb, (B) interscapular BAT temperature, and (C) the differential between BAT temperature and Tb in chow-fed C57BL/6J mice after MTII (red) or vehicle (black) treatment. Temperatures were measured at the indicated times after dosing. Data are mean ±SEM; n=6/group; * indicates p<0.05 vs vehicle (mean weight 27.5 g). (D) Infrared images of chow-fed mice taken 5 minutes before and 20 minutes after vehicle or MTII treatment using a Flir T400 camera and analyzed using Flir QuickReport 1.2 SP2 for measurement of temperatures within images. (E) Response of Ucp1−/− mice to MTII at 22 °C (body weight 26.1 ±1.6 g, n=8–10/group, crossover design, 8 male and 2 female). Every tenth SEM is shown for visual clarity. (F) Rate of heat loss in DIO mice. Mice were euthanized by carbon dioxide (black dashed line) or cervical dislocation (black solid line) at time 0. As a concurrent control, live mice were administered MTII (red). Ambient temperature was 21.6 °C (data are mean ±SEM; n=6–7/group; body weight 52.1 ±1.3 g). For visual clarity, every fifth SEM is graphed. (G) Choice of environmental temperature. Mice on a chow diet were treated with MTII (red) or vehicle (black) and immediately placed in a thermal gradient, with position monitored by video (n=8, crossover design). Ambient temperature was 21.6 °C (dashed line).
Next, we compared the rate of MTII-induced Tb reduction to the rate of heat loss after death (Figure 3F). The times for Tb to fall from 35.0 to 33.5 °C were 8.5 ±1.0 and 7.3 ±0.2 minutes in two euthanasia groups, compared with 9.0 ±1.3 minutes in MTII-treated mice (not statistically different). Thus, the rate of Tb reduction by MTII approaches that seen with cessation of metabolism.
When mice were allowed to choose their environment after MTII administration, the preferred environmental temperature was ~5 °C cooler than after treatment with vehicle and the duration of the cool preference was similar to the duration of the hypothermia (Figure 3G). After moving to the cool region, the mice were largely immobile. No shivering was observed. Taken together, these data suggest that the mouse uses all available mechanisms in order to achieve profound hypometabolism/ hypothermia.
Hypothermic effect attenuates with repeated dosing of MTII
When MTII was administered daily for five days, the late increase in Tb showed little or no attenuation, while the hypothermic effect was greatly attenuated by the second dose and not detectable subsequently (data not shown). The kinetics of the attenuation were explored by injecting two doses of MTII two hours apart. There was no hypothermia with the second MTII dose (Figure S2). This suggests that MTII hypometabolism/hypothermia is a time-limited response followed by an extended refractory period.
Hypotension accompanies MTII hypometabolism/hypothermia
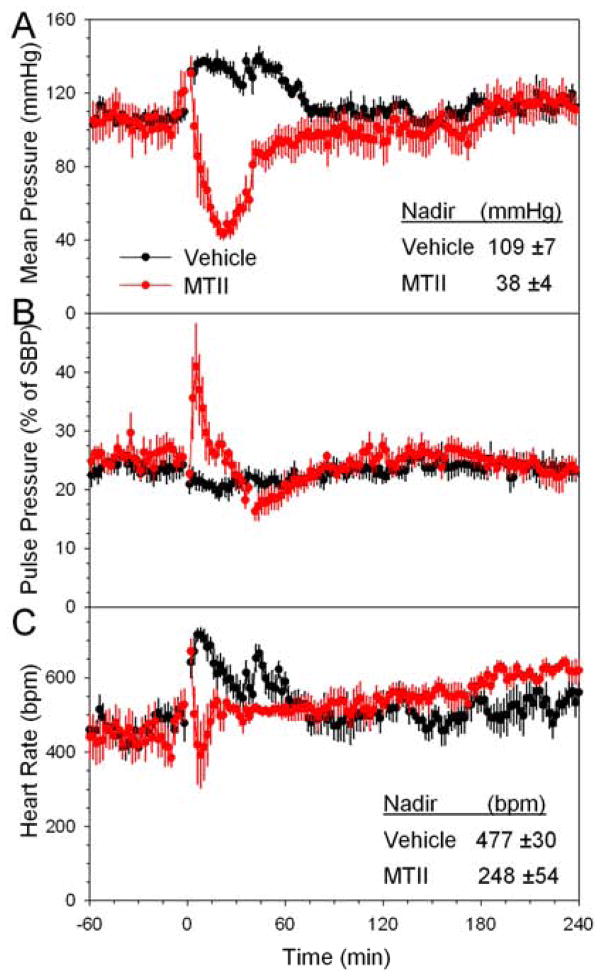
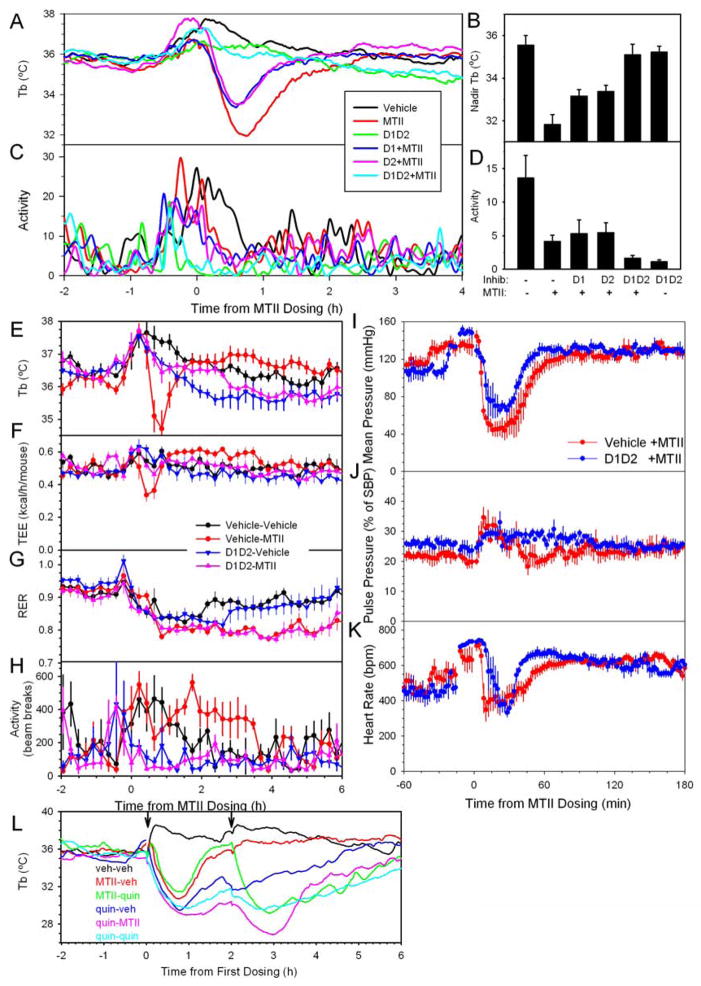
Melanocortin activation causes an increase in blood pressure (Greenfield et al., 2009; Hall et al., 2010; Sohn et al., 2013). However, at early times after MTII dosing we observed profound hypotension, with a nadir arterial pressure of 109 ±7 mmHg in the vehicle group vs 38 ±4 mmHg after MTII (p<0.001) (Figure 4A). Similar nadir changes were observed in the systolic (127 ±6 vs 48 ±11 mmHg, p<0.001) and diastolic blood pressure (99 ±5 vs 32 ±8 mmHg, p<0.001). The pulse pressure increased, suggesting that vasodilation contributes to the hypotension (Figure 4B). The blood pressure nadir was at 28 minutes (median), similar in time course and duration similar to the drop in TEE. MTII treatment also decreased heart rate, but this effect was smaller, more variable, and of shorter duration than the effect on blood pressure (Figure 4C). Interestingly, the MTII hypotension was not accompanied by tachycardia at any time; the heart rate actually remained below that of the vehicle-treated controls, which exhibited the expected increase due to handling stress. At later times after MTII dosing, there was a suggestion of an increase in heart rate but no clear increase in blood pressure.
Figure 4.
MTII effect on blood pressure and heart rate. (A) Mean arterial pressure, (B) pulse pressure (as a % of systolic blood pressure), and (C) heart rate were measured in ambulating telemetered mice at 22 °C treated with vehicle or MTII in a crossover design, n=5–6/group. Tb was measured just prior to MTII injection (35.5 ±0.3°C) and again at 40 minutes (vehicle, 36.1 ±0.3 °C; MTII, 31.1 ±0.3°C). Data are mean ±SEM.
MTII hypometabolism and hypermetabolism in melanocortin mutant mice
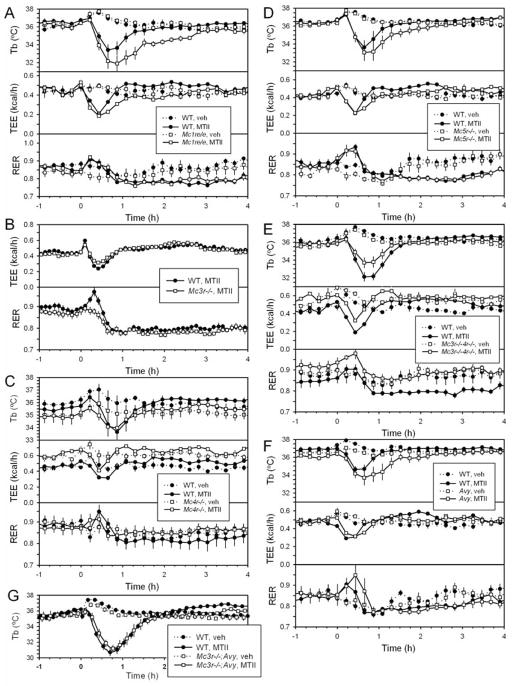
Since MTII binds four melanocortin receptors (MC1R, MC4R > MC3R > MC5R (Haskell-Luevano et al., 1997); see also (Conde-Frieboes et al., 2012)), we examined which receptors mediate the TEE and Tb effects in chow-fed mice. An initial hypometabolism/hypothermia was observed in Mc1r e/e, Mc3r−/−, Mc4r−/−, and Mc5r−/− mice (Figure 5A–D). It was also observed in Mc3r−/−;Mc4r−/− double null mice, Avy/+ mice (Avy/+ mice have reduced Mc1r and Mc4r signaling), and Mc3r−/−;Avy/+ mice (Figure 5E,F,G). These results indicate that none of Mc1r, Mc3r, Mc4r, or Mc5r is individually required for the initial hypometabolic/hypothermic response. The late increase in TEE and reduction in RER (indicating increased fat oxidation) were lost in the Mc4r−/− and Mc3r−/−;Mc4r−/− mice, and retained in Mc1r e/e, Mc3r−/−, and Mc5r−/− mice, demonstrating that these late effects are dependent on Mc4r. These data demonstrate that different receptors control the hypometabolism/hypothermia vs the hypermetabolism/hyperthermia.
Figure 5.
Tb, TEE, and RER response of melanocortin receptor mutant mice to MTII. (A) Mc1r e/e (21.6 ±0.3 g) and wild type (23.3 ±0.8 g) male mice, n=6/group. (B) Mc3r−/− (27.3 ±0.6 g) and wild type (27.0 ±0.5 g) male mice, n=12/group. (C) Mc4r−/− (54.1 ±1.0 g) and wild type (29.3 ±0.6 g) male and female mice, n=4–6/group. (D) Mc5r−/− (23.3 ±0.6 g) and wild type (22.3 ±0.6 g) male mice, n=6/group. (E) Mc3r−/−;Mc4r−/− (42.7 ±1.6 g) and wild type (25.6 ±0.8 g) male and female mice, n=6/ group. (F) Avy/+ (30.3 ±1.5 g) and wild type (22.5 ±0.6 g) female mice, n=5/group. (G) Mc3r−/−; Avy/+ (37.6 ±2.0 g, n=5) and wild type (28.5 ±0.5 g, n=9) male mice, crossover design. Every tenth symbol and SEM are shown for visual clarity.
To further explore receptor specificity, we measured the effect of a MC1R agonist, BMS-470539 (Kang et al., 2006). While BMS-470539 at 30 mg/kg ip caused hypothermia in wild type mice, hypothermia also occurred in Mc1r e/e mice (Figure S3A,B), indicating that this BMS-470539 effect does not require MC1R. We found that the small molecule MC4R agonist compound 2B (Guo et al., 2008) caused hypothermia and hypometabolism in both wild type and Mc4r−/− mice (Figure S3C,D), indicating that hypothermia/hypometabolism with 40 mg/kg ip compound 2B does not require MC4R. Another peptide melanocortin agonist, NDP-MSH ([Nle4,D-Phe7]-α-MSH) (Haskell-Luevano et al., 1997) also caused hypothermia with doses at the high end of those reported (Hoggard et al., 2004) and the hypothermia also occurred in Mc3r−/−;Avy/+ mice (Figure S3E).
Dopamine receptor antagonists block MTII hypometabolism/hypothermia
To investigate possible mechanisms by which MTII causes hypothermia, we selectively blocked neurotransmitters that can cause hypothermia. Specifically, naloxone (10 mg/kg ip) blocks mu/kappa opioid receptor-mediated hypothermia (Baker and Meert, 2002), naltrindole (5 mg/kg ip) blocks delta opioid receptor-mediated hypothermia (Rawls and Cowan, 2006), WAY100635 (1 mg/kg sc) blocks serotonin 5-HT1A receptor-mediated hypothermia (Cryan et al., 2000; Rawls and Cowan, 2006), and AM251 (10 mg/kg ip) blocks cannabinoid-1 receptor-mediated hypothermia (McMahon and Koek, 2007). Each inhibitor was tested by itself and had no effect on Tb in the first hour after dosing. When dosed prior to MTII, none of the inhibitors ameliorated MTII hypothermia (data not shown). These data indicate that MTII hypothermia does not require signaling via opioid (mu, kappa, or delta), serotonin 5-HT1A, or cannabinoid-1 receptors.
Brain-penetrant dopamine agonists cause hypothermia via both D1-like and D2-like receptors (Carboni et al., 1986; Nunes et al., 1991), referred to hereafter as D1 and D2. Individually, a D1 antagonist (SCH23390, 2 mg/kg ip) or a D2 antagonist (sulpiride, 30 mg/kg ip) partially inhibited MTII hypothermia, while the combined inhibitors completely blocked MTII hypothermia (Figure 6A,B). D1/D2 combined inhibition also inhibited MTII-induced hypometabolism (Figure 6E–H). MTII is not acting directly on either D1 or D2 receptors, as MTII does not bind with high affinity to these receptors (Figure S4). Physical activity was not affected by D2 antagonism and only modestly inhibited by D1 antagonism. However, activity was greatly inhibited by combined D1/D2 inhibition, irrespective of MTII treatment (Figure 6C,D). Decreased physical activity typically decreases Tb, thus the prevention of MTII hypothermia by D1/D2 inhibition is in the opposite direction expected for an activity effect. The later decrease in RER caused by MTII is not inhibited by dopamine antagonists. Taken together, these data demonstrate that the hypothermia caused by MTII is reversed by D1/D2 blockers and is largely independent of physical activity. In contrast, combined D1/D2 inhibition did not ablate the hypotensive effect of MTII, despite slight increases in mean arterial pressure and reduction in pulse pressure (Figure 6I–K). These results mechanistically distinguish the hypometabolism/hypothermia from the hypotension.
Figure 6.
Role of dopamine in mediating the hypothermic response to MTII. (A) Tb, (B) nadir Tb, (C) activity, and (D) mean activity (mean of 10–60 minutes) were measured in chow-fed mice (30.0 ±0.3 g) at 22 °C pre-treated with vehicle, D1 antagonist (SCH23390, 2 mg/kg), or D2 antagonist (sulpiride, 30 mg/kg) fifteen minutes before treatment with vehicle or MTII, n=7–8/group. Activity is in arbitrary units via Mini Mitter. (E) Tb, (F) TEE, (G) RER, and (H) activity were measured at 22 °C in chow-fed mice (27.2 ±0.3 g) pre-treated with vehicle, D1and D2 antagonists (SCH23390, 2 mg/kg and sulpiride, 30 mg/kg) fifteen minutes before treatment with vehicle or MTII, n=6/group. (I) Mean arterial pressure, (J) pulse pressure, and (K) heart rate were measured in ambulating telemetered mice at 22 °C pre-treated with vehicle or D1 and D2 antagonists (SCH23390, 2 mg/kg ip and sulpiride, 30 mg/kg ip) fifteen minutes before treatment with MTII at t=0 in a crossover design, n=3–4/group. (L) chow-fed mice (29.2 ±0.3 g) at 22 °C were treated with vehicle, MTII or quinpirole at 0 h and again 2 hours later, as indicated, n=4/group. Data are mean ±SEM.
The hypothermic effect of the D2 agonist, quinpirole (1 mg/kg ip) attenuates with daily dosing (Buck et al., 2000). However, when given 2 hours apart, quinpirole-induced hypothermia did not attenuate and there was no cross–attenuation of MTII and quinpirole (Figure 6L). Thus, attenuation of MTII hypometabolism/hypothermia does not appear to be a direct D2 effect.
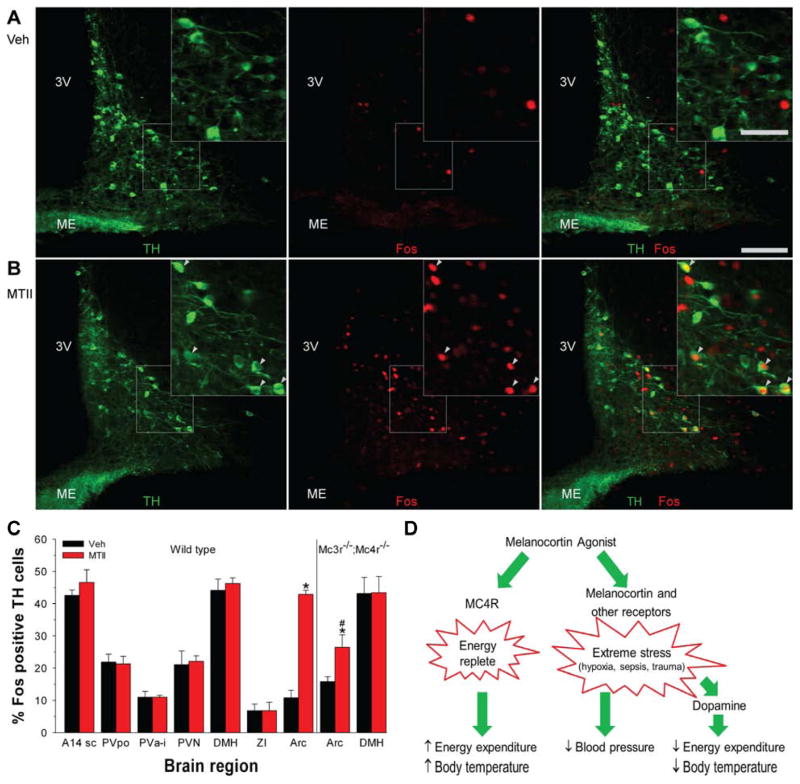
MTII selectively activates dopaminergic neurons in the arcuate nucleus
The dopamine antagonist data suggest that MTII acts via dopaminergic neurons to cause hypothermia/hypometabolism. To identify such neurons, we looked in brain regions implicated in the regulation of Tb and EE for dopaminergic (tyrosine hydroxylase (TH)-immunoreactive) neurons activated (Fos-immunoreactive) at one hour after treatment with MTII. Of seven candidate regions, only the arcuate nucleus showed increased Fos staining after MTII treatment in TH-immunoreactive neurons (Figure 7). Since melanocortins can act via arcuate dopaminergic neurons to suppress prolactin secretion (Dutia et al., 2012), we measured Fos activation in Mc3r−/−;Mc4r−/− mice, which presumably lack this function. In Mc3r−/−;Mc4r−/− mice treated with MTII, the percentage of TH-immunoreactive neurons that stained for Fos, while reduced compared to wild type mice, was significantly greater than in vehicle-treated Mc3r−/−;Mc4r−/− mice (Figure 7). These data suggest that arcuate dopaminergic neurons may mediate the MTII-induced hypothermia.
Figure 7.
MTII activates dopaminergic neurons selectively in the arcuate nucleus. A, B) Immunohistochemistry for TH (green; left panel) and Fos (red; middle panel) after vehicle (A) or MTII (B) treatment of C57BL/6J mice. Scale bar is 100 μm in main image and 50 μm in insert. Arrowheads indicate Fos-positive TH neurons; 3V, third ventricle; ME, median eminence. (C) Percentage TH-immunoreactive neurons staining for Fos after vehicle or MTII administration in wild type (n = 3/group) and Mc3r−/−;Mc4r−/− mice (n = 4/group). Neurons were counted in the subcommissural part of A14 dopaminergic cell group (A14sc; 2–3 sections), preoptic part of the periventricular hypothalamic nucleus (PVpo; 3 sections), paraventricular nucleus of the hypothalamus (PVN; 6–7 sections), anterior and intermediate part of the periventricular hypothalamic nucleus (PVa-i; 6–7 sections), dorsomedial hypothalamic nucleus (DMH; 2–3 sections), zona incerta (ZI; 3 sections), and arcuate nucleus (Arc; 6–7 sections). The number of TH neurons per region were not statistically different between the MTII and vehicle groups and the analyzed areas had expected distributions of TH-immunoreactive neurons (Lookingland and Moore, 2005). Data are mean ±SEM; * indicates P<0.05 vs vehicle. # indicates P<0.05, wild type vs Mc3r−/−;Mc4r−/−. (D) Actions of melanocortin agonists. Melanocortin agonists act via Mc4r to signal that the body is in an ‘energy replete’ state, detectable for example as an increase in energy expenditure and Tb during fasting. Melanocortin agonists also act likely via both melanocortin and other receptors to signal ‘extreme stress’ such as caused by hypoxia, sepsis, and severe trauma. This triggers a time-limited set of responses including hypometabolism and hypothermia mediated by dopaminergic pathways and also hypotension, which appears not to occur via dopaminergic pathways.
Discussion
The current studies aimed to unravel the contradictory observations that melanocortin agonists can both increase and decrease both metabolic rate and Tb. We found that MTII, the most commonly used melanocortin agonist, causes hypermetabolism/ hyperthermia in mice, which is preceded by a transient hypometabolism/ hypothermia/hypotension, with both effects occurring at similar doses. Immediately after MTII administration there are dramatic reductions in metabolic rate (by ~50%), Tb (by ~4 °C), and blood pressure (by ~65%) in awake mice at room temperature. Characteristics of the hypometabolic/hypothermic state include turning off BAT thermogenesis, rapid heat loss, active seeking of a cool environment, reduced physical activity, and no compensatory shivering. The TEE nadir at ~24 minutes precedes the Tb nadir at ~40 minutes, suggesting that hypometabolism drives the hypothermia. Thus, the hypometabolism is not simply a consequence of the reduced temperature per se causing the reduction in metabolic rate processes (known as a ‘Q10’ or temperature coefficient effect). While melanocortins cause heat loss via the tail in rat (Sinha et al., 2003) and ears in rabbit (Lipton et al., 1981), heat loss in the mouse appears generalized. Indeed, the hypotension and increased pulse pressure suggest that vasodilation is facilitating the heat loss. It is notable that the hypotension is not accompanied by a compensatory tachycardia, as occurs with some causes of hypotension, such as hypovolemia. The inactive BAT, hypotension, and lack of tachycardia are all evidence of reduced sympathetic tone. These observations demonstrate that reaching the hypothermic state is an active, coordinately regulated process, with reduced heat generation and without induction of heat-conserving mechanisms.
The hypometabolic/hypothermic response to MTII is preserved in mice lacking function of any one of Mc1r, Mc3r, Mc4r, or Mc5r. Three other melanocortin ligands (NDP-MSH, compound 2B, and BMS-470539) also caused hypothermia and these responses were also preserved in various melanocortin mutant mice, despite using doses previously believed to be selective. Thus, the hypometabolic/hypothermic response to MTII and other ligands appears to be mediated redundantly via melanocortin and/or other unidentified receptors. It is noteworthy that the MTII ED50s for both the hypothermic and hyperthermic responses are comparable and smaller than the 4 to 20 mg/kg doses typically used, e.g., (Balthasar et al., 2005; Chen et al., 2000b; Chen et al., 2009).
Our working model (Figure 7D) is that MTII causes hypometabolism/ hypothermia via dopamine neurons, possibly in the arcuate nucleus. The dopamine acts on D1 and D2 receptors to lower Tb. It is not known if MTII is acting directly on the dopaminergic neurons or on more upstream neurons. We focused on dopamine because MTII hypothermia is inhibited by dopamine antagonists, but not by other inhibitors of pharmacologic hypothermia (antagonists of mu, kappa, and delta opioid, 5HT1A, and cannabinoid-1 receptors). Dopamine regulates Tb in the preoptic area/anterior hypothalamus (Boulay et al., 1999; Brown et al., 1982; Cox and Lee, 1977). It is possible that the arcuate nucleus dopaminergic neurons that are still activated by MTII in Mc3r−/−;Mc4r−/− mice are part of the circuit between MTII-responding cells and the hypometabolism/hypothermia.
The physical site of MTII action causing hypometabolism/hypothermia is not known (Warne and Xu, 2013). Since hypermetabolism is a centrally-mediated effect, the similar ED50s for hyperthermia and hypothermia are consistent with hypometabolism/ hypothermia also being a central effect. In rabbits, melanocortin injection into the preoptic and septal regions (which regulate Tb (Nakamura, 2011)) inhibited fever (Feng et al., 1987; Glyn-Ballinger et al., 1983). However a more anatomically diverse regulation of MTII effects has been proposed since local MTII injections into the nucleus tractus solitarius, paraventricular nucleus, rostral ventrolateral medulla, parabrachial nucleus, and the retrochiasmatic area all increased Tb (Skibicka and Grill, 2009). Mc4r reactivation in paraventricular nucleus (PVN) neurons restores the food intake response to MTII, but not the metabolic rate effects (Balthasar et al., 2005). Metabolic rate and blood pressure effects of MC4R are mediated by cholinergic preganglionic neurons in the dorsal motor nucleus of the vagus (DMV) and intermediolateral column (IML) (Rossi et al., 2011; Sohn et al., 2013).
After its rapid onset, the hypothermic/hypometabolic state is self-limited, being largely reversed by ~1 hour and refractory to a second dose of MTII. These kinetics are not a simple function of MTII pharmacokinetics since the Tb changes are independent of MTII dose over a 4-fold range and thus likely independent of plasma MTII levels (MTII plasma elimination t½ is ~20 minutes (Hatziieremia et al., 2007)). The intact rewarming in Ucp1−/− mice demonstrates that the backup mechanisms invoked to maintain Tb in these mutant mice (Bal et al., 2012; Liu et al., 2003) are also sufficient for rewarming after MTII hypothermia. These data suggest that the hypothermic/hypometabolic response is a triggered process of limited duration that is followed by a refractory period; the hypermetabolism does not exhibit a refractory period.
What is the physiologic significance of the hypometabolism/hypothermia? All five of the melanocortin receptors have anti-inflammatory activities (Caruso et al., 2007; Catania et al., 2004; Catania et al., 2010; Getting et al., 2008; Lee and Taylor, 2013; Li and Taylor, 2008). It seems plausible that the hypometabolism/hypothermia/hypotension effects are part of a physiologic response to limit stress/inflammation and increase survival. Indeed, melanocortin agonism increases survival in a mouse model of multiple organ dysfunction (Bitto et al., 2011). In humans, hypothermia is endogenously generated in ominous clinical situations including severe trauma (Shafi et al., 2005) and sepsis (Clemmer et al., 1992). Hypothermia/ hypometabolism is also induced therapeutically to improve clinical outcomes, such as with hypoperfusion surgery (Lampe and Becker, 2011) and after hypoxic/hypoperfusion injury (Holzer, 2010). We hypothesize that hypometabolism/ hypothermia is a conserved, organized, and regulated physiologic response to dire situations, and not dysregulated thermal regulation. Just as fever increases the inflammatory response to fight infection (Mackowiak, 1998), hypothermia puts a brake on it, with beneficial effect in some situations.
Melanocortin hypermetabolism/hyperthermia is distinguished from the hypometabolism/ hypothermia by its timing, lack of attenuation, and selectivity for MC4R. The metabolism/obesity field has focused on the hypermetabolism/hyperthermia as this melanocortin physiology is relevant for understanding food intake, metabolic rate, body weight and adiposity, and insulin sensitivity. Melanocortin agonism is a potential treatment for obesity, but has been hampered by autonomic effects including increased blood pressure (Greenfield et al., 2009). Our observations suggest that there may be other effects of high doses of such drugs. The hypometabolic/ hypothermic and hypermetabolic/hyperthermic responses are distinct physiologic processes with distinguishable drivers and biologic effects. Further understanding of the hypometabolic/ hypothermic response could lead to better understanding of when to use clinical hypothermia, improved methods for inducing, maintaining, and reversing clinical hypothermia, and optimized treatment of accidental hypothermia.
Experimental Procedures
Mice
C57BL/6 male mice (Jackson Laboratories) were singly-housed at ~22 °C, fed NIH-07 chow (15 kcal % from fat, Harlan) or D12492 (60 kcal% from fat, Research Diets), and were studied between 14 and 37 weeks of age. Mice were maintained at 21–22 °C with lights on 6am–6pm. Food and water were available ad libitum including during drug treatments and indirect calorimetry. Mice were studied under approved protocols. Mc4r−/− mice (loxTB Mc4r; Mc4rtm1Lowl/J) (Balthasar et al., 2005), Mc3r−/− mice (loxTB Mc3r; Mc3rtmButl/J) (Begriche et al., 2011), and Mc3r−/−;Mc4r−/− mice were generated by heterozygote mating and studied on mixed genetic backgrounds using littermates as controls. Mc1re/e mice (81% C57BL/6J) (Robbins et al., 1993) were provided by Dr. David Fisher (MGH). Mc5r−/− mice (Chen et al., 1997) were supplied by Dr. Andrew Taylor (Boston University Medical Center, C57BL/6J background). Avy/+ mice (B6.C3-Avy/J) (Yen et al., 1994) and Ucp1−/− (B6.129-Ucp1tm1Kz/J) (Enerback et al., 1997) were purchased from Jackson Laboratories. Mice were studied ≥7 days after any operation or prior treatment. Reuse of mice tends to reduce physical activity levels, presumably due to acclimatization. No specific effort was made to acclimatize mice to handling in individual experiments.
Drugs
Drugs (Bachem for MTII; otherwise Tocris or Sigma) were administered at 10 to 11 am with the indicated dosing concentration, dose, route, and vehicle: MTII (up to 2 mg/ml ip, saline; dosed at 10 mg/kg ip when not otherwise specified), NDP-MSH (100 and 400 μg/mouse ip, saline), sulpiride (3 mg/ml, 30 mg/kg ip, 100% DMSO, heated to 60 °C, then 9 volumes of saline), SCH23390 (0.4 mg/ml, 2 mg/kg ip, saline), quinpirole (0.1 mg/ml, 1 mg/kg ip, water), AM251 (1 mg/ml, 10 mg/kg po, 100% DMSO, heated to 60 °C, then 1 volume Tween 80 and 8 volumes of saline), naloxone (1 mg/ml, 10 mg/kg ip, saline), naltrindole (0.5 mg/ml, 5 mg/kg ip, saline), and WAY100635 (0.1 mg/ml, 1 mg/kg sc, water).
Tb telemetry
Tb and activity were continuously measured by telemetry (Mini Mitter/Philips Respironics) using ER4000 energizer/receivers, G2 E-mitters implanted intraperitoneally, and VitalView software with data collected each minute. The hypothermia metric is the nadir Tb at 20–50 minutes after melanocortin dosing. The hyperthermia metric is the mean Tb at 120–300 minutes minus the mean Tb at −150 to −30 minutes relative to dosing. ED50s were calculated by fitting to a four parameter logistic curve using SigmaPlot v12.5. Brown adipose tissue (BAT) temperature and Tb were measured simultaneously in mice carrying two IPTT-300 transponders (Bio-Medic Data Systems), one sutured to the omentum and the other sutured underneath the interscapular BAT.
Blood pressure telemetry
Chronic ambulatory arterial blood pressure and heart rate were measured with radio transmitters (model TA11PA-C10; Data Sciences International, St. Paul, MN) implanted during ketamine and xylazine anesthesia in the carotid artery as described (Oppermann et al., 2009). Data were sampled for 10 s every 2 min and processed using a model RPC-1 receiver, a 20-channel data exchange matrix, APR-1 ambient pressure monitor, and a Data Quest ART Silver 2.3 acquisition system.
Temperature preference test
Mice were placed in a stainless steel pan (64 × 15 × 20 cm, length x width x height) spanning two hot/cold plates such that a temperature gradient from 18 °C to 36 °C was established across the floor of the pan. Mice were placed into this pan for 90 minutes and their position was tracked with an overhead camera and video tracking software (Ethovision 9.0, Noldus, Leesburg, VA). The temperature gradient was calibrated with a Flir T400 infrared camera, and these images were used to transform the position data into temperature.
Calorimetry
An OxyMax/CLAMS (Columbus Instruments) was used to measure Tb, energy expenditure (O2 consumption and CO2 production), and activity by beam break simultaneously in mice implanted with G2 E-mitters. RER, respiratory exchange ratio is the ratio of CO2 produced to O2 consumed. Experiments were performed at 22 °C or 30 °C, as indicated. Sampling was typically every thirteen minutes measuring from twelve chambers. When better time resolution was required, only six chambers were studied, allowing sampling every six minutes.
Immunohistochemistry
Male mice were housed individually from day −7 and during the four days preceding the experiment, were habituated to handling and ip injection. On the day of the experiment mice received MTII (10 mg/kg, ip) or vehicle, returned to their home cage, and sixty minutes later were anesthetized, had their rectal temperature measured, and were perfused with 10% formalin solution. Brains were fixed for 2 hours, transferred to 30% sucrose solution for two days, frozen, and sectioned (Leica SM2010R). A 1-in-3 series of coronal 40 μm sections was incubated (overnight, room temperature, constant agitation) with antibodies to TH (Millipore, MAB318; 1:1000 dilution) and Fos (Calbiochem, PC38; 1:7000 dilution). After rinsing, the sections were incubated in Alexafluor-488 anti mouse and Alexafluor-555 anti rabbit (1:500 dilution) for two hours, rinsed, and mounted using Prolong Gold antifade medium. Images were captured (Olympus VS120 Slide Scanner microscope) and analyzed with OlyVIA software (version 2.6) or with a Zeiss LSM 510 confocal microscope. A neuron was scored TH-immunoreactive only if its nucleus was visible and surrounded by a rim of TH immunofluorescence. Anatomy was defined according to (Franklin and Paxinos, 2008), with periventricular hypothalamic nucleus subdivisions per Allen Mouse Brain Atlas (http://mouse.brain-map.org/static/atlas). We did not use a stereological procedure or counting correction factor, since we are testing for changes between genotype and/or treatment. The percentage of MTII treatment-activated TH neurons is the number of Fos positive TH-immunoreactive neurons divided by the total number of TH-immunoreactive neurons.
Statistics
Data are reported as mean ±SEM. Significance (two-tailed p <0.05) was determined using SigmaPlot using t-test or 2-way ANOVA.
Supplementary Material
Highlights.
MTII causes a transient hypometabolism/hypothermia followed by hypermetabolism
The hypothermia is prevented by dopamine antagonists
The hypothermia is an organized response, not a failure of thermoregulation
Acknowledgments
We thank Jurgen Schnermann and David R. Sibley for insightful discussions and Anna Panyutin for technical assistance. This research was supported by the Intramural Research Program (DK075062, DK075063) and DK073189 of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), NIH.
Abbreviations
- Tb
core body temperature
- BAT
brown adipose tissue
- TEE
total energy expenditure
- RER
respiratory exchange ratio
- DIO
diet-induced obesity
- ED50
dose producing 50% of the maximal effect
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alharbi KK, Spanakis E, Tan K, Smith MJ, Aldahmesh MA, O’Dell SD, Sayer AA, Lawlor DA, Ebrahim S, Davey Smith G, et al. Prevalence and functionality of paucimorphic and private MC4R mutations in a large, unselected European British population, scanned by meltMADGE. Hum Mutat. 2007;28:294–302. doi: 10.1002/humu.20404. [DOI] [PubMed] [Google Scholar]
- Baker AK, Meert TF. Functional effects of systemically administered agonists and antagonists of mu, delta, and kappa opioid receptor subtypes on body temperature in mice. J Pharmacol Exp Ther. 2002;302:1253–1264. doi: 10.1124/jpet.102.037655. [DOI] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Begriche K, Levasseur PR, Zhang J, Rossi J, Skorupa D, Solt LA, Young B, Burris TP, Marks DL, Mynatt RL, et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane G-protein-coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J Biol Chem. 2011;286:40771–40781. doi: 10.1074/jbc.M111.278374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Polito F, Altavilla D, Irrera N, Giuliani D, Ottani A, Minutoli L, Spaccapelo L, Galantucci M, Lodi R, et al. Melanocortins protect against multiple organ dysfunction syndrome in mice. Br J Pharmacol. 2011;162:917–928. doi: 10.1111/j.1476-5381.2010.01098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Gisolfi CV, Mora F. Temperature regulation and dopaminergic systems in the brain: does the substantia nigra play a role? Brain Res. 1982;234:275–286. doi: 10.1016/0006-8993(82)90868-x. [DOI] [PubMed] [Google Scholar]
- Buck K, Lischka T, Dorow J, Crabbe J. Mapping quantitative trait loci that regulate sensitivity and tolerance to quinpirole, a dopamine mimetic selective for D(2)/D(3) receptors. Am J Med Genet. 2000;96:696–705. doi: 10.1002/1096-8628(20001009)96:5<696::aid-ajmg17>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- Carboni E, Longoni R, Deidda S, Di Chiara G. SCH 23390 antagonizes apomorphine- and ergot-induced hypothermia. Eur J Pharmacol. 1986;125:17–22. doi: 10.1016/0014-2999(86)90078-6. [DOI] [PubMed] [Google Scholar]
- Caruso C, Durand D, Schioth HB, Rey R, Seilicovich A, Lasaga M. Activation of melanocortin 4 receptors reduces the inflammatory response and prevents apoptosis induced by lipopolysaccharide and interferon-gamma in astrocytes. Endocrinology. 2007;148:4918–4926. doi: 10.1210/en.2007-0366. [DOI] [PubMed] [Google Scholar]
- Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- Catania A, Lipton JM. alpha-Melanocyte stimulating hormone in the modulation of host reactions. Endocr Rev. 1993;14:564–576. doi: 10.1210/edrv-14-5-564. [DOI] [PubMed] [Google Scholar]
- Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. Scientific World Journal. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000a;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000b;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O, Weinstein LS. Central nervous system imprinting of the G protein G(s)alpha and its role in metabolic regulation. Cell Metab. 2009;9:548–555. doi: 10.1016/j.cmet.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- Clemmer TP, Fisher CJ, Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1992;20:1395–1401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Conde-Frieboes K, Thogersen H, Lau JF, Sensfuss U, Hansen TK, Christensen L, Spetzler J, Olsen HB, Nilsson C, Raun K, et al. Identification and in vivo and in vitro characterization of long acting and melanocortin 4 receptor (MC4-R) selective alpha-melanocyte-stimulating hormone (alpha-MSH) analogues. J Med Chem. 2012;55:1969–1977. doi: 10.1021/jm201489a. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- Cox B, Lee TF. Do central dopamine receptors have a physiological role in thermoregulation? Br J Pharmacol. 1977;61:83–86. doi: 10.1111/j.1476-5381.1977.tb09742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Harkin A, Naughton M, Kelly JP, Leonard BE. Characterization of D-fenfluramine-induced hypothermia: evidence for multiple sites of action. Eur J Pharmacol. 2000;390:275–285. doi: 10.1016/s0014-2999(00)00012-1. [DOI] [PubMed] [Google Scholar]
- Dutia R, Kim AJ, Mosharov E, Savontaus E, Chua SC, Jr, Wardlaw SL. Regulation of prolactin in mice with altered hypothalamic melanocortin activity. Peptides. 2012;37:6–12. doi: 10.1016/j.peptides.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Feng JD, Dao T, Lipton JM. Effects of preoptic microinjections of alpha-MSH on fever and normal temperature control in rabbits. Brain Res Bull. 1987;18:473–477. doi: 10.1016/0361-9230(87)90111-0. [DOI] [PubMed] [Google Scholar]
- Feng N, Young SF, Aguilera G, Puricelli E, Adler-Wailes DC, Sebring NG, Yanovski JA. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54:2663–2667. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Sterotaxic Coordinates. New York, NY: Elsevier; 2008. [Google Scholar]
- Getting SJ, Riffo-Vasquez Y, Pitchford S, Kaneva M, Grieco P, Page CP, Perretti M, Spina D. A role for MC3R in modulating lung inflammation. Pulm Pharmacol Ther. 2008;21:866–873. doi: 10.1016/j.pupt.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Glyn-Ballinger JR, Bernardini GL, Lipton JM. alpha-MSH injected into the septal region reduces fever in rabbits. Peptides. 1983;4:199–203. doi: 10.1016/0196-9781(83)90114-6. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Temperature regulation in laboratory rodents. New York: Cambridge University Press; 1993. [Google Scholar]
- Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Thermal Biol. 2012;37:654–685. [Google Scholar]
- Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- Guo L, Ye Z, Ujjainwalla F, Sings HL, Sebhat IK, Huber J, Weinberg DH, Tang R, MacNeil T, Tamvakopoulos C, et al. Synthesis and SAR of potent and orally bioavailable tert-butylpyrrolidine archetype derived melanocortin subtype-4 receptor modulators. Bioorg Med Chem Lett. 2008;18:3242–3247. doi: 10.1016/j.bmcl.2008.04.049. [DOI] [PubMed] [Google Scholar]
- Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Nikiforovich G, Sharma SD, Yang YK, Dickinson C, Hruby VJ, Gantz I. Biological and conformational examination of stereochemical modifications using the template melanotropin peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp-Ala-Lys]-NH2, on human melanocortin receptors. J Med Chem. 1997;40:1738–1748. doi: 10.1021/jm960845e. [DOI] [PubMed] [Google Scholar]
- Hatziieremia S, Kostomitsopoulos N, Balafas V, Tamvakopoulos C. A liquid chromatographic/tandem mass spectroscopic method for quantification of the cyclic peptide melanotan-II. Plasma and brain tissue concentrations following administration in mice. Rapid Commun Mass Spectrom. 2007;21:2431–2438. doi: 10.1002/rcm.3106. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Rayner DV, Johnston SL, Speakman JR. Peripherally administered [Nle4,D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol. 2004;33:693–703. doi: 10.1677/jme.1.01632. [DOI] [PubMed] [Google Scholar]
- Holzer M. Targeted Temperature Management for Comatose Survivors of Cardiac Arrest. New Engl J Med. 2010;363:1256–1264. doi: 10.1056/NEJMct1002402. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Kang L, McIntyre KW, Gillooly KM, Yang Y, Haycock J, Roberts S, Khanna A, Herpin TF, Yu G, Wu X, et al. A selective small molecule agonist of the melanocortin-1 receptor inhibits lipopolysaccharide-induced cytokine accumulation and leukocyte infiltration in mice. J Leukoc Biol. 2006;80:897–904. doi: 10.1189/jlb.1204748. [DOI] [PubMed] [Google Scholar]
- Kievit P, Halem H, Marks DL, Dong JZ, Glavas MM, Sinnayah P, Pranger L, Cowley MA, Grove KL, Culler MD. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 2013;62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Becker LB. State of the art in therapeutic hypothermia. Annu Rev Med. 2011;62:79–93. doi: 10.1146/annurev-med-052009-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Taylor AW. Both MC5r and A2Ar Are Required for Protective Regulatory Immunity in the Spleen of Post-Experimental Autoimmune Uveitis in Mice. J Immunol. 2013 doi: 10.4049/jimmunol.1300182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1394–1398. doi: 10.1152/ajpregu.00306.2004. [DOI] [PubMed] [Google Scholar]
- Leoni G, Voisin MB, Carlson K, Getting S, Nourshargh S, Perretti M. The melanocortin MC(1) receptor agonist BMS-470539 inhibits leucocyte trafficking in the inflamed vasculature. Br J Pharmacol. 2010;160:171–180. doi: 10.1111/j.1476-5381.2010.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Taylor AW. Diminishment of alpha-MSH anti-inflammatory activity in MC1r siRNA-transfected RAW264.7 macrophages. J Leukoc Biol. 2008;84:191–198. doi: 10.1189/jlb.0707463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton JM, Glyn JR. Central administration of peptides alters thermoregulation in the rabbit. Peptides. 1980;1:15–18. doi: 10.1016/0196-9781(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Lipton JM, Glyn JR, Zimmer JA. ACTH and alpha-melanotropin in central temperature control. Fed Proc. 1981;40:2760–2764. [PubMed] [Google Scholar]
- Liu X, Rossmeisl M, McClaine J, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lookingland KJ, Moore KE. Handbook of Chemical Neuroanatomy. Elsevier; 2005. Functional neuroanatomy of hypothalamic dopaminergic neuroendocrine systems; pp. 435–523. [Google Scholar]
- Loos RJ, Lindgren CM, Li S, Wheeler E, Zhao JH, Prokopenko I, Inouye M, Freathy RM, Attwood AP, Beckmann JS, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40:768–775. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak PA. Concepts of fever. Arch Intern Med. 1998;158:1870–1881. doi: 10.1001/archinte.158.17.1870. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007;569:70–76. doi: 10.1016/j.ejphar.2007.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Gagen K, Raustad KA, Yang L, White A, Wang SP, Craw S, Liu P, Lanza T, Lin LS, et al. Body temperature as a mouse pharmacodynamic response to bombesin receptor subtype-3 agonists and other potential obesity treatments. Am J Physiol Endocrinol Metab. 2010;299:E816–824. doi: 10.1152/ajpendo.00404.2010. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, Wallace MR, Romberg RR, Bijl H, Sarton EY, Fillingim RB, et al. Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet. 2005;42:583–587. doi: 10.1136/jmg.2004.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MT, Richards DB, Lipton JM. Antipyretic potency of centrally administered alpha-melanocyte stimulating hormone. Science. 1983;221:192–193. doi: 10.1126/science.6602381. [DOI] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1207–1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Ni XP, Pearce D, Butler AA, Cone RD, Humphreys MH. Genetic disruption of gamma-melanocyte-stimulating hormone signaling leads to salt-sensitive hypertension in the mouse. J Clin Invest. 2003;111:1251–1258. doi: 10.1172/JCI16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–982. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- Nunes JL, Sharif NA, Michel AD, Whiting RL. Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem Res. 1991;16:1167–1174. doi: 10.1007/BF00966597. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Qin Y, Lai EY, Eisner C, Li L, Huang Y, Mizel D, Fryc J, Wilcox CS, Briggs J, et al. Enhanced tubuloglomerular feedback in mice with vascular overexpression of A1 adenosine receptors. Am J Physiol Renal Physiol. 2009;297:F1256–1264. doi: 10.1152/ajprenal.00264.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible LH, Knickerbocker D. Alpha-melanocyte-stimulating hormone (MSH) and [Nle4,D-Phe7]-alpha-MSH: effects on core temperature in rats. Pharmacol Biochem Behav. 1993;44:533–538. doi: 10.1016/0091-3057(93)90163-n. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Cowan A. Modulation of delta opioid-evoked hypothermia in rats by WAY 100635 and fluoxetine. Neurosci Lett. 2006;398:319–324. doi: 10.1016/j.neulet.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Renquist BJ, Lippert RN, Sebag JA, Ellacott KL, Cone RD. Physiological roles of the melanocortin MC(3) receptor. Eur J Pharmacol. 2011;660:13–20. doi: 10.1016/j.ejphar.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resch GE, Simpson CW. Effects of central alpha-MSH injections on performance in a cued discrimination task. Peptides. 1991;12:929–936. doi: 10.1016/0196-9781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi S, Elliott AC, Gentilello L. Is hypothermia simply a marker of shock and injury severity or an independent risk factor for mortality in trauma patients? Analysis of a large national trauma registry. J Trauma. 2005;59:1081–1085. doi: 10.1097/01.ta.0000188647.03665.fd. [DOI] [PubMed] [Google Scholar]
- Silva AA, Kuo JJ, Tallam LS, Liu J, Hall JE. Does obesity induce resistance to the long-term cardiovascular and metabolic actions of melanocortin 3/4 receptor activation? Hypertension. 2006;47:259–264. doi: 10.1161/01.HYP.0000198458.70351.e0. [DOI] [PubMed] [Google Scholar]
- Sinha PS, Schioth HB, Tatro JB. Activation of central melanocortin-4 receptor suppresses lipopolysaccharide-induced fever in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1595–1603. doi: 10.1152/ajpregu.00581.2002. [DOI] [PubMed] [Google Scholar]
- Sinha PS, Schioth HB, Tatro JB. Roles of the melanocortin-4 receptor in antipyretic and hyperthermic actions of centrally administered alpha-MSH. Brain Res. 2004;1001:150–158. doi: 10.1016/j.brainres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O’Rahilly S, et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes. 2008;57:2511–2518. doi: 10.2337/db08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, et al. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci U S A. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne JP, Xu AW. Metabolic transceivers: in tune with the central melanocortin system. Trends Endocrinol Metab. 2013;24:68–75. doi: 10.1016/j.tem.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse BE, Ogimoto K, Schwartz MW. Role of hypothalamic interleukin-1beta (IL-1beta) in regulation of energy homeostasis by melanocortins. Peptides. 2006;27:265–273. doi: 10.1016/j.peptides.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/− mice: ectopic expression of the agouti gene. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20:111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.