Abstract
Cerebral ischemia caused by loss of blood supply to the brain during cardiac arrest or stroke are major causes of death and disability. Biological sex is an important factor in predicting vulnerability of the brain to an ischemic insult, with males being at higher risk for cardio-cerebrovascular events than females of the same age. However, relative incidence of stroke between the genders appears to normalize at advanced ages. Therefore, many scientists have focused on the mechanisms of sex differences in outcome following brain ischemic injury, with a particular emphasis on the role of sex steroids. The majority of studies indicate that female sex steroids, such as estrogen and progesterone, play important roles in the relative neuroprotection following cerebral ischemia observed in females. However, less is known about male sex steroids and brain damage. This review describes the state of our knowledge of androgen-related contributions to neurological injury and recovery following cerebral ischemia that occurs following stroke. Experimental studies examining the effects of castration, androgenic agonists and antagonists and aging provide valuable insights into the role of androgens in clinical outcome following cerebrovascular events.
Introduction
Stroke is a leading cause of death and disability in the United States and clinical literature readily recognizes male gender as a significant risk factor. Experimental stroke models recapitulate the robust epidemiological gender differences in prevalence, demonstrating gender differences in tissue (histological) outcomes. The experimental stroke field has focused largely on the role of female sex steroids, such as the estrogens (for review see (McCullough & Hurn, 2003; Herson et al., 2009; Lebesgue et al., 2009; Liu et al., 2010). The topic of the current review is the role of androgens in shaping the male brain response to ischemic injury. Emerging experimental studies indicate that male sex steroids, or androgens (testosterone or dihydrotestosterone; DHT), can have both deleterious and protective effects on the central nervous system (CNS) following cerebrovascular insults such as stroke or cardiac arrest (Cheng et al., 2007; Uchida et al., 2009; Nakano et al., 2010; Cheng et al., 2011). Levels of circulating androgens change with normal development and aging and these changes may contribute to vulnerability of the male brain to an ischemic event. Correlation between testosterone levels and incidence of cerebrovascular disease has been reported, with low plasma testosterone in aging men being associated with increased risk for cardiovascular disease and stroke. In contrast, androgens in the young may contribute to injury, such that increased levels of androgens in young adults increases ischemic injury. This complex age-dependent and dose-dependent role of androgens in incidence and vulnerability of males to ischemic injury is discussed in the current review. The combination of clinical and basic science literature leads to the conclusion that while high levels of androgens enhance ischemic brain injury, the natural decline in physiological levels of androgens contributes to increased incidence and worsened outcome with aging. Therefore, it appears the most logical approach to take clinically would be to attempt to maintain men within the normal physiological range of androgens throughout the lifespan.
Androgens and stroke incidence
Ischemic stroke and cardio/cerebrovascular disease (CVD) are multifactor diseases that involve several predisposing risk factors that contribute to incidence and severity of injury. Male sex is a recognized risk factor for stroke, albeit non-modifiable. Indeed, several retrospective studies examining gender differences in stroke incidence have demonstrated that males are at a higher risk of stroke across most of the lifespan (Hollander et al., 2003; Rothwell et al., 2005; Reeves et al., 2008; Appelros et al., 2009). The relatively high levels of testosterone in young adult men may contribute to increased incidence of cerebrovascular disease, as shown by the South London Stroke register which shows males have a higher incidence of stroke in the young adult population (15–54y; 58.5%) (Wang et al., 2013). Interestingly, the incidence of stroke between males and females are similar in the >54y population (Wang et al., 2013). Similarly, the large North Manhattan Stroke Study observed that stroke rates in men and women equalized at older ages (Sacco, 1998; Reeves et al., 2008). The gender differences that are observed in young adulthood are generally attributed to the protective role of estrogens in female, which reduce dramatically following menopause (McCullough & Hurn, 2003). However, the relatively high levels of androgens in young adult males likely also contribute to increased vulnerability. Clinical evidence for the detrimental role of androgens in ischemic injury is difficult to obtain, however testosterone supplementation has been used for some purposes, such as the treatment of androgen deficiency in men or to improve performance in athletics. Testosterone levels in men may contribute to the relatively increased incidence of stroke via altering various known risk factors of stroke. Specifically, anabolic steroid use is associated with cardiovascular pathologies including increased vascular tone/arterial tension and promoting platelet aggregation (Littleton-Kearney & Hurn, 2004). There are several case reports observing stroke and sudden cardiac arrest in otherwise healthy young men with a history of anabolic steroid abuse (Fineschi et al., 2007; Youssef et al., 2011). However, anabolic steroid use and incidence/severity of stroke remains to be determined in large scale studies.
Aging and loss of androgens
The role of endogenous levels of androgens in determining incidence and severity of injury points to a protective role of androgens, such that acute and chronic (aging) decreases in circulating levels of androgens increase incidence of stroke and likely worsen outcome. There are several studies that indicate that lower testosterone levels in young and older males is associated with increased cardiovascular risk factors and stroke incidence (Muller et al., 2004; Yeap et al., 2009; Chock et al., 2012; Firtser et al., 2012). While the mechanism of increased CVD incidence remains unclear, evidence indicates that low testosterone in men is associated with increased blood glucose level and metabolic syndrome, which may predispose men to higher risk of CVD and mortality (Haffner et al., 1994; Kaplan et al., 2006; Kapoor & Jones, 2008; Kupelian et al., 2008). Similarly, acute androgen removal, such as occurs during androgen deprivation therapy (ADT) in prostate cancer patients increases risk of stroke. Azoulay L. et al. prospectively studied 22,310 prostate cancer patients for a mean of 3.9 years and demonstrated that the risk of stroke/TIA are increased with all types of ADTs, with the highest risk observed in patients who underwent bilateral orchiectomy (Azoulay et al., 2011). Moreover, ADT was associated with an increased incidence of diabetes and other cardiovascular-related risk factors, which may increase stroke risk and increase ischemic injury (Taylor et al., 2009; Bain, 2010). Thus, ADT provides a unique insight into the role of androgen removal on ischemic brain injury, indicating that removal of endogenous androgens appears to be detrimental. There is evidence that testosterone therapy is beneficial for overall quality of life in young men (<50 years) with low testosterone levels and has been shown to reduce mortality (Shores et al., 2012; Spitzer et al., 2013). However recent studies in old men or those with a history of cardiovascular disease which demonstrate increased mortality and stroke incidence in patients receiving testosterone therapy (Basaria et al., 2010). In summary of the adult clinical literature, androgens are capable of both detrimental and beneficial effects and that maintaining androgen levels within the young adult ‘normal’ physiological range is likely the optimal condition to minimize male risk for stroke. Future studies are needed to characterize the benefits and risks of testosterone therapy on cerebrovascular health in in the middle and old-aged populations and with comorbidities. The cellular and molecular mechanisms of these contradictory roles of androgens in ischemic injury are discussed further below.
Androgens and childhood stroke
Childhood stroke, while relatively rare, has devastating consequences on quality of life. The level of androgens varies throughout life, thus it is likely that androgen related effects on brain ischemia would vary with development and age. Incidence of ischemic stroke is higher in neonates and infants compared to older children of the same gender (Fullerton et al., 2003; Steinlin et al., 2005; Lo et al., 2009). In general, androgen levels are very low in pre-pubertal children and likely have little impact on stroke risk. Neonates and infant boys are the exception, however, as there are two postnatal surges in testosterone levels that occurs at 1 week and 4–6 months after birth (McIntyre, 2006; Hill, Threlkeld, et al., 2011; Hill & Fitch, 2012). Therefore, it is likely that androgen-related effects following brain ischemia is more influential to outcome during these periods. Consistent with this hypothesis, recent studies demonstrate increased incidence of stroke in boy neonates and infants (<4 years old) compared to girls of the same age (Fullerton et al., 2003; Golomb et al., 2003; 2009; Lo et al., 2009). During the pre-pubertal adolescent ages (4–15) no gender differences are observed (Lo et al., 2009) when females have a slightly higher level of circulating androgens (Courant et al., 2010). In contrast, the male predominance in stroke incidence is evident in children over 15, after the onset of puberty (Lo et al., 2009). These data implicate relative levels of androgens in enhancing the rate of stroke in boys, as the ages associated with increased levels of testosterone (infants and post-pubertal) are the ages associated with increased risk of stroke compared to girls. However, viewed in light of the adult literature which indicates that androgens in young adults are likely beneficial, it is equally possible that the post-pubertal effect is mediated by an increase in protective female steroids. Nonetheless, the enhanced vulnerability in neonates and infants (<4 years) is an important link between ischemic insult and androgens. Research scientists have focused on this enhanced risk of childhood stroke in neonates and children in their first years of life by taking advantage of animal models of neonatal stroke. Studies using neonatal animal models indicate that male neonates exhibit greater long-term deficits following injury than age matched females, including increased brain volume loss, enhanced dysmyelination and increased behavioral deficits following hypoxia and hypoxic-ischemic brain injury (Mayoral et al., 2009; Hill, Alexander, et al., 2011; Hill, Threlkeld, et al., 2011; Lan et al., 2011). Similar to the epidemiological data described above for 4–15 year old pre-pubertal children, a mouse model of pediatric stroke demonstrated equivalent injury in male and female pediatric mice following MCAO (Herson et al., 2013). These observations are consistent with a role for androgens in sensitizing the male brain to ischemic injury as neonates/infants, which is lost as androgen levels decline during early childhood.
Animal Models to assess the role of androgens in acute injury
While patient studies can provide some insight into androgens as risk factors for stroke and cardiovascular disease, there is very little known in the clinical population regarding what impact androgen levels have on infarct size and outcome in those patients that do have an ischemic brain injury. Animal models of cerebral ischemia have been used extensively to examine the role of sex steroids in gender differences in neurological injury. Animal models of cerebral ischemia reliably demonstrate that male animals suffer larger ischemic injury compared with age-matched females (Hall et al., 1991; Liu et al., 2009; Manwani et al., 2013). Extensive literature links estrogen to the relative protection observed in females (For review see (McCullough & Hurn, 2003; Herson et al., 2009; Lebesgue et al., 2009) and is the focus of an accompanying article in the issue. However, the effects of androgens in cerebral ischemia are controversial and remain understudied. Testosterone is the most abundant male sex steroid and generally the focus of clinical investigation. However, experimentally the use of testosterone is complicated by the fact that testosterone can be converted into estrogen by the enzyme aromatase (Roselli et al., 2009). To avoid this possible complication, experimental studies generally use the potent metabolite of testosterone, 5α-dihydrotestosterone (DHT) which binds with high affinity to the androgen receptor and is not able to be converted by aromatase to estrogen. The mechanistic studies described below have ruled out the possible confounding effects of aromatase by either using DHT experimentally or inhibiting aromatase. Thus, for the purpose of the current review we focus only on studies that have directly implicated androgen signaling in the effects reported. Multiple studies using the middle cerebral artery occlusion (MCAO) model of focal ischemic stroke have examined the effects of androgen removal and replacement in cerebral infarction. The majority of studies focused on the role of androgens in ischemic outcome demonstrate that castration of young males to remove endogenous androgens prior to MCAO reduced infarct size and androgen replacement increases infarct to levels similar to that observed in hormonally intact males (Yang et al., 2002; Cheng et al., 2007; 2009; Uchida et al., 2009; Vagnerova et al., 2010). These studies led to the conclusion that androgens enhance injury in male animals (Table 1). However, careful dose-response relations of androgen replacement prior to MCAO indicates that low doses of androgens (testosterone or DHT) further reduces infarct size compared to castration alone and that high doses exacerbate infarcts (Uchida et al., 2009). The dose-dependent effects on outcome, coupled with fluctuating androgen levels across the lifespan make it difficult to define a simple role for androgens in injury.
Table 1. Summary of testosterone manipulation in animal models.
Summary of effects of androgen manipulation in experimental stroke models.
| Species/Model | Age | Manipulation | Effect on outcome | Reference |
|---|---|---|---|---|
| Rat/MCAO | Adult (12 weeks) | CAST; CAST+T | Cast ↓infarct; CAST+T same as intact | Hawk, 1998 |
| Gerbil/unilateral carotid occlusion | Adult | n/a | Males incidence/injury > female | Hall, 1991 |
| Rat/MCAO | Adult (9–10weeks) | CAST+/− T or DHT | Cast ↓infarct; CAST+T same as intact; CAST+DHT↑ infarct | Cheng, 2007 |
| Rat/MCAO | Adult | CAST+/− T (T removed for depletion) | T depletion >6hrs protected | Yang, 2002 |
| Rat/MCAO | Adult (8–9 weeks) | CAST+/− T replacement 1 wk post-MCAO | T accelerated recovery | Pan, 2005 |
| Mouse/MCAO | Adult (9–10 weeks) | CAST+/− T or DHT | Low doses ↓ infarct; high doses ↑ infarct; both blocked by Flut | Uchida, 2009 |
| Young and aged rat and mouse/MCAO | 3-month-old Wistar rats and C57/BL6 mice, 14-month-old Wistar rats and 12-month-old C57/BL6 mice | CAST (in young) +/− T | T in young ↑ infarct; T in middle-aged ↓ infarct; both blocked by Flut | Cheng, 2009 |
| Mouse/MCAO | Adult (8–10 weeks) | CAST +/− T | PARP inhibitor protection lost in CAST; restored in CAST+T | Vagnerova, 2010 |
| Mouse/CA/CPR | Adult, 12 weeks | +/− T | Low and high doses ↑injury; intermediate had no effect | Nakano, 2010 |
| Rat/Mouse/MCAO | Adult male Wistar rats and C57/BL6 mice (12 weeks) | CAST+/− DHT | DHT protective; increases SIK1 expression | Cheng, 2011 |
| Mouse/MCAO | Adult (8–10 weeks) | CAST+/− DHT | DHT increased post-stroke immunosuppression | Dziennis, 2011 |
| Mouse/MCAO | Adult (9 to 10 weeks) | CAST+/− DHT | TRPM2 inhibitor protection lost in CAST; restored in +DHT | Shimizu, 2013 |
| Rat/Kainate | Adult (10–12 weeks) | CAST+/− DHT | CAST↑ injury; CAST+DHT same as intact | Ramsden, 2003 |
| Rat/MCAO | Adult (12 weeks) | CAST; Flut; letrozole | CAST and Flut↓ infarct; letrozole ↑ | Fanaei, 2013 |
Middle cerebral artery occlusion (MCAO); Cardiac arrest/cardiopulmonary resuscitation (CA/CPR); Castration/gonadectomy (CAST); Testosterone (T); Dihydrotestosterone (DHT)
Recent studies suggest that the age-dependent decline in circulating androgens is associated with worsened outcome in aging mice following MCAO (Liu et al., 2009) and androgen replacement is protective in middle aged mice (Cheng et al., 2009). Thus, experimental models of stroke demonstrate that androgens are capable of playing both a protective and damaging role in ischemic injury. Most importantly, data obtained from young adult male mice does not reliably model the human data; that is removal of androgens appears to be detrimental in people and beneficial in experimental animals. In contrast, data obtained using middle aged and aging animals more faithfully model the clinical situation. Therefore, it is strongly recommended that future studies aimed at unraveling the role of androgens in stroke outcomes take advantage of aging animals to provide more reliable pre-clinical data.
Mechanisms of androgen influence on ischemic injury
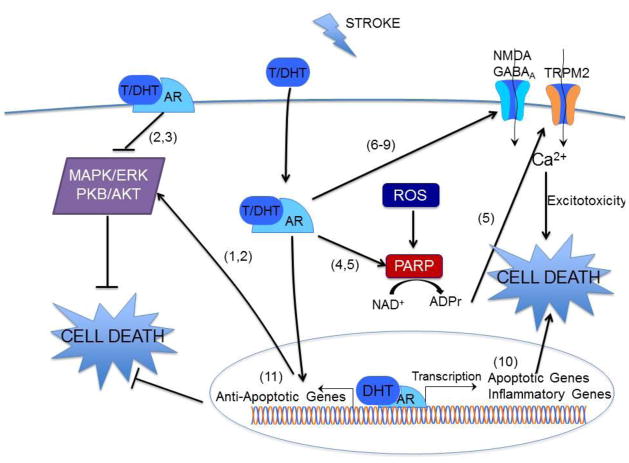
The deleterious and protective effects of testosterone are predominantly androgen receptor dependent (Cheng et al., 2007; Uchida et al., 2009; Nakano et al., 2010). Canonical androgen signaling involves androgen binding to cytoplasmic androgen receptors, translocation to the nucleus and transcription of androgen responsive genes (for review see (Bennett et al., 2010)). Recent evidence is emerging that androgens interact with rapid signaling pathways, although the presence of a membrane associated androgen receptor remains controversial. Nonetheless, extensive evidence exists demonstrating that androgens interact with a variety of signaling pathways involved in cell death and survival, thereby exhibiting both neurotoxic and neuroprotective effects (Figure 1). Several mechanisms have been identified by which androgens increase injury. Exogenous testosterone can increase glutamate-induced calcium influx, thereby increasing excitotoxic injury in cultured hippocampal, oligodendrocyte and glial cells (Caruso et al., 2004; Foradori et al., 2007; Holmes et al., 2013). Similarly, in immature neuronal cultures which use GABA as an excitatory neurochemical, testosterone increases GABA induced calcium influx and injury (Nuñez & McCarthy, 2008). Testosterone was also observed to induce pro-apoptotic p53 target genes (Bax, MDM2, p21) in cultured oligodendrocytes (Caruso et al., 2004). A number of androgen gene targets have been identified in mouse brain following MCAO that likely increase injury through increased inflammation, dysregulation of the blood brain barrier or altered cell signaling (Cheng et al., 2007). The use of BSA conjugated testosterone and DHT have revealed the possibility of a non-classical membrane-associated androgen receptor that decreases cell survival, causing a reduction in ERK and AKT phosphorylation (Nguyen et al., 2005; Gatson et al., 2006; Gatson & Singh, 2007).
Figure 1.
Model of mechanisms of androgen neurotoxicity and neuroprotection. ADPr, adenine dinucleotide ribose; AR, androgen receptor; GABAA receptor; DHT, 5α-dihydrotestosterone; MAPK/ERK, mitogen activated protein kinases; NAD+, nicotine adenine dinucleotide; NMDA receptor; PARP, poly(ADP ribose) polymerase; PKB/AKT, protein kinase B; ROS, reactive oxygen species; T, testosterone. Numbers in parentheses indicate source reference for each signaling pathway, (1) Nguyen et al. 2005 (2) Gatson et al., 2006 (3) Gatson et al., 2007 (4) Vagnerova et al., 2010 (5) Shimizu et al., 2013 (6) Caruso et al., 2004 (7) Foradori et al., 2007 (8) Holmes et al., 2013 (9) Nunez & McCarthy, 2008 (10) Cheng et al., 2007 (11) Nguyen et al., 2009
In contrast, several studies have demonstrated that androgens can be neuroprotective (Ahlbom et al., 2001; Nguyen et al., 2005; Pike et al., 2008). Interestingly, ERK and AKT appear to be differentially phosphorylated in a concentration and agonist-dependent manner, with low doses of androgens increasing phosphorylation and promoting cell survival (Nguyen et al., 2005; Gatson et al., 2006; Gatson & Singh, 2007). Other pathways which may contribute to the neuroprotective properties of androgens include increased antioxidant catalase activity, increased expression of salt induced kinase 1, and CREB activation (Ahlbom et al., 2001; Nguyen et al., 2009; Cheng et al., 2011). Figure 1 is a schematic illustrating the various signaling pathways observed to be modified by androgens in the context of injury. Similar to the observations made in experimental animal models of stroke, signaling studies demonstrate that androgens can exert opposing effects on cell survival. The relative balance of each of these signaling pathways is likely determined by the timing and dose of androgen exposure as well as the specific cell type and differential activation of intracellular versus membrane associated receptors.
In addition to the role of sex steroids in determining ischemic sensitivities, emerging data indicates that hormone-independent sex differences in cell death signaling exists. The majority of these data are obtained using primary cell cultures obtained from embryos grown in the absence of sex steroids and then exposed to various injurious stimuli. Several studies have converged to provide the overarching observation that male cell death after ischemia-reperfusion injury is mediated by oxidative stress, subsequent over-activation of poly(ADP)ribose polymerase (PARP) and activation of transient receptor potential M2 (TRPM2) cation channels (Figure 1), while female cell death is predominantly mediated by apoptosis involving caspase activation (petere et al., 2008). However, recent work has begun to consider the influence of androgens on the PARP-TRPM2 cell death pathway in adult animals. It appears that early development provides a sex-stratified signaling foundation such that male-specific ischemic injury is mediated by over-activation of PARP and TRPM2, however in adulthood the presence of androgens adds an additional level of complexity. Specifically, castration of adult mice reverses ischemia-induced activation of PARP (Vagnerova et al., 2010) and TRPM2 (Shimizu et al., 2013) and thus alters cell death mechanisms. Thus, sex-specific cell death pathways laid down early in development are modified during adulthood by the presence of circulating sex steroids.
Chronic androgen treatment and recovery
Clinical data demonstrates that serum testosterone levels are reduced in patients following stroke and reduced testosterone levels are associated with poor outcome in men (Dash et al., 1991; Jeppesen et al., 1996). Animal studies have primarily focused on the effects of pre-stroke levels of androgens on acute injury, with less attention paid to whether manipulating post-stroke levels of androgens will alter the rate of recovery and long-term outcomes. An exception to this is the work from Pan et al, which examined whether delayed testosterone replacement (7 days after MCAO) in castrated males altered functional recovery compared to androgen deficient males (Pan et al., 2005). This study observed that testosterone-treated males had accelerated recovery of motor function compared to testosterone deficient castrated controls. In addition, there are a few studies that point towards a role for delayed and chronic androgen replacement as a potential restorative therapy following stroke. For example, testosterone replacement after stroke has been associated with less reactive astrocyte hypertrophy in peri-infarct areas, a process that can prevent cortical remodeling (Moon et al., 2000; Chen et al., 2002; Tan et al., 2005). The critical role testosterone plays on neuronal viability during development and its impact on neurogenesis, axonal sprouting and plasticity make it likely that low testosterone following stroke would be detrimental to the repair process following injury. The role of androgens in post-ischemic neurogenesis was examined in intact, castrated and androgen treated mice and revealed that endogenous androgen receptor signaling did not alter post-ischemic neurogenesis, but that supraphysiological androgen levels suppress neurogenesis after stroke (Zhang et al., 2013). Effects of testosterone depletion and replacement on neuronal structure and function have been examined in rodents, although not in the context of brain injury. Therefore, consideration of the role of androgens in determining acute ischemic injury as well as chronic repair and plasticity should be considered.
In addition to plasticity and repair in the CNS following cerebral ischemia, alterations in immune function is a major contributor to morbidity and long-term recovery after stroke. Thus, it may be important to consider the impact of testosterone levels on immune function following brain injury, particularly following stroke. Clinical studies have shown that peripheral immunosuppression is a major consequence of stroke and can increase susceptibility of patients to a secondary infection, resulting in longer hospital stays and poorer prognosis of recovery. Indeed, fatal infection is the most common cause of death in patients who survive the acute phase of stroke (Meisel et al., 2005; Chamorro et al., 2007; Dirnagl et al., 2007). Animal studies have demonstrated post-stroke peripheral immunosuppression, characterized by splenic atrophy, reduced T cell activation and splenocyte proliferation (for review see (Offner et al., 2009) ). A recent study by Dziennis and co-workers observed that androgens exacerbated stroke-induced immunosuppression compared to castrated male mice (Dziennis et al., 2011). This observation is consistent with previous studies in peripheral disease models which indicate that androgens can have immunosuppressive effects (Olsen & Kovacs, 1996; Palaszynski et al., 2004). Similarly, it has been observed that testosterone depletion or androgen receptor antagonism prevents immunosuppression following shock or trauma (Angele et al., 2000). However, direct comparisons of intact versus castrated male mice following experimental stroke have not been reported. Nonetheless, these data clearly indicate the necessity of considering the physiological effects of androgens when designing future ischemic stroke studies.
Conclusion
The research and clinical community has been aware of the fact that stroke is a sexually dimorphic disease for several years, with extensive focus on the role of estrogens in providing females with relative protection. However, less attention has been given to the role of male sex steroids, androgens, in altering the male brain’s response to ischemia. Epidemiological data is emerging suggesting that levels of circulating testosterone in men is correlated with incidence of cardiovascular disease and stroke, with low testosterone levels associated with increased incidence and worse outcome. In contrast, experimental studies generally observe androgens contributing to increased injury. Importantly, the vast majority of experimental stroke studies utilize young adult animals. A few stroke studies using aging animals observed a potentially beneficial effect of androgens similar to the clinical observations. Thus, it is clear that future basic science studies would benefit from the use of aging animals and consider the effects of androgens both within and outside the central nervous system.
Acknowledgments
Project funded by NIH grant NS080851 and the Walter S. and Lucienne Driskill Foundation grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock (Augusta, Ga) 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–90. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Azoulay L, Yin H, Benayoun S, Renoux C, Boivin J, Suissa S. Androgen-deprivation therapy and the risk of stroke in patients with prostate cancer. European urology. 2011;60:1244–50. doi: 10.1016/j.eururo.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Bain J. Testosterone and the aging male: to treat or not to treat? Maturitas. 2010;66:16–22. doi: 10.1016/j.maturitas.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363:109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. The international journal of biochemistry & cell biology. 2010;42:813–27. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Caruso A, Di Gerevini VG, Castiglione M, Marinelli F, Tomassini V, Pozzilli C, Caricasole A, Bruno V, Caciagli F, Moretti A, Nicoletti F, Melchiorri D. Testosterone amplifies excitotoxic damage of cultured oligodendrocytes. J Neurochem. 2004;88:1179–85. doi: 10.1046/j.1471-4159.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Ughrin Y, Levine JM. Inhibition of axon growth by oligodendrocyte precursor cells. Molecular and cellular neurosciences. 2002;20:125–39. doi: 10.1006/mcne.2002.1102. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–62. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Age-dependent effects of testosterone in experimental stroke. J Cereb Blood Flow Metab. 2009;29:486–94. doi: 10.1038/jcbfm.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Uchida M, Zhang W, Grafe MR, Herson PS, Hurn PD. Role of salt-induced kinase 1 in androgen neuroprotection against cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:339–50. doi: 10.1038/jcbfm.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chock B, Lin T, Li C, Swislocki A. Plasma testosterone is associated with Framingham risk score. The aging male: the official journal of the International Society for the Study of the Aging Male. 2012;15:134–9. doi: 10.3109/13685538.2011.654369. [DOI] [PubMed] [Google Scholar]
- Courant F, Aksglaede L, Antignac J, Monteau F, Sorensen K, Andersson A, Skakkebaek NE, Juul A, Le Bizec B. Assessment of circulating sex steroid levels in prepubertal and pubertal boys and girls by a novel ultrasensitive gas chromatography-tandem mass spectrometry method. The Journal of clinical endocrinology and metabolism. 2010;95:82–92. doi: 10.1210/jc.2009-1140. [DOI] [PubMed] [Google Scholar]
- Dash RJ, Sethi BK, Nalini K, Singh S. Circulating testosterone in pure motor stroke. Functional neurology. 1991;6:29–34. [PubMed] [Google Scholar]
- Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, Prass K, Meisel A. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–3. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Akiyoshi K, Subramanian S, Offner H, Hurn PD. Role of dihydrotestosterone in post-stroke peripheral immunosuppression after cerebral ischemia. Brain, behavior, and immunity. 2011;25:685–95. doi: 10.1016/j.bbi.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineschi V, Riezzo I, Centini F, Silingardi E, Licata M, Beduschi G, Karch SB. Sudden cardiac death during anabolic steroid abuse: morphologic and toxicologic findings in two fatal cases of bodybuilders. International journal of legal medicine. 2007;121:48–53. doi: 10.1007/s00414-005-0055-9. [DOI] [PubMed] [Google Scholar]
- Firtser S, Juonala M, Magnussen CG, Jula A, Loo B, Marniemi J, Viikari JS, Toppari J, Perheentupa A, Hutri-Kähönen N, Raitakari OT. Relation of total and free testosterone and sex hormone-binding globulin with cardiovascular risk factors in men aged 24–45 years. The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2012;222:257–62. doi: 10.1016/j.atherosclerosis.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Werner SB, Sandau US, Clapp TR, Handa RJ. Activation of the androgen receptor alters the intracellular calcium response to glutamate in primary hippocampal neurons and modulates sarco/endoplasmic reticulum calcium ATPase 2 transcription. Neuroscience. 2007;149:155–64. doi: 10.1016/j.neuroscience.2007.06.054. [DOI] [PubMed] [Google Scholar]
- Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–94. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–64. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–34. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40:52–7. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- Golomb MR, deVeber GA, MacGregor DL, Domi T, Whyte H, Stephens D, Dick PT. Independent walking after neonatal arterial ischemic stroke and sinovenous thrombosis. Journal of child neurology. 2003;18:530–6. doi: 10.1177/08830738030180080901. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Valdez RA, Mykkänen L, Stern MP, Katz MS. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metabolism: clinical and experimental. 1994;43:599–603. doi: 10.1016/0026-0495(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–8. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Herson P, Koerner I, Hurn P. Sex, Sex Steroids, and Brain Injury. Semin Reprod Med. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ. Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling. Stroke. 2013;44:759–63. doi: 10.1161/STROKEAHA.112.675124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Fitch RH. Sex differences in mechanisms and outcome of neonatal hypoxia-ischemia in rodent models: implications for sex-specific neuroprotection in clinical neonatal practice. Neurology research international. 2012;2012:867531. doi: 10.1155/2012/867531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Alexander ML, McCullough LD, Fitch RH. Inhibition of X-linked inhibitor of apoptosis with embelin differentially affects male versus female behavioral outcome following neonatal hypoxia-ischemia in rats. Developmental neuroscience. 2011;33:494–504. doi: 10.1159/000331651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CA, Threlkeld SW, Fitch RH. Reprint of “Early testosterone modulated sex differences in behavioral outcome following neonatal hypoxia ischemia in rats”. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2011;29:621–8. doi: 10.1016/j.ijdevneu.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. Journal of neurology, neurosurgery, and psychiatry. 2003;74:317–21. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative Stress Defines the Neuroprotective or Neurotoxic Properties of Androgens in Immortalized Female Rat Dopaminergic Neuronal Cells. Endocrinology. 2013:1–12. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen LL, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arteriosclerosis, thrombosis, and vascular biology. 1996;16:749–54. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- Kaplan SA, Meehan AG, Shah A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? The Journal of urology. 2006;176:1524–7. doi: 10.1016/j.juro.2006.06.003. discussion 1527–8. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Jones TH. Androgen deficiency as a predictor of metabolic syndrome in aging men: an opportunity for intervention? Drugs & aging. 2008;25:357–69. doi: 10.2165/00002512-200825050-00001. [DOI] [PubMed] [Google Scholar]
- Kupelian V, Hayes FJ, Link CL, Rosen R, McKinlay JB. Inverse association of testosterone and the metabolic syndrome in men is consistent across race and ethnic groups. The Journal of clinical endocrinology and metabolism. 2008;93:3403–10. doi: 10.1210/jc.2008-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan WJ, Priestley M, Mayoral SR, Tian L, Shamloo M, Penn AA. Sex-specific cognitive deficits and regional brain volume loss in mice exposed to chronic, sublethal hypoxia. Pediatric research. 2011;70:15–20. doi: 10.1203/PDR.0b013e31821b98a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: Multiple cellular pathways of neuroprotection. Steroids. 2009;74:555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton-Kearney M, Hurn PD. Testosterone as a modulator of vascular behavior. Biological research for nursing. 2004;5:276–85. doi: 10.1177/1099800403262927. [DOI] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kelley MH, Herson PS, Hurn PD. Neuroprotection of sex steroids. Minerva endocrinologica. 2010;35:127–43. [PMC free article] [PubMed] [Google Scholar]
- Lo W, Stephens J, Fernandez S. Pediatric stroke in the United States and the impact of risk factors. Journal of child neurology. 2009;24:194–203. doi: 10.1177/0883073808322665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental neurology. 2013;249:120–31. doi: 10.1016/j.expneurol.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatric research. 2009;66:248–53. doi: 10.1203/PDR.0b013e3181b1bc34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends in endocrinology and metabolism: TEM. 2003;14:228–35. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reproductive biology and endocrinology: RB&E. 2006;4:10. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–86. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Moon LD, Brecknell JE, Franklin RJ, Dunnett SB, Fawcett JW. Robust regeneration of CNS axons through a track depleted of CNS glia. Experimental neurology. 2000;161:49–66. doi: 10.1006/exnr.1999.7230. [DOI] [PubMed] [Google Scholar]
- Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation. 2004;109:2074–9. doi: 10.1161/01.CIR.0000125854.51637.06. [DOI] [PubMed] [Google Scholar]
- Nakano T, Hurn PD, Herson PS, Traystman RJ. Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res. 2010;1357:124–130. doi: 10.1016/j.brainres.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639–51. doi: 10.1111/j.1471-4159.2005.03318.x. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Dihydrotestosterone activates CREB signaling in cultured hippocampal neurons. Brain Res. 2009;1298:1–12. doi: 10.1016/j.brainres.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Androgens predispose males to GABAA-mediated excitotoxicity in the developing hippocampus. Experimental neurology. 2008;210:699–708. doi: 10.1016/j.expneurol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocrine reviews. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Loo KK, Ashouri JF, Liu H, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Journal of neuroimmunology. 2004;146:144–52. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Hormones and behavior. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet neurology. 2008;7:915–26. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–17. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–83. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- Sacco RL. Identifying patient populations at high risk for stroke. Neurology. 1998;51:S27–30. doi: 10.1212/wnl.51.3_suppl_3.s27. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Macey T, Quillinan N, Klawitter J, Perraud A, Traystman R, Herson P. Androgen and PARP-1 regulation of TRPM2 channels after ischemic injury. J Cereb Blood Flow Metab. 2013:1–7. doi: 10.1038/jcbfm.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. The Journal of clinical endocrinology and metabolism. 2012;97:2050–8. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nature reviews Endocrinology. 2013;9:414–24. doi: 10.1038/nrendo.2013.73. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Pfister I, Pavlovic J, Everts R, Boltshauser E, Mori AC, Mercati DG, Hänggeli C, Keller E, Luetschg J, Marcoz J, Ramelli G, Perez ER, Schmitt-Mechelke T, Weissert M. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36:90–7. doi: 10.1055/s-2005-837658. [DOI] [PubMed] [Google Scholar]
- Tan AM, Zhang W, Levine JM. NG2: a component of the glial scar that inhibits axon growth. Journal of anatomy. 2005;207:717–25. doi: 10.1111/j.1469-7580.2005.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LG, Canfield SEL, du X. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–99. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–62. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, Herson PS. Poly (ADP-ribose) polymerase-1 initiated neuronal cell death pathway--do androgens matter? Neuroscience. 2010;166:476–81. doi: 10.1016/j.neuroscience.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rudd AG, Wolfe CD. Age and Ethnic Disparities in Incidence of Stroke Over Time: The South London Stroke Register. Stroke. 2013:1–10. doi: 10.1161/STROKEAHA.113.002604. [DOI] [PubMed] [Google Scholar]
- Yang S, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. Journal of applied physiology (Bethesda, Md: 1985) 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. The Journal of clinical endocrinology and metabolism. 2009;94:2353–9. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- Youssef MY, Alqallaf A, Abdella N. Anabolic androgenic steroid-induced cardiomyopathy, stroke and peripheral vascular disease. BMJ case reports. 2011 doi: 10.1136/bcr.12.2010.3650. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cheng J, Vagnerova K, Ivashkova Y, Young J, Cornea A, Grafe MR, Murphy SJ, Hurn PD, Brambrink AM. Effects of Androgens on Early Post-ischemic Neurogenesis in Mice. Transl Stroke Res. 2013 doi: 10.1007/s12975-013-0298-6. [DOI] [PubMed] [Google Scholar]
- petere, Lang JT, McCullough LD. Pathways to ischemic neuronal cell death: are sex differences relevant? Journal of Translational Medicine. 2008;6:33. doi: 10.1186/1479-5876-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]